Abstract
The stability of papillomavirus E2 proteins is regulated by proteasomal degradation, and regulation of degradation could contribute to the higher expression levels E2 proteins observed in suprabasal layers of differentiated skin. We have recently shown that the E2 proteins are modified by sumoylation [Wu Y-C, Roark AA, Bian X-L, Wilson, VG (2008) Virol 378:329–338], and that sumoylation levels are up-regulated during keratinocyte differentiation [Deyrieux AF, Rosas-Acosta G, Ozbun MA, Wilson VG (2007) J Cell Sci 120:125–136]. These observations, coupled with the known ability of sumoylation to prevent proteasomal degradation of certain proteins, suggested that this modification might contribute to stabilizing E2 proteins in suprabasal keratinocytes. Conditions that increased overall sumoylation were found to increase the intracellular amounts of the HPV11, 16, and 18 E2 proteins. No effect of sumoylation was seen on E2 transcripts, and the increased levels of E2 proteins resulted from a greatly increased half-life for the E2 proteins. In vitro studies confirmed that sumoylation could block the proteasomal degradation of the 16E2 protein. Interestingly, this stabilization effect was indirect as it did not require sumoylation of 16 E2 itself and must be acting through sumoylation of a cellular target(s). This sumoylation-dependent, indirect stabilization of E2 proteins is a novel process that may couple E2 levels to changes in the cellular environment. Specifically, our results suggest that the levels of papillomavirus E2 protein could be upregulated in differentiating keratinocytes in response to the increased overall sumoylation that accompanies differentiation.
Keywords: HPV, SUMO, Proteasome, Post-translational modification
Introduction
Papillomavirus E2 early proteins are key regulators of many viral and cellular processes, including viral replication (Chiang et al., 1992; Demeret et al., 1995; Stubenrauch, Colbert, and Laimins, 1998), genome segregation (McPhillips et al., 2006; You et al., 2004), and transcriptional control (Demeret et al., 1997; Kovelman et al., 1996). The critical role of E2 in viral transcription requires binding of E2 to the upstream regulatory region (URR) in a concentration-dependent manner that regulates early promoter expression. The regulation is positive when the E2 concentration is low, while early transcription is repressed when the E2 concentration is high (Demeret et al., 1997). Therefore, changes in E2 steady-state level during viral growth and persistence would be expected to modulate the viral transcription program.
The life cycle of papillomaviruses is closely linked to the epithelial differentiation scheme of the infected host keratinocyte (Longworth and Laimins, 2004), and E2 proteins are found to accumulate to higher levels in the suprabasal layers (Penrose and McBride, 2000; Stevenson et al., 2000). Increased transcription during differentiation likely contributes to at least part of the increase in E2 protein levels (Klumpp and Laimins, 1999; Ozbun and Meyers, 1998), though regulation at the level of protein stability may also be involved. The stability of E2 proteins is regulated through the proteasome degradation pathway (Bellanger et al., 2001; Penrose and McBride, 2000), and for the bovine papillomavirus (BPV) E2 protein, phosphorylation of E2 by casein kinase II signals degradation of E2 (Penrose et al., 2004). The critical phosphorylation event occurs within a PEST sequences in E2, and phosphorylation of this PEST sequence reduces its conformational stability which then targets E2 for proteasomal degradation (Garcia-Alai et al., 2006). While HPV E2 proteins also are proteasomally degraded, less is known about signals and regulation of this process. Recently, we demonstrated that HPV E2 proteins are modified by sumoylation, and that this modification affects transcriptional activity (Wu et al., 2008). Interesting, sumoylation has also been shown to affect protein stability both positively and negatively. For proteins such as IκBα (Desterro, Rodriguez, and Hay, 1998), Smad4 (Lin et al., 2003), and Huntingtin (Steffan et al., 2004), sumoylation stabilizes the protein through direct competition with ubiquitin for a common lysine residue. Alternatively, several proteins including APA-1 (Benanti et al., 2002), HIF-1α (Bae et al., 2004), c-Myb (Bies, Markus, and Wolff, 2002), and Ku70 (Yurchenko et al., 2008), are protected from degradation by sumoylation through poorly defined mechanisms that do not involve competition with ubiquitin for a common lysine residue. In contrast to these examples of stabilization, for many proteins sumoylation may promote proteasomal degradation by increasing ubiquitinylation through recruitment of SUMO-dependent ubiquitin ligases [reviewed in (Wilson and Heaton, 2008)]. As overall sumoylation is up-regulated during keratinocyte differentiation (Deyrieux et al., 2007), a possible link between E2 protein levels and sumoylation changes was examined in this study.
Sumoylation is a post-translational modification, prevalent among transcription factors, where the small ubiquitin-related modifier (SUMO) is attached to the substrate proteins in a reversible manner (Matunis, Coutavas, and Blobel, 1996). There are three principle SUMO proteins: SUMO1, SUMO2, and SUMO3. The process and enzymes involved in sumoylation are functionally similar to those enzymes catalyzing the attachment of ubiquitin to its substrates (Hay, 2005). In the present study, we demonstrate that increased in vivo sumoylation leads to elevated levels of papillomavirus E2 protein. The increased E2 levels were not due to transcriptional effects and did not require sumoylation of E2 itself, indicating an indirect effect. Using a reconstituted proteosomal degradation assay, sumoylation also blocked HPV16 E2 degradation in vitro. These results reveal a novel mechanism for sumoylation-dependent stabilization of E2 proteins that may tie E2 levels to host cell sumoylation changes during the cell cycle, in response to environmental signals, or during keratinocyte differentiation.
Results
Exogenous expression of the sumoylation components in vivo increases intracellular levels of HPV E2 proteins
We recently demonstrated the papillomavirus E2 proteins are sumoylated and showed that for HPV 16 E2 there is a single sumoylation site at lysine 292 (Wu et al., 2008). Those studies utilized proteasome inhibitors to stabilize E2 and allow detection of the low level of the sumoylated form of E2. However, during those studies we observed that in the absence of proteasome inhibitor the steady state level of 16E2 protein increased during co-expression with sumoylation components, and the basis for that observation is explored in this current study. Under the transfection conditions used, co-expression of SUMO with Ubc9 led to increased overall intracellular sumoylation (Fig. 1A) and specific sumoylation of known target proteins, as exemplified by C/EBP (Fig. 1B). Similarly, transfection of HPV16 E2 along with SUMO3 and Ubc9 resulted in sumoylation of E2 (Fig. 1C, lane 2), and also resulted in a large increase in the amount of unmodified E2 compared to transfection of E2 without SUMO and Ubc9 (Fig. 1C, lane 1), suggesting that E2 expression is affected by sumoylation levels. To further evaluate the effect of Ubc9 and various SUMO types, combination transfections experiments were performed (Fig. 2A). With the transfection and immunoblot exposure conditions used throughout this study, the sumoylated E2 band was quite faint so only the unmodified E2 form is shown in this and subsequent figures. Likewise, the short-lived unmodified E2 protein expressed without SUMO and Ubc9 was just barely detectable under these conditions (see first lane in each panel of. 2 A–C). In contrast, co-expression of SUMOs 1, 2, or 3 with Ubc9 significantly increased the level of HPV16 E2 compared to HPV16 E2 expressed alone (Fig. 2A, left panel). In addition, transfection of any of the SUMOs alone or of Ubc9 alone can also increase overall intracellular sumoylation, though to a lesser extent than the combination of SUMO + Ubc9 (unpublished observations). Consequently, we tested the effect of the individual SUMOs alone and Ubc9 alone on E2 levels. Each of the SUMOs was sufficient to enhance E2 levels, and there was no difference in the E2 levels observed with any of the 3 SUMO types (Fig. 2A, right panel). Likewise, Ubc9 alone also increased E2 levels (Fig. 2A, lower panel).
Fig. 1.
Expression of exogenous SUMO1 and Ubc9 enhances overall sumoylation as well as sumoylation of specific targets. (A) HeLa cells were transfected with 3 μg of pcDNA5 (lane 1) or were co-transfected with 1.5 μg of pcDNA5/FRT/TO/SUMO1 and 1.5 μg of Ubc9 (lane 2). Two day after transfection, total protein extracts were harvested and then analyzed by immunoblotting with rabbit anti-SUMO1 serum. Introduction of SUMO1 and Ubc9 led to a large increase in sumoylated products. Molecular weight marker positions are indicated on the right. (B) The transfections in part (A) were repeated with the additional transfection of 2:g pcDNA3.1-C/EBPβ to each sample. The immunoblot was analyzed with rabbit anti-C/EBPβ (Santa Cruz; 1:10,000 dilution). (C) As in (B) except that 3:g of pWEB-16E2 DNA was used rather than the pcDNA3.1-C/EBPβ DNA. Immunoblotting for E2 was as described in Materials and Methods.
Fig. 2.
Sumoylation up-regulates the protein levels of papillomavirus E2. (A) SUMO1, SUMO2, and SUMO3 are able to increase the protein expression of HPV16E2. Left panel: HeLa cells were transfected with 3 μg of pWEB-16E2 alone (lane 1) or in combination with 1.5 μg of pcDNA5/FRT/TO/HA-Ubc9 and 1.5 ug of either pcDNA5/FRT/TO/His-S-SUMO1, pcDNA3.1-SUMO2, or pcDNA3.1-SUMO3 as indicated; Upper right panel: HeLa cells were transfected with 3 μg of pWEB-16E2 alone (lane 1) or together with 3 μg of either pcDNA5/FRT/TO/His-S-SUMO1, pcDNA3.1-SUMO2, or pcDNA3.1-SUMO3 as indicated. Bottom right panel: HeLa cells were transfected with 3 μg of pWEB-16E2 alone (lane 1) or together with 3 μg of pcDNA5/FRT/TO/HA-Ubc9. (B) Functional SUMO and Ubc9 are necessary for the stabilization of E2. Left panel: HeLa cells were transfected with 5 μg of pWEB-16E2 alone or together with 1 μg of either pCS2-GFP-SUMO1WT or pCS2-GFP-SUMO1ΔGG as shown. The position of molecular weight markers is indicated on left side of the panel. Right panel: HeLa cells were transfected with 3 μg of pWEB-16E2 together with 1.5 μg of pcDNA3.1-SUMO3 alone, or with 1.5 μg of pcDNA3.1-SUMO3 and 1.5 μg of either pcDNA5/FRT/TO/HA-Ubc9 or pcDNA5/FRT/TO/HA-Ubc9(C93S) [labeledUbc9* in the figure] as indicated. (C) Sumoylation increased the expression of HPV11E2 and HPV18E2. HeLa cells were transfected as indicated using 3 μg of pSG5/HA-11E2, 3 μg pSG5/HA-18E2, 1.5 μg of pcDNA5/FRT/TO/HA-Ubc9, and 1.5 μg of pcDNA5/FRT/TO/His-S-SUMO1. For (A), (B), and (C), carrier pcDNA plasmid DNA was used to equalize the total DNA amount in all samples. All cells were collected two days after transfection, and samples were analyzed by immunoblotting with the indicated antibodies.
The results in Fig. 2A could reflect an influence of enzymatic sumoylation on E2 protein or some non-enzymatic effect mediated through mere expression of the SUMOs or Ubc9 protein. To address whether or not the effect on E2 levels requires active sumoylation, two mutants, SUMO1ΔGG and Ubc9(C93S), were tested. SUMO1ΔGG has a deletion of the C-terminal diglycine motif and is incapable of being conjugated to substrates (Johnson, 2004) (Fig. 2B, left lower panel), while Ubc9(C93S) is a catalytically inactive form of Ubc9 (Poukka et al., 1999). Neither of these mutations abrogates interaction between the mutant proteins and sumoylation substrates (Ahn et al., 2001; Chen et al., 2006; Tomoiu et al., 2006), and both of these mutant proteins were expressed at levels comparable to their wild-type forms (Fig. 2B). However, in contrast to their wild-type counterparts, neither SUMO1ΔGG nor Ubc9(C93S) were able to increase the level of HPV16 E2 (Fig. 2B), consistent with the effect on E2 requiring active sumoylation and not simply expression of the sumoylation components.
Since the majority of our studies have been with HPV16 E2, it was important to determine if this effect on E2 levels was restricted to 16E2 (clade A9) or was a more general phenomenon affecting other HPV type E2 proteins. Consequently, we examined two other mucosal HPV types, the high risk 18E2 (clade A7) and the low risk 11E2 (clade A10). As shown in Fig. 2C, levels of both HPV 11E2 and 18E2 were also increased in the presence of SUMO1 and Ubc9. These positive results for 3 different clades suggest a general role for sumoylation in modulating HPV E2 expression levels, at least for mucosal alpha-HPV types. Whether or not the effect of sumoylation extends to all alpha types, or to cutaneous HPVs, remains to be determined.
Sumoylation increases E2 levels post-transcriptionally
Next, the mechanism by which sumoylation increased the level of E2 proteins was investigated. To examine transcriptional effects, semi-quantitative RT-PCR experiments were performed. E2 transcript levels were evaluated after 20, 25, 30, and 35 cycles of amplification. Fig. 3A shows the results at 25 cycles, and the level of HPV16 E2 mRNA was unchanged in the presence of the SUMO components. No E2 RNA signal was detectable at 20 cycles, and the signal intensity for all samples increased proportionally at both 30 and 35 cycles indicating that 25 cycles was not saturating (not shown). These results are consistent with the conclusion that sumoylation was not affecting E2 transcription. Alternatively, E2 proteins are known to be relatively unstable, so the effect of proteasome inhibition was examined. The level of HPV16 E2 rescued from degradation by a proteasome inhibitor, ALLN, was similar to that observed with SUMO plus Ubc9 in the absence of ALLN (Fig. 3B). Additionally, combined treatment of ALLN with SUMO+Ubc9 did not further increase E2 levels above ALLN alone, consistent with each of these treatments acting through a similar pathway; identical results were obtained with MG132 (not shown). These combined results suggest that sumoylation may protect E2 from the proteasome degradation pathway.
Fig. 3.
The stabilization of 16E2 by sumoylation is post-transcriptional. (A) The RNA level of 16E2 does not respond to increased sumoylation. HeLa cells were transfected with 3 μg of 16E2 alone, or together with 1.5 μg of the SUMO plasmids and 1.5 μg of either Ubc9 or Ubc9* (the Ubc9 [C93S] mutant) as indicated. Two days after transfection, total RNA were extracted and subjected to RT-PCR with either 16E2 or β-actinin primers. (B) Protein degradation inhibitors mimic the stabilization effect of SUMO expression. HeLa cells were transfected with 3 μg of 16E2 alone or together with 1.5 μg of SUMO3 and 1.5 μg of Ubc9 as indicated. One day after transfection, two of the four cell cultures were treated with 40 μg/ml of ALLN. At 24 h after the ALLN treatment, cells were collected, extracts prepared, and the proteins resolved by SDS-PAGE. Western blotting was performed with the indicated antibodies. For (A) and (B), pcDNA plasmid DNA was used in these experiments to equalize the total DNA amount of transfected DNA in each sample.
The stabilization of E2 by sumoylation is indirect
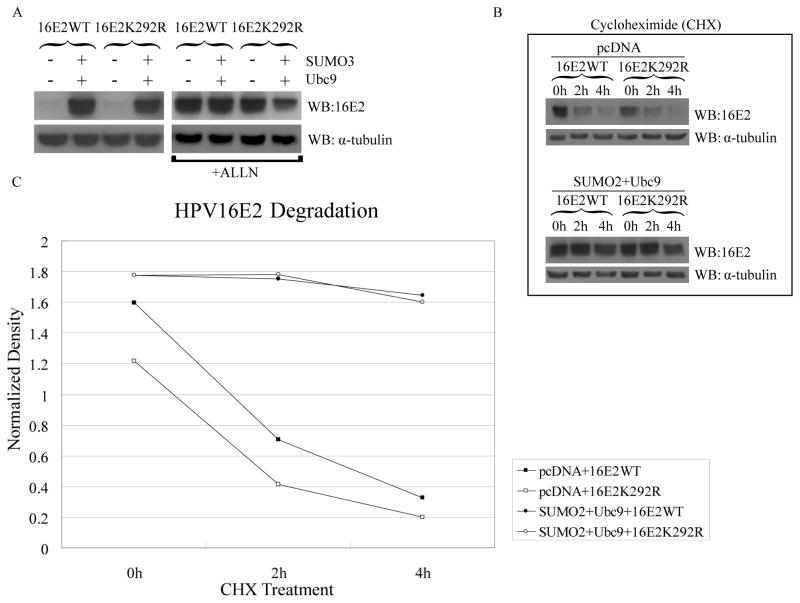
One mechanism by which sumoylation could prevent proteosomal degradation of 16E2 is via competition for the lysine residue targeted for ubiquitinylation. While the lysine(s) targeted for ubiquitinylation of 16E2 is unknown, HPV16 E2 has a single, predominant sumoylation site at lysine 292 (Wu et al., 2008), and increased sumoylation levels might enhance sumoylation of this residue, blocking its usage as an ubiquitinylation site. To address this question, we examined the effect of increased sumoylation on wild-type 16E2 versus the E2 K292R mutant. In the absence of increased sumoylation, the levels of wild-type and K292R E2 were similarly low (Fig. 4A, left panel). Surprisingly, co-transfection of SUMO and Ubc9 increased the levels of the K292R protein comparable to wild-type E2 (Fig. 4A, left panel) even though we were unable to detect sumoylation of this mutant protein (Wu et al., 2008). Additionally, treatment with ALLN (Fig. 4A, right panel) or MG132 (not shown) also increased the wild-type and mutant E2 proteins to similar levels, indicating that the K292R mutant was still susceptible to proteosomal degradation and that lysine 292 was not involved in ubiquitinylation of E2 under normal conditions. We conclude from these results that the elevated levels of E2 are an indirect result of increased cellular sumoylation rather than the result of direct sumoylation of E2 itself.
Fig. 4.
SUMO modification of 16E2 is not required for stabilization. (A) Sumoylation had similar stabilizing effects on both 16E2WT and it sumoylation-defective mutant, 16E2K292R. HeLa cells were co-transfected with 3 μg of pWEB-16E2WT or pWEB-16E2K292R, together with 3 μg of pcDNA or with a combination of 1.5 μg of pcDNA5/FRT/TO/HA-Ubc9 and 1.5 μg of pcDNA3.1-SUMO3 as indicated. One day after transfection, half the samples (right panel) were treated with 40 μg/ml of ALLN and the other half were untreated. Total cell extracts were collected after another 24 h and were processed for immunoblotting as in Fig. 2. (B) HeLa cells were transfected with 3 μg of pWEB-16E2WT or pWEB-16E2K292R as shown and were also co-transfected with 3 μg of pcDNA (upper panel) or with a combination of 1.5 μg of pcDNA5/FRT/TO/HA-Ubc9 and 1.5 μg of pcDNA3.1-SUMO2 (bottom panel). Two days after transfection, cells were treated with 200 μg/ml of cycloheximide (CHX), and total cell extracts were collected at the indicated times. Samples were analyzed by immunoblotting with anti-16E2 or anti-α-tubulin antibodies. The immunoblot for E2 in the upper panel was exposed for a longer time than those in the previous figures and in the lower panel in order to allow visible detection of E2 in the absence of SUMO plus Ubc9; absolute levels of E2 at time 0 in the upper panel are actually much less than in the lower panel. (C) The samples shown in part (B) were quantitated by densitometry and the 16E2 amounts were normalized to α-tubulin.
To further explore the effect of sumoylation on E2 protein, we also performed in vitro degradation studies to compare the stabilities of wild-type and K292R mutant 16 E2 proteins. After transfection, cells were treated with cycloheximide to stop further protein synthesis, and the amount of E2 was determined over time (Figs. 4B and 4C). Both wild-type and the K292R mutant 16E2 degraded rapidly at similar rates and had half-lives of less than 2 hours. In contrast, co-expression of SUMO plus Ubc9 rendered both E2 proteins quite stable with half-lives well in excess of 4 hours. Thus, sumoylation appears to increase E2 levels by protecting E2 from proteosomal degradation via a mechanism that does not require direct sumoylation of E2 at lysine 292. This does not appear to be a nonspecific, general response to increased sumoylation as no significant effect on protein levels was observed for two other viral proteins, E6 (Supplemental Fig. 1) and E1 (Rangasamy et al., 2000), and for two cellular proteins, C/EBP (see Fig. 1B) and p14ARF (Supplemental Fig. 1), tested under similar conditions.
Sumoylation blocks the proteasomal degradation of HPV16 E2 in vitro
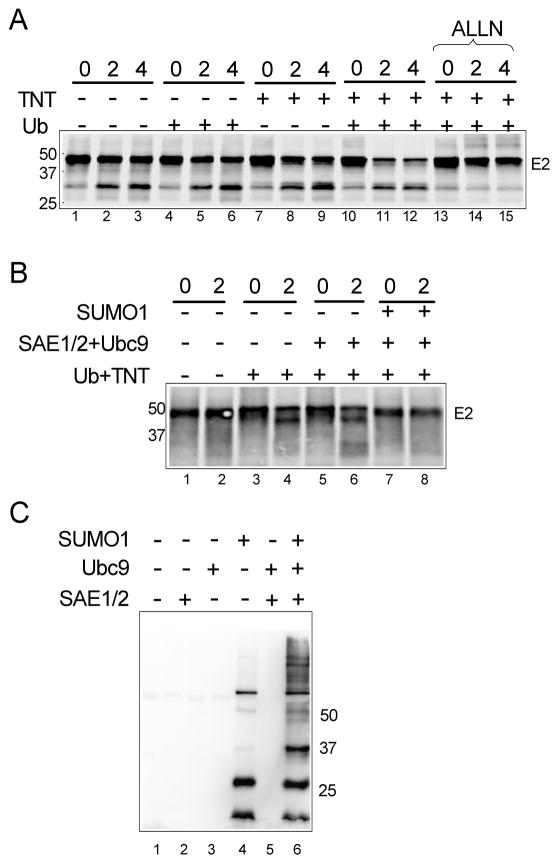
The in vivo observations suggested that sumoylation stabilized E2 by preventing proteasomal degradation. To confirm this conclusion, we utilized an in vitro ubiquitin and proteasome-dependent degradation assay. When in vitro translated E2 was subsequently incubated for an additional 4 hrs in the translation reaction, E2 was relatively stable, with the majority of the sample remaining as full-length E2 (Fig. 5A, lanes 1–3). Addition of fresh TNT cell lysate (providing proteasomes and ubiquitinylation enzymes) reduced the amount of full-length E2 somewhat (lanes 7–9) as did addition of exogenous ubiquitin alone (lanes 4–6). However, addition of both fresh TNT and exogenous ubiquitin lead to significant decrease in full-length E2 within 2 hrs (lanes 10–12, and this decrease was blocked by proteasome inhibitors (ALLN, lanes 13–15 and MG132, not shown), consistent with the loss of full-length E2 resulting from ubiquitin-dependent proteasomal degradation. As seen in vivo, addition of the complete sumoylation reaction components (Sae2/1+Ubc9+SUMO1) to the in vitro system prevented this degradation of HPV16 E2 (Fig. 5B, lanes 7 and 8). No protection of E2 was observed when only the sumoylation enzymes without SUMO were added (lanes 5 and 6), consistent with a requirement for actual sumoylation of some target and not simply the presence of the enzymatic components. Note that under these reaction conditions the sumoylation of E2 is undetectable (not shown), while overall sumoylation of cellular components is efficiently enhanced only when the complete set of sumoylation enzymes plus SUMO1 are added (Fig. 5C). These data strongly support the conclusion that sumoylation’s role in stabilizing papillomavirus E2 proteins is mediated through the ubiquitin-proteasome pathway via targeting a factor(s) other than E2 itself. The availability of the sumoylation-inhibited in vitro degradation system should allow identification of the relevant factor(s) and elucidation of the mechanism for this effect.
Fig. 5.
The degradation of HPV16E2 was inhibited by sumoylation. (A) The in vitro ubiquitination assay of 16E2 was performed as described in Materials and methods. In vitro translated 16E2 was incubated at 30°C for 0, 2, or 4 hr with buffer alone (lanes 1–3) or the indicated components (lanes 4–15). After incubation, each reaction was terminated with SDS-sample buffer, the samples were resolved by 10% SDS-PAGE, and then transferred to PVDF membrane for visualization with a phosphorimager. Molecular weight marker positions are indicated on the left side of the figure. (B) The reaction and analysis was performed as in part (A) except that the samples were incubated for 0 or 2 h prior to termination and analysis as indicated. Addition of the Ub+TNT and purified sumoylation components is as indicated. The position of molecular weight markers is shown on left side of the figure. Samples in this experiment were resolved on an 8% SDS-PAGE gel and run longer to resolve the E2 cleavage species that runs just below full-length E2. (C) The in vitro degradation reaction samples were analyzed by immunoblotting with anti-SUMO1. All samples contained Ub, TNT, and E2 comparable to lanes 7 and 8 in panel B. Samples were incubated for 2 hr at 30°C prior to termination and immunoblot analysis.
Elevation of endogenous sumoylation stabilizes HPV16 E2
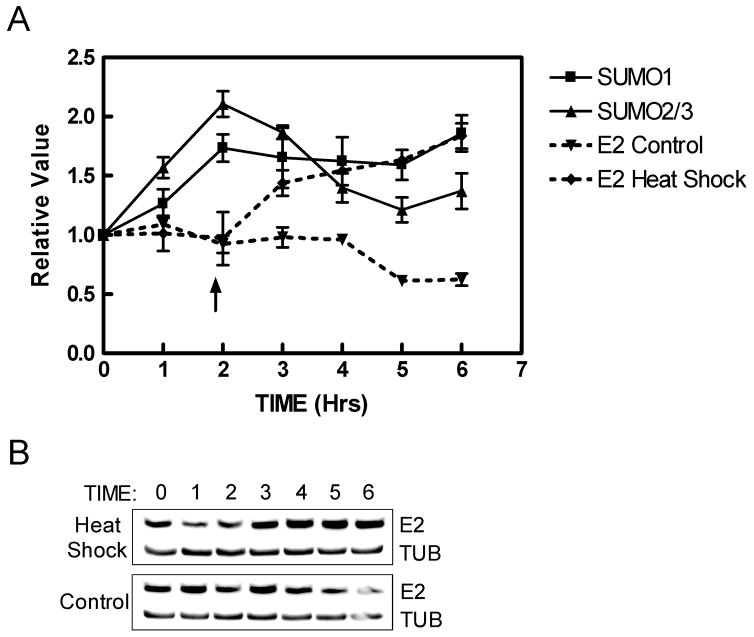
The results in the previous sections were observed with exogenous expression of the sumoylation system both in vivo and in vitro. However, it would be more biologically relevant to demonstrate that E2 could be stabilized by endogenous sumoylation under physiological conditions. It has been reported that heat shock stress can rapidly increase the level of SUMO conjugates in cultured mammalian cells (Mao, Desai, and Liu, 2000; Saitoh and Hinchey, 2000), and we used this approach to evaluate the effect of a physiologic sumoylation increase on E2 protein. Two days after transfection of HPV16 E2, C33A cells were incubated briefly at 42°C to increase endogenous sumoylation. Total cell extracts were collected after various time points and then analyzed by immunoblotting with antibodies against E2, SUMO1, and SUMO2/3. Quantitation of the blots indicated that overall endogenous sumoylation with both SUMO1 and SUMO2/3 increased, reaching a maximum of approximately 2-fold over basal levels by two hours after heat treatment and then slowly diminished (Fig. 6A). As the sumoylation levels reached their maximum, the amount of HPV16 E2 in the shocked cells began to increase while E2 levels in the unshocked control sample remained unchanged (Fig. 6A and B). By six hours post-heat shock, E2 levels had increased 2-fold while in the control cells E2 levels had declined slightly. These results are consistent with modest physiological increases in endogenous sumoylation levels being sufficient to stabilize the HPV16 E2 protein.
Fig. 6.
HPV16 E2 is stabilized by elevated endogenous sumoylation. (A) C33A cells were transfected with pWEB-16E2 and then heat-shocked by incubation at 42°C for 1 hour at two days after transfection or left continuously at the normal temperature of 37°C. Protein samples were collected at 1-hour time intervals and then analyzed by immunoblotting with anti-16E2 or SUMO-specific antibodies. The E2 samples were quantified by densitometry and the 16E2 amounts were normalized to α-tubulin. The zero time point E2 values for the heat-shocked (◆) and unshocked (▼) samples were set as 1.0, and the subsequent E2 amounts are relative to the zero time point values. A similar analysis was performed using anti-SUMO1 (■) and anti-SUMO2/3 (▲) antibodies to measure total sumoylation by SUMO1 and SUMO2/3 in the heat-shocked samples. The data shown are the average of three experiments. The arrow indicates the time when sumoylation has reached maximum levels. (B) A representative immunoblot for E2 and tubulin for heat shocked (upper panel) and non-shocked (lower panel) cultures.
Discussion
Both ubiquitination and sumoylation are dynamic post-translational modification processes regulated by functionally equivalent classes of enzymes. Ubiquitination of BPV and HPV18 E2 proteins causes their rapid turnover in transfected and infected cells (Bellanger et al., 2001; Penrose and McBride, 2000), however, the effect of sumoylation on papillomavirus E2 protein stability had not been examined. In this work, we identified a novel effect of sumoylation on papillomavirus E2 proteins and found that augmented extrinsic or intrinsic sumoylation indirectly stabilized E2 proteins; a similar observation of indirect stabilization by sumoylation was recently reported for the Ku70 protein (Yurchenko et al., 2008). Together with our previous observation that overall sumoylation levels are increased during keratinocyte differentiation (Deyrieux et al., 2007), we propose that sumoylation contributes to the reported higher levels of E2 in the upper layers of differentiated epithelium. Furthermore, in addition to differentiation-dependent changes in sumoylation, sumoylation levels are influenced by a variety of cellular stresses (Agbor and Taylor, 2008; Gill, 2004), raising the intriguing possibility that E2 levels in infected skin could be affected by such things as trauma, UV exposure, hypoxia, or hormonal changes. Regulating E2 stability by overall sumoylation levels could provide a means to couple viral replication and transcription to environmental conditions that affect the cellular state. Whether or not such effects contribute to HPV persistence, disease manifestation, or malignant progression with high-risk viruses remains to be tested.
SUMOs have been shown to conjugate covalently to a variety of proteins that have significant roles in gene expression, chromatin structure, signal transduction, and genome maintenance, and can influence the activity and intracellular localization of these proteins (Gill, 2004). In addition, sumoylation can also modulate the turnover of certain substrates, and in some cases increases the stability of target proteins by direct modification of these substrates. However, for E2 proteins we observed that the K292R mutant, which is not detectably sumoylated, was efficiently stabilized in vivo, indicating that sumoylation was acting indirectly and not through direct modification of E2. The stabilizing effect of sumoylation could be recapitulated in an in vitro degradation system under conditions where wild-type E2 was not detectably sumoylated. Since E2 itself does not need to be sumoylated either in vivo or in vitro, this indicates that sumoylation must be acting on some other cellular substrate(s) that influences E2 stability, possibly members of the ubiquitin pathway itself. The ubiquitinylation machinery consists of two activating enzymes, approximately thirty ubiquitin conjugating enzymes, numerous ubiquitin ligases, and a variety of deubiquitinylating enzymes; the specific components involved in E2 degradation have not been defined. Any of the components involved in the ubiquitinylation pathway could be targets for sumoylation, and their modification by SUMO could impair their ability to utilize papillomavirus E2 proteins as substrates for ubiquitinylation, leading to increased E2 stability. Consistent with this possibility, sumoylation of both a ubiquitin conjugating enzyme (Pichler et al., 2005) and a ubiquitin ligase (Xirodimas et al., 2002) has already been reported, and there is growing evidence of extensive cross-talk between the ubiquitin and SUMO systems (Wilson and Heaton, 2008). Conversely, sumoylation could be targeting a deubiquitinylating enzyme causing enhanced activity towards ubiquitinylated E2 and leading to increased removal of ubiquitin with subsequent stabilization of E2.
An alternative to targeting the ubiquitin pathway directly would be for sumoylation to affect another modification of E2 that is necessary for ubiquitinylation. While relatively little is known about phosphorylation of HPV E2 proteins, it is clear that BPV E2 degradation is triggered by phosphorylation via casein kinase II (Penrose et al., 2004). Therefore, another possible mechanism for sumoylation to prevent HPV E2 protein degradation would be to modify and inactivate a requisite kinase (or conversely activate an appropriate phosphatase), and thus reduce a critical degradation signal. Interestingly, pilot experiments have shown that casein kinase II can be sumoylated in vivo (Wu and Wilson, unpublished observations), which is consistent with the possibility that SUMO addition could regulate the enzyme activity of casein kinase II and possibly other kinases. Lastly, E2 has many cellular binding partners, including Brd4 (McPhillips et al., 2006; You et al., 2004), p53 (Massimi et al., 1999), TopBP1 (Donaldson, Boner, and Morgan, 2007), and many transcriptional factors such as YY1 (Lee, Broker, and Chow, 1998) and C/EBP (Hadaschik et al., 2003). Sumoylation of any of the E2 partners could influence complex formation and lead to reduced E2 accessibility to the proteasomal machinery. Both of this mechanism and the phosphoregulatory model would of course require that the appropriate factors were present in the in vitro degradation reactions. Given the complexity of the TNT mixture, this is certainly a possibility, though it remains untested.
In summary, there are multiple potential targets through which sumoylation could prevent E2 degradation, both within the ubiquitin pathway and external to it. Regardless of the target(s), it is clear from the work presented here that changes in sumoylation levels can dramatically impact the stability of E2 proteins. Much additional work will be required to resolve the mechanism of action and to determine if this is a general mechanism that can regulate stability of proteins other than E2.
Materials and methods
Plasmids
Plasmids utilized in this study were generously provided by the following: pSG5/HA-11E2, pSG5/HA-16E2 and pSG5/HA-18E2 by Dr. S.M. Huang; pWEB-16E2 by Dr. K. Gaston; pcDNA3.1/HA-SUMO2, pcDNA3.1/HA-SUMO3, and the GST-SAE1/SAE2 expression plasmid by Dr. Ronald T. Hay; pCS2-GFP-SUMO1WT and pCS2-GFP-SUMO1ΔGG by Dr. Ryan Potts; and pcDNA3.1-C/EBPβ by Dr. Linda Sealy. The pRSET-SUMO1 plasmid (Rosas-Acosta et al., 2005a), and the pcDNA5/FRT/TO/His-S-SUMO1, pcDNA5/FRT/TO/HA-Ubc9 and pcDNA5/FRT/TO/HA-Ubc9(C93S) plasmids have all been described previously (Rosas-Acosta et al., 2005b).
Cell culture, transfection and reagents
HeLa cells were maintained and grown in Dulbecco MEM (DMEM) supplemented with 10% Fetal Bovine Serum (FBS) and 45 mM of sodium bicarbonate. C33A cells were maintained and grown in DMEM supplemented with 10% FBS and 18 mM of sodium bicarbonate. Both cell lines were maintained at 37°C and 5% CO2 in a humidified incubator. For transfections, 90% confluent cells in 6-well plates were transfected using a total of 6 μg of DNA and 6 μl of Lipofectamine 2000 (Invitrogen) according to the supplier’s recommendations. Two days after transfection, the HeLa or C33A cells were collected by addition of 500 μl of SDS-sample buffer (150 mM Tris-HCl, pH 6.8, 12% SDS, 30% glycerol). These samples were passed through a 26 gauge syringe and denatured at 95°C for 7 min. Then, 30 μl of each sample were resolved by SDS-PAGE followed by immunoblot analysis as previously described (Wu et al., 2008). Cycloheximide (Sigma) and the calpain protease inhibitor, ALLN (Calbiochem), were used at the concentrations indicated in the figure legends.
Immunoblotting
Primary antibodies used in this manuscript were: mouse anti-α-tubulin (1:5000, Santa Cruz), mouse anti-HPV16 E2 (1:1000, Santa Cruz), rabbit anti-SUMO1 and anti-Ubc9 serum [1:1000, (Deyrieux et al., 2007)], mouse anti-HA (1:10,000, Santa Cruz), mouse anti-GFP (1:5000, Santa Cruz), and rabbit anti-SUMO3 (1:200, Zymed). Samples were analyzed by SDS-PAGE and were immunoblotted as previously described (Wu et al., 2008).
RT-PCR
Total RNAs were extracted using the RNAqueous kit (Ambion) and were stored at −80°C until use. The Superscript III RT-PCR was performed with a mixture containing 50 ng of RNA in a final reaction volume of 25 μl according to the manufacturer’s instructions (Invitrogen). RT-PCR was performed for 30 minutes at 55°C for the reverse transcription step, followed by 2 minutes at 94°C, and 20, 25, 30, or 35 cycles of amplification (94°C for 15 seconds; 60°C for 30 seconds; 68°C for 20 seconds). Amplified products were analyzed on 1% agarose gels and visualized with an Innotech Alphaimager system (Alpha Innotech).
In vitro degradation assay
The HPV16 E2 protein was expressed from the pSG5/HA-16E2 plasmid using the T7-coupled rabbit reticulocyte lysatesystem in the presence of [35S] methionine according to the manufacturer’s instructions (Promega). All sumoylation components were expressed and purified as described previously (Rosas-Acosta et al., 2005a). For the degradation assay, 2 μl of 35S-labeled protein was mixed with or without 10 μg of ubiquitin (Sigma), and 4 μl of reticulocyte lysate (TNT, Promega). Where indicted in the figures, some samples also received 2 μg of SAE2/SAE1, 1 μg of Ubc9, and 5 μg of SUMO1. All the reactions were performed for the indicated times at 30°C in a final buffer volume of 25 μl containing 25 mM Tris, pH 7.5, 60 mM NaCl, 8 mM MgCl2, 4 mM ATP, and 1 mM DTT. Ten μl of SDS-sample buffer were added to each sample to stop the reaction. For the degradation assay, mixtures were prepared as for the ubiquitinylation assay and then divided into two halves. One of half was mixed immediately with 12.5 μl of SDS-sample buffer, and the other half was further incubated at 30°C for another 2 hours before addition of the SDS-sample buffer. All samples were incubated at 95°C for 7 min and then were analyzed by SDS-PAGE. Radiolabeled bands were visualized by phosphorimaging with a Storm 860 (General Electric).
Heat shock experiment
C33A cells grown at 37°C were transferred to 42°C, incubated for 1 hour at this elevated temperature, and then returned to the 37°C incubator. Total cell extracts were collected with 500 μl of SDS-sample buffer every hour for six hours, and the samples were analyzed by immunoblotting for total sumoylation and for E2 protein. Relative quantities of E2 and total sumoylation were obtained by densitometry of the X-ray films using α-tubulin as the internal standard. For the total sumoylation quantification, the signal for each entire lane was summed using the Alpha Innotech 1D-Multi software.
Supplementary Material
Acknowledgments
This work was supported by grant CA89298 from the NIH. We thank Dr. Adeline Deyrieux for the experiments shown in Supplemental Figure 1.
References
- Agbor TA, Taylor CT. SUMO, hypoxia and the regulation of metabolism. Biochem Soc Trans. 2008;36(3):445–448. doi: 10.1042/BST0360445. [DOI] [PubMed] [Google Scholar]
- Ahn JH, Xu Y, Jang WJ, Matunis MJ, Hayward GS. Evaluation of interactions of human cytomegalovirus immediate-early IE2 regulatory protein with small ubiquitin-like modifiers and their conjugation enzyme Ubc9. J Virol. 2001;75(8):3859–3872. doi: 10.1128/JVI.75.8.3859-3872.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae SH, Jeong JW, Park JA, Kim SH, Bae MK, Choi SJ, Kim KW. Sumoylation increases HIF-1alpha stability and its transcriptional activity. Biochem Biophys Res Commun. 2004;324(1):394–400. doi: 10.1016/j.bbrc.2004.09.068. [DOI] [PubMed] [Google Scholar]
- Bellanger S, Demeret C, Goyat S, Thierry F. Stability of the human papillomavirus type 18 E2 protein is regulated by a proteasome degradation pathway through its amino-terminal transactivation domain. J Virol. 2001;75(16):7244–7251. doi: 10.1128/JVI.75.16.7244-7251.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benanti JA, Williams DK, Robinson KL, Ozer HL, Galloway DA. Induction of extracellular matrix-remodeling genes by the senescence-associated protein APA-1. Mol Cell Biol. 2002;22(21):7385–7397. doi: 10.1128/MCB.22.21.7385-7397.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bies J, Markus J, Wolff L. Covalent attachment of the SUMO-1 protein to the negative regulatory domain of the c-Myb transcription factor modifies its stability and transactivation capacity. J Biol Chem. 2002;277(11):8999–9009. doi: 10.1074/jbc.M110453200. [DOI] [PubMed] [Google Scholar]
- Chen A, Wang PY, Yang YC, Huang YH, Yeh JJ, Chou YH, Cheng JT, Hong YR, Li SS. SUMO regulates the cytoplasmonuclear transport of its target protein Daxx. J Cell Biochem. 2006;98(4):895–911. doi: 10.1002/jcb.20703. [DOI] [PubMed] [Google Scholar]
- Chiang CM, Ustav M, Stenlund A, Ho TF, Broker TR, Chow LT. Viral E1 and E2 proteins support replication of homologous and heterologous papillomaviral origins. Proc Natl Acad Sci USA. 1992;89(13):5799–5803. doi: 10.1073/pnas.89.13.5799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demeret C, Desaintes C, Yaniv M, Thierry F. Different mechanisms contribute to the E2-mediated transcriptional repression of human papillomavirus type 18 viral oncogenes. J Virol. 1997;71(12):9343–9349. doi: 10.1128/jvi.71.12.9343-9349.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demeret C, Le Moal M, Yaniv M, Thierry F. Control of HPV 18 DNA replication by cellular and viral transcription factors. Nucleic Acids Res. 1995;23(23):4777–4784. doi: 10.1093/nar/23.23.4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desterro JM, Rodriguez MS, Hay RT. SUMO-1 modification of IkappaBalpha inhibits NF-kappaB activation. Mol Cell. 1998;2(2):233–239. doi: 10.1016/s1097-2765(00)80133-1. [DOI] [PubMed] [Google Scholar]
- Deyrieux AF, Rosas-Acosta G, Ozbun MA, Wilson VG. Sumoylation dynamics during keratinocyte differentiation. J Cell Sci. 2007;120(1):125–136. doi: 10.1242/jcs.03317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson MM, Boner W, Morgan IM. TopBP1 regulates human papillomavirus type 16 E2 interaction with chromatin. J Virol. 2007;81(8):4338–4342. doi: 10.1128/JVI.02353-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Alai MM, Gallo M, Salame M, Wetzler DE, McBride AA, Paci M, Cicero DO, de Prat-Gay G. Molecular basis for phosphorylation-dependent, PEST-mediated protein turnover. Structure. 2006;14(2):309–319. doi: 10.1016/j.str.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Gill G. SUMO and ubiquitin in the nucleus: different functions, similar mechanisms? Genes Dev. 2004;18(17):2046–2059. doi: 10.1101/gad.1214604. [DOI] [PubMed] [Google Scholar]
- Hadaschik D, Hinterkeuser K, Oldak M, Pfister HJ, Smola-Hess S. The Papillomavirus E2 protein binds to and synergizes with C/EBP factors involved in keratinocyte differentiation. J Virol. 2003;77(9):5253–5265. doi: 10.1128/JVI.77.9.5253-5265.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay RT. SUMO: a history of modification. Mol Cell. 2005;18(1):1–12. doi: 10.1016/j.molcel.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Johnson ES. Protein modification by SUMO. Annu Rev Biochem. 2004;73:355–382. doi: 10.1146/annurev.biochem.73.011303.074118. [DOI] [PubMed] [Google Scholar]
- Klumpp DJ, Laimins LA. Differentiation-induced changes in promoter usage for transcripts encoding the human papillomavirus type 31 replication protein E1. Virology. 1999;257(1):239–246. doi: 10.1006/viro.1999.9636. [DOI] [PubMed] [Google Scholar]
- Kovelman R, Bilter GK, Glezer E, Tsou AY, Barbosa MS. Enhanced transcriptional activation by E2 proteins from the oncogenic human papillomaviruses. J Virol. 1996;70(11):7549–7560. doi: 10.1128/jvi.70.11.7549-7560.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KY, Broker TR, Chow LT. Transcription factor YY1 represses cell-free replication from human papillomavirus origins. J Virol. 1998;72(6):4911–4917. doi: 10.1128/jvi.72.6.4911-4917.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Liang M, Liang YY, Brunicardi FC, Feng XH. SUMO-1/Ubc9 promotes nuclear accumulation and metabolic stability of tumor suppressor Smad4. J Biol Chem. 2003;278(33):31043–31048. doi: 10.1074/jbc.C300112200. [DOI] [PubMed] [Google Scholar]
- Longworth MS, Laimins LA. Pathogenesis of human papillomaviruses in differentiating epithelia. Microbiol Mol Biol Rev. 2004;68(2):362–372. doi: 10.1128/MMBR.68.2.362-372.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Y, Desai SD, Liu LF. SUMO-1 conjugation to human DNA topoisomerase II isozymes. J Biol Chem. 2000;275(34):26066–26073. doi: 10.1074/jbc.M001831200. [DOI] [PubMed] [Google Scholar]
- Massimi P, Pim D, Bertoli C, Bouvard V, Banks L. Interaction between the HPV-16 E2 transcriptional activator and p53. Oncogene. 1999;18(54):7748–7754. doi: 10.1038/sj.onc.1203208. [DOI] [PubMed] [Google Scholar]
- Matunis MJ, Coutavas E, Blobel G. A novel ubiquitin-like modification modulates the partitioning of the Ran-GTPase-activating protein RanGAP1 between the cytosol and the nuclear pore complex. J Cell Biol. 1996;135(6 Pt 1):1457–1470. doi: 10.1083/jcb.135.6.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPhillips MG, Oliveira JG, Spindler JE, Mitra R, McBride AA. Brd4 is required for e2-mediated transcriptional activation but not genome partitioning of all papillomaviruses. J Virol. 2006;80(19):9530–9543. doi: 10.1128/JVI.01105-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozbun MA, Meyers C. Human papillomavirus type 31b E1 and E2 transcript expression correlates with vegetative viral genome amplification. Virology. 1998;248(2):218–230. doi: 10.1006/viro.1998.9285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penrose KJ, Garcia-Alai M, de Prat-Gay G, McBride AA. Casein Kinase II phosphorylation-induced conformational switch triggers degradation of the papillomavirus E2 protein. J Biol Chem. 2004;279(21):22430–22439. doi: 10.1074/jbc.M314340200. [DOI] [PubMed] [Google Scholar]
- Penrose KJ, McBride AA. Proteasome-mediated degradation of the papillomavirus E2-TA protein is regulated by phosphorylation and can modulate viral genome copy number. J Virol. 2000;74(13):6031–6038. doi: 10.1128/jvi.74.13.6031-6038.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichler A, Knipscheer P, Oberhofer E, van Dijk WJ, Korner R, Olsen JV, Jentsch S, Melchior F, Sixma TK. SUMO modification of the ubiquitin-conjugating enzyme E2–25K. Nat Struct Mol Biol. 2005;12(3):264–269. doi: 10.1038/nsmb903. [DOI] [PubMed] [Google Scholar]
- Poukka H, Aarnisalo P, Karvonen U, Palvimo JJ, Janne OA. Ubc9 interacts with the androgen receptor and activates receptor-dependent transcription. J Biol Chem. 1999;274(27):19441–19446. doi: 10.1074/jbc.274.27.19441. [DOI] [PubMed] [Google Scholar]
- Rangasamy D, Woytek K, Khan SA, Wilson VG. SUMO-1 modification of bovine papillomavirus E1 protein is required for intranuclear accumulation. J Biol Chem. 2000;275(48):37999–38004. doi: 10.1074/jbc.M007777200. [DOI] [PubMed] [Google Scholar]
- Rosas-Acosta G, Langereis MA, Deyrieux A, Wilson VG. Proteins of the PIAS family enhance the sumoylation of the papillomavirus E1 protein. Virology. 2005a;331(1):190–203. doi: 10.1016/j.virol.2004.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas-Acosta G, Russell WK, Deyrieux A, Russell DH, Wilson VG. A universal strategy for proteomic studies of SUMO and other ubiquitin-like modifiers. Mol Cell Proteomics. 2005b;4(1):56–72. doi: 10.1074/mcp.M400149-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh H, Hinchey J. Functional heterogeneity of small ubiquitin-related protein modifiers SUMO-1 versus SUMO-2/3. J Biol Chem. 2000;275(9):6252–6258. doi: 10.1074/jbc.275.9.6252. [DOI] [PubMed] [Google Scholar]
- Steffan JS, Agrawal N, Pallos J, Rockabrand E, Trotman LC, Slepko N, Illes K, Lukacsovich T, Zhu YZ, Cattaneo E, Pandolfi PP, Thompson LM, Marsh JL. SUMO modification of Huntingtin and Huntington’s disease pathology. Science. 2004;304(5667):100–104. doi: 10.1126/science.1092194. [DOI] [PubMed] [Google Scholar]
- Stevenson M, Hudson LC, Burns JE, Stewart RL, Wells M, Maitland NJ. Inverse relationship between the expression of the human papillomavirus type 16 transcription factor E2 and virus DNA copy number during the progression of cervical intraepithelial neoplasia. J Gen Virol. 2000;81(7):1825–1832. doi: 10.1099/0022-1317-81-7-1825. [DOI] [PubMed] [Google Scholar]
- Stubenrauch F, Colbert AM, Laimins LA. Transactivation by the E2 protein of oncogenic human papillomavirus type 31 is not essential for early and late viral functions. J Virol. 1998;72(10):8115–8123. doi: 10.1128/jvi.72.10.8115-8123.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomoiu A, Gravel A, Tanguay RM, Flamand L. Functional interaction between human herpesvirus 6 immediate-early 2 protein and ubiquitin-conjugating enzyme 9 in the absence of sumoylation. J Virol. 2006;80(20):10218–10228. doi: 10.1128/JVI.00375-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson VG, Heaton PR. Ubiquitin proteolytic system: focus on SUMO. Expert Rev Proteomics. 2008;5(1):121–135. doi: 10.1586/14789450.5.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YC, Roarke AA, Bian XL, Wilson VG. Modification of Papillomavirus E2 proteins by the Small Ubiquitin-like Modifier Family Members (SUMOs) Virology. 2008;378:329–338. doi: 10.1016/j.virol.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xirodimas DP, Chisholm J, Desterro JM, Lane DP, Hay RT. P14ARF promotes accumulation of SUMO-1 conjugated (H)Mdm2. FEBS Lett. 2002;528(1–3):207–211. doi: 10.1016/s0014-5793(02)03310-0. [DOI] [PubMed] [Google Scholar]
- You J, Croyle JL, Nishimura A, Ozato K, Howley PM. Interaction of the bovine papillomavirus E2 protein with Brd4 tethers the viral DNA to host mitotic chromosomes. Cell. 2004;117(3):349–360. doi: 10.1016/s0092-8674(04)00402-7. [DOI] [PubMed] [Google Scholar]
- Yurchenko V, Xue Z, Gama V, Matsuyama S, Sadofsky MJ. Ku70 is stabilized by increased cellular SUMO. Biochem Biophys Res Commun. 2008;366(1):263–268. doi: 10.1016/j.bbrc.2007.11.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.