Abstract
Parthenium dermatitis is a distressing dermatitis caused by the air borne allergen of the Compositae weed Parthenium hysterophorus. Uncommon presentations, newer insights in pathogenesis and management of this “scourge” are discussed in this article.
Keywords: Atopic dermatitis, parthenium dermatitis, patch and prick test, type I hypersensitivity, type IV hypersensitivity
INTRODUCTION
Parthenium hysterophorus (synonyms: Congress grass, Congress weed, Santa Maria, bitter weed, carrot grass, false ragweed, fever few, white top, gajar ghas, the “Scourge of India”) is an annual herb that was accidentally introduced into Pune, India in 1956 through contaminated wheat shipments from the USA. Parthenium sensitivity was first reported by Lonkar et al., from Pune.[1] The epithet “Congress weed” may have two interpretations: Its white flowers which resemble the white cap worn by members of the Indian National Congress.[2] Another report refers to the US Congress which allocated the shipment to Pune, India.[3] Parthenos in Greek means virgin; “Parthenium” could refer to only the female flowers being fertile.[2] In Latin, “parthenice” suggests medicinal uses and the herb has been used as a tonic, febrifuge and emmenagogue. “Parth” in Sanskrit is another name for Arjuna, an indestructible and invincible character in the Indian epic, Mahabharata. P. hysterophorus is thus inadvertently, aptly named.[4] It is the leading cause of plant-induced airborne contact dermatitis (ABCD) in India.[5] This “scourge of India” has infested most of her rural and urban areas, causing epidemics of dermatitis.[6] The weed has serious impact on human health, and consumption by livestock can cause tainted meat. It can cause marked decrease in crop production, destroy grasslands and pastures, and degrade natural ecosystems.[7] A recent review has covered various aspects of parthenium dermatitis.[5] This review is an update of the previous article.
DISTRIBUTION
The weed is a native of the West Indies and North East Mexico, but has spread worldwide in the last hundred years [Table 1].[8,9] P. hysterophorus is thought to be a natural hybrid of Parthenium confertum and Parthenium bipinnatifidum[9] and belongs to the family Asteraceae (Compositae), one of the largest (about 25,000 species) and most important families in the plant kingdom that contains weeds, ornamental annuals, herbaceous perennials, medicinal and food plants.[5]
Table 1.
Distribution of P. hysterophorus L. worldwide
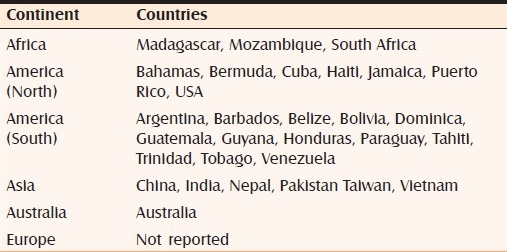
Fifteen species of Parthenium L. are reported from America and the West Indies. P. hysterophorus L., has spread like wildfire throughout India (wastelands, disturbed lands, degraded pastures, croplands, forests, along railway tracks and roadside, along streams and rivers) and across a wide range of habitats (hot, arid, semiarid, humid high altitude areas).
ALLERGENICITY
The allergens in Asteraceae are sesquiterpene lactones (SQLs). SQLs are characterized by the presence of a γ-butyrolactone ring bearing an exocylic α-methylene group. Related plant families like Magnoliaceae, Winteraceae, Jubulaceae and Lauraceae also contain SQLs.
SQLs are biologically active plant chemicals identified in many plant families, with Compositae having the greatest numbers (more than 3000 different SQLs). They are classified on the basis of their carbocyclic skeletons into germanocranolides, guaianalides, eudesmanolides, pseudoguaianolides and xanthonolides. The suffix “olide” refers to the lactone function and is based on costunolide, a germanocranolide which is related to the 10-membered carbocyclic sesquiterpene, germacrone. An individual plant species generally produces one skeletal type of SQL concentrated primarily in the leaves and flower heads. The percentage of SQL per dry weight may vary from 0.01 to 8%. Certain SQLs derived from several different plant species have significant bactericidal and fungicidal activity in vitro.[10]
While parthenin, a pseudoguaianolide, is the major constituent of P. hysterophorus from the USA, Mexico, West Indies and India, it is replaced by hymenin in populations from southern Bolivia and central Argentina and in one population from Texas.[11] Based on SQL composition, samples from 31 populations of P. hysterophorus were divided into seven chemical types using thin layer chromatography (TLC) and/or nuclear magnetic resonance (NMR) in crude extracts.[11] All samples from North and Central America, Venezuela, South Africa, India, Australia and one from Jamaica belong to type I which contains parthenin and possibly all these samples have the same origin. Samples from Argentina do not contain parthenin but contain hymenin which is a diastereomer of parthenin.[12] Other SQLs detected were coronopilin, hysterin, tetraneurin-A, and dihydroisoparthenin. In India, the plant contains large amounts of parthenin and ambrosin.[12] No cross-reaction exists between parthenin and hymenin in humans and guinea pigs.[13] Parthenium dermatitis is not reported from South America possibly because hymenin is the major SQL and parthenin is absent, whereas in regions like India where the plant contains large amounts of parthenin, the dermatitis is severe and distressing.[14,15]
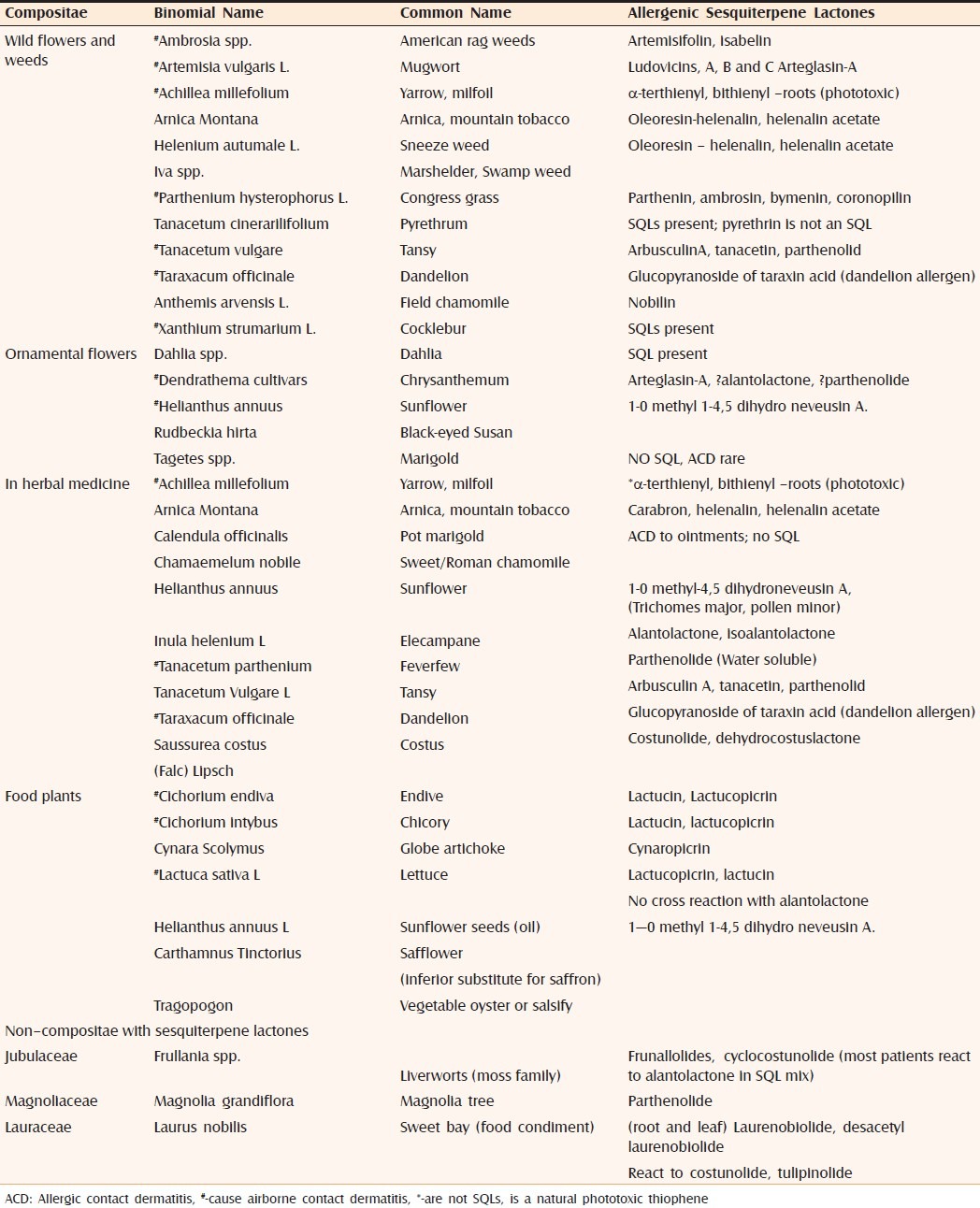
Patients with contact dermatitis to Compositae can react to many other non-Compositae SQL-containing plants [Table 2].[5] Cross-reactivity between SQLs does not follow any rules. Neither any single SQL nor the commonly used “SQL mix” of three common SQLs (alantolactone, dehydrocostus lactone and costunolide) serves as a reliable screen for SQL allergy. This mix which has been widely tested detects only 30% cases of sensitization.[16] Compositae mix is an alternative preparation comprising a mixture of plant extracts (arnica, yarrow, tansy, German chamomile, and feverfew) and detects a higher proportion of cases.[17] Therefore, the samples of the suspect plant should always be used while patch testing a patient.
Table 2.
Allergic Compositae and non Compositae with sesquiterpene lactones[5]
BOTANY
The plants of Compositae family have many tiny flowers (florets) clustered to form a flower head (capitulum). This flower head is surrounded by bracts (modified leaves) that form an involucre beneath or around a flower cluster [Figure 1].
Figure 1.

Star shaped flowers with 5 petal-like ray florets; tiny disc florets clustered to form a flower head (capitulum)
P. hysterophorus has two stages in its life cycle:[9]
Juvenile or rosette stage
Mature or adult stage
Juvenile stage
It has a rosette with large, dark green, simple, radical, pinnatisect, small leaves, and flowering is absent. The large lower leaves are spread on the ground like a carpet, not allowing any vegetation underneath it [Figure 2].
Figure 2.

Juvenile with deeply lobed leaves spread on the ground
Adult stage
It is procumbent (trailing along the ground but not rooting), profusely branched, leafy herb resembling a bush or shrub because of its height (1–2.5 m) [Figure 3]. The stem becomes tough and woody as the plant matures into a hardy bush. An enormous number of pollen grains (624 million per plant) are produced by anemophilous (by wind) pollination. It is an extremely prolific seed producer with up to 25,000 seeds (achenes) per plant. The plant is thermo- and photo-insensitive; hence, it grows round the year except in severe winters; in other words, it survives environmental extremes. It is a rapid colonizer and competes out other vegetation in its vicinity within two growing seasons. It grows in almost all types of soil except near the seashore as the saline soil is not conducive to parthenium flowering. to be necessary.
Figure 3.

Mature plant (shrub) with many branches and flowers
CLINICAL PRESENTATIONS
The presentation may be hay fever, asthma or dermatitis caused by dust and debris from the plant as well as pollen.[18]
Sex
Adult males bear the brunt of involvement both in USA and India, with a male to female ratio of 20:1.[19] Since Indian women and children also work in fields, this cannot be attributed to exposure alone. Possibly, women and children are less frequently sensitized.[19,20]
Sites of involvement
Initially, the exposed sites of the face, neck and flexures are affected with erythema, blistering and intense pruritus, resulting later in skin thickening, hyperpigmentation and development of a leonine facies.[1] Unexposed sites may get involved late in the course of the disease. A seasonal variation is initially observed with the dermatitis flaring in the summers corresponding to the growing season and disappearing in winters. After several years, persistent pruritic lichenified dermatitis develops without seasonal variation.[21] Winter exacerbation is seen in southern India during the months of September, October and November and may be due to the increased growth of parthenium following the North-East monsoon showers.[22] The skin of the upper eyelids, the retro-auricular and submental areas, which are spared in photodermatitis, are involved in weed dermatitis (pseudophotodermatitis). The dermatitis can become generalized to produce an erythroderma.
Various clinical patterns of dermatitis[5,21,23–25]
ABCD [Figure 4]
Pseudophotodermatitis [Figure 5]
Atopic dermatitis [Figure 6]
Seborrheic dermatitis
Exfoliative dermatitis
Photosensitive lichenoid dermatitis
Hand dermatitis
Lichen nitidus-like[25]
Figure 4.
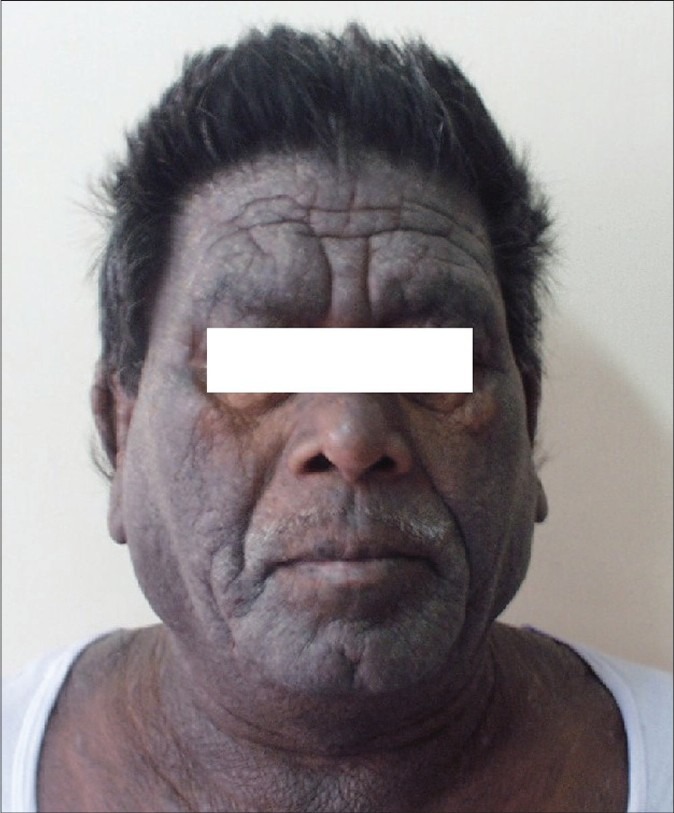
Airborne contact dermatitis
Figure 5.
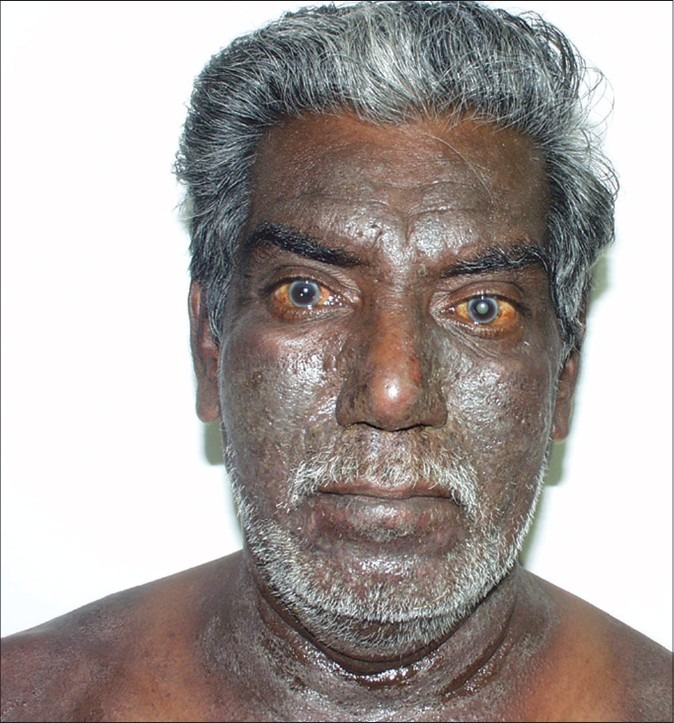
Pseudophotodermatitis
Figure 6.
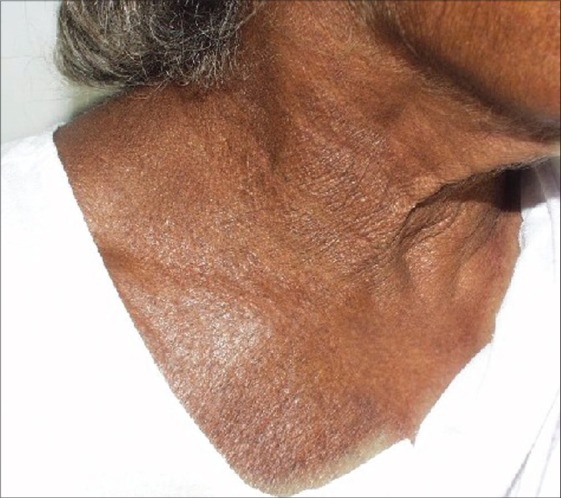
Atopic dermatitis
Figure 7.
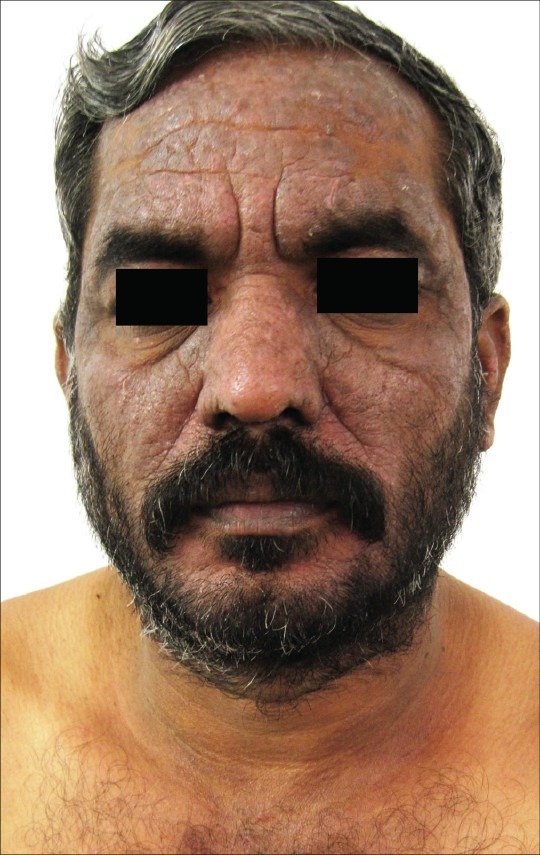
Chronic actinic dermatitis
Figure 8.
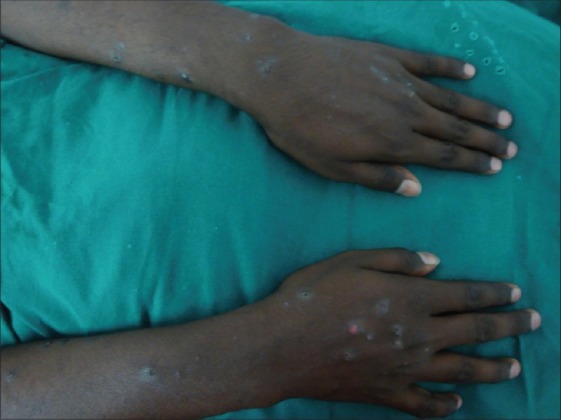
Prurigo nodularis-like lesions over the forearms of an atopic patient
Figure 9.
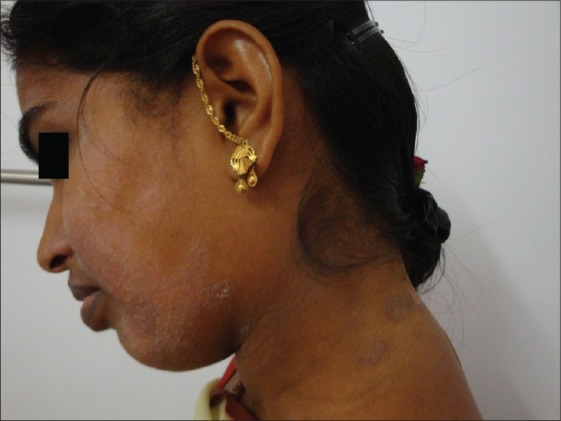
PLE-like lesions over the face and neck
Hand dermatitis is observed in gardeners after contact with the weed.[19] Vitiliginous skin is thought to be spared due to the vacuolization of Langerhans cells of the area.[26] However a patient with vitiligo who patch tested positive to parthenium developed PLE-like lesions over the forehead and forearms which depigmented by the Koebner phenomenon [Figure 10]. The dermatitis in the depigmented area did not resolve until potent topical corticosteroids and systemic azathioprine were instituted.[23]
Figure 10.
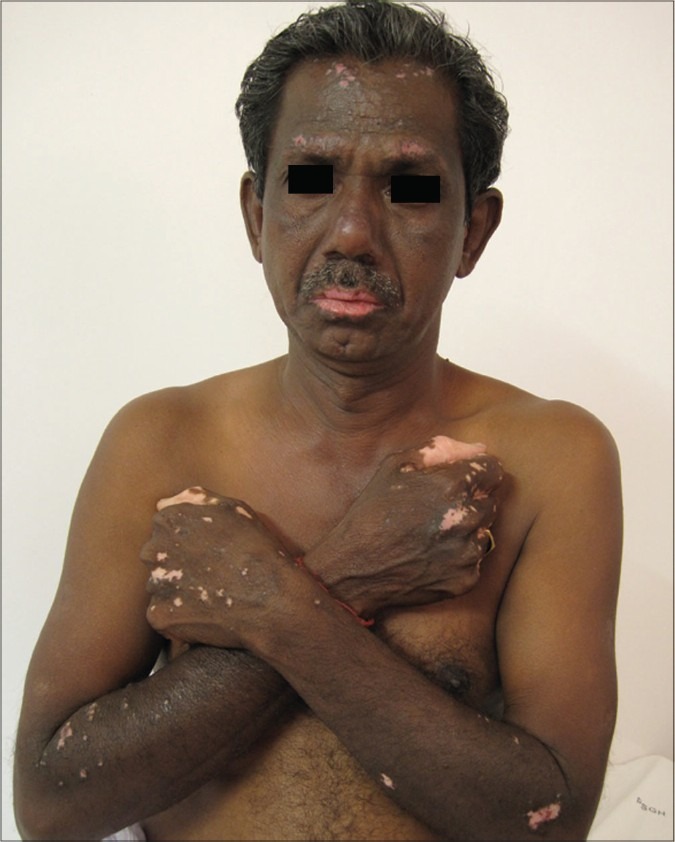
PLE-like lesions over the forearms and forehead showing depigmentation
CROSS-SENSITIVITY
Other members of the Compositae family causing ABCD in North India include Xanthium strumarium, Chrysanthemum morifolium (chrysanthemums), Dahlia pinnata (dahlia) and Tagetes indica (marigold).[27,28] Cross-reactions may occur between the various sesquiterpene allergens. P. hysterophorus and X. strumarium have shown a high rate of cross-sensitivity in Indian patients,[29] whereas the prevalence of cross-reaction with chrysanthemum is generally low.[30,31] It is also important to distinguish between true cross-sensitization and polysensitivity.[32] If a patient develops independent allergies to more than one agent that do not share any chemical groups (antigenic determinants), then such a situation is called polysensitivity and not cross-sensitivity. Cross-reactivity between paraphenylene diamine (PPD) and SQL has also been postulated.[33] Compositae and Frullania cross-react because of the content of related SQLs. Compositae-sensitive patients may cross-react to plants which are not present in their environment; this has immunological relevance.
The severity of dermatitis in a parthenium-sensitive patient depends on the degree of contact hypersensitivity in the patient at that time and the quantity of antigen in contact with the patient.[34] Inhalation of pollens can cause allergic rhinitis and bronchitis or asthma if the pollens enter the lungs during respiration.[35] The degree of contact hypersensitivity to an agent can be determined by the titer of contact hypersensitivity (TCH).[36] Increased dilutions of the causative antigen in addition to the standard concentration recommended for the antigen are applied on the sensitized patient. The highest dilution (or the lowest concentration of the antigen) that still produced a distinct positive patch test reaction is labeled as the TCH in that patient.[37] The TCH was found to be a reliable indicator of the degree of contact hypersensitivity, and the results have been shown to be reproducible.[38] However, other reports have found that the TCH does not correlate with the clinical severity of contact dermatitis or response to treatment.[39]
Aeropollen sampling in Bangalore, southern India over a 6-year period revealed that 40–60% of the total pollen count was from P. hysterophorus.[7] Allergenicity to P. hysterophorus pollen extracts was recorded in 34% allergic rhinitis and 12% bronchial asthma patients from Bangalore.[40] Parthenium pollen is now a major cause of allergic rhinitis in Bangalore, affecting 7% of the population and 40% showing sensitivity to the pollen.[41] Such a high incidence of allergic rhinitis to a specific pollen has not been reported from any other part of the world.[7] Subsequent studies in northern India (Punjab) showed sensitization to P. hysterophorus in bronchial asthma patients.[42] In New Delhi, out of 63 patients with ABCD, 62 showed a positive reaction to the parthenium weed.[43] Studies on cross-reactivity between ragweed (Ambrosia) and parthenium pollen suggest that individuals sensitized to parthenium may develop type I hypersensitivity reactions to ragweed and vice versa when they travel to regions infested with the weed, to which they have not been previously exposed.[44]
PHOTOSENSITIVITY AND PARTHENIUM (COMPOSITAE) DERMATITIS
The distribution of parthenium dermatitis, primarily affecting the exposed skin surfaces, resembles photodermatitis, but is actually pseudophotodermatitis since the upper eyelids, submental and retroauricular areas are also involved (areas characteristically spared in photodermatitis). But it is well known that affected persons recover when they are relocated away from the weed even though they are still exposed to sunlight.[1]
Lowered minimal erythema dose (MED) to UVB and positive reactions to UVA have been reported in an atopic patient.[45] Other reports have confirmed the reduction in MED to UVB and reduction in the minimum phototoxic dose (MPD) to UVA.[46,47] The relationship between photosensitivity and parthenium dermatitis has been a mystery. SQLs are not photosensitizers, they have neither phototoxic nor photoallergic properties.[19,48,49] This puzzle is solved by atopy and concomitant type I hypersensitivity. Although Compositae dermatitis is reported to spare children in various studies, a history of atopy in children may pose a risk factor for developing contact sensitization to Compositae.[50]
Pathogenesis of parthenium dermatitis – Atopy, the missing link
Delayed (type IV) hypersensitivity alone does not explain the varying clinical patterns and photoaggravation. Parthenium dermatitis has been thought to be mediated solely by type IV hypersensitivity, but recently a combined immediate (type I) and delayed (type IV) hypersensitivity mechanism has been postulated in the initiation and perpetuation of parthenium dermatitis, especially in sensitized subjects with an atopic diathesis.[51] The patch test detects delayed hypersensitivity (type IV), while the skin prick test (SPT) detects immediate hypersensitivity (type I) [Figure 11]. A positive SPT comprises two responses – an immediate reaction seen within 15–20 minutes characterized by a wheal and flare, and a delayed reaction or the late phase reaction (LPR) that occurs at about 5–6 hours and may persist for 1–2 days. It presents as a nodule that is painful rather than itchy. The LPR is mediated by newly formed mast cell mediators (leukotrienes, prostaglandins, cytokines) in concert with inflammatory cells and is thought to be responsible for the chronic skin and bronchial hypersensitivity in atopic patients with dermatitis and bronchial asthma, respectively.[52] The LPR of the type I hypersensitivity reaction shows histopathology consistent with leukocytoclastic vasculitis.[53] Leukocytoclastic vasculitis has been reported from the LPR of the prick tested site in parthenium dermatitis and from the lesional skin of parthenium dermatitis.[22,23,54] Photoaggravation, heat intolerance and flexural involvement are the features of atopic dermatitis.[55] Various clinical patterns of parthenium dermatitis such as flexural eczema, prurigo nodularis, chronic actinic dermatitis can be observed in patients with an atopic diathesis.[56,57] Earlier, a combination of type III and type IV hypersensitivity had also been postulated,[58] but this has been questioned since IgG antibodies that mediate type III hypersensitivity have not been detected.[54] The additional involvement of atopy (type I hypersensitivity) would explain the varied clinical presentation.[23] The serum IgE is usually elevated in an atopic patient with parthenium dermatitis.
Figure 11.
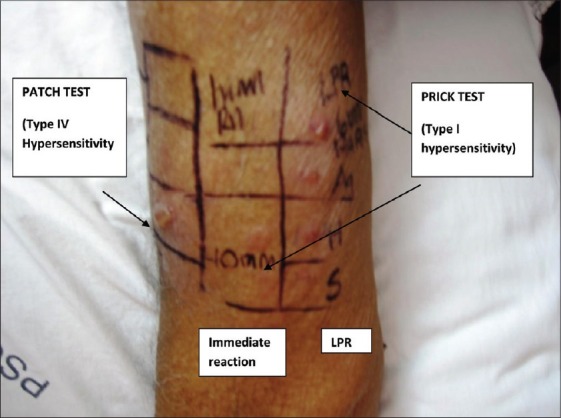
Type I and type IV hypersensitivity in parthenium dermatitis
The LPR in parthenium dermatitis
Leukocytoclastic vasculitis has been reported from the LPR of the prick tested site in parthenium dermatitis and from the lesional skin of parthenium dermatitis.[22,23,54] An LPR has been observed after 24 h in several cases of parthenium dermatitis, even though the immediate reaction at 15 min is less than that elicited by histamine. One patient who patch tested positive to parthenium showed an immediate reaction to parthenium leaf of 3 mm diameter and an LPR of 6.5 mm diameter after 24 h [Figures 12 and 13]. Histamine and saline elicited an immediate wheal of 6 mm and 3 mm, respectively, with no induration after 24 h; only erythema of about 1 mm diameter could be seen. LPR for histamine and saline was thus negative. In this patient, in order to visualize both reactions simultaneously, prick testing was repeated after 24 h to elicit the immediate reaction adjacent to the LPR. He was an inpatient in whom antihistamines had been withheld 5 days prior to testing. He had not been administered systemic corticosteroids or immunosuppressants for more than 2 months. Although he denied a family and personal history of atopy, his serum IgE was elevated [1661 IU/ml (normal value <100 IU/ml)].
Figure 12.
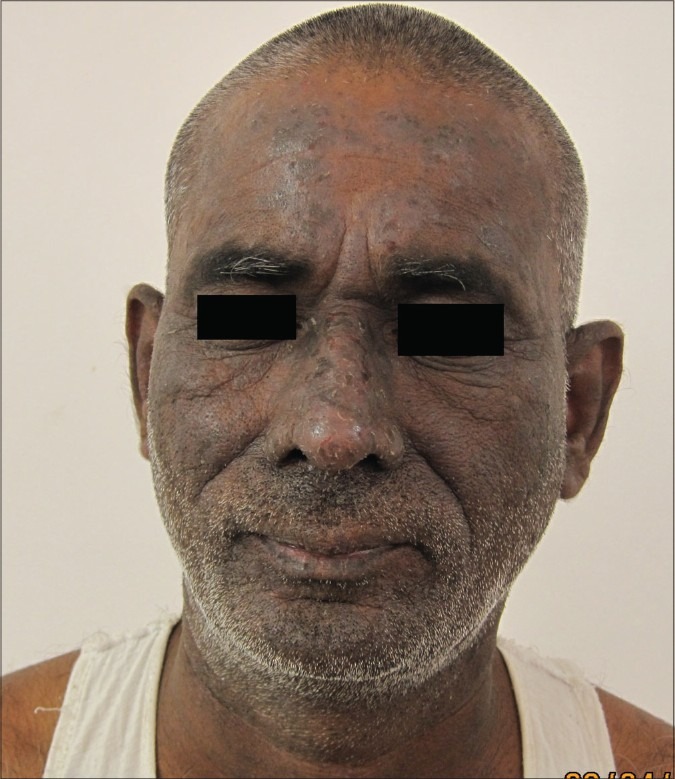
Involvement of sun exposed areas in parthenium dermatitis
Figure 13.
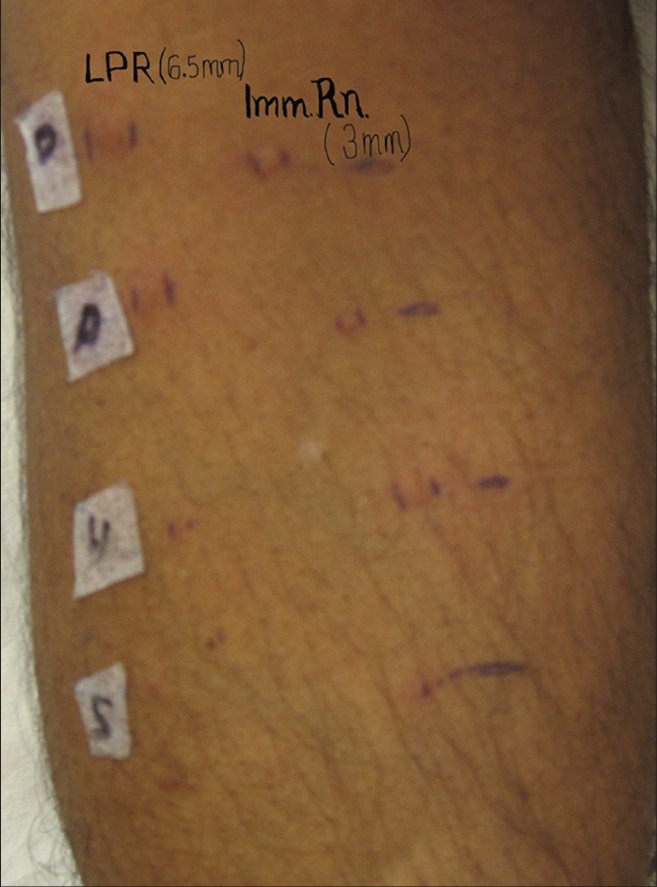
LPR greater than the immediate reaction in parthenium dermatitis
This finding is interesting since it has been reported that a immediate wheal of at least 8 mm is required to stimulate a clinically observable LPR.[59] This is of clinical significance since a negative immediate reaction at 15 min does not rule out type I hypersensitivity to parthenium; the LPR reading has to be taken 24 h later to confirm presence or absence of type I hypersensitivity.
Investigations in parthenium dermatitis
The following tests are recommended when a patient presents with features of parthenium dermatitis. Of these, patch and prick tests are mandatory to detect type IV and type I hypersensitivity, respectively. The clinical severity may be scored as described by Verma et al.[60]
Patch test is carried out with the plant material “as is.” In addition, parthenium antigen from the Indian Standard Series (ISS) may also be applied. Readings are interpreted as recommended by the International Contact Dermatitis Research Group (ICDRG). Patches are applied using Finn Chambers® on Scanpor tape® and removed after 2 days. The Indian Standard Series (ISS) containing 1% parthenium antigen was earlier produced by Systopic Labs, India. At present, Chemotechnique Diagnosics (Sweden), is producing the ISS and this contains parthenolide (0.1%) a sesquiterpene lactone from Tanacetum parthenium (fever few plant) but does not contain the sesquiterpene lactones parthenium and ambrosin (present in Parthenium hysterophorus). Parthenolide does not always pick up sensitivity to parthenium.
Prick test is performed with the parthenium leaf solution and antigen extract containing 500 PNU, with glycerinated saline as negative control and histamine as positive control (Creative Drug Industries, Allergology Division, Navi Mumbai, India). Plant materials can be crushed and diluted with saline (1:9 parts) in order to obtain a parthenium leaf solution that can be easily pricked. Both the immediate reaction at 15 min and the LPR at 24–48 h should be recorded.
Radioallergosorbent test (RAST) can be carried out for parthenium-specific antibodies. However, RAST is less sensitive than prick testing.[61,62]
Serum IgE estimation by chemiluminescence immunoassay (ADVIA Centaur, Bayer Health Care, Mumbai, India). A detailed history of atopy must be taken.
Photopatch testing is done by applying Finn Chambers® in duplicate over the upper back. After 24-48 hours, both sets are removed; one set is covered with UV opaque material and the other side is irradiated with 10J/cm2 UV A. Both sides are then occluded for 48 hours. Positive reaction on the irradiated side and negative reaction on the non-irradiated side indicates photocontact allergy. If both the sides show positive reactions of equal intensity, then contact allergy is present. If the positive reaction on the irradiated side is more than the non-irradiated side, with a difference of more than 1+, then both contact and photocontact allergies are present. If the positive reaction on the irradiated side is just more than the non-irradiated side (difference of less than 1+), then it is interpreted as contact allergy with photoaggravation.[63]
Atopic patch test (APT) is performed if the patient has an atopic diathesis. This is carried out with prick test allergens including parthenium. They are applied as a patch and occluded for 48 hours. Readings are taken at 48 and 72 hours.[64]
Preparation of plant extracts
Plant allergens are low-molecular-weight secondary metabolites and are usually soluble in acetone, ethanol or ether. A filtered acetone or ethanol extract of dried plant material or a short ether extract of fresh material produces a solution suitable for patch testing. Aqueous extracts degrade rapidly and lose their sensitizing power within a month.[65] Acetone extract of P. hysterophorus (1%) is reported to be more sensitive than the water extract.[66] Although extracts in organic solvents are more stable, with time, evaporation of the solvent may increase the concentration and the sensitizing effect of the allergen(s).[67] Incorporating evaporated extract into petrolatum represents a standard means of retaining material for patch testing.
Prevention and protective measures
Though weed eradication is the ideal form of prevention, this has been unsuccessful. Burning the leaf and patch testing with the residue resulted in a positive patch test.[5] Efforts have been made to utilize parthenium as a green leaf manure, biopesticide, compost for agricultural purposes and additive with cattle manure in biogas production.[68] Patch testing with the compost in a sensitive patient yielded positive result, thereby confirming that the allergenicity is retained.[4] Parthenium is indeed indestructible as mentioned earlier.[4] Since P. hysterophorus is ubiquitous, a change of residence or job is not a suitable option. This would also lead to social and economic consequences.
Hence, prevention is aimed at the reduction in the quantity of the antigen to which the patient is exposed. These measures include the following:[69]
To remove as much of the causative plant as possible from the immediate environment of the patient.
To cover as much of the skin as possible by clothing.
To frequently use a barrier cream to slow the penetration of the antigen into the skin and to wash before reapplication.
To avoid the exposure to sunlight; sunscreen lotions may serve as barrier creams.
Drying of clothes indoors also helps in reducing the quantity of antigen. Clothes dried outdoors gather the airborne parthenium allergen.[45] Pieces of cloth dried outside were reported to elicit a positive patch test in a sensitive patient.[70]
Gloves may not offer protection since the SQL permeates vinyl, polyethylene and latex gloves.[71]
Treatment of parthenium dermatitis
Oral hyposensitization is claimed to be successful. Patients showed clinical improvement and the patch test reading became less severe or negative.[19,48] This could explain why chrysanthemum allergy, which is the commonest Compositae allergy in Europe, is rare in Japan, where chrysanthemum leaves and flowers are eaten with sushi, salad and soups.
However, oral hyposensitization with parthenium leaves does not yield consistent results.[72] Adverse effects are similar to those seen with Toxicodendron hyposensitization and include pruritus ani, a widespread urticarial or eczematous eruption, dyspepsia and toxicity.[73,74]
In non-atopics, type IV hypersensitivity is operational, whereas combined type I and type IV hypersensitivity is operational in atopics with parthenium dermatitis.[51] Treatment is based on targeting type IV hypersensitivity, or combined type IV and type I hypersensitivity (both the immediate reaction and the LPR).[51]
Corticosteroids have both immunosuppressive and anti-inflammatory actions. They suppress delayed hypersensitivity and also the LPR of the type I hypersensitivity reaction.[22] Corticosteroids are not usually thought of being capable of protecting against immediate allergic reactions after antigen challenge.[75] However, it has been observed that even a brief application of corticosteroid cream could diminish the immediate reaction.[76] Similarly, the preventive application of corticosteroid to the nasal mucosa of an allergic patient is capable of diminishing the symptoms produced by an antigen challenge.[77] Application of corticosteroid topically for several days depleted the mast cells in the skin and reduced the response to codeine, a histamine-releasing agent.[78]
Acute dermatitis has to be treated immediately. Once daily application of potent topical steroids is as effective as twice daily.[79] Potent topical steroids and oral prednisone are relatively ineffective unless employed early and if further exposure to SQLs is prevented.[19,42,80] Systemic corticosteroids have been the mainstay of treatment in the acute phase. Long-term use may lead to adrenocortical axis suppression with attendant complications.[81] A trial with dexamethasone–cyclophosphamide pulse (DCP) therapy was unsuccessful.[82] Antihistamines suppress only the immediate reaction of type I hypersensitivity; the LPR remains unaffected.[83] Olopatadine, a selective histamine H1-receptor antagonist with action against the late phase of allergy, was found to suppress the LPR in three patients with parthenium dermatitis.[84] Leukotriene receptor antagonists like montelukast play an important role in the treatment of asthma and other allergic conditions such as allergic rhinitis, atopic dermatitis, and chronic urticaria.[85] Montelukast did not inhibit the LPR in a patient with parthenium dermatitis[86] and also another patient with HIV infection on rupatidine and colchicine.[87] Montelukast may be of benefit in atopic patients with parthenium dermatitis who also have allergic rhinitis, allergic conjunctivitis or bronchial asthma.
Chloroquine 200 mg TID for 1 week and ethinyl estradiol 0.5 mg for 3 weeks have also been used;[80] however, they are not currently recommended. Psoralen and ultraviolet A (PUVA) therapy has reportedly helped Compositae dermatitis. A protocol developed by Storrs et al., combines PUVA with oral prednisone.[19]
Azathioprine has immunosuppressive, anti-inflammatory and steroid-sparing properties and is effective in the treatment of parthenium dermatitis in a dose of 1–2 mg/kg/day.[5,60,67] A weekly pulse dose of 300 mg is also reported to be effective with better compliance and reduced cost of therapy.[60] The safety of a bolus dose has been questioned.[88] Its limitation is the slow onset of action, taking 2–3 months to achieve a clinical effect.[22]
Cyclosporine, an immunosuppressive drug with potent anti-inflammatory actions, has been reported to be effective in the acute phase of parthenium dermatitis as a crisis intervention measure.[22] It also overcomes the disadvantages of systemic corticosteroid usage. It suppresses the delayed hypersensitivity reaction as well as the LPR.[22] Leukocytoclastic vasculitis due to LPR disappeared following the initiation of cyclosporine.[22] Methotrexate has also been reported to be effective at a dose of 15 mg/week along with topical corticosteroids and sunscreens.[89]
Parthenium dermatitis is a severe dermatitis causing significant morbidity in the productive age group. Inspite of being severe and affecting all parts of India, there is no data on the prevalence of this distressing dermatitis in India. A multicentric project involving 5 centres aims address this by studying the profile of parthenium dermatitis during a one year period. Parthenium antigen (1% extract in petrolatum) for the study has been prepared at PSG Institute of Medical Sciences and Research (PSGIMSR) by the method described by Rowan et al.[90]
Parthenium terminates crop productivity and natural flora as it invades and destroys agricultural land and natural ecosystems. It poses a severe health hazard in India. Legislative measures such as the Weeds Management Act 2001 of Australia[91] would be required to exterminate this alien.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Lonkar A, Mitchell JC, Calnan CD. Contact dermatitis from Parthenium hysterophorus. Trans St Johns Hosp Dermatol Soc. 1974;60:43–53. [PubMed] [Google Scholar]
- 2.Motooka P. Weeds of Hawa’I’s’ Pastures and Natural Areas; An Identification and Management Guide. College of Tropical Agriculture and Human Resources. Manoa: University of Hawa’I’; 2003. [Google Scholar]
- 3.Guin JD. Sesquiterpene-lactone dermatitis. Immunol Allergy Clin North Am. 1989;9:447–61. [Google Scholar]
- 4.Lakshmi C, Srinivas CR, Chinnusamy C. Retention of allergic potential of parthenium following composting. Contact Dermatitis. 2007;57:348–9. doi: 10.1111/j.1600-0536.2007.01137.x. [DOI] [PubMed] [Google Scholar]
- 5.Lakshmi C, Srinivas CR. Parthenium: A wide angle view. Indian J Dermatol Venereol Leprol. 2007;73:296–306. doi: 10.4103/0378-6323.35732. [DOI] [PubMed] [Google Scholar]
- 6.Mitchell JC, Calnan CD. Scourge of India: Parthenium dermatitis. Int J Dermatol. 1978;17:303–4. doi: 10.1111/j.1365-4362.1978.tb06080.x. [DOI] [PubMed] [Google Scholar]
- 7.Evans HC. Parthenium hysterophorus: A review of its weed status and the possibilities for biological control. Biocontrol News Information. 1997;18:89N–98N. [Google Scholar]
- 8.Aneja KR, Dhawan SR, Sharma AB. Deadly weed Parthenium hysterophorus L. and its distribution. Indian J Weed Sci. 1991;23:14–8. [Google Scholar]
- 9.Narwal SS, Palaniraj R, Sati SC, Kadian HS, Dahiya DS. Allelopathic plants; 8. Parthenium hysterophorus L. Allelopathy Jl. 2003;11:151–70. [Google Scholar]
- 10.Neerman ME. Sesquiterpene lactones: a diverse class of compounds found in essential oils possessing antibacterial and antifungal properties. Int J Aromatherapy. 2003;13:114–20. [Google Scholar]
- 11.Picman AK, Towers GH. Sesquiterpene Lactones in various populations of Parthenium hysterophorus. Biochemical Syst Ecol. 1982;10:145–53. [Google Scholar]
- 12.Rao PV, Mangala A, Towers GH, Rodriguez E. Immunological activity of parthenin and its diastereomer in persons sensitized by Parthenium hysterophorus L. Contact Dermatitis. 1978;4:199–203. doi: 10.1111/j.1600-0536.1978.tb03789.x. [DOI] [PubMed] [Google Scholar]
- 13.Picman AK, Picman J, Towers GH. Cross-reactivity between sesquiterpene lactones and parthenin in parthenin-sensitized guinea pigs. Contact Dermatitis. 1982;8:294–301. doi: 10.1111/j.1600-0536.1982.tb04234.x. [DOI] [PubMed] [Google Scholar]
- 14.Lonkar A, Nagasampagi BA, Narayanan CR, Landge AB, Sawaikar DD. An antigen from Parthenium hysterophorus Linn. Contact Dermatitis. 1976;2:151–4. doi: 10.1111/j.1600-0536.1976.tb03015.x. [DOI] [PubMed] [Google Scholar]
- 15.Towers GH, Mitchell JC, Rodriguez E. Biology and chemistry of Parthenium hysterophorus L. a problem weed in India. J Sci Ind Res. 1997;36:672–84. [Google Scholar]
- 16.Green C, Ferguson J. Sesquiterpene lactone mix is not an adequate screen for Compositae allergy. Contact Dermatitis. 1994;31:151–3. doi: 10.1111/j.1600-0536.1994.tb01954.x. [DOI] [PubMed] [Google Scholar]
- 17.Paulsen E, Andersen KE, Hausen BM. Compositae dermatitis in a Danish dermatology department in one year. I. Results of routine patch testing with the sesquiterpene lactone mix supplemented with aimed patch testing with extracts and sesquiterpene lactones of Compositae plants. Contact Dermatitis. 1993;29:6–10. doi: 10.1111/j.1600-0536.1993.tb04528.x. [DOI] [PubMed] [Google Scholar]
- 18.McFadyen RE. Parthenium weed and human health in Queensland. Aust Fam Physician. 1995;24:1455–9. [PubMed] [Google Scholar]
- 19.Warshaw EM, Zug KA. Sesquiterpene lactone allergy. Am J Contact Dermat. 1996;7:1–23. doi: 10.1016/s1046-199x(96)90028-7. [DOI] [PubMed] [Google Scholar]
- 20.Sharma SC, Kaur S. Airborne contact dermatitis from Composite plants in Northern India. Contact Dermatitis. 1989;21:1–5. doi: 10.1111/j.1600-0536.1989.tb04676.x. [DOI] [PubMed] [Google Scholar]
- 21.Shenoi SD, Srinivas CR. Changing clinical patterns of Parthenium dermatitis. Contact Dermatitis. 1997;37:128. doi: 10.1111/j.1600-0536.1997.tb00318.x. [DOI] [PubMed] [Google Scholar]
- 22.Lakshmi C, Srinivas CR, Jayaraman A. Ciclosporin in parthenium dermatitis - a report of 2 cases. Contact Dermatitis. 2008;59:245–8. doi: 10.1111/j.1600-0536.2007.01208.x. [DOI] [PubMed] [Google Scholar]
- 23.Lakshmi C, Srinivas CR, Pillai SB, Shanthakumari S. Parthenium dermatitis manifesting clinically as polymorphic light eruption and prurigo nodularis-like lesions with vasculitis-like picture on histopathology. Indian Dermatol Online J. 2011 doi: 10.4103/2229-5178.86002. [In press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharma VK, Sahoo B. Prurigo-nodularis-like lesions in parthenium dermatitis. Contact Dermatitis. 2000;42:235–48. doi: 10.1034/j.1600-0536.2000.042004235.x. [DOI] [PubMed] [Google Scholar]
- 25.De D, Jindal R, Kanwar AJ. Contact dermatitis to parthenium simulating lichen nitidus. Indian J Dermatol Venereol Leprol. 2010;76:286–7. doi: 10.4103/0378-6323.62978. [DOI] [PubMed] [Google Scholar]
- 26.Singh KK, Srinivas CR, Balachandran C, Menon S. Parthenium dermatitis sparing vitiliginous skin. Contact Dermatitis. 1987;16:174. doi: 10.1111/j.1600-0536.1987.tb01415.x. [DOI] [PubMed] [Google Scholar]
- 27.Pasricha JS, Verma KK, D’Souza P. Air borne contact dermatitis caused exclusively by Xanthium strumarium. Indian J Dermatol Venereol Leprol. 1995;61:354–5. [PubMed] [Google Scholar]
- 28.Sharma SC, Kaur S. Contact dermatitis from composite plants. Indian J Dermatol Venereol Leprol. 1990;56:27–30. [Google Scholar]
- 29.Pasricha JS, Bhaumik P, Agarwal A. Contact Dermatitis due to Xanthium strumarium. Indian J Dermatol Venereol Leprol. 1990;56:319–21. [Google Scholar]
- 30.Pasricha JS, Nandakishore T. Air borne contact dermatitis due to chrysanthemum with true cross sensitivity to Parthenium hysterophorus and Xanthium strumarium. Indian J Dermatol Venereol Leprol. 1992;58:268–71. [Google Scholar]
- 31.Sharma SC, Tanwar RC, Kaur S. Contact dermatitis from chrysanthemums in India. Contact Dermatitis. 1989;21:69–71. doi: 10.1111/j.1600-0536.1989.tb04699.x. [DOI] [PubMed] [Google Scholar]
- 32.Benezra X, Maibach H. True cross-sensitization, false cross-sensitization and otherwise. Contact Dermatitis. 1984;11:65–9. doi: 10.1111/j.1600-0536.1984.tb00928.x. [DOI] [PubMed] [Google Scholar]
- 33.Paulsen E, Christensen LP, Andersen KE. Possible cross-reactivity between para-phenylenediamine and sesquiterpene lactones. Contact Dermatitis. 2008;58:120–2. doi: 10.1111/j.1600-0536.2007.01192.x. [DOI] [PubMed] [Google Scholar]
- 34.Verma KK, Pasricha JS. Azathioprine as a corticosteroid-sparing agent in air-borne contact dermatitis. Indian J Dermatol Venereol Leprol. 1996;62:30–2. [PubMed] [Google Scholar]
- 35.Towers GH, Subba Rao PV. Impact of the pan topical weed. Parthenium hysterophorus L on human affairs. In: Richardson RG, editor. Proceedings of the 1st International Weed Control Congress. Melbourne, Australia: Weed Science Society of Victoria; pp. 134–8. [Google Scholar]
- 36.Pasricha JS. Titre of contact hypersensitivity (TCH) as a means of determining the degree of hypersensitivity in contact dermatitis. Indian J Dermatol Venereol Leprol. 1986;52:195–7. [PubMed] [Google Scholar]
- 37.Pasricha JS. Contact Dermatitis in India. 2nd ed. New Delhi: Department of Science and Technology; 1988. [Google Scholar]
- 38.Ramam M, Manchanda Y, Verma KK, Sharma VK. Reproducibility of titre of contact hypersensitivity to Parthenium hysterophorus. Contact Dermatitis. 2000;42:366. [PubMed] [Google Scholar]
- 39.Verma KK, Manchanda Y, Dwivedi SN. Failure of titre of contact hypersensitivity to correlate with clinical severity and therapeutic response in contact dermatitis caused by parthenium. Indian J Dermatol Venereol Leprol. 2004;70:210–3. [PubMed] [Google Scholar]
- 40.Rao M, Prakash O, Subba Rao PV. Reaginic allergy to Parthenium pollen: evaluation by skin test and RAST. Clin Allergy. 1985;15:449–54. doi: 10.1111/j.1365-2222.1985.tb02294.x. [DOI] [PubMed] [Google Scholar]
- 41.Sriramarao P, Nagpal S, Rao BS, Prakash O, Rao PV. Immediate hypersensitivity to Parthenium hysterophorus. II Clinical studies on the prevalence of Parthenium rhinitis. Clin Exp Allergy. 1991;21:55–62. doi: 10.1111/j.1365-2222.1991.tb00804.x. [DOI] [PubMed] [Google Scholar]
- 42.Suresh PV, Gupta D, Behera D, Jindal SK. Bronchial provocation with Parthenium pollen extract in bronchial asthma. Indian J Chest Dis Allied Sci. 1994;36:104. [Google Scholar]
- 43.Nandhakishore T, Pasricha JS. Pattern of cross-sensitivity between 4 Compositae plants, Parthenium hysterophorus, Xanthium strumarium, Helianthus annuus and Chrysanthemum coronarium, in Indian patients. Contact Dermatitis. 1994;30:162–7. doi: 10.1111/j.1600-0536.1994.tb00698.x. [DOI] [PubMed] [Google Scholar]
- 44.Sriramarao P, Rao PV. Allergenic cross-infectivity between Parthenium and ragweed pollen allergens. Int Arch Allergy Immunol. 1993;100:79–85. doi: 10.1159/000236391. [DOI] [PubMed] [Google Scholar]
- 45.Arlette J, Mitchell JC. Compositae dermatitis. Current aspects. Contact dermatitis. 1981;7:129–36. doi: 10.1111/j.1600-0536.1981.tb04584.x. [DOI] [PubMed] [Google Scholar]
- 46.Srinivas CR, Shenoi SD. Minimal erythema dose to ultra violet light in parthenium dermatitis. Indian J Dermatol Venereol Leprol. 1994;60:149–50. [Google Scholar]
- 47.Murphy GM, White IR, Hawk JL. Allergic airborne contact dermatitis to composite with photosensitivity chronic actinic dermatitis in evolution. Photodermatol Photoimmunol Photomed. 1990;7:38–9. [PubMed] [Google Scholar]
- 48.Wrangsjo K, Ros AM. Compositae allergy. Semin Dermatol. 1996;15:87–94. doi: 10.1016/s1085-5629(96)80027-7. [DOI] [PubMed] [Google Scholar]
- 49.Towers GH, Mitchell JC. The current status of the weed Parthenium hysterophorus L. as a cause of allergic contact dermatitis. Contact Dermatitis. 1983;9:465–9. doi: 10.1111/j.1600-0536.1983.tb04465.x. [DOI] [PubMed] [Google Scholar]
- 50.Paulsen E, Otkjaer A, Andersen KE. Sesquiterpene lactone dermatitis in the young: is atopy a risk factor? Contact Dermatitis. 2008;59:1–6. doi: 10.1111/j.1600-0536.2008.01328.x. [DOI] [PubMed] [Google Scholar]
- 51.Lakshmi C, Srinivas CR. Type I Hypersensitivity to Parthenium hysterophorus in patients with parthenium dermatitis. Indian J Dermatol Venereol Leprol. 2007;73:103–5. doi: 10.4103/0378-6323.31895. [DOI] [PubMed] [Google Scholar]
- 52.Coico R, Sunshine G, Benjamini E, editors. Hypersensitivity reactions type I In: Immunology a Short Course. 5th ed. New Jersey: Wiley Liss; 2003. pp. 199–211. [Google Scholar]
- 53.Samolitis N, Leiferman KM, Gleich GJ. The IgE-mediated cutaneous late-phase reaction. In: Greaves MW, Kaplan AP, editors. Urticaria and Angioedema. New York: Marcel Dekker; 2006. pp. 119–40. [Google Scholar]
- 54.Lakshmi C, Srinivas CR. Parthenium dermatitis caused by immediate and delayed hypersensitivity. Contact Dermatitis. 2007;57:64–5. doi: 10.1111/j.1600-0536.2007.01145.x. [DOI] [PubMed] [Google Scholar]
- 55.Kunz B, Ring J. Clinical features and diagnostic criteria of atopic dermatitis. In: Harper J, Orange A, Prose N, editors. Textbook of Pediatric Dermatology. Vol. 1. Oxford: Blackwell Science; 2000. pp. 199–214. [Google Scholar]
- 56.Uehara M. Prurigo reaction in atopic dermatitis. Acta Derm Venereol (Stockh) 1980;92:109–10. [Google Scholar]
- 57.Russell SC, Dave RS, Collins, Man I, Ferguson J. The photosensitivity dermatitis and the actinic reticuloid syndrome (chronic actinic dermatitis) occurring in seven young atopic dermatitis patients. Br J Dermatol. 1998;138:496–501. doi: 10.1046/j.1365-2133.1998.02132.x. [DOI] [PubMed] [Google Scholar]
- 58.Mahajan VK, Sharma NL, Sharma RC. Parthenium dermatitis: is it a systemic contact dermatitis or an airborne contact dermatitis? Contact Dermatitis. 2004;51:231–4. doi: 10.1111/j.0105-1873.2004.00400.x. [DOI] [PubMed] [Google Scholar]
- 59.Dolovich J, Hargreave FE, Chalmers R, Shier KJ, Gauldie J, Bienenstock J. Late cutaneous allergic responses in isolated IgE-dependent reactions. J Allergy Clin immunol. 1973;52:38–46. doi: 10.1016/0091-6749(73)90119-x. [DOI] [PubMed] [Google Scholar]
- 60.Verma KK, Bansal A, Sethuraman G. Parthenium dermatitis treated with azathioprine weekly pulse doses. Indian J Dermatol Venereol Leprol. 2006;72:24–7. doi: 10.4103/0378-6323.19713. [DOI] [PubMed] [Google Scholar]
- 61.Warner MR, Taylor JS, Leow YH. Agents causing contact urticaria. Clin Dermatol. 1997;15:623–35. doi: 10.1016/s0738-081x(97)00027-8. [DOI] [PubMed] [Google Scholar]
- 62.Lakshmi C, Srinivas CR. Type I hypersensitivity to Parthenium hysterophorus in patients with parthenium dermatitis. Indian J Dermatol Venereol Leprol. 2007;73:265–6. doi: 10.4103/0378-6323.31895. [DOI] [PubMed] [Google Scholar]
- 63.Sharma VK, Sethuraman G, Bhat R. Evolution of clinical pattern of parthenium dermatitis: a study of 74 cases. Contact Dermatitis. 2005;53:84–88. doi: 10.1111/j.0105-1873.2005.00652.x. [DOI] [PubMed] [Google Scholar]
- 64.Krupa Shankar DS, Chakravarthi M. Atopic patch testing. Indian J Dermatol Venereol Leprol. 2008;74:467–70. doi: 10.4103/0378-6323.44301. [DOI] [PubMed] [Google Scholar]
- 65.Shelmire B. Contact dermatitis from weeds: Patch testing with their oleoresins. J Am Med Assoc. 1939;113:1085–90. [Google Scholar]
- 66.Sharma VK, Sethuraman G, Tejasvi T. Comparison of patch test contact sensitivity to acetone and aqueous extracts of Parthenium hysterophorus in patients with air borne contact dermatitis. Contact Dermatitis. 2004;50:230–2. doi: 10.1111/j.0105-1873.2004.0318.x. [DOI] [PubMed] [Google Scholar]
- 67.Le Coz CJ, Ducombs G. Plants and plant products. In: Frosch PJ, Menne T, Leppoittevin JP, editors. Contact Dermatitis. 4th ed. 2006. pp. 751–800. [Google Scholar]
- 68.Gunaseelan VN. Parthenium as an additive with cattle manure in biogas production. Biol Wastes. 1987;21:1095–2002. [Google Scholar]
- 69.Srinivas CR, Balachandran C, Shenoi SD, Acharya S. Azathioprine in the treatment of Parthenium dermatitis. Br J Dermatol. 1991;124:394–5. doi: 10.1111/j.1365-2133.1991.tb00610.x. [DOI] [PubMed] [Google Scholar]
- 70.Srinivas CR. Transmission of parthenium dermatitis by clothing. Arch Dermatol. 2005;141:1605. doi: 10.1001/archderm.141.12.1605. [DOI] [PubMed] [Google Scholar]
- 71.Goncalo M, Mascarenhas R, Vieira R, Figueiredo A. Permeability of gloves to plant allergens. Contact Dermatitis. 2004;50:200–1. [Google Scholar]
- 72.Handa S, Sahoo B, Sharma VK. Oral hyposensitization in patients with contact dermatitis from Parthenium hysterophorus. Contact Dermatitis. 2001;44:279–82. doi: 10.1034/j.1600-0536.2001.440505.x. [DOI] [PubMed] [Google Scholar]
- 73.Srinivas CR, Krupashankar DS, Singh KK, Balachandran C, Shenoi SD. Oral hyposensitisation in parthenium dermatitis. Contact Dermatitis. 1998;18:242–3. doi: 10.1111/j.1600-0536.1988.tb02814.x. [DOI] [PubMed] [Google Scholar]
- 74.Watson ES. Toxicodendron hyposensitization programs. Clin Dermatol. 1986;4:160–70. doi: 10.1016/0738-081x(86)90075-1. [DOI] [PubMed] [Google Scholar]
- 75.Gronneberg R, Strandberg K, Stalenheim G, Zettertrom O. Effect in man of anti-allergic drugs on the immediate and late phase cutaneous allergic reactions induced by anti-IgE. Allergy. 1981;36:201–8. doi: 10.1111/j.1398-9995.1981.tb01835.x. [DOI] [PubMed] [Google Scholar]
- 76.Andersson M, Andersson P, Pipkorn U. Topical glucocorticosteroids and allergen-induced increase in nasal reactivity: relationship between treatment time and inhibitory effect. J Allergy Clin Immunol. 1988;82:1019–26. doi: 10.1016/0091-6749(88)90139-x. [DOI] [PubMed] [Google Scholar]
- 77.Andersson M, Pipkorn U. Inhibition of the dermal immediate allergic reaction through prolonged treatment with topical glucocorticoids. J Allergy Clin Immunol. 1987;79:345–9. doi: 10.1016/0091-6749(87)90153-9. [DOI] [PubMed] [Google Scholar]
- 78.Pipkorn U, Hammarlund A, Enerback L. Prolonged treatment with topical glucocorticoids results in an inhibition of the allergen induced weal-and-flare response and a reduction in skin mast cell numbers and histamine content. Clin Exp Allergy. 1989;19:19–25. doi: 10.1111/j.1365-2222.1989.tb02338.x. [DOI] [PubMed] [Google Scholar]
- 79.Narasimha SK, Srinivas CR, Mathew AC. Effect of topical corticosteroid application frequency on histamine-induced wheals. Int J Dermatol. 2005;44:425–7. doi: 10.1111/j.1365-4632.2005.02482.x. [DOI] [PubMed] [Google Scholar]
- 80.Fisher AA. Esoteric contact dermatitis. Part IV: Devastating contact dermatitis in India produced by American parthenium weed. (the scourge of India) Cutis. 1996;57:297–8. [PubMed] [Google Scholar]
- 81.Gallant C, Kenny P. Oral glucocorticoids and their complications. A review. J Am Acad Dermatol. 1986;14:161–77. doi: 10.1016/s0190-9622(86)70018-2. [DOI] [PubMed] [Google Scholar]
- 82.Pasricha JS. Story of pulse therapy in pemphigus and other dermatoses. In: Shankar PS, Biradar PM, editors. Advances in Dermato-Venereo-Leprology. Gulbarga: South Zone conference of IADVL; 1992. pp. 53–60. [Google Scholar]
- 83.Smith JA, Mansfield LE, DeShazo RD, Nelson HS. An evaluation of the pharmacologic inhibition of the immediate and late cutaneous reactions to allergen. J Allergy Clin Immunol. 1980;65:185. [Google Scholar]
- 84.Raj A, Lakshmi C, Srinivas CR. Olopatadine suppresses the late phase reaction in parthenium dermatitis. Indian J Dermatol Venereol Leprol. doi: 10.4103/0378-6323.86491. (in press) [DOI] [PubMed] [Google Scholar]
- 85.Scow DT, Luttermoser GK, Dickerson KS. Leukotriene inhibitors in the treatment of allergy and asthma. Am Fam Physician. 2007;75:65–70. [PubMed] [Google Scholar]
- 86.Lakshmi C, Srinivas CR. Montelukast does not inhibit the late phase reaction in Parthenium dermatitis. Indian J Dermatol. 2009;54:94. doi: 10.4103/0019-5154.49006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shanmuga SC, Shanmugasundaram V, Srinivas CR. Parthenium dermatitis in an HIV patient: A case report. Indian J Dermatol. 2009;54:41–2. [Google Scholar]
- 88.Srinivas CR. Parthenium dermatitis treated with azathioprine weekly pulse doses. Indian J Dermatol Venereol Leprol. 2006;72:234. doi: 10.4103/0378-6323.25791. [DOI] [PubMed] [Google Scholar]
- 89.Sharma VK, Bhat R, Sethuraman G, Manchanda Y. Treatment of parthenium dermatitis with methotrexate. Contact Dermatitis. 2007;57:118–9. doi: 10.1111/j.1600-0536.2006.00950.x. [DOI] [PubMed] [Google Scholar]
- 90.Rowan MG. Preparation of plant extracts. In: Lovell CR, editor. In: Plants and the Skin. 1st edition. Oxford: Blackwell Scientific Publications; 1993. pp. 101–105. [Google Scholar]
- 91. [Accessed on May 8, 2012]. http://www.weeds.gov.au/government/roles/state.html .


