Recommendations developed by an international panel of multidisciplinary experts for the management of patients with liver metastases from colorectal cancer are presented.
Keywords: Biochemotherapy, Chemotherapy, Colorectal cancer, Hepatectomy, Hepatobiliary multidisciplinary teams, Imaging, Liver metastases, Resection, Targeted treatment
Abstract
An international panel of multidisciplinary experts convened to develop recommendations for the management of patients with liver metastases from colorectal cancer (CRC). The aim was to address the main issues facing the CRC hepatobiliary multidisciplinary team (MDT) when managing such patients and to standardize the treatment patients receive in different centers. Based on current evidence, the group agreed on a number of issues including the following: (a) the primary aim of treatment is achieving a long disease-free survival (DFS) interval following resection; (b) assessment of resectability should be performed with high-quality cross-sectional imaging, staging the liver with magnetic resonance imaging and/or abdominal computed tomography (CT), depending on local expertise, staging extrahepatic disease with thoracic and pelvic CT, and, in selected cases, fluorodeoxyglucose positron emission tomography with ultrasound (preferably contrast-enhanced ultrasound) for intraoperative staging; (c) optimal first-line chemotherapy—doublet or triplet chemotherapy regimens combined with targeted therapy—is advisable in potentially resectable patients; (d) in this situation, at least four courses of first-line chemotherapy should be given, with assessment of tumor response every 2 months; (e) response assessed by the Response Evaluation Criteria in Solid Tumors (conventional chemotherapy) or nonsize-based morphological changes (antiangiogenic agents) is clearly correlated with outcome; no imaging technique is currently able to accurately diagnose complete pathological response but high-quality imaging is crucial for patient management; (f) the duration of chemotherapy should be as short as possible and resection achieved as soon as technically possible in the absence of tumor progression; (g) the number of metastases or patient age should not be an absolute contraindication to surgery combined with chemotherapy; (h) for synchronous metastases, it is not advisable to undertake major hepatic surgery during surgery for removal of the primary CRC; the reverse surgical approach (liver first) produces as good an outcome as the conventional approach in selected cases; (i) for patients with resectable liver metastases from CRC, perioperative chemotherapy may be associated with a modestly better DFS outcome; and (j) whether initially resectable or unresectable, cure or at least a long survival duration is possible after complete resection of the metastases, and MDT treatment is essential for improving clinical and survival outcomes. The group proposed a new system to classify initial unresectability based on technical and oncological contraindications.
Introduction
Colorectal cancer (CRC) is the third leading cause of cancer death in the world [1]. In 2008, >1.2 million new cases were recorded worldwide, with estimated age-standardized incidence and mortality rates, respectively, of 29.6% and 12.4% in Europe, 29.2% and 8.8% in the U.S., and 12.9% and 6.6% in Asia [1]. In some countries, the mortality rate from CRC is continuing to rise [1]. In Asia, many countries, including China, Japan, South Korea, and Singapore, have experienced a two- to fourfold increase in the incidence of CRC in the past couple of decades [2]. In many European Union countries, however, there has been a slight decrease in mortality from CRC [1], probably related to the introduction of screening programs but possibly also influenced by the greater efficacy of treatment and higher resection rates. In the U.K., following the introduction of screening, detection of stage I and II CRC increased compared with stage III/IV disease in the unscreened population. It is estimated that screening may reduce mortality from CRC by 40% and will also have the benefit of reducing treatment costs. Not all countries, however, currently have screening programs.
About 25% of patients present with stage IV CRC (synchronous metastases) and ∼50% of patients overall develop liver metastases. About 85% of patients with stage IV CRC have liver disease considered unresectable at presentation [3–5]. The 10-year survival rate for patients with stage I disease is ∼90%, but for patients with inoperable stage IV disease, it is currently only ∼5% [4]. For patients with liver metastases, the treatment strategy should be directed toward resectability [6]. A need has been recognized for a new staging system that acknowledges the improvements in surgical techniques for resectable metastases and the impact of modern chemotherapy on rendering initially unresectable liver metastases from CRC resectable while distinguishing between patients with a chance for cure at presentation and those for whom only palliative treatment is possible [5].
An international group of experts in managing liver metastases from CRC convened to discuss strategies to optimize treatment outcomes. The aims of this meeting were to address certain management issues that are currently under debate and to reach a consensus on the contraindications to initial liver resection so that patients presenting with such disease may be offered a similar standard of treatment wherever they are treated.
Methods
The international consensus panel from the U.S., Europe, and Asia comprised one coordinator, five medical oncologists (including two hepatogastroenterologists), five hepatic surgeons, three radiologists, and a pathologist, with experience in the management of liver metastases from CRC. The coordinator selected the experts based on their experience. All important items related to multidisciplinary team (MDT) management of liver metastases from CRC were selected prior to the meeting by the coordinator and referred to an expert for presentation at the meeting. Meta-analyses, randomized controlled trials, and studies evaluating clinical practice in the management of liver metastases from CRC were identified and reviewed before and discussed during the meeting. After discussion, specific controversial issues were submitted to a vote of each expert to reflect the state of consensus. Recommendations were formulated when approved by all or a large majority of the panel members (Table 1). The strength of the recommendations was attributed based on the Strength of Recommendation Taxonomy [7]. The meeting was supported by Serono Symposia International Foundation, Rome, Italy.
Table 1.
Questions about liver metastases from colorectal cancer addressed by the panel and recommendations from the discussions

aAttributed based on the Strength of Recommendation Taxonomy [7]: A, recommendation based on consistent and good-quality patient-oriented evidence; B, recommendation based on inconsistent or limited-quality patient-oriented evidence; C, recommendation based on consensus, usual practice, opinion, disease-oriented evidence, or case series for studies of diagnosis, treatment, prevention or screening.
Abbreviations: CEUS, contrast-enhanced ultrasound; CRC, colorectal cancer; CT, computed tomography; FDG-PET, 18F-fluorodeoxyglucose positron emission tomography; MRI, magnetic resonance imaging; RECIST, Response Evaluation Criteria in Solid Tumors; US, ultrasound.
Role of Imaging in the Diagnosis of Liver Metastases
In the management of liver metastases from CRC, imaging is used to detect and characterize liver lesions, to aid surgical decision making, and to help choose the best treatment option. Practice varies among institutions, but evidence suggests that the best methods for detection of liver metastases from CRC are computed tomography (CT) and magnetic resonance imaging (MRI) [8–10]. However, many teams alternate liver ultrasonography (US) and CT for detection to decrease irradiation resulting from repeated CT. A meta-analysis of 39 articles (3,391 patients) published in 2010 comparing CT, MRI, and 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET) showed sensitivity estimates of 74.4%, 80.3%, and 81.4%, respectively, on a per lesion basis, and 83.6%, 88.2%, and 94.1%, respectively, on a per patient basis [9]. Data on FDG-PET–CT were too limited for comparison with other modalities. For lesions <10 mm, MRI was a more sensitive modality than CT. Similar results were reported in another meta-analysis published in 2010, which showed that MRI had better sensitivity than CT both in per patient (81.1% versus 74.8%; p = .05) and in per lesion (86.3% versus 82.6%; p < .0001) analyses [8]. However, these conclusions should be subject to caution because, as a result of the stringent inclusion criteria, only a few studies were derived from a large body of work. Many of the articles cited either did not indicate the CT technique or used suboptimal parameters. In general, meta-analyses reflect average practices and not state-of-the-art techniques; consequently, many of the technical parameters of the cited studies have been superseded.
For characterization of focal liver lesions, CT, contrast-enhanced ultrasound (CEUS), and MRI can be used [11]. MRI is the best technique for characterizing lesions and is often used as a means of additional imaging assessment. The serial change in liver lesions after neoadjuvant chemotherapy is also a very helpful means of tumor characterization. Resectability is dependent on multiple factors, including the number and location of metastases, volume of the future liver remnant (FLR), and quality of the nontumoral liver. All lesions identified on the prechemotherapy scan need to be accounted for on the presurgical scan to limit the risk for missed metastases. It is recognized that chemotherapy can induce toxic injury to the liver, primarily steatohepatitis and sinusoidal injury. Noncontrast CT and MRI may be used to assess steatosis [12–14], but steatohepatitis cannot be diagnosed with imaging. Sinusoidal injury can be judged by indirect signs of portal hypertension, particularly spleen size [15], or by using the liver-specific MRI contrast agent gadoxetic acid [16].
Complete resection is possible if liver vascularity can be preserved, the FLR is adequate with reference to body weight and total liver volume, and the quality of the remnant liver parenchyma is acceptable [17]. MRI and high-quality CT can be used for preoperative imaging; the choice of modality is dependent on the precise point in the clinical pathway and local expertise. US is indicated for intraoperative assessment of resectability [18]. When used intraoperatively, CEUS has been shown to reveal more lesions than conventional US [18], and was significantly more sensitive (p < .05) than CT and/or MRI and conventional US in detecting liver metastases [19].
For the detection of extrahepatic metastases and local recurrence at the site of the initial colorectal surgery, CT and FDG-PET are available. Whereas high-quality CT can detect the majority of extrahepatic disease, FDG-PET may reveal additional disease or high metabolic activity of concern in indeterminate lesions. Initial studies showed a change in management in 10%–20% of patients following FDG-PET [20–23], whereas a recent prospective randomized trial evaluating high-quality CT and FDG-PET involving 263 patients showed only a 7.6% change in management following FDG-PET [24]. Thus, the size of the advantage of FDG-PET is uncertain, and the most cost-effective role for FDG-PET remains to be defined. It may be used for patients at higher risk for extrahepatic disease or as a problem-solving modality. It should be borne in mind that the sensitivity of FDG-PET is lower following neoadjuvant chemotherapy.
Consensus Recommendations
The group recommended high-quality MRI and/or CT for mapping liver metastases preoperatively, depending on local expertise.
US is useful for intraoperative staging and contrast-enhancement may improve the sensitivity of intraoperative US.
High-quality CT of the chest and pelvis is recommended for detecting extrahepatic metastases.
FDG-PET may provide additional information, mainly in patients with a high risk for extrahepatic disease, but there is currently no consensus as to the patient population with the most to gain, and current evidence is not considered strong enough to recommend its use in all patients.
Optimal Chemotherapy
Chemotherapy may be used for both unresectable and resectable disease. Chemotherapy given to convert unresectable liver metastases to resectable is referred to as “conversion chemotherapy.” The term “neoadjuvant chemotherapy” is reserved for chemotherapy for resectable and potentially resectable disease prior to surgery with or without adjuvant chemotherapy after surgery. One randomized trial demonstrated a higher progression-free survival (PFS) rate 3 years after liver resection using perioperative chemotherapy (i.e., before or after surgery) than with surgery alone [25], and it has been recommended that most patients with liver metastases from CRC be treated up front with chemotherapy, irrespective of the initial resectability of their metastases. However, in that study, all patients had resectable disease at the time of diagnosis and, although the results are encouraging, issues such as the true benefit of perioperative chemotherapy in terms of the overall survival (OS) time and its role in liver injury remain to be addressed. In addition, a recent multicentric retrospective study has not revealed any benefit in terms of OS or PFS of neoadjuvant chemotherapy in single metachronous liver metastases as opposed to a positive effect of adjuvant chemotherapy [26].
Without treatment, surgical resection is not possible in 70%–90% of patients with liver metastases from CRC. The prognosis of CRC patients is poor if metastases cannot be removed surgically; the aim of conversion chemotherapy is to achieve resectability rather than a complete response. When chemotherapy is initiated to achieve resectability, the aim should be for as short a treatment course as possible and surgery performed as soon as the metastases become resectable [27].
In the last 10 years, the overall survival (OS) times of patients with metastatic CRC have improved substantially [28] and largely reflect the increased number of available therapies. Before 2000, 5-fluorouracil (5-FU) was the only chemotherapeutic option. From 2000 onward, oxaliplatin and irinotecan became available and doublet cytotoxic regimens became standard therapy. Greater response rates have been reported with chemotherapy with doublet regimens than with single fluoropyrimidine-based regimens [4, 29], with complete resection (R0) rates of 11%–33% reported following chemotherapy with the leucovorin, 5-FU, and oxaliplatin (FOLFOX) or with leucovorin, 5-FU, and irinotecan (FOLFIRI) regimens [30–33]. Among 131 consecutive patients who underwent liver resection for multiple (four or more) liver metastases from CRC after systemic chemotherapy in 1993–2000, the 5-year survival rate of patients who received preoperative chemotherapy with 5-FU and leucovorin, combined with either oxaliplatin or irinotecan or both, was better in those achieving an objective response (37%) than in those with stable disease (30%) or disease progression (8%) [34]; 23% of patients in that study received two or three lines of chemotherapy and 24% had extrahepatic metastases. Similar results were observed in 4,851 patients from a prospective multi-institutional database—the LiverMetSurvey (Fig. 1). The use of triplet cytotoxic combinations with 5-FU plus both oxaliplatin and irinotecan (FOLFOXIRI) has translated into higher response rates and resectability rates, at least in one randomized study [35]. Hepatic arterial infusion (HAI) of chemotherapy may also provide a second chance to remove initially unresectable liver metastases from CRC [36, 37], even in patients heavily pretreated with chemotherapy [38].
Figure 1.
Overall survival probability in relation to response to preoperative chemotherapy in 4,851 patients undergoing a first resection of colorectal liver metastases from the LiverMetSurvey [78]. Reproduced with permission from http://www.livermetsurvey.org, June 2011.
More recently, treatment for metastatic CRC has moved toward more targeted therapy, with the availability of monoclonal antibodies targeting the epidermal growth factor receptor, for example, cetuximab and panitumumab, for which wild-type KRAS is a predictive biomarker, and vascular endothelial growth factor, for example, bevacizumab, which acts to inhibit angiogenesis. Studies (Cetuximab Combined With Irinotecan in First-Line Therapy for Metastatic Colorectal Cancer and Oxaliplatin and Cetuximab in First-Line Treatment of mCRC) have shown that the addition of cetuximab to doublet cytotoxic chemotherapy results in a significantly higher response rate in patients with wild-type KRAS tumors [39–41], and it was associated with a relevant greater R0 resection rate [39] without a higher rate of surgical or postoperative complications. Although the Medical Research Council Continuous Chemotherapy plus Cetuximab or Intermittent Chemotherapy trial did not confirm a benefit of the addition of cetuximab to oxaliplatin plus fluoropyrimidine chemotherapy in the first-line treatment of patients with advanced CRC, cetuximab resulted in a higher response rate in patients with wild-type KRAS tumors [42]. In the Cetuximab in Neoadjuvant Treatment of Non-Resectable Colorectal Liver Metastases trial, the rate of resectability was higher in patients with wild-type KRAS tumors when treated with the combination of FOLFOX or FOLFIRI plus cetuximab, although the R0 resection rates were similar in patients with wild-type KRAS and mutated KRAS tumors (34% versus 33%, respectively) [43]. According to a retrospective review, resectability rates in that study increased from 32% (22 of 68 patients) at baseline to 60% (41 of 68 patients) after chemotherapy with cetuximab (p < .0001) [43]. In the Preoperative Chemotherapy for Hepatic Resection study of FOLFOXIRI plus cetuximab in 41 patients with liver metastases from CRC, the overall response rate was 79% and resections were performed in 60% of patients [44]. Other recent studies, such as the NORDIC VII study, however, have shown less positive results with cetuximab as first-line therapy, even in patients with wild-type KRAS disease [45]. Studies with panitumumab have also shown slightly higher response rates with first- and second-line therapy, but the R0 resection rate was not higher [46–48].
Efficacy data on bevacizumab conversion treatment are also available. In the NO16966 trial, a slightly higher hepatic R0 resection rate (12.3% versus 11.6%) than with placebo was reported when bevacizumab was added to 5-FU or capecitabine plus oxaliplatin in patients with liver-only disease [49]. In the first Bevacizumab Expanded-Access Trial, the numbers of patients undergoing surgery with curative intent were 153 of 949 (16.1%) patients receiving oxaliplatin plus bevacizumab chemotherapy and 64 of 662 (9.7%) patients receiving irinotecan plus bevacizumab chemotherapy; R0 hepatic resection was performed in 76 of 949 (8.0%) and 34 of 662 (5.1%) patients, respectively [49]. In the Bevacizumab, Oxaliplatin, Xeloda® in Unresectable Liver Metastases study, in which bevacizumab was given with capecitabine plus oxaliplatin in patients with borderline, technically unresectable, liver-only metastases from CRC, 40% of patients were converted to resectability [50]. Furthermore, bevacizumab added to FOLFOXIRI achieved promising results in terms of the response rate and PFS interval, without unforeseen adverse events [51]. In addition, bevacizumab added to FOLFOX has been associated with a significantly higher frequency of complete or major response in patients undergoing hepatic resection following neoadjuvant chemotherapy [52].
Intra-arterial chemotherapy has been in use for some time, has clear efficacy, at least in tumor control [53, 54], and is associated with a lower risk for hepatic recurrence [55, 56]. In patients with unresectable liver metastases from CRC and a history of systemic chemotherapy failure, resection became possible in seven of 44 patients following bimonthly HAI of oxaliplatin combined with leucovorin and 5-FU [57]. After multidrug chronomodulated HAI as rescue treatment in heavily pretreated patients, resection became possible in four of 29 patients [38]. HAI may also be used as adjuvant therapy following resection [58]. However, HAI is not commonly used, and trials in progress will better define the place of HAI and, similarly, drug-eluting beads and radioembolization as bridges to surgery for liver metastases from CRC.
There is a need for further clinical trials studying the combination of targeted agents with effective triplet chemotherapy regimens. In addition, studies are warranted in patients with CRC and initially unresectable liver metastases that focus on different clinical presentations, such as patients with nonresectable disease that is potentially resectable if tumor shrinkage occurs, compared with those assessed as never resectable.
Following resection, in patients who have received a limited number of preoperative chemotherapy cycles, adjuvant chemotherapy should be considered. Because trials of adjuvant chemotherapy in patients with stage III CRC have not shown benefits of targeted agents [59–61] or irinotecan [62], such therapies cannot be recommended. Furthermore, there is no consensus for adjuvant chemotherapy in patients with resection of metachronous metastases following oxaliplatin-based adjuvant chemotherapy for the primary cancer.
Consensus Recommendations
The group agreed that the aim of conversion chemotherapy is to achieve resectability rather than a complete response. When there is a potential for resectability, at least four courses of chemotherapy should be given first line and, if progression occurs during first-line therapy or only stable disease is achieved after 4 months, second-line treatment should be considered.
The optimal timing for assessment of response to chemotherapy was considered by the group to be every 2 months.
The group agreed that preoperative treatment to induce resectability should be as short as possible and that postoperative chemotherapy should continue.
Using oxaliplatin regimens is possible for patients who do not progress during adjuvant oxaliplatin-based treatment after resection of the primary CRC.
Overall, a total duration of 6 months of perioperative (preoperative and adjuvant) chemotherapy is recommended.
Role of Imaging in Evaluation of Response to Chemotherapy
In the clinical setting, response to chemotherapy using imaging modalities can be judged from three perspectives: change in tumor size, morphological changes unrelated to size, and metabolic activity. Size-based criteria have included the World Health Organization criteria [63], original Response Evaluation Criteria in Solid Tumors (RECIST) [64], and revised RECIST [65], which use unidimensional criteria to formulate a response rate. One of the shortcomings of the RECIST is the arbitrary choice of cutoff values that define a response [66]. Two recent studies in patients with liver metastases from CRC showed that an early decrease in size by 10% correlates better with outcome than the required 30% decrease using the RECIST [66, 67], suggesting that the cutoff value and optimal time for evaluation need to be reappraised. Of interest, a 10% decrease in size is also the cutoff value used for the Choi criteria in the evaluation of gastrointestinal stromal tumors [68].
Morphological changes that are nonsize-based have been described for assessing response to biological agents, which have a predominantly cytostatic mechanism of action. These observations form the basis of the Choi criteria and the recently described morphological response criteria for hepatic metastasis from CRC [69]. These new criteria are based on the subjective evaluation of changes in tumor texture and margins. Responding tumors evolve from a complex, heterogeneous solid mass to a pseudocystic mass, and response is classified as optimal, incomplete, or absent. In a study of 234 liver metastases from CRC from 50 patients who underwent hepatic resection after neoadjuvant chemotherapy that included bevacizumab, morphological criteria correlated with the pathological response and OS outcomes and were a better surrogate for pathological response than the RECIST [69]. In a validation cohort of 82 patients with unresectable diseases, an optimal morphological response was associated with a median OS duration of 31 months (95% confidence interval [CI], 26.8–35.2 months), compared with 19 months (95% CI, 14.6–23.4 months) in patients with an incomplete or no morphological response (p = .009), whereas the RECIST did not correlate with the survival outcome in either the surgical or the validation cohort [69].
Metabolic activity is currently evaluated primarily with FDG-PET–CT. Preliminary studies suggest that the degree of chemotherapy-induced change in tumor glucose metabolism is predictive of patient outcome, and that the use of FDG-PET for therapy monitoring is clinically feasible [70, 71]. The role of PET in monitoring the response to treatment for patients with liver metastases from CRC, however, remains to be further defined. Other biomarkers that can detect response to treatment prior to change in lesion size, such as perfusion CT and diffusion-weighted MRI, are also under evaluation for assessing treatment response of liver metastases from CRC [72].
The disappearance of metastases on CT and MRI scans qualifies as a complete radiographic response using the RECIST but is not an indication of a complete pathological response [73]. Similarly, the lack of FDG uptake or complete response using PET does not imply complete pathological response [74]. Because some lesions may disappear or may be hardly detectable with intraoperative ultrasonography (IOUS) following neoadjuvant chemotherapy, a technique to mark small lesions with coils before chemotherapy has been successful [75].
Consensus Recommendations
Changes in tumor size (RECIST) adequately evaluate the efficacy of conventional chemotherapy, but morphological response criteria correlate better with pathological response and OS outcomes than the RECIST in patients who have received antiangiogenic agents. Factors that are important for the evaluation of response to chemotherapy and for preoperative staging include high-quality prechemotherapy imaging and careful comparison of prechemotherapy and presurgical scans.
Surgical Management
Resectable Patients
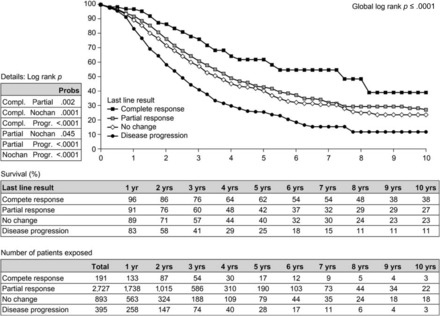
Surgical resection remains the only treatment associated with a long survival time in patients with liver metastases from CRC, with a 40% survival at 5 years and almost 25% of patients demonstrating a postoperative survival duration up to 10 years in specialized centers [76, 77]. Results from the LiverMetSurvey, involving 13,334 patients from 330 centers in 58 countries who underwent surgery for liver metastases, show a better survival outcome in patients who undergo first resection of liver metastases than in those who do not (Fig. 2) [78]. A recent systematic review of 142 studies published in 1999–2010 confirmed these results and revealed 5-year survival rates for patients with liver metastases in the range of 16%–71% (median, 38%) after liver resection [79]. Although advances in diagnostic techniques and treatment strategies have improved resectability rates and surgical outcomes over the past 10 years, most patients with hepatic metastases from CRC remain ineligible for potentially curative surgical resection [80].
Figure 2.
Overall survival probability after a first resection for colorectal liver metastases in 14,774 patients from the LiverMetSurvey [78]. Reproduced with permission from http://www.livermetsurvey.org, June 2011.
Initially Unresectable Patients
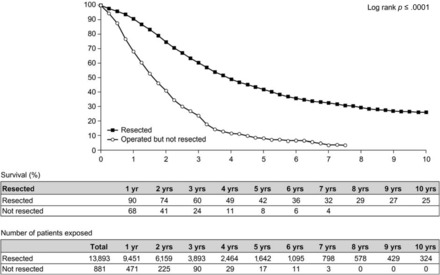
Until relatively recently, patients with unresectable disease were treated with palliative chemotherapy [81]. A range of strategies has now been developed to render a patient's disease surgically resectable (e.g., portal vein embolization, neoadjuvant chemotherapy, hepatectomy combined with radiofrequency ablation, two-stage hepatectomy) [82, 83]. Portal vein embolization is indicated when the FLR as a proportion of total liver volume is 20%–30% in patients with a normal liver and ≤40% in patients who have had extensive chemotherapy [17, 84]. The efficacy of conversion chemotherapy has increased dramatically in recent years, and it has become the best means of downsizing tumoral disease and converting patients with unresectable disease to resectability [30]. Although survival times following conversion chemotherapy and surgery in patients with initially unresectable disease are lower than those in patients undergoing primary liver resection [30, 78] (Fig. 3), they are greater than when resection is not performed [30]. However, the tumor eradication process generally involves more than one procedure in the majority of patients [30].
Figure 3.
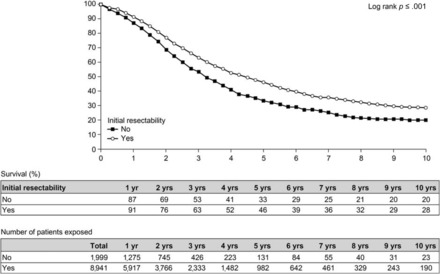
Overall survival probability after resection of initially unresectable versus nonresectable liver metastases. Data from 10,940 patients in the LiverMetSurvey [78]. Reproduced with permission from http://www.livermetsurvey.org, June 2011.
The current definition of resectability includes the potential for complete resection with tumor-free margins (R0 resection), with preservation of at least two disease-free liver segments with viable vascular inflow, outflow, and biliary drainage and an FLR volume of 30% [85]. Some authors, however, set the safety limit to a 20%–25% FLR volume [80, 86]. Further clarification of categories of nonresectability is crucial; for example, differentiation between disease that is technically nonresectable and disease that is oncologically nonresectable (e.g., presence of other metastases in the lung). Furthermore, many patients with liver metastases from CRC who now satisfy the revised resection criteria are in high-risk groups associated with poor outcomes. As the number of high-risk patients undergoing liver resection continues to increase, a better understanding of the associated risk–benefit ratio is needed to prevent unnecessary surgery. Several independent prognostic risk factors have been identified, including the number of hepatic metastases, node-positive primary tumors, poorly differentiated primary tumors, extrahepatic disease, tumor diameter, carcinoembryonic antigen levels, and positive resection margins [77, 79]. However, none of these risk factors represents an absolute contraindication to hepatic resection provided that surgery is able to remove all the tumoral disease. In particular, a large number of liver metastases should not be a contraindication because resection of multiple bilobar hepatic metastases has demonstrated survival benefits in selected cases [87]. Increasing interest in predictive molecular markers, such as KRAS and BRAF, will also help stratify patient populations to targeted therapy [67, 88–91]. However, more data are needed before widespread use of such biomarkers is implemented.
Approximately 20%–30% of newly diagnosed cases of CRC have identifiable synchronous liver metastases [92]. There is currently no standard of care for treating synchronous liver metastases from CRC, and no consensus has been reached concerning the timing of primary and metastatic tumor resections. Although synchronous liver metastases from CRC are traditionally treated with a two-stage resection, recent improvements in perioperative care and postsurgical morbidity and mortality favor simultaneous resection in cases in which the primary tumor is in the colon and the number of hepatic metastases is limited. Resection of liver metastases first (reverse treatment) is considered the best option when hepatic disease is predominant and when the primary tumor is asymptomatic or symptoms are easy to manage [93, 94]. This approach can be performed with acceptable perioperative mortality and morbidity rates. Although this represents a highly selected group of patients with synchronous liver metastases from CRC, particularly when the primary tumor is predominantly in the rectum, requiring a neoadjuvant treatment strategy, a considerable OS benefit can be achieved [95].
Separate resections are generally favored in cases of rectal primary tumors and cases of multiple metastatic sites [96–98]. The surgical mortality rate is significantly higher when surgery of extensive hepatic resections is combined with colorectal resection [87].
In patients with extensive bilateral (bilobar) hepatic metastases with some parenchymal sparing, chemotherapy should be followed by consideration of two-stage hepatectomy, a procedure first described in 2000 [99]. With this approach, left liver metastases are usually resected separately (first-stage resection) with or without portal vein embolization followed by right or extended right hepatectomy (second-stage resection) [100, 101]. Resection of the primary can be combined with the left hemiliver clearance during the first stage because the hepatectomy is usually limited to fewer than three segments. The two-stage approach has been shown to be associated with a better survival outcome than with chemotherapy without surgery in patients with liver-only disease and an objective response to chemotherapy: the 5-year OS rates were 51% versus 15%, respectively (p = .005) [102]. The surgical mortality rate is significantly higher when surgery of extensive hepatic resections is combined with colorectal resection [87, 103].
Increasingly more patients with unresectable liver metastases from CRC are being treated with a combination of systemic chemotherapy and radiofrequency ablation (RFA). A randomized phase II trial of 119 patients with liver-limited disease demonstrated a significantly longer median PFS interval with than without RFA (p = .025) [104]. However, the survival results with RFA or RFA combined with resection remain inferior to those with resection in patients with solitary or multiple liver metastases [105, 106].
Consensus Recommendations
Surgery remains the best treatment option for a long survival duration when R0 resection can be achieved with preservation of a functioning liver remnant of 25%–30%.
Conversion chemotherapy results in a higher resectability rate and greater survival probability in patients with initially unresectable tumors.
Strategies to help achieve R0 resection include portal vein embolization, staged hepatectomies, and hepatectomies with RFA. The safety of major hepatic resection during surgery for the primary CRC is still controversial.
Separate resections are favored over synchronous resections with rectal surgery because of the higher morbidity and mortality rates associated with the latter. The reverse surgical approach (liver first) produces as good an outcome as the conventional approach.
A large number of liver metastases should not be an absolute contraindication to surgery combined with chemotherapy provided that resection can be complete.
Surgery may be performed in more than one procedure (two-stage hepatectomy) when resection of all liver metastases cannot be achieved in a single procedure. Repeated hepatectomy or thermal ablation of liver recurrences can prolong the hepatic DFS interval.
Pathological Response
Pathological response to neoadjuvant chemotherapy has shown prognostic potential in patients with liver metastases from CRC, and consequently it has been integrated as an endpoint in several studies [107–109]. The degree of pathological response has been shown to vary depending on the chemotherapy regimen used [107, 108].
In addition to a pathological complete response, in which no residual tumor cells can be observed, other ways of scoring pathological response have been proposed, including tumor regression grades [107, 110] and a pathological response grade [108, 111, 112]. The first evaluates the ratio of residual viable tumor cells to fibrous tissue within the tumor and has the advantage of being the same scoring system as the one used for primary colorectal tumors by the American Joint Committee on Cancer (the tumor–node–metastasis staging system), allowing easy comparison of response between the primary tumor and metastases. The latter is based on the percentage of viable tumor cells. Both scoring systems evaluate the amount of residual tumor cells rather than the change in tumor size; even if a tumor appears to shrink in size, the number of viable tumor cells may not decrease proportionally. Both scoring systems have shown prognostic value [107, 110–112].
Pathological examinations can also reveal the mechanisms underlying chemotherapy-associated liver injuries, which can potentially result in higher postoperative morbidity rates and, in rare cases, patient death [113]. For example, oxaliplatin-based regimens have been linked to toxic injury to endothelial cells, resulting in sinusoidal obstruction syndrome (SOS, formerly known as veno-occlusive disease), characterized by sinusoidal wall disruption, and can be complicated by fibrosis or nodular regenerative hyperplasia (NRH) and a greater risk for bleeding during surgery or poor liver function or reserve [114, 115]. The addition of bevacizumab has been associated with a lower incidence of oxaliplatin-induced SOS and NRH lesions [116]. Similarly, irinotecan has been linked to the development of steatohepatitis, a type of nonalcoholic fatty liver disease [117, 118], and 5-FU plus leucovorin and/or irinotecan are associated with a higher incidence of hepatic steatosis, particularly in patients with a body mass index >25 kg/m2 [119, 120]. Many patients with liver metastases from CRC are only referred for surgical resection following numerous prior chemotherapy regimens, but accumulated hepatic toxicity may mean that curative surgery is no longer possible in some of these patients, highlighting the importance of coordinated efforts among medical oncologists, surgeons, and patients to optimize disease management [121].
Tumor margins for resection can be difficult to define. Most residual tumor cells after chemotherapy are seen in the periphery of the tumor at the interface between the tumor and non-neoplastic liver tissue [107]. A recent study of 22 patients with advanced bilateral liver metastases from CRC found that half of the patients had tumor regrowth at the periphery of the metastasis when neoadjuvant chemotherapy was interrupted, and this occurred regardless of tumor response. This peripheral tumor growth was found to be caused by a “dangerous halo” of proliferating tumor cells infiltrating the parenchyma surrounding the metastasis [122]. Tumor-specific DNA has also been detected up to 4 mm beyond the visible tumor margin [123]. However, surgical resection margins are currently selected using preoperative radiology, IOUS, and palpation, none of which take into account the possibility of a halo, increasing the risk for disease recurrence [122]. The use of two-stage hepatectomies may help to reduce the risk for recurrence by allowing pathological assessment between resections [102]. Lesions that have disappeared on imaging after chemotherapy have been found to contain viable tumor cells when resected [73]. The development of a fatty liver while being treated with chemotherapy may contribute to the disappearance of liver metastases on multidetector CT, particularly if a portal venous phase acquisition alone is obtained; in these circumstances, MRI is particularly advantageous [124]. Surgical oncologists are often faced with the dilemma of whether to resect all areas that previously contained definite metastases in order to prevent missing undetected metastases or to resect only those areas where metastatic lesions remain visible [121]. MRI, particularly with diffusion sequences, can currently detect small remnant metastases that are not visible with other imaging modalities. Although a consensus emerged to attempt resection of previous metastatic sites, recent experience in highly chemosensitive patients, especially after adjuvant HAI, showed that about two thirds of disappearing lesions did not recur [125]. When disappearing metastases occur, adjuvant intra-arterial chemotherapy is probably the best means to avoid them recurring [126].
Consensus Recommendations
Resection is best performed on the basis of the site of metastasis on the prechemotherapy CT or MRI scan. As an alternative, when disappearing metastases are deep and undetectable even intraoperatively, a wait-and-see policy may be employed with follow-up every 2 months to see if the metastases become visible, and then resect. The management decision depends on the initial extent of the disease.
Assessments should include histological evaluation of tumor margins, evaluation of the nontumoral liver parenchyma, and evaluation of the pathological response to preoperative chemotherapy.
Is Cure Possible?
The group discussed the definition of cure and agreed that a precise definition is needed. Cure is usually defined by a 10-year survival time because relapse is unusual after 10 years from an R0 resection [127]. However, a DFS duration of 3 or 5 years should be explored as a marker for potential cure. It was also agreed that, in patients with metastatic CRC, cure is only possible after hepatic resection [128]. In patients with initially unresectable disease, chemotherapy can convert 16% of patients to resectability [129].
Consensus Recommendations
Cure is possible after complete resection regardless of whether the initial disease was resectable or unresectable.
MDTs
MDTs are an increasingly favored management approach for cancer care [130, 131], and it has been proposed that patients with liver metastases from CRC should be treated by an MDT whenever possible [27]. The MDT model is a patient-centered approach requiring a team of specialists, including at least surgeons, oncologists, radiologists, and pathologists, and depending on the institution size and availability of expertise, nurses, nutritionists, and a cancer coordinator may ideally form part of the MDT [132]. The team works together to identify appropriate tests and the best treatment options available under the guidance of a nominated team leader in regularly scheduled MDT meetings [132, 133]. This model also allows communication between local and more specialized physicians, whether in the form of a second opinion or patient referral. An essential part of managing an MDT involves regular assessment of team effectiveness to help optimize patient care [134]. Although there are few published data on the barriers to an effective MDT, potential barriers may include: (a) a lack of (or conflict regarding) leadership and coordination, including ambiguous team roles; (b) insufficient administrative support and implementation; (c) limited resources (e.g., expertise, time, associated costs, availability of meeting facilities, data management, and a coordinator); and (d) a lack of commitment and/or interest from team members and hospital staff [133]. However, despite the obstacles facing MDTs, several important patient benefits have been reported, including greater accuracy of disease staging [135], fewer treatment and referral delays [136, 137], individualized evidence-based practice for patients, greater continuity of care [138], enhanced quality of life [139], and better clinical and survival outcomes [140–146]. In addition, health care professionals and health services may benefit from MDTs through strengthened awareness, communication, and relationships among members and disciplines, increasing the opportunities for knowledge sharing and professional development (e.g., review sessions providing learning opportunities for staff) [136, 147, 148]. Other advantages include facilitation of clinical trial recruitment [149], minimization of duplication of effort, opportunities to pool resources and encourage crosstraining [150], and the potential to alleviate regional disparities in health care services.
Consensus Recommendations
Patients with liver metastases from CRC are best managed with a multidisciplinary approach. MDTs contribute many advantages and benefits in cancer care for the patient, health care professionals, and health care service providers. However, MDTs also involve several challenges.
Although there is a fundamental need to establish specialized MDTs, universal guidelines on implementation, dynamics, and monitoring are currently lacking, and recommendations from scientific societies would prove particularly valuable to sustain a performing MDT.
Conclusions
Resectability and survival rates are better in specialized centers that employ multidisciplinary specialists, including radiologists, pathologists, oncologists, and liver surgeons, than in nonspecialized centers. Thus, it is recommended that all patients with liver metastases be managed by specialized hepatobiliary MDTs to decide the best strategy, the main objective of which is to achieve surgical resection. The group considered that multidisciplinary treatment is essential for better clinical and survival outcomes. It is hoped that the proposed new system for deciding whether or not a patient is eligible for resection described in Table 2 will help to simplify treatment decisions and standardize treatment care across centers. In addition, the recommendations on a number of key issues—managing potentially resectable synchronous metastases, whether or not the prospect of surgery should influence the choice of first-line chemotherapy, whether or not there is a maximum number of metastases for achieving potentially curative surgery, the recommended action to be taken when there is a complete clinical response, guidance on the minimum number of chemotherapy cycles before surgery, and the respective role of preoperative and postoperative chemotherapy—should also help to raise the standard of care for patients with liver metastases from CRC.
Table 2.
Contraindications to hepatic resection in patients with colorectal cancer liver metastases

Any patient should be categorized as A1 or A2/B1, B2, or B3. This classification may help to clearly define the type of unresectable patients included in all clinical trials.
aIncludes all methods, including radiofrequency ablation.
Acknowledgments
The authors acknowledge Jane Davies, Laura McDonagh, and Catherine Kidd (Caudex Medical Ltd, Oxford, U.K., supported by Serono Symposia International Foundation, Rome, Italy) for editorial assistance in the development of the manuscript.
The consensus meeting from which this publication was written was supported by Serono Symposia International Foundation (SSIF), Rome, Italy.
Footnotes
- (C/A)
- Consulting/advisory relationship
- (RF)
- Research funding
- (E)
- Employment
- (H)
- Honoraria received
- (OI)
- Ownership interests
- (IP)
- Intellectual property rights/inventor/patent holder
- (SAB)
- Scientific advisory board
Author Contributions
Conception/Design: René Adam, Aimery de Gramont, Joan Figueras, Ashley Guthrie, Norihiro Kokudo, Francis Kunstlinger, Evelyne Loyer, Graeme Poston, Philippe Rougier, Laura Rubbia-Brandt, Alberto Sobrero, Josep Tabernero, Catherine Teh, Eric van Cutsem, Jean-Nicolas Vauthey
Provision of study material or patients: René Adam, Aimery de Gramont, Joan Figueras, Ashley Guthrie, Norihiro Kokudo, Francis Kunstlinger, Evelyne Loyer, Graeme Poston, Philippe Rougier, Laura Rubbia-Brandt, Alberto Sobrero, Josep Tabernero, Catherine Teh, Eric van Cutsem, Jean-Nicolas Vauthey
Collection and/or assembly of data: René Adam, Aimery de Gramont, Joan Figueras, Ashley Guthrie, Norihiro Kokudo, Francis Kunstlinger, Evelyne Loyer, Graeme Poston, Philippe Rougier, Laura Rubbia-Brandt, Alberto Sobrero, Josep Tabernero, Catherine Teh, Eric van Cutsem, Jean-Nicolas Vauthey
Data analysis and interpretation: René Adam, Aimery de Gramont, Joan Figueras, Ashley Guthrie, Norihiro Kokudo, Francis Kunstlinger, Graeme Poston, Philippe Rougier, Laura Rubbia-Brandt, Alberto Sobrero, Josep Tabernero, Catherine Teh, Eric van Cutsem, Jean-Nicolas Vauthey
Manuscript writing: René Adam, Aimery de Gramont, Joan Figueras, Ashley Guthrie, Norihiro Kokudo, Francis Kunstlinger, Evelyne Loyer, Graeme Poston, Philippe Rougier, Laura Rubbia-Brandt, Alberto Sobrero, Josep Tabernero, Catherine Teh, Eric van Cutsem, Jean-Nicolas Vauthey
Final approval of manuscript: René Adam, Aimery de Gramont, Joan Figueras, Ashley Guthrie, Norihiro Kokudo, Francis Kunstlinger, Evelyne Loyer, Graeme Poston, Philippe Rougier, Laura Rubbia-Brandt, Alberto Sobrero, Josep Tabernero, Catherine Teh, Eric van Cutsem, Jean-Nicolas Vauthey
References
- 1.Ferlay J, Shin HR, Bray F, et al. GLOBOCAN 2008, Version 1.2, Cancer Incidence and Mortality Worldwide. IARC CancerBase No. 10. [accessed November 23, 2011]. Available at http://globocan.iarc.fr.
- 2.Sung JJ, Lau JY, Goh KL, et al. Increasing incidence of colorectal cancer in Asia: Implications for screening. Lancet Oncol. 2005;6:871–876. doi: 10.1016/S1470-2045(05)70422-8. [DOI] [PubMed] [Google Scholar]
- 3.Nordlinger B, Van Cutsem E, Rougier P, et al. Does chemotherapy prior to liver resection increase the potential for cure in patients with metastatic colorectal cancer? A report from the European Colorectal Metastases Treatment Group. Eur J Cancer. 2007;43:2037–2045. doi: 10.1016/j.ejca.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 4.Van den Eynde M, Hendlisz A. Treatment of colorectal liver metastases: A review. Rev Recent Clin Trials. 2009;4:56–62. doi: 10.2174/157488709787047558. [DOI] [PubMed] [Google Scholar]
- 5.Poston GJ, Figueras J, Giuliante F, et al. Urgent need for a new staging system in advanced colorectal cancer. J Clin Oncol. 2008;26:4828–4833. doi: 10.1200/JCO.2008.17.6453. [DOI] [PubMed] [Google Scholar]
- 6.Aloia TA, Adam R, Azoulay D, et al. Outcome following hepatic resection of metastatic renal tumors: The Paul Brousse Hospital experience. HPB (Oxford) 2006;8:100–105. doi: 10.1080/13651820500496266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ebell MH, Siwek J, Weiss BD, et al. Strength of recommendation taxonomy (SORT): A patient-centered approach to grading evidence in the medical literature. Am Fam Physician. 2004;69:548–556. [PubMed] [Google Scholar]
- 8.Floriani I, Torri V, Rulli E, et al. Performance of imaging modalities in diagnosis of liver metastases from colorectal cancer: A systematic review and meta-analysis. J Magn Reson Imaging. 2010;31:19–31. doi: 10.1002/jmri.22010. [DOI] [PubMed] [Google Scholar]
- 9.Niekel MC, Bipat S, Stoker J. Diagnostic imaging of colorectal liver metastases with CT, MR imaging, FDG PET, and/or FDG PET/CT: A meta-analysis of prospective studies including patients who have not previously undergone treatment. Radiology. 2010;257:674–684. doi: 10.1148/radiol.10100729. [DOI] [PubMed] [Google Scholar]
- 10.Selzner M, Hany TF, Wildbrett P, et al. Does the novel PET/CT imaging modality impact on the treatment of patients with metastatic colorectal cancer of the liver? Ann Surg. 2004;240:1027–1034. doi: 10.1097/01.sla.0000146145.69835.c5. discussion 1035–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bartolotta TV, Taibbi A, Midiri M, et al. Characterisation of focal liver lesions undetermined at grey-scale US: Contrast-enhanced US versus 64-row MDCT and MRI with liver-specific contrast agent. Radiol Med. 2010;115:714–731. doi: 10.1007/s11547-010-0506-3. [DOI] [PubMed] [Google Scholar]
- 12.Kodama Y, Ng CS, Wu TT, et al. Comparison of CT methods for determining the fat content of the liver. AJR Am J Roentgenol. 2007;188:1307–1312. doi: 10.2214/AJR.06.0992. [DOI] [PubMed] [Google Scholar]
- 13.Reeder SB, Sirlin CB. Quantification of liver fat with magnetic resonance imaging. Magn Reson Imaging Clin N Am. 2010;18:337–357. ix. doi: 10.1016/j.mric.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Werven JR, Marsman HA, Nederveen AJ, et al. Assessment of hepatic steatosis in patients undergoing liver resection: Comparison of US, CT, T1-weighted dual-echo MR imaging, and point-resolved 1H MR spectroscopy Radiology. 2010;256:159–168. doi: 10.1148/radiol.10091790. [DOI] [PubMed] [Google Scholar]
- 15.Overman MJ, Maru DM, Charnsangavej C, et al. Oxaliplatin-mediated increase in spleen size as a biomarker for the development of hepatic sinusoidal injury. J Clin Oncol. 2010;28:2549–2555. doi: 10.1200/JCO.2009.27.5701. [DOI] [PubMed] [Google Scholar]
- 16.Shin NY, Kim MJ, Lim JS, et al. Accuracy of gadoxetic acid-enhanced magnetic resonance imaging for the diagnosis of sinusoidal obstruction syndrome in patients with chemotherapy-treated colorectal liver metastases. Eur Radiol. 2012;22:864–871. doi: 10.1007/s00330-011-2333-x. doi: 10.1007/s00330–011-2333-x. [DOI] [PubMed] [Google Scholar]
- 17.Zorzi D, Laurent A, Pawlik TM, et al. Chemotherapy-associated hepatotoxicity and surgery for colorectal liver metastases. Br J Surg. 2007;94:274–286. doi: 10.1002/bjs.5719. [DOI] [PubMed] [Google Scholar]
- 18.Chami L, Lassau N, Malka D, et al. Benefits of contrast-enhanced sonography for the detection of liver lesions: Comparison with histologic findings. AJR Am J Roentgenol. 2008;190:683–690. doi: 10.2214/AJR.07.2295. [DOI] [PubMed] [Google Scholar]
- 19.Leen E, Ceccotti P, Moug SJ, et al. Potential value of contrast-enhanced intraoperative ultrasonography during partial hepatectomy for metastases: An essential investigation before resection? Ann Surg. 2006;243:236–240. doi: 10.1097/01.sla.0000197708.77063.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Necib H, Garcia C, Wagner A, et al. Detection and characterization of tumor changes in 18F-FDG PET patient monitoring using parametric imaging. J Nucl Med. 2011;52:354–361. doi: 10.2967/jnumed.110.080150. [DOI] [PubMed] [Google Scholar]
- 21.Hendlisz A, Golfinopoulos V, Garcia C, et al. Serial FDG-PET/CT for early outcome prediction in patients with metastatic colorectal cancer undergoing chemotherapy. Ann Oncol. 2012;23:1687–1693. doi: 10.1093/annonc/mdr554. doi: 10.1093/annonc/mdr554. [DOI] [PubMed] [Google Scholar]
- 22.Ruers TJ, Wiering B, van der Sijp JR, et al. Improved selection of patients for hepatic surgery of colorectal liver metastases with (18)F-FDG PET: A randomized study. J Nucl Med. 2009;50:1036–1041. doi: 10.2967/jnumed.109.063040. [DOI] [PubMed] [Google Scholar]
- 23.Wiering B, Adang EM, van der Sijp JR, et al. Added value of positron emission tomography imaging in the surgical treatment of colorectal liver metastases. Nucl Med Commun. 2010;31:938–944. doi: 10.1097/MNM.0b013e32833fa9ba. [DOI] [PubMed] [Google Scholar]
- 24.Moulton C, Levine MN, Law C, et al. An Ontario Clinical Oncology Group (OCOG) randomized controlled trial (RCT) assessing FDG PET/CT in resectable liver colorectal adenocarcinoma metastases (CAM) J Clin Oncol. 2011;29(15 suppl):3520. [Google Scholar]
- 25.Nordlinger B, Sorbye H, Glimelius B, et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): A randomised controlled trial. Lancet. 2008;371:1007–1016. doi: 10.1016/S0140-6736(08)60455-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adam R, Bhangui P, Poston G, et al. Is perioperative chemotherapy useful for solitary, metachronous, colorectal liver metastases? Ann Surg. 2010;252:774–787. doi: 10.1097/SLA.0b013e3181fcf3e3. [DOI] [PubMed] [Google Scholar]
- 27.Nordlinger B, Vauthey JN, Poston G, et al. The timing of chemotherapy and surgery for the treatment of colorectal liver metastases. Clin Colorectal Cancer. 2010;9:212–218. doi: 10.3816/CCC.2010.n.031. [DOI] [PubMed] [Google Scholar]
- 28.Kopetz S, Chang GJ, Overman MJ, et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol. 2009;27:3677–3683. doi: 10.1200/JCO.2008.20.5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Golfinopoulos V, Salanti G, Pavlidis N, et al. Survival and disease-progression benefits with treatment regimens for advanced colorectal cancer: A meta-analysis. Lancet Oncol. 2007;8:898–911. doi: 10.1016/S1470-2045(07)70281-4. [DOI] [PubMed] [Google Scholar]
- 30.Adam R, Delvart V, Pascal G, et al. Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: A model to predict long-term survival. Ann Surg. 2004;240:644–657. doi: 10.1097/01.sla.0000141198.92114.f6. discussion 657–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alberts SR, Horvath WL, Sternfeld WC, et al. Oxaliplatin, fluorouracil, and leucovorin for patients with unresectable liver-only metastases from colorectal cancer: A North Central Cancer Treatment Group phase II study. J Clin Oncol. 2005;23:9243–9249. doi: 10.1200/JCO.2005.07.740. [DOI] [PubMed] [Google Scholar]
- 32.Ho WM, Ma B, Mok T, et al. Liver resection after irinotecan, 5-fluorouracil, and folinic acid for patients with unresectable colorectal liver metastases: A multicenter phase II study by the Cancer Therapeutic Research Group. Med Oncol. 2005;22:303–312. doi: 10.1385/MO:22:3:303. [DOI] [PubMed] [Google Scholar]
- 33.Pozzo C, Basso M, Cassano A, et al. Neoadjuvant treatment of unresectable liver disease with irinotecan and 5-fluorouracil plus folinic acid in colorectal cancer patients. Ann Oncol. 2004;15:933–939. doi: 10.1093/annonc/mdh217. [DOI] [PubMed] [Google Scholar]
- 34.Adam R, Pascal G, Castaing D, et al. Tumor progression while on chemotherapy: A contraindication to liver resection for multiple colorectal metastases? Ann Surg. 2004;240:1052–1061. doi: 10.1097/01.sla.0000145964.08365.01. discussion 1061–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Falcone A, Ricci S, Brunetti I, et al. Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: The Gruppo Oncologico Nord Ovest. J Clin Oncol. 2007;25:1670–1676. doi: 10.1200/JCO.2006.09.0928. [DOI] [PubMed] [Google Scholar]
- 36.Elias D, Lasser P, Rougier P, et al. Frequency, technical aspects, results, and indications of major hepatectomy after prolonged intra-arterial hepatic chemotherapy for initially unresectable hepatic tumors. J Am Coll Surg. 1995;180:213–219. [PubMed] [Google Scholar]
- 37.Goéré D, Deshaies I, De Baere T, et al. Prolonged survival of initially unresectable hepatic colorectal cancer patients treated with hepatic arterial infusion of oxaliplatin followed by radical surgery of metastases. Ann Surg. 2010;251:686–691. doi: 10.1097/SLA.0b013e3181d35983. [DOI] [PubMed] [Google Scholar]
- 38.Bouchahda M, Adam R, Giacchetti S, et al. Rescue chemotherapy using multidrug chronomodulated hepatic arterial infusion for patients with heavily pretreated metastatic colorectal cancer. Cancer. 2009;115:4990–4999. doi: 10.1002/cncr.24549. [DOI] [PubMed] [Google Scholar]
- 39.Bokemeyer C, Bondarenko I, Makhson A, et al. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2009;27:663–671. doi: 10.1200/JCO.2008.20.8397. [DOI] [PubMed] [Google Scholar]
- 40.Van Cutsem E, Köhne CH, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408–1417. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 41.Van Cutsem E, Köhne CH, Láng I, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: Updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol. 2011;29:2011–2019. doi: 10.1200/JCO.2010.33.5091. [DOI] [PubMed] [Google Scholar]
- 42.Maughan TS, Adams RA, Smith CG, et al. Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: Results of the randomised phase 3 MRC COIN trial. Lancet. 2011;377:2103–2114. doi: 10.1016/S0140-6736(11)60613-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Folprecht G, Gruenberger T, Bechstein WO, et al. Tumour response and secondary resectability of colorectal liver metastases following neoadjuvant chemotherapy with cetuximab: The CELIM randomised phase 2 trial. Lancet Oncol. 2010;11:38–47. doi: 10.1016/S1470-2045(09)70330-4. [DOI] [PubMed] [Google Scholar]
- 44.Garufi C, Torsello A, Tumolo S, et al. Cetuximab plus chronomodulated irinotecan, 5-fluorouracil, leucovorin and oxaliplatin as neoadjuvant chemotherapy in colorectal liver metastases: POCHER trial. Br J Cancer. 2010;103:1542–1547. doi: 10.1038/sj.bjc.6605940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tveit K, Guren T, Glimelius B, et al. Randomized phase III study of 5-fluorouracil/folinate/oxaliplatin given continuously or intermittently with or without cetuximab, as first-line treatment of metastatic colorectal cancer: The NORDIC VII study ( NCT00145314), by the Nordic Colorectal Cancer Biomodulation Group. J Clin Oncol. 2011;29(suppl 4):365. [Google Scholar]
- 46.Douillard JY, Siena S, Cassidy J, et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: The PRIME study. J Clin Oncol. 2010;28:4697–4705. doi: 10.1200/JCO.2009.27.4860. [DOI] [PubMed] [Google Scholar]
- 47.Peeters M, Cohn A, Köhne CH, et al. Panitumumab in combination with cytotoxic chemotherapy for the treatment of metastatic colorectal carcinoma. Clin Colorectal Cancer. 2012;11:14–23. doi: 10.1016/j.clcc.2011.06.010. doi: 10.1016/j.clcc.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 48.Van Cutsem E, Peeters M, Siena S, et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol. 2007;25:1658–1664. doi: 10.1200/JCO.2006.08.1620. [DOI] [PubMed] [Google Scholar]
- 49.Okines A, Puerto OD, Cunningham D, et al. Surgery with curative-intent in patients treated with first-line chemotherapy plus bevacizumab for metastatic colorectal cancer First BEAT and the randomised phase-III NO16966 trial. Br J Cancer. 2009;101:1033–1038. doi: 10.1038/sj.bjc.6605259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wong R, Cunningham D, Barbachano Y, et al. A multicentre study of capecitabine, oxaliplatin plus bevacizumab as perioperative treatment of patients with poor-risk colorectal liver-only metastases not selected for upfront resection. Ann Oncol. 2011;22:2042–2048. doi: 10.1093/annonc/mdq714. [DOI] [PubMed] [Google Scholar]
- 51.Masi G, Loupakis F, Salvatore L, et al. Bevacizumab with FOLFOXIRI (irinotecan, oxaliplatin, fluorouracil, and folinate) as first-line treatment for metastatic colorectal cancer: A phase 2 trial. Lancet Oncol. 2010;11:845–852. doi: 10.1016/S1470-2045(10)70175-3. [DOI] [PubMed] [Google Scholar]
- 52.Kishi Y, Zorzi D, Contreras CM, et al. Extended preoperative chemotherapy does not improve pathologic response and increases postoperative liver insufficiency after hepatic resection for colorectal liver metastases. Ann Surg Oncol. 2010;17:2870–2876. doi: 10.1245/s10434-010-1166-1. [DOI] [PubMed] [Google Scholar]
- 53.Sullivan RD, Norcross JW, Watkins E., Jr Chemotherapy of metastatic liver cancer by prolonged hepatic-artery infusion. N Engl J Med. 1964;270:321–327. doi: 10.1056/NEJM196402132700701. [DOI] [PubMed] [Google Scholar]
- 54.Bouchahda M, Lévi F, Adam R, et al. Modern insights into hepatic arterial infusion for liver metastases from colorectal cancer. Eur J Cancer. 2011;47:2681–2690. doi: 10.1016/j.ejca.2011.06.037. [DOI] [PubMed] [Google Scholar]
- 55.Kemeny N, Huang Y, Cohen AM, et al. Hepatic arterial infusion of chemotherapy after resection of hepatic metastases from colorectal cancer. N Engl J Med. 1999;341:2039–2048. doi: 10.1056/NEJM199912303412702. [DOI] [PubMed] [Google Scholar]
- 56.Kemeny N. Management of liver metastases from colorectal cancer. Oncology (Williston Park) 2006;20:1161–1176. 1179. discussion 1179–1180, 1185–1186. [PubMed] [Google Scholar]
- 57.Boige V, Malka D, Elias D, et al. Hepatic arterial infusion of oxaliplatin and intravenous LV5FU2 in unresectable liver metastases from colorectal cancer after systemic chemotherapy failure. Ann Surg Oncol. 2008;15:219–226. doi: 10.1245/s10434-007-9581-7. [DOI] [PubMed] [Google Scholar]
- 58.Elias D, Goere D, Boige V, et al. Outcome of posthepatectomy-missing colorectal liver metastases after complete response to chemotherapy: Impact of adjuvant intra-arterial hepatic oxaliplatin. Ann Surg Oncol. 2007;14:3188–3194. doi: 10.1245/s10434-007-9482-9. [DOI] [PubMed] [Google Scholar]
- 59.Alberts SR, Sargent DJ, Smyrk TC, et al. Adjuvant mFOLFOX6 with or without cetuxiumab (Cmab) in KRAS wild-type (WT) patients (pts) with resected stage III colon cancer (CC): Results from NCCTG Intergroup Phase III Trial N0147. Proc Am Soc Clin Oncol. 2010;28:CRA3507. [Google Scholar]
- 60.Allegra CJ, Yothers G, O'Connell MJ, et al. Phase III trial assessing bevacizumab in stages II and III carcinoma of the colon: Results of NSABP protocol C-08. J Clin Oncol. 2011;29:11–16. doi: 10.1200/JCO.2010.30.0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Andre T, Van Cutsem E, Schmoll H, et al. A multinational, randomized phase III study of bevacizumab (Bev) with FOLFOX4 or XELOX versus FOLFOX4 alone as adjuvant treatment for colon cancer (CC): Subgroup analyses from the AVANT trial. Proc Am Soc Clin Oncol. 2011;29:3509. [Google Scholar]
- 62.Ychou M, Raoul JL, Douillard JY, et al. A phase III randomised trial of LV5FU2 + irinotecan versus LV5FU2 alone in adjuvant high-risk colon cancer (FNCLCC Accord02/FFCD9802) Ann Oncol. 2009;20:674–680. doi: 10.1093/annonc/mdn680. [DOI] [PubMed] [Google Scholar]
- 63.Miller AB, Hoogstraten B, Staquet M, et al. Reporting results of cancer treatment. Cancer. 1981;47:207–214. doi: 10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 64.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 65.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 66.Suzuki C, Blomqvist L, Sundin A, et al. The initial change in tumor size predicts response and survival in patients with metastatic colorectal cancer treated with combination chemotherapy. Ann Oncol. 2012;23:948–954. doi: 10.1093/annonc/mdr350. doi: 10.1093/annonc/mdr350. [DOI] [PubMed] [Google Scholar]
- 67.De Roock W, Piessevaux H, De Schutter J, et al. KRAS wild-type state predicts survival and is associated to early radiological response in metastatic colorectal cancer treated with cetuximab. Ann Oncol. 2008;19:508–515. doi: 10.1093/annonc/mdm496. [DOI] [PubMed] [Google Scholar]
- 68.Choi H, Charnsangavej C, Faria SC, et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: Proposal of new computed tomography response criteria. J Clin Oncol. 2007;25:1753–1759. doi: 10.1200/JCO.2006.07.3049. [DOI] [PubMed] [Google Scholar]
- 69.Chun YS, Vauthey JN, Boonsirikamchai P, et al. Association of computed tomography morphologic criteria with pathologic response and survival in patients treated with bevacizumab for colorectal liver metastases. JAMA. 2009;302:2338–2344. doi: 10.1001/jama.2009.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Byström P, Berglund A, Garske U, et al. Early prediction of response to first-line chemotherapy by sequential [18F]-2-fluoro-2-deoxy-D-glucose positron emission tomography in patients with advanced colorectal cancer. Ann Oncol. 2009;20:1057–1061. doi: 10.1093/annonc/mdn744. [DOI] [PubMed] [Google Scholar]
- 71.de Geus-Oei LF, Vriens D, van Laarhoven HW, et al. Monitoring and predicting response to therapy with 18F-FDG PET in colorectal cancer: A systematic review. J Nucl Med. 2009;50(suppl 1):43S–54S. doi: 10.2967/jnumed.108.057224. [DOI] [PubMed] [Google Scholar]
- 72.Anzidei M, Napoli A, Zaccagna F, et al. Liver metastases from colorectal cancer treated with conventional and antiangiogenetic chemotherapy: Evaluation with liver computed tomography perfusion and magnetic resonance diffusion-weighted imaging. J Comput Assist Tomogr. 2011;35:690–696. doi: 10.1097/RCT.0b013e318230d905. [DOI] [PubMed] [Google Scholar]
- 73.Benoist S, Brouquet A, Penna C, et al. Complete response of colorectal liver metastases after chemotherapy: Does it mean cure? J Clin Oncol. 2006;24:3939–3945. doi: 10.1200/JCO.2006.05.8727. [DOI] [PubMed] [Google Scholar]
- 74.Tan MC, Linehan DC, Hawkins WG, et al. Chemotherapy-induced normalization of FDG uptake by colorectal liver metastases does not usually indicate complete pathologic response. J Gastrointest Surg. 2007;11:1112–1119. doi: 10.1007/s11605-007-0218-8. [DOI] [PubMed] [Google Scholar]
- 75.Zalinski S, Abdalla EK, Mahvash A, et al. A marking technique for intraoperative localization of small liver metastases before systemic chemotherapy. Ann Surg Oncol. 2009;16:1208–1211. doi: 10.1245/s10434-009-0328-5. [DOI] [PubMed] [Google Scholar]
- 76.Fong Y, Fortner J, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: Analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–318. doi: 10.1097/00000658-199909000-00004. discussion 318–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rees M, Tekkis PP, Welsh FK, et al. Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: A multifactorial model of 929 patients. Ann Surg. 2008;247:125–135. doi: 10.1097/SLA.0b013e31815aa2c2. [DOI] [PubMed] [Google Scholar]
- 78.LiverMetSurvey. International Registry of Patients Operated for Colorectal Liver Metastasis. [accessed November 23, 2011]. Available at http://www.livermetsurvey.org.
- 79.Taylor A, Kanas G, Langeberg W, et al. Survival after surgical resection of hepatic metastases from colorectal cancer: A systematic review and meta-analysis. Ann Oncol. 2010;21(suppl 8):632P. [Google Scholar]
- 80.Misiakos EP, Karidis NP, Kouraklis G. Current treatment for colorectal liver metastases. World J Gastroenterol. 2011;17:4067–4075. doi: 10.3748/wjg.v17.i36.4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rougier P, Milan C, Lazorthes F, et al. Prospective study of prognostic factors in patients with unresected hepatic metastases from colorectal cancer. Fondation Frana̧ise de Cancérologie Digestive. Br J Surg. 1995;82:1397–1400. doi: 10.1002/bjs.1800821034. [DOI] [PubMed] [Google Scholar]
- 82.Penna C, Nordlinger B. Colorectal metastasis (liver and lung) Surg Clin North Am. 2002;82:1075–1090. doi: 10.1016/s0039-6109(02)00051-8. [DOI] [PubMed] [Google Scholar]
- 83.Vibert E, Canedo L, Adam R. Strategies to treat primary unresectable colorectal liver metastases. Semin Oncol. 2005;32(suppl 8):33–39. doi: 10.1053/j.seminoncol.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 84.Wicherts DA, de Haas RJ, Andreani P, et al. Impact of portal vein embolization on long-term survival of patients with primarily unresectable colorectal liver metastases. Br J Surg. 2010;97:240–250. doi: 10.1002/bjs.6756. [DOI] [PubMed] [Google Scholar]
- 85.Clavien PA, Petrowsky H, DeOliveira ML, et al. Strategies for safer liver surgery and partial liver transplantation. N Engl J Med. 2007;356:1545–1559. doi: 10.1056/NEJMra065156. [DOI] [PubMed] [Google Scholar]
- 86.Charnsangavej C, Clary B, Fong Y, et al. Selection of patients for resection of hepatic colorectal metastases: Expert consensus statement. Ann Surg Oncol. 2006;13:1261–1268. doi: 10.1245/s10434-006-9023-y. [DOI] [PubMed] [Google Scholar]
- 87.Bolton JS, Fuhrman GM. Survival after resection of multiple bilobar hepatic metastases from colorectal carcinoma. Ann Surg. 2000;231:743–751. doi: 10.1097/00000658-200005000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Maithel SK, Gonen M, Ito H, et al. Improving the clinical risk score: An analysis of molecular biomarkers in the era of modern chemotherapy for resectable hepatic colorectal cancer metastases. Surgery. 2012;151:162–170. doi: 10.1016/j.surg.2011.07.020. [DOI] [PubMed] [Google Scholar]
- 89.D'Angelica M, Ammori J, Gonen M, et al. Folate receptor-α expression in resectable hepatic colorectal cancer metastases: Patterns and significance. Mod Pathol. 2011;24:1221–1228. doi: 10.1038/modpathol.2011.82. [DOI] [PubMed] [Google Scholar]
- 90.Bruin SC, He Y, Mikolajewska-Hanclich I, et al. Molecular alterations associated with liver metastases development in colorectal cancer patients. Br J Cancer. 2011;105:281–287. doi: 10.1038/bjc.2011.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stremitzer S, Maresch J, Aschacher T, et al. Influence of KRAS status of colorectal cancer liver metastases in patients receiving neoadjuvant chemotherapy including bevacizumab prior liver resection. J Clin Oncol. 2011;29(15 suppl):10620. [Google Scholar]
- 92.Resection of the liver for colorectal carcinoma metastases: A multi-institutional study of indications for resection. Registry of Hepatic Metastases. Surgery. 1988;103:278–288. [PMC free article] [PubMed] [Google Scholar]
- 93.Mentha G, Majno PE, Andres A, et al. Neoadjuvant chemotherapy and resection of advanced synchronous liver metastases before treatment of the colorectal primary. Br J Surg. 2006;93:872–878. doi: 10.1002/bjs.5346. [DOI] [PubMed] [Google Scholar]
- 94.Brouquet A, Mortenson MM, Vauthey JN, et al. Surgical strategies for synchronous colorectal liver metastases in 156 consecutive patients: Classic, combined or reverse strategy? J Am Coll Surg. 2010;210:934–941. doi: 10.1016/j.jamcollsurg.2010.02.039. [DOI] [PubMed] [Google Scholar]
- 95.de Jong MC, van Dam RM, Maas M, et al. The liver-first approach for synchronous colorectal liver metastasis: A 5-year single-centre experience. HPB (Oxford) 2011;13:745–752. doi: 10.1111/j.1477-2574.2011.00372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tanaka K, Shimada H, Matsuo K, et al. Outcome after simultaneous colorectal and hepatic resection for colorectal cancer with synchronous metastases. Surgery. 2004;136:650–659. doi: 10.1016/j.surg.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 97.Martin R, Paty P, Fong Y, et al. Simultaneous liver and colorectal resections are safe for synchronous colorectal liver metastasis. J Am Coll Surg. 2003;197:233–241. doi: 10.1016/S1072-7515(03)00390-9. discussion 241–242. [DOI] [PubMed] [Google Scholar]
- 98.Bipat S, van Leeuwen MS, Comans EF, et al. Colorectal liver metastases: CT, MR imaging, and PET for diagnosis–meta-analysis. Radiology. 2005;237:123–131. doi: 10.1148/radiol.2371042060. [DOI] [PubMed] [Google Scholar]
- 99.Adam R, Laurent A, Azoulay D, et al. Two-stage hepatectomy: A planned strategy to treat irresectable liver tumors. Ann Surg. 2000;232:777–785. doi: 10.1097/00000658-200012000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jaeck D, Oussoultzoglou E, Rosso E, et al. A two-stage hepatectomy procedure combined with portal vein embolization to achieve curative resection for initially unresectable multiple and bilobar colorectal liver metastases. Ann Surg. 2004;240:1037–1049. doi: 10.1097/01.sla.0000145965.86383.89. discussion 1049–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wicherts DA, Miller R, de Haas RJ, et al. Long-term results of two-stage hepatectomy for irresectable colorectal cancer liver metastases. Ann Surg. 2008;248:994–1005. doi: 10.1097/SLA.0b013e3181907fd9. [DOI] [PubMed] [Google Scholar]
- 102.Brouquet A, Abdalla EK, Kopetz S, et al. High survival rate after two-stage resection of advanced colorectal liver metastases: Response-based selection and complete resection define outcome. J Clin Oncol. 2011;29:1083–1090. doi: 10.1200/JCO.2010.32.6132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Reddy SK, Pawlik TM, Zorzi D, et al. Simultaneous resections of colorectal cancer and synchronous liver metastases: A multi-institutional analysis. Ann Surg Oncol. 2007;14:3481–3491. doi: 10.1245/s10434-007-9522-5. [DOI] [PubMed] [Google Scholar]
- 104.Ruers T, Punt CJ, van Coevorden F, et al. Final results of the EORTC intergroup randomized study 40004 (CLOCC) evaluating the benefit of radiofrequency ablation (RFA) combined with chemotherapy for unresectable colorectal liver metastases (CRC LM) J Clin Oncol. 2010;28(15 suppl):3526. [Google Scholar]
- 105.Abdalla EK, Vauthey JN, Ellis LM, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg. 2004;239:818–825. doi: 10.1097/01.sla.0000128305.90650.71. discussion 825–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Aloia TA, Vauthey JN, Loyer EM, et al. Solitary colorectal liver metastasis: Resection determines outcome. Arch Surg. 2006;141:460–466. doi: 10.1001/archsurg.141.5.460. discussion 466–467. [DOI] [PubMed] [Google Scholar]
- 107.Rubbia-Brandt L, Giostra E, Brezault C, et al. Importance of histological tumor response assessment in predicting the outcome in patients with colorectal liver metastases treated with neo-adjuvant chemotherapy followed by liver surgery. Ann Oncol. 2007;18:299–304. doi: 10.1093/annonc/mdl386. [DOI] [PubMed] [Google Scholar]
- 108.Blazer DG, 3rd, Kishi Y, Maru DM, et al. Pathologic response to preoperative chemotherapy: A new outcome end point after resection of hepatic colorectal metastases. J Clin Oncol. 2008;26:5344–5351. doi: 10.1200/JCO.2008.17.5299. [DOI] [PubMed] [Google Scholar]
- 109.Adam R, Wicherts DA, de Haas RJ, et al. Complete pathologic response after preoperative chemotherapy for colorectal liver metastases: Myth or reality? J Clin Oncol. 2008;26:1635–1641. doi: 10.1200/JCO.2007.13.7471. [DOI] [PubMed] [Google Scholar]
- 110.Aloysius MM, Zaitoun AM, Beckingham IJ, et al. The pathological response to neoadjuvant chemotherapy with FOLFOX-4 for colorectal liver metastases: A comparative study. Virchows Arch. 2007;451:943–948. doi: 10.1007/s00428-007-0497-1. [DOI] [PubMed] [Google Scholar]
- 111.Ribero D, Wang H, Donadon M, et al. Bevacizumab improves pathologic response and protects against hepatic injury in patients treated with oxaliplatin-based chemotherapy for colorectal liver metastases. Cancer. 2007;110:2761–2767. doi: 10.1002/cncr.23099. [DOI] [PubMed] [Google Scholar]
- 112.Chan G, Hassanain M, Chaudhury P, et al. Pathological response grade of colorectal liver metastases treated with neoadjuvant chemotherapy. HPB (Oxford) 2010;12:277–284. doi: 10.1111/j.1477-2574.2010.00170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chun YS, Laurent A, Maru D, et al. Management of chemotherapy-associated hepatotoxicity in colorectal liver metastases. Lancet Oncol. 2009;10:278–286. doi: 10.1016/S1470-2045(09)70064-6. [DOI] [PubMed] [Google Scholar]
- 114.Rubbia-Brandt L, Audard V, Sartoretti P, et al. Severe hepatic sinusoidal obstruction associated with oxaliplatin-based chemotherapy in patients with metastatic colorectal cancer. Ann Oncol. 2004;15:460–466. doi: 10.1093/annonc/mdh095. [DOI] [PubMed] [Google Scholar]
- 115.Aloia T, Sebagh M, Plasse M, et al. Liver histology and surgical outcomes after preoperative chemotherapy with fluorouracil plus oxaliplatin in colorectal cancer liver metastases. J Clin Oncol. 2006;24:4983–4990. doi: 10.1200/JCO.2006.05.8156. [DOI] [PubMed] [Google Scholar]
- 116.Rubbia-Brandt L, Lauwers GY, Wang H, et al. Sinusoidal obstruction syndrome and nodular regenerative hyperplasia are frequent oxaliplatin-associated liver lesions and partially prevented by bevacizumab in patients with hepatic colorectal metastasis. Histopathology. 2010;56:430–439. doi: 10.1111/j.1365-2559.2010.03511.x. [DOI] [PubMed] [Google Scholar]
- 117.Fernandez FG, Ritter J, Goodwin JW, et al. Effect of steatohepatitis associated with irinotecan or oxaliplatin pretreatment on resectability of hepatic colorectal metastases. J Am Coll Surg. 2005;200:845–853. doi: 10.1016/j.jamcollsurg.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 118.Vauthey JN, Pawlik TM, Ribero D, et al. Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J Clin Oncol. 2006;24:2065–2072. doi: 10.1200/JCO.2005.05.3074. [DOI] [PubMed] [Google Scholar]
- 119.Peppercorn PD, Reznek RH, Wilson P, et al. Demonstration of hepatic steatosis by computerized tomography in patients receiving 5-fluorouracil-based therapy for advanced colorectal cancer. Br J Cancer. 1998;77:2008–2011. doi: 10.1038/bjc.1998.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Parikh AA, Gentner B, Wu TT, et al. Perioperative complications in patients undergoing major liver resection with or without neoadjuvant chemotherapy. J Gastrointest Surg. 2003;7:1082–1088. doi: 10.1016/j.gassur.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 121.Bilchik AJ, Poston G, Curley SA, et al. Neoadjuvant chemotherapy for metastatic colon cancer: A cautionary note. J Clin Oncol. 2005;23:9073–9078. doi: 10.1200/JCO.2005.03.2334. [DOI] [PubMed] [Google Scholar]
- 122.Mentha G, Terraz S, Morel P, et al. Dangerous halo after neoadjuvant chemotherapy and two-step hepatectomy for colorectal liver metastases. Br J Surg. 2009;96:95–103. doi: 10.1002/bjs.6436. [DOI] [PubMed] [Google Scholar]
- 123.Holdhoff M, Schmidt K, Diehl F, et al. Detection of tumor DNA at the margins of colorectal cancer liver metastasis. Clin Cancer Res. 2011;17:3551–3557. doi: 10.1158/1078-0432.CCR-10-3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kulemann V, Schima W, Tamandl D, et al. Preoperative detection of colorectal liver metastases in fatty liver: MDCT or MRI? Eur J Radiol. 2011;79:e1–e6. doi: 10.1016/j.ejrad.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 125.Goèré D, Gaujoux S, Deschamp F, et al. Patients operated on for initially unresectable colorectal liver metastases with missing metastases experience a favorable long-term outcome. Ann Surg. 2011;254:114–118. doi: 10.1097/SLA.0b013e31821ad704. [DOI] [PubMed] [Google Scholar]
- 126.House MG, Kemeny NE, Gönen M, et al. Comparison of adjuvant systemic chemotherapy with or without hepatic arterial infusional chemotherapy after hepatic resection for metastatic colorectal cancer. Ann Surg. 2011;254:851–856. doi: 10.1097/SLA.0b013e31822f4f88. [DOI] [PubMed] [Google Scholar]
- 127.Tomlinson JS, Jarnagin WR, DeMatteo RP, et al. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol. 2007;25:4575–4580. doi: 10.1200/JCO.2007.11.0833. [DOI] [PubMed] [Google Scholar]
- 128.Van Cutsem E, Nordlinger B, Adam R, et al. Towards a pan-European consensus on the treatment of patients with colorectal liver metastases. Eur J Cancer. 2006;42:2212–2221. doi: 10.1016/j.ejca.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 129.Adam R, Wicherts DA, de Haas RJ, et al. Patients with initially unresectable colorectal liver metastases: Is there a possibility of cure? J Clin Oncol. 2009;27:1829–1835. doi: 10.1200/JCO.2008.19.9273. [DOI] [PubMed] [Google Scholar]
- 130.Fennell ML, Das IP, Clauser S, et al. The organization of multidisciplinary care teams: Modeling internal and external influences on cancer care quality. J Natl Cancer Inst Monogr. 2010;2010:72–80. doi: 10.1093/jncimonographs/lgq010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Rabinowitz B. Interdisciplinary breast cancer care: Declaring and improving the standard. Oncology (Williston Park) 2004;18:1263–1268. discussion 1268–1270, 1275. [PubMed] [Google Scholar]
- 132.Wright FC, De Vito C, Langer B, et al. Multidisciplinary cancer conferences: A systematic review and development of practice standards. Eur J Cancer. 2007;43:1002–1010. doi: 10.1016/j.ejca.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 133.Look Hong NJ, Gagliardi AR, Bronskill SE, et al. Multidisciplinary cancer conferences: Exploring obstacles and facilitators to their implementation. J Oncol Pract. 2010;6:61–68. doi: 10.1200/JOP.091085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jefferies H, Chan KK. Multidisciplinary team working: Is it both holistic and effective? Int J Gynecol Cancer. 2004;14:210–211. doi: 10.1111/j.1048-891X.2004.014201.x. [DOI] [PubMed] [Google Scholar]
- 135.Davies AR, Deans DA, Penman I, et al. The multidisciplinary team meeting improves staging accuracy and treatment selection for gastro-esophageal cancer. Dis Esophagus. 2006;19:496–503. doi: 10.1111/j.1442-2050.2006.00629.x. [DOI] [PubMed] [Google Scholar]
- 136.Fleissig A, Jenkins V, Catt S, et al. Multidisciplinary teams in cancer care: Are they effective in the UK? Lancet Oncol. 2006;7:935–943. doi: 10.1016/S1470-2045(06)70940-8. [DOI] [PubMed] [Google Scholar]
- 137.Gabel M, Hilton NE, Nathanson SD. Multidisciplinary breast cancer clinics. Do they work? Cancer. 1997;79:2380–2384. [PubMed] [Google Scholar]
- 138.Carter S, Garside P, Black A. Multidisciplinary team working, clinical networks, and chambers; opportunities to work differently in the NHS. Qual Saf Health Care. 2003;12(suppl 1):i25–i28. doi: 10.1136/qhc.12.suppl_1.i25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rummans TA, Clark MM, Sloan JA, et al. Impacting quality of life for patients with advanced cancer with a structured multidisciplinary intervention: A randomized controlled trial. J Clin Oncol. 2006;24:635–642. doi: 10.1200/JCO.2006.06.209. [DOI] [PubMed] [Google Scholar]
- 140.Forrest LM, McMillan DC, McArdle CS, et al. An evaluation of the impact of a multidisciplinary team, in a single centre, on treatment and survival in patients with inoperable non-small-cell lung cancer. Br J Cancer. 2005;93:977–978. doi: 10.1038/sj.bjc.6602825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Birchall M, Bailey D, King P. South West Cancer Intelligence Service Head and Neck Tumour Panel. Effect of process standards on survival of patients with head and neck cancer in the south and west of England. Br J Cancer. 2004;91:1477–1481. doi: 10.1038/sj.bjc.6602118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Westin T, Stalfors J. Tumour boards/multidisciplinary head and neck cancer meetings: Are they of value to patients, treating staff or a political additional drain on healthcare resources? Curr Opin Otolaryngol Head Neck Surg. 2008;16:103–107. doi: 10.1097/MOO.0b013e3282f6a4c4. [DOI] [PubMed] [Google Scholar]
- 143.Stephens MR, Lewis WG, Brewster AE, et al. Multidisciplinary team management is associated with improved outcomes after surgery for esophageal cancer. Dis Esophagus. 2006;19:164–171. doi: 10.1111/j.1442-2050.2006.00559.x. [DOI] [PubMed] [Google Scholar]
- 144.Du CZ, Li J, Cai Y, et al. Effect of multidisciplinary team treatment on outcomes of patients with gas-trointestinal malignancy. World J Gastroenterol. 2011;17:2013–2018. doi: 10.3748/wjg.v17.i15.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.MacDermid E, Hooton G, MacDonald M, et al. Improving patient survival with the colorectal cancer multi-disciplinary team. Colorectal Dis. 2009;11:291–295. doi: 10.1111/j.1463-1318.2008.01580.x. [DOI] [PubMed] [Google Scholar]
- 146.Obias VJ, Reynolds HL., Jr Multidisciplinary teams in the management of rectal cancer. Clin Colon Rectal Surg. 2007;20:143–147. doi: 10.1055/s-2007-984858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Devitt B, Philip J, McLachlan SA. Team dynamics, decision making, and attitudes toward multidisciplinary cancer meetings: Health professionals' perspectives. J Oncol Pract. 2010;6:e17–e20. doi: 10.1200/JOP.2010.000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ruhstaller T, Roe H, Thr̈limann B, et al. The multidisciplinary meeting: An indispensable aid to communication between different specialities. Eur J Cancer. 2006;42:2459–2462. doi: 10.1016/j.ejca.2006.03.034. [DOI] [PubMed] [Google Scholar]
- 149.McNair AG, Choh CT, Metcalfe C, et al. Maximising recruitment into randomised controlled trials: The role of multidisciplinary cancer teams. Eur J Cancer. 2008;44:2623–2626. doi: 10.1016/j.ejca.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 150.Siggins Miller. CanNET National Support and Evaluation Service. Final National Evaluation Report. [accessed November 23, 2011]. Available at http://www.canceraustralia.gov.au/sites/default/files/user-upload/cannet/about/natl_cannet_eval_report.pdf.


