Differences in the therapeutic approach to and tumor-related mortality of young and elderly colorectal cancer patients were analyzed. In comparison with younger patients, elderly patients were found to be undertreated, mainly because of their age and not because of their tumor type or comorbidity.
Keywords: Elderly, Cancer, Colorectal, Therapy, Assessment
Learning Objectives
After completing this course, the reader will be able to:
Use patient age as only one consideration, along with tumor status and comorbidities, in deciding on treatment strategies for elderly colorectal cancer patients.
Obtain and apply information regarding the medical, functional, mental, and social status of colorectal cancer elderly patients in order to make appropriate therapeutic decisions.
This article is available for continuing medical education credit at CME.TheOncologist.com
Abstract
Purpose.
To analyze differences in the therapeutic approach to and tumor-related mortality of young and elderly colorectal cancer (CRC) patients.
Patients and Methods.
This was a descriptive study of a retrospective cohort, based on administrative databases, of all patients with CRC diagnosed or treated in our institution. We extracted data on sociodemographic characteristics, comorbidity, type of cancer, type of treatment received, survival time, and cause of death. We compared differences between a young group (YG) (age <75 years) and an older group (OG) (age ≥75 years) and assessed the variables associated with receiving different therapeutic options (multivariate analysis) and with survival time (Cox proportional hazards models).
Results.
The study included 503 patients (YG, 320; OG, 183), with mean ages of 63.1 years in the YG and 81.8 years in the OG. No differences were observed between the groups in degree of differentiation, extension, tumor stage, or comorbidity. After adjustment for gender, comorbidity, and tumor localization and extension, YG patients were more likely than OG patients to receive surgery, radiotherapy, and chemotherapy and less likely to receive palliative care. After a median follow-up of 36.5 months, YG patients had a longer tumor-specific survival time than OG patients (36.41 months vs 26.05 months). After further adjustment, the YG had a lower tumor-specific mortality risk (hazard ratio, 0.66) than the OG.
Conclusion.
In comparison with younger patients, elderly CRC patients are undertreated, mainly because of their age and not because of their tumor type or comorbidity. Elderly patients have a significantly shorter tumor-specific survival time, partially because of this undertreatment.
Introduction
Colorectal cancer (CRC) is a disease of the elderly. The mean age at diagnosis is ∼72 years, with 70% of cases occurring in patients aged >65 years and 40% of cases occurring in patients aged >75 years [1–3]. Based on the aging demographics [4], we can expect an increase in the number of elderly CRC patients in the coming years; in addition, patients will be older (>85 years). The geriatric CRC population is a very heterogeneous group, including patients with excellent health status and others with comorbid conditions, functional dependency, and limited life expectancy [5]. These issues could account for the fact that a significant proportion of elderly CRC patients is undertreated, at least partially, in comparison with younger patients [6–13].
Scientific evidence in this field is scarce. In leading intervention studies on CRC, <20% of patients included are >70 years old [14–16]. However, there is considerable evidence that most elderly patients tolerate cancer treatment fairly well and can benefit from it in the same way as younger patients [17, 18], even from the newest therapies [19, 20]. Some authors question extrapolation of results to “real-world” geriatric patients because of the small populations included in trials and because these patients are fit and aged, but not geriatric. On the other hand, our increasing life expectancy makes an active therapeutic approach for CRC patients potentially more valuable in terms of years of life gained [21–24].
The main challenge is to identify the right patient for the right treatment. However, the factors influencing the therapeutic approach in elderly patients with CRC are not well known. Furthermore, the consequences of decisions on therapy in terms of survival outcomes are unclear [25].
We designed a retrospective cohort study to analyze differences in the therapeutic approach to CRC in young patients (aged <75 years) and elderly patients (aged ≥75 years) from a Spanish institution. The rates of surgery, radiotherapy (RT), chemotherapy (CT), and palliative care were recorded to assess which factors were associated with receiving any of these options. We also assessed tumor-related mortality in both groups.
Methods
This was a descriptive study of a retrospective cohort based on administrative databases from a tertiary care hospital, the Hospital General Universitario “Gregorio Marañón” (HGUGM), Madrid, Spain.
Patients
The study population was comprised of all patients diagnosed with CRC (2009 International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] diagnosis code 153.0–153.9 for malignant neoplasm of the colon and ICD-9-CM code 154.0–154.9 for malignant neoplasm of the rectum) in our hospital and those referred from other centers for treatment in our institution in January 1, 2003 to December 31, 2005. Patients who had received all or part of their treatment at another center and those diagnosed using necropsy were excluded.
Sources of Data
We used data from the Central Cancer Registry (CCR) of the Cancer Data Exchange System of Madrid (Spain), which has been collecting cases of patients newly diagnosed with cancer since 1992. The registry is notified by all hospital and health centers of the Regional Health System of Madrid and collects data on age, gender, diagnosis, anatomical location, treatment, and mortality. Following the usual criteria [26], we extracted data on different variables: age, gender, cancer characteristics (localization, degree of differentiation, extension, and stage), time from first consultation to diagnosis and to first treatment, type of treatment received, survival time, and cause of death. Comorbid conditions and hospitalization data were extracted from our Minimum Basic Data Set (MBDS), which records the main diagnostic features of patients at discharge. Both databases were merged for some descriptive analyses.
Study Variables and Follow-Up
The different therapeutic options (surgery, RT, CT, and palliative care) were considered dependent variables. Four dichotomous variables were created to show whether or not the patient had received any of these treatments during follow-up. Patients were categorized into two groups based on age: a young group (YG), aged <75 years, and an older group (OG), aged ≥75 years. Comorbidity was assessed using a modified Charlson index [27], which included neither cancer nor age, because age was the comparison group of interest and all patients had cancer. Tumor-related independent variables included localization (rectum or colon), degree of differentiation (I, II, or III), extension (in situ, localized, regional, or disseminated), and tumor stage at diagnosis (Union for International Cancer Control or American Joint Committee on Cancer stage 0, I, II, III, or IV) as the usual criteria [28]. We also analyzed time from first consultation to diagnosis and to first treatment received.
Statistical Analysis
Data were compared using frequency distributions, summary statistics, and univariate analysis. Differences between the YG and OG were compared using two-tailed independent t-tests for continuous variables and the χ2 statistic for categorical variables. To assess the effect of confounders, four multivariate logistic regression models were used to measure the association between age group and the four therapeutic options (surgery, RT, CT, and palliative care) after adjusting for location, extension, gender, and the modified Charlson index.
The tumor-associated mortality probability was estimated using a Kaplan–Meier survival analysis considering the time to event in months and stratifying by age group. The tumor-specific survival time was calculated from the time of the primary diagnosis. Tumor-related death was defined as death resulting from tumor progression, tumor-related clinical complications, or treatment-associated toxicity, censoring those who died as a result of other causes. The effects of the different therapeutic options, age group, gender, comorbidity, and tumor location and extension on survival were studied using Cox proportional hazards models.
Finally, we applied multivariate analysis to assess the variables associated with receiving any of the different therapeutic options during the follow-up period.
Statistical analysis was performed using Predictive Analytics Software Statistics, Version 18 (SPSS Inc., Chicago. IL). Estimates were made using the complex sample functions. Statistical significance was set at p < .05 (p-values were two tailed).
Results
Study Population and Therapeutic Approach
The study population was comprised of 503 patients (YG, 320; OG, 183), with mean ages of 63.1 years (standard deviation [SD], 9.9 years; range, 27–74 years) in the YG and 81.8 years (SD, 4.9 years; range, 75–100 years) in the OG. Table 1 shows the sociodemographic and tumor characteristics and comorbid conditions for the whole group and for the individual age groups. No differences were observed between groups in degree of differentiation, extension, or tumor stage at the time of diagnosis. At all times during follow-up, YG patients were more likely than OG patients to receive surgery, RT, and CT and less likely to receive palliative care. As shown in Table 1, the score of the original Charlson index, including age and tumor-related items, and the number of secondary diagnoses were greater in the OG.
Table 1.
Study population and therapeutic approach
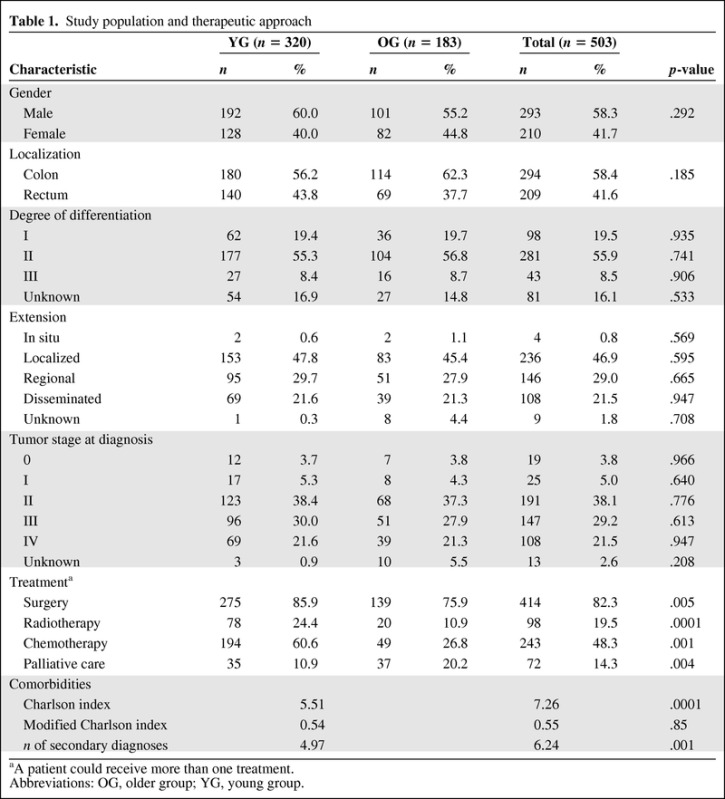
aA patient could receive more than one treatment.
Abbreviations: OG, older group; YG, young group.
In order to assess comorbid conditions, the CCR and MBDS were merged. We were unable to identify 26% of the patients (85 in the YG and 47 in the OG), thus leaving 371 patients for assessment (YG, 235; OG, 136). The modified Charlson index revealed no significant difference between the YG (0.54; SD, 1.0) and the OG (0.55; SD, 0.79) (p = .855).
There was no difference in the time from first consultation to diagnosis between the YG (median, 8 days; interquartile range [IQR], 31.25 days) and the OG (median, 9 days; IQR, 24 days) and no difference in time to first antitumor treatment between the YG (median, 35 days; IQR, 39 days) and the OG (median, 29 days; IQR, 48.25 days).
Multivariate Analysis of Therapeutic Options
Table 2 shows the variables related to the different therapeutic options in the multivariate analysis. After adjustment for gender, comorbidity, and tumor localization and extension, YG patients and patients with a tumor in the colon were more likely to receive surgery than OG patients and patients with rectal tumors. Treatment with RT was more probable in YG patients, patients with rectal tumors, and patients with regional extension than in OG patients, patients with a colon tumor, and patients with localized or disseminated disease. YG patients and patients with regional tumors received CT more frequently than OG patients and those with localized tumors. OG patients and those with disseminated tumors were more frequently treated with palliative care.
Table 2.
Variables associated with therapeutic options
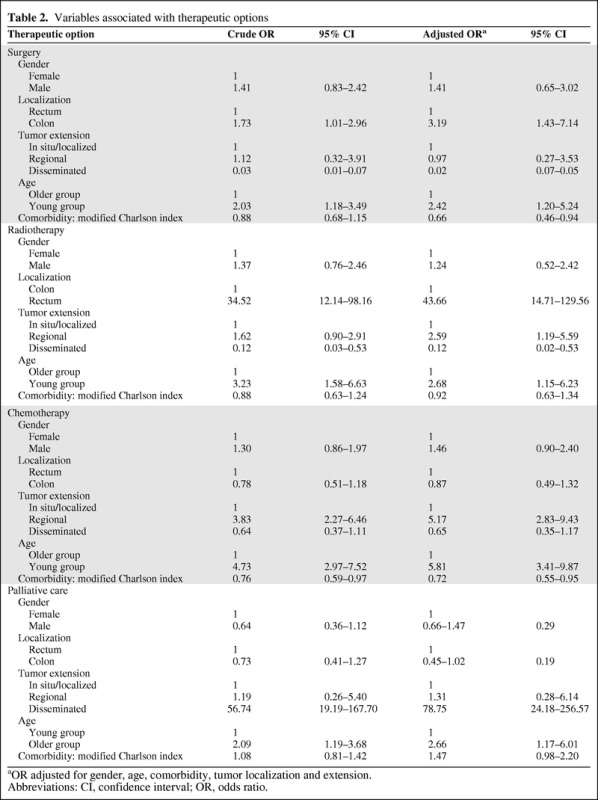
aOR adjusted for gender, age, comorbidity, tumor localization and extension.
Abbreviations: CI, confidence interval; OR, odds ratio.
As shown in Table 2, the score of the Charlson index was inversely related to the amount of therapy received, mainly surgery and CT.
Cancer-Specific Survival Analysis
We assessed tumor-related mortality using Kaplan–Meier survival analysis (Fig. 1). After a median follow-up of 36.5 months, YG patients had a longer tumor-specific survival duration than OG patients—36.41 months (95% confidence interval [CI], 33.53–39.33 months) versus 26.05 months (95% CI, 31.05–35.93 months).
Figure 1.
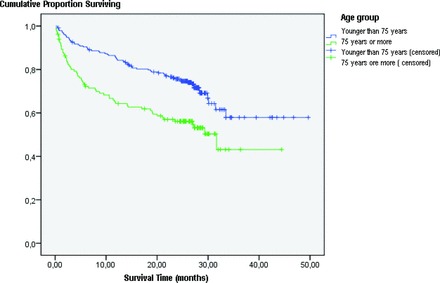
Cancer-specific survival curve.
In order to assess the effect of each therapeutic option on tumor-related mortality risk, we performed a Cox multivariate analysis adjusted for gender, tumor localization and extension, age, comorbidity, and therapeutic options (Table 3). YG patients had a lower tumor-specific mortality risk than OG patients. Patients with regional and disseminated disease, a higher Charlson comorbidity index, no surgical treatment, and no CT had a higher tumor-related mortality risk.
Table 3.
Tumor-related mortality hazard ratio
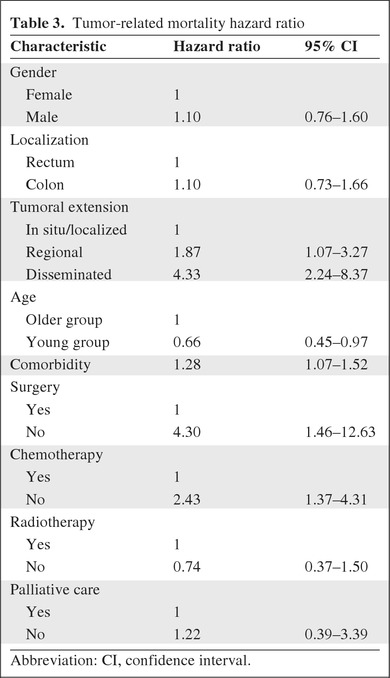
Abbreviation: CI, confidence interval.
Discussion
We found that age was the main reason for the different therapeutic approaches in young and elderly CRC patients. YG patients were more likely to receive surgery, RT, and CT and OG patients were more likely to receive palliative care. YG patients had a longer tumor-related survival time than OG patients, in part because of the suboptimal antitumor therapy in the latter group. We also found an inverse relationship between the Charlson index and the amount of surgery and CT received.
Multiple comorbidities are common in elderly CRC patients and can affect cancer stage and survival outcomes. A systematic review of surgical treatment of 35,000 elderly CRC patients demonstrated a higher frequency of associated comorbidities and a higher incidence of advanced tumors [8]. Another recent study was unable to find differences between young and elderly patients in tumor location, degree of differentiation, and staging [29].
We used the Charlson index because it is the most extensively studied comorbidity index [30], has been adapted for use with ICD-9 databases [31], and has predictive value for tolerance of treatment [32] and survival outcomes [33]. The lack of differences in our study could be a result of the limitations of the Charlson index in elderly patients, namely, the weight assigned to each condition does not always correspond to the true impact on the patient (dementia is weighted as one point, as is peptic ulcer) and it does not take into account prevalent conditions and disabilities in elderly patients (Parkinson disease, depression) [34]. Moreover, we excluded age and cancer from the Charlson index, because age was the comparison group of interest and all patients had cancer. We believe these results are not related to the low quality or bias of the discharge data collected in the MBDS. In our country, encoding rules require including all the patient's comorbidities. According to the data from an external and independent audit conducted by the Ministry of Health in 2011, the average number of diagnoses encoded is higher in our hospital than the average of the other university hospitals (6.3 vs 5.8).
Surgery is the treatment of choice for stages I–III disease and several subsets of stage IV colon cancer [24]. Despite advances in surgical techniques and postoperative care that make surgery safer in the elderly, age continues to affect treatment choice, and curative cancer-directed surgery is less frequent in older patients [8, 35]. However, several studies show that surgery is feasible to treat CRC in elderly patients [36, 37], even in octogenarians [38].
Two important aspects of surgery to treat CRC in elderly patients are the overall survival time after surgery and operative morbidity and mortality rates. Some studies describe a shorter overall survival duration in elderly patients [8, 35], whereas others do not [39, 40]. This discrepancy might be a result of the type of surgery (older patients less frequently receive curative surgery than younger ones) and the fact that older patients are more likely to undergo emergency surgery, with a much worse prognosis [24].
Published results for operative morbidity and mortality rates are conflicting: some studies show an association between age and postoperative complications [8, 35, 41] whereas others do not [42]. This difference could also be a result of the different types of elderly patients included in the studies (fit or frail). Those with more comorbid conditions could have more perioperative complications and a higher mortality risk [43, 44], but clinically healthy patients have results similar to those of younger patients [45].
RT for rectal cancer is one of the best evidence-based treatments in oncology [18, 46]. Tolerability is dose and volume dependent, but older people are more susceptible than younger patients. The Stockholm II trial showed that a reduction in the target volume to the posterior pelvis only was sufficient to eliminate this risk and probably contributed to a survival benefit and fewer pelvic recurrences [46].
The results of studies on adjuvant CT are inconsistent. In the Surveillance, Epidemiology, and End Results registry and Medicare Database, which include >4,500 elderly patients, those who received adjuvant CT with 5-fluorouracil (5-FU) had significantly better survival outcome (hazard ratio, 0.66). The authors concluded that adjuvant CT in the older population is associated with a lower mortality probability, similar to that observed in younger patients [47]. However a subanalysis of that study investigating stage III disease in older patients treated with 5-FU showed that >30% of those in whom CT was initiated discontinued treatment early and that the mortality rate among these patients was nearly twice as high as in patients who completed 5–7 months of treatment [48]. Jessup et al. [49], who analyzed data from 85,934 patients with stage III colon cancer, found that older patients received the same benefit as younger ones, although they were less frequently treated. In a population-based study, Bouvier et al. [50] concluded that adjuvant CT did not have a negative impact on quality of life in older colon cancer patients. Several specific retrospective pooled analyses show that older patients benefit from adjuvant CT in the same way as younger ones, without significantly greater toxicity [10, 45, 51].
In relation to CT for metastatic disease, a pooled analysis of 22 clinical trials including 3,285 patients (14% aged >70 years) assessed the efficacy of 5-FU and found no differences between age groups in terms of the overall survival time, overall response rate, and progression-free survival interval [52].
In view of those results, the European Organization for Research and Treatment of Cancer (EORTC) Elderly Task Force experts recommended that older patients with stage III disease not be denied adjuvant CT only on the basis of chronological age. Similarly, CT should not be denied to older patients with advanced CRC. Treatment decisions should take into account the estimated absolute benefit, life expectancy, treatment tolerance, cognition, comorbidities, and patient preferences [24].
In our study, older patients and those with disseminated tumors were more frequently treated with palliative care. This decision was not based on tumor-related aspects but on age itself. Some of these patients could have benefited from more aggressive therapy.
By assessing tumor-related mortality risk, we demonstrated that YG patients had longer tumor-related survival times than OG patients, probably because of suboptimal antitumor therapy in OG patients. Multivariate analysis revealed that the YG had a lower mortality risk than the OG. Other factors associated with a higher tumor-related mortality risk were regional and disseminated disease versus local disease, higher comorbidity index, lack of surgical treatment, and lack of CT.
These results are consistent with those of other authors who evaluated cancer-specific survival outcomes and found that age-related differences were much less pronounced, thus clearly underlining the importance of taking into account the issue of deaths not related to cancer when evaluating survival in older patients [24].
The hallmark of aging is a gradual loss of physiologic reserve (the body's ability to compensate when exposed to stressors such as infection and cancer), which in turn produces a decline in the function of some organs [53–55]. This decline varies among individuals and among organs, making elderly people a very heterogeneous population. These changes also have the potential to increase the risk for severe toxicity and decrease tolerance to the adverse effects of cancer treatments [5]. Thus, the spectrum of elderly CRC patients ranges from fit elderly with outcomes similar to those of younger patients to frail patients with a high risk for suffering adverse clinical outcomes such as hospitalization and death. The middle of the spectrum is comprised of the vast majority of patients, who are neither frail nor fit. The biggest challenge is to learn how to identify each group and treat each patient appropriately [5, 56, 57].
Frailty, defined as vulnerability, weakness, instability, and functional limitations, is very common in geriatric cancer patients, placing them at risk for complications and death [58, 59]. With a suitable approach, frailty is preventable, treatable, and even reversible, making prognoses much better [60, 61].
We showed that older patients with CRC are undertreated. It is a matter of debate whether this trend represents reticence by patients, relatives, and physicians associated with comorbid disease and frailty or is an inappropriate reflection of ageism [5]. The finding of an inverse relationship between the Charlson index and the amount surgery and CT received suggests that, independent of age group, a person with more comorbidities is less likely to receive surgery and CT. However, this finding should be interpreted with caution. The effect size is small, with the 95% CI of the odds ratios approaching 1, and this effect was not shown for palliative care or RT.
Not all elderly CRC patients are referred for oncologic therapy, and not all those referred for therapy have a comprehensive geriatric assessment (CGA), which collects data on medical, functional, mental, and social capabilities. As with other cancers, assessment of elderly CRC patients must include an evaluation of comorbidity, functional and cognitive status, emotional status, nutrition, social support, and life expectancy. The CGA is the most validated and sensitive instrument for classifying patients as fit, vulnerable, or frail [59, 62–66]. Therefore, therapeutic decisions must be made on an individual basis, taking into account the overall clinical status (assessed with the CGA) and patient preferences, and not merely chronological age [17].
Despite its many advantages, the CGA has limitations for oncologists: it is not standardized and it is time consuming [67]. Thus, experts in CGA (geriatricians) and oncologists should cooperate [68]. However, only a minority of cancer units have staff who care specifically for elderly patients and who receive assessment from geriatricians [25].
Geriatricians attending elderly patients with cancer could apply the CGA, help in the decision-making process, optimize the clinical status so that the patient can better tolerate treatment, and minimize the more frequent clinical complications (delirium, undernutrition, functional impairment, depression) [69, 70].
The recommendations of the International Society of Geriatric Oncology for elderly CRC patients state that elderly patients should be exposed to more aggressive management than they are currently receiving, namely, management that is closer to that received by younger patients. Treatment should be intensive, appropriate, safe, and effective and should be adjusted to take account of biological age and comorbidities in order to maximize survival while minimizing toxicity [18]. In view of these recommendations, it is evident that our patients are not treated appropriately.
The EORTC Elderly Task Force [24] adds that clinical trials enrolling an adequate number of older patients are mandatory in order to provide evidence-based recommendations for the treatment of this population.
The main strength of our work is the number of patients included and the variables assessed, both of which allow us to draw conclusions.
The main weakness is the scarcity of medical and functional data from patients. It would be interesting to compare outcome in relation to activities of daily living and medical complications. Furthermore, the Charlson index may be insufficiently sensitive to reveal differences in comorbidities among these patients. Finally, this is not a population-based study. It is a single-site study and, consequently, the findings may not apply to all patients with CRC. However, our hospital is the reference hospital of a population of >700,000 people (14% of the population of the Community of Madrid),with >17% of them being >65 years of age. In addition, because we excluded 19 of 521 (3.6%) patients who were treated, at least in part, outside our institution, there may be a small selection bias. However, this exclusion similarly affected both age groups, and there is no differential bias.
In conclusion, we show that elderly CRC patients are undertreated, mainly because of their age and not because of their tumor status or comorbidities. Because of this undertreatment, elderly patients have a significantly shorter tumor-specific survival duration.
Acknowledgment
This study was presented in part at 2010 Annual Scientific Meeting of the American Geriatrics Society, Florida, May 2010.
Footnotes
- (C/A)
- Consulting/advisory relationship
- (RF)
- Research funding
- (E)
- Employment
- (H)
- Honoraria received
- (OI)
- Ownership interests
- (IP)
- Intellectual property rights/inventor/patent holder
- (SAB)
- Scientific advisory board
Author Contributions
Conception/Design: José A. Serra-Rexach, Javier Ortiz, Maite Vidán, Paz Rodríguez, Miguel Martin, Ana B. Jimenez, Rosa Pla
Provision of study material or patients: Ana B. Jimenez, Rosa Pla
Collection and/or assembly of data: María A. García-Alhambra, José A. Serra, Ana B. Jimenez, Rosa Pla
Data analysis and interpretation: José A. Serra-Rexach, Javier Ortiz, Maite Vidán, Paz Rodráguez, Miguel Martin, Ana B. Jimenez, Pilar García-Alfonso, Rosa Pla
Manuscript writing: José A. Serra-Rexach
Final approval of manuscript: José A. Serra-Rexach, Javier Ortiz, Maite Vidán, Paz Rodráguez, Miguel Martin, Ana B. Jimenez, Pilar García-Alfonso, Rosa Pla
References
- 1.Köhne CH, Folprecht G, Goldberg RM, et al. Chemotherapy in elderly patients with colorectal cancer. The Oncologist. 2008;13:390–402. doi: 10.1634/theoncologist.2007-0043. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM, Bray F, Ferlay J, et al. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94:153–156. doi: 10.1002/ijc.1440. [DOI] [PubMed] [Google Scholar]
- 3.Boyle P, Ferlay J. Cancer incidence and mortality in Europe, 2004. Ann Oncol. 2005;16:481–488. doi: 10.1093/annonc/mdi098. [DOI] [PubMed] [Google Scholar]
- 4.Christensen K, Doblhammer G, Rau R, et al. Ageing populations: The challenges ahead. Lancet. 2009;374:1196–1208. doi: 10.1016/S0140-6736(09)61460-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanoff HK, Bleiberg H, Goldberg RM. Managing older patients with colorectal cancer. J Clin Oncol. 2007;25:1891–1897. doi: 10.1200/JCO.2006.10.1220. [DOI] [PubMed] [Google Scholar]
- 6.Rougier P, Mitry E, Aranda E, et al. Elderly colorectal cancer patients are under treated. Eur J Cancer Suppl. 2004;2:8–13. [Google Scholar]
- 7.Passeto LM, Basso U, Friso ML, et al. Determining therapeutic approaches in the elderly with rectal cancer. Drugs Aging. 2007;24:781–790. doi: 10.2165/00002512-200724090-00006. [DOI] [PubMed] [Google Scholar]
- 8.Colorectal Cancer Collaborative Group. Surgery for colorectal cancer in elderly patients: A systematic review. Lancet. 2000;356:968–974. [PubMed] [Google Scholar]
- 9.Schrag D, Cramer LD, Bach PB, et al. Age and adjuvant chemotherapy use after surgery for stage III colon cancer. J Natl Cancer Inst. 2001;93:850–857. doi: 10.1093/jnci/93.11.850. [DOI] [PubMed] [Google Scholar]
- 10.Sargent DJ, Goldberg RM, Jacobson SD, et al. A pooled analysis of adjuvant chemotherapy for resected colon cancer in elderly patients. N Engl J Med. 2001;345:1091–1097. doi: 10.1056/NEJMoa010957. [DOI] [PubMed] [Google Scholar]
- 11.Ayanian JZ, Zaslavsky AM, Fuchs CS, et al. Use of adjuvant chemotherapy and radiation therapy for colorectal cancer in a population-based cohort. J Clin Oncol. 2003;21:1293–1300. doi: 10.1200/JCO.2003.06.178. [DOI] [PubMed] [Google Scholar]
- 12.Lemmens VE, van Halteren AH, Janssen-Heijnen ML, et al. Adjuvant treatment for elderly patients with stage III colon cancer in southern Netherlands is affected by socioeconomic status, gender, and comorbidity. Ann Oncol. 2005;16:767–772. doi: 10.1093/annonc/mdi159. [DOI] [PubMed] [Google Scholar]
- 13.Potosky AL, Harlan LC, Kaplan RS, et al. Age, sex, and racial differences in the use of standard adjuvant therapy for colorectal cancer. J Clin Oncol. 2002;20:1192–1202. doi: 10.1200/JCO.2002.20.5.1192. [DOI] [PubMed] [Google Scholar]
- 14.Fenitman IS. Are the elderly receiving appropriate treatment for cancer? Ann Oncol. 1996;7:657–658. doi: 10.1093/oxfordjournals.annonc.a010712. [DOI] [PubMed] [Google Scholar]
- 15.Hutchins LF, Unger JM, Crowley JJ, et al. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med. 1999;341:2061–2067. doi: 10.1056/NEJM199912303412706. [DOI] [PubMed] [Google Scholar]
- 16.Honecker F, Köhne CH, Bokemeyer C. Colorectal cancer in the elderly: Is palliative chemotherapy of value? Drugs Aging. 2003;20:1–11. doi: 10.2165/00002512-200320010-00001. [DOI] [PubMed] [Google Scholar]
- 17.Golfinopoulos V, Pentheroudakis G, Pavlidis N. Treatment of colorectal cancer in the elderly: A review of the literature. Cancer Treat Rev. 2006;32:1–8. doi: 10.1016/j.ctrv.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 18.Papamichael D, Audisio R, Horiot JC, et al. Treatment of the elderly colorectal cancer patients: SIOG expert recommendations. Ann Oncol. 2009;20:5–16. doi: 10.1093/annonc/mdn532. [DOI] [PubMed] [Google Scholar]
- 19.Boehm S, Rothermundt C, Hess D, et al. Antiangiogenic drugs in oncology: A focus on drug safety and the elderly—a mini-review. Gerontology. 2010;56:303–309. doi: 10.1159/000262450. [DOI] [PubMed] [Google Scholar]
- 20.Kozloff MF, Berlin J, Flynn PJ, et al. Clinical outcomes in elderly patients with metastatic colorectal cancer receiving bevacizumab and chemotherapy: Results from the BRiTE observational cohort study. Oncology. 2010;78:329–339. doi: 10.1159/000320222. [DOI] [PubMed] [Google Scholar]
- 21.Feliu J, Sereno M, Castro DJ, et al. Chemotherapy for colorectal cancer in the elderly: Whom to treat and what to use. Cancer Treat Rev. 2009;35:246–254. doi: 10.1016/j.ctrv.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 22.Saif MW, Lichtman SM. Chemotherapy options and outcomes in older adult patients with colorectal cancer. Crit Rev Oncol Hematol. 2009;72:155–169. doi: 10.1016/j.critrevonc.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 23.Shahir MA, Lemmens VE, van de Poll-Franse LV, et al. Elderly patients with rectal cancer have a higher risk of treatment-related complications and a poorer prognosis than younger patients: A population-based study. Eur J Cancer. 2006;42:3015–3021. doi: 10.1016/j.ejca.2005.10.032. [DOI] [PubMed] [Google Scholar]
- 24.Pallis AG, Papamichael D, Audisio R, et al. EORTC Elderly Task Force experts' opinion for the treatment of colon cancer in older patients. Cancer Treat Rev. 2010;36:83–90. doi: 10.1016/j.ctrv.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 25.Pasetto LM, Falci C, Basso U, et al. Colorectal cancer treatment in elderly patients: Results of a retrospective analysis addressed to the chiefs of medical oncology units in Italy. Anticancer Res. 2007;27:3601–3608. [PubMed] [Google Scholar]
- 26.Regional Desk of Oncology Coordination, editor. Madrid, Spain: Oficina Regional de Coordinación Oncológica; 2006. Handbook of Codification Criteria and Registry Process of Cancer Cases; pp. 11–63. [Google Scholar]
- 27.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 28.Greene F, Page D, Fleming I, et al. Cancer Staging Handbook: TNM Classification of Malignant Tumors. Sixth Edition. New York: Springer; 2002. American Joint Committee on Cancer; pp. 127–139. [Google Scholar]
- 29.Araujo SE, de Paris Caravatto PP, de Campos FG, et al. Colorectal cancer among patients aged 75 years or over. Hepatogastroenterology. 2007;54:427–430. [PubMed] [Google Scholar]
- 30.de Groot V, Beckerman H, Lankhorst GJ, et al. How to measure comorbidity: A critical review of available methods. J Clin Epidemiol. 2003;56:221–229. doi: 10.1016/s0895-4356(02)00585-1. [DOI] [PubMed] [Google Scholar]
- 31.D'Hoore W, Bouckaert A, Tilquin C. Practical considerations on the use of the Charlson Comorbidity Index with administrative data bases. J Clin Epidemiol. 1996;49:1429–1433. doi: 10.1016/s0895-4356(96)00271-5. [DOI] [PubMed] [Google Scholar]
- 32.Extermann M. Measurement and impact of comorbidity in older cancer patients. Crit Rev Oncol Hematol. 2000;35:181–200. doi: 10.1016/s1040-8428(00)00090-1. [DOI] [PubMed] [Google Scholar]
- 33.Yancik R, Wesley MN, Ries LA, et al. Comorbidity and age as predictors of risk for early mortality of male and female colon carcinoma patients: A population-based study. Cancer. 1998;82:2123–2134. [PubMed] [Google Scholar]
- 34.Abizanda Soler P, Paterna Mellinas G, Martínez Sánchez E, et al. [Comorbidity in the elderly: Utility and validity of assessment tools.] Rev Esp Geriatr Gerontol. 2010;45:219–228. doi: 10.1016/j.regg.2009.10.009. In Spanish. [DOI] [PubMed] [Google Scholar]
- 35.van Leeuwen BL, Påhlman L, Gunnarsson U, et al. The effect of age and gender on outcome after treatment for colon carcinoma. A population-based study in the Uppsala and Stockholm region. Crit Rev Oncol Hematol. 2008;67:229–236. doi: 10.1016/j.critrevonc.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 36.Mäkelä JT, Kiviniemi H, Laitinen S. Survival after operations for colorectal cancer in patients aged 75 years or over. Eur J Surg. 2000;166:473–479. doi: 10.1080/110241500750008790. [DOI] [PubMed] [Google Scholar]
- 37.Ong ES, Alassas M, Dunn KB, et al. Colorectal cancer surgery in the elderly: Acceptable morbidity? Am J Surg. 2008;195:344–348. doi: 10.1016/j.amjsurg.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 38.Louis DJ, Hsu A, Brand MI, et al. Morbidity and mortality in octogenarians and older undergoing major intestinal surgery. Dis Colon Rectum. 2009;52:59–63. doi: 10.1007/DCR.0b013e31819754d4. [DOI] [PubMed] [Google Scholar]
- 39.Chiappa A, Zbar AP, Bertani E, et al. Surgical treatment of advanced colorectal cancer in the elderly. Chir Ital. 2005;57:589–596. [PubMed] [Google Scholar]
- 40.Quaglia A, Capocaccia R, Micheli A, et al. A wide difference in cancer survival between middle aged and elderly patients in Europe. Int J Cancer. 2007;120:2196–2201. doi: 10.1002/ijc.22515. [DOI] [PubMed] [Google Scholar]
- 41.Lee L, Jannapureddy M, Albo D, et al. Outcomes of Veterans Affairs patients older than age 80 after surgical procedures for colon malignancies. Am J Surg. 2007;194:646–651. doi: 10.1016/j.amjsurg.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 42.Schiffmann L, Ozcan S, Schwarz F, et al. Colorectal cancer in the elderly: Surgical treatment and long-term survival. Int J Colorectal Dis. 2008;23:601–610. doi: 10.1007/s00384-008-0457-5. [DOI] [PubMed] [Google Scholar]
- 43.Morel P, Egeli RA, Wachtl S, et al. Results of operative treatment of gastrointestinal tract tumours in patients over 80 years of age. Arch Surg. 1989;124:662–664. doi: 10.1001/archsurg.1989.01410060024004. [DOI] [PubMed] [Google Scholar]
- 44.Hosking MP, Warner MA, Lobdell CM, et al. Outcomes of surgery in patients 90 years of age and older. JAMA. 1989;261:1909–1915. [PubMed] [Google Scholar]
- 45.Popescu RA, Norman A, Ross PJ, et al. Adjuvant or palliative chemotherapy for colorectal cancer in patients 70 years or older. J Clin Oncol. 1999;17:2412–2418. doi: 10.1200/JCO.1999.17.8.2412. [DOI] [PubMed] [Google Scholar]
- 46.Folkesson J, Birgisson H, Pahlman L, et al. Swedish Rectal Cancer Trial: Long lasting benefits from radiotherapy on survival and local recurrence rate. J Clin Oncol. 2005;23:5644–5650. doi: 10.1200/JCO.2005.08.144. [DOI] [PubMed] [Google Scholar]
- 47.Sundararajan V, Mitra N, Jacobson JS, et al. Survival associated with 5-fluorouracil-based adjuvant chemotherapy among elderly patients with node-positive colon cancer. Ann Intern Med. 2002;136:349–357. doi: 10.7326/0003-4819-136-5-200203050-00007. [DOI] [PubMed] [Google Scholar]
- 48.Neugut AI, Matasar M, Wang X, et al. Duration of adjuvant chemotherapy for colon cancer and survival among the elderly. J Clin Oncol. 2006;24:2368–2375. doi: 10.1200/JCO.2005.04.5005. [DOI] [PubMed] [Google Scholar]
- 49.Jessup JM, Stewart A, Greene FL, et al. Adjuvant chemotherapy for stage III colon cancer: Implications of race/ethnicity, age, and differentiation. JAMA. 2005;294:2703–2711. doi: 10.1001/jama.294.21.2703. [DOI] [PubMed] [Google Scholar]
- 50.Bouvier AM, Jooste V, Bonnetain F, et al. Adjuvant treatments do not alter the quality of life in elderly patients with colorectal cancer: A population-based study. Cancer. 2008;113:879–886. doi: 10.1002/cncr.23629. [DOI] [PubMed] [Google Scholar]
- 51.Fata F, Mirza A, Craig G, et al. Efficacy and toxicity of adjuvant chemotherapy in elderly patients with colon carcinoma: A 10-year experience of the Geisinger Medical Center. Cancer. 2002;94:1931–1938. doi: 10.1002/cncr.10430. [DOI] [PubMed] [Google Scholar]
- 52.Folprecht G, Cunningham D, Ross P, et al. Efficacy of 5-fluorouracil-based chemotherapy in elderly patients with metastatic colorectal cancer: A pooled analysis of clinical trials. Ann Oncol. 2004;15:1330–1338. doi: 10.1093/annonc/mdh344. [DOI] [PubMed] [Google Scholar]
- 53.Ferrucci L, Guralnik JM, Cavazzini C, et al. The frailty syndrome: A critical issue in geriatric oncology. Crit Rev Oncol Hematol. 2003;46:127–137. doi: 10.1016/s1040-8428(02)00177-4. [DOI] [PubMed] [Google Scholar]
- 54.Sawhney R, Sehl M, Naeim A. Physiologic aspects of aging: Impact on cancer management and decision making, part I. Cancer J. 2005;11:449–460. doi: 10.1097/00130404-200511000-00004. [DOI] [PubMed] [Google Scholar]
- 55.Sehl M, Sawhney R, Naeim A. Physiologic aspects of aging: Impact on cancer management and decision making, part II. Cancer J. 2005;11:461–473. doi: 10.1097/00130404-200511000-00005. [DOI] [PubMed] [Google Scholar]
- 56.Merlin F, Prochilo T, Tondulli L, et al. Colorectal cancer treatment in elderly patients: An update on recent clinical studies. Clin Colorectal Cancer. 2008;7:357–363. doi: 10.3816/CCC.2008.n.047. [DOI] [PubMed] [Google Scholar]
- 57.Balducci L, Extermann M. Management of cancer in the older person: A practical approach. The Oncologist. 2000;5:224–237. doi: 10.1634/theoncologist.5-3-224. [DOI] [PubMed] [Google Scholar]
- 58.Walston J, Hadley EC, Ferrucci L, et al. Research agenda for frailty in older adults: Toward a better understanding of physiology and etiology. Summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc. 2006;54:991–1001. doi: 10.1111/j.1532-5415.2006.00745.x. [DOI] [PubMed] [Google Scholar]
- 59.Rodin MB, Mohile SG. A practical approach to geriatric assessment in oncology. J Clin Oncol. 2007;25:1936–1944. doi: 10.1200/JCO.2006.10.2954. [DOI] [PubMed] [Google Scholar]
- 60.Xue QL. The frailty syndrome: Definition and natural history. Clin Geriatr Med. 2011;27:1–15. doi: 10.1016/j.cger.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ahmed N, Mandel R, Fain MJ. Frailty: An emerging geriatric syndrome. Am J Med. 2007;120:748–753. doi: 10.1016/j.amjmed.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 62.Marenco D, Marinello R, Berruti A, et al. Multidimensional geriatric assessment in treatment decision in elderly cancer patients: 6-year experience in an outpatient geriatric oncology service. Crit Rev Oncol Hematol. 2008;68:157–164. doi: 10.1016/j.critrevonc.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 63.Maas H, Janssen-Heijnen ML, Olde Rikkert MG, et al. Comprehensive geriatric assessment and its clinical impact in oncology. Eur J Cancer. 2007;43:2161–2169. doi: 10.1016/j.ejca.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 64.Pal SK, Katheria V, Hurria A. Evaluating the older patient with cancer: Understanding frailty and the geriatric assessment. CA Cancer J Clin. 2010;60:120–132. doi: 10.3322/caac.20059. [DOI] [PubMed] [Google Scholar]
- 65.Balducci L, Colloca G, Cesari M, et al. Assessment and treatment of elderly patients with cancer. Surg Oncol. 2010;19:117–123. doi: 10.1016/j.suronc.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 66.Terret C, Zulian GB, Naiem A, et al. Multidisciplinary approach to the geriatric oncology patient. J Clin Oncol. 2007;25:1876–1881. doi: 10.1200/JCO.2006.10.3291. [DOI] [PubMed] [Google Scholar]
- 67.Carreca I, Balducci L, Extermann M. Cancer in the older person. Cancer Treat Rev. 2005;31:380–402. doi: 10.1016/j.ctrv.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 68.Xiao H, Lichtman SM. Management of colorectal cancer in older patients. Oncology (Williston Park) 2006;20:741–750. discussion 750, 755–756. [PubMed] [Google Scholar]
- 69.Girre V, Falcou MC, Gisselbrecht M, et al. Does a geriatric oncology consultation modify the cancer treatment plan for elderly patients? J Gerontol A Biol Sci Med Sci. 2008;63:724–730. doi: 10.1093/gerona/63.7.724. [DOI] [PubMed] [Google Scholar]
- 70.Flood KL, Carroll MB, Le CV, et al. Geriatric syndromes in elderly patients admitted to an oncology-acute care for elders unit. J Clin Oncol. 2006;24:2298–2303. doi: 10.1200/JCO.2005.02.8514. [DOI] [PubMed] [Google Scholar]


