This article presents a pilot randomized controlled trial that examined the feasibility and potential efficacy of brief cognitive-behavioral therapy to reduce anxiety in patients with terminal cancer.
Keywords: Cancer, Anxiety, Cognitive behavior therapy, Quality of life
Learning Objectives
After completing this course, the reader will be able to:
Explain the current state of evidence-based treatment for anxiety in patients with cancer and the need for tailored intervention, especially for those with terminal cancer.
Discuss and utilize methods for increasing access to psychosocial intervention for patients with cancer who suffer significant physical and psychological morbidity.
Describe the effect of a brief cognitive-behavioral therapy intervention tailored to the needs of patients with terminal cancer and comorbid anxiety symptoms.
This article is available for continuing medical education credit at CME.TheOncologist.com
Abstract
Introduction.
Patients with terminal cancer often experience marked anxiety that is associated with poor quality of life. Although cognitive-behavioral therapy (CBT) is an evidence-based treatment for anxiety disorders, the approach needs to be adapted to address realistic concerns related to having cancer, such as worries about disease progression, disability, and death. In this pilot randomized controlled trial (clinicaltrials.gov identifier NCT00706290), we examined the feasibility and potential efficacy of brief CBT to reduce anxiety in patients with terminal cancer.
Methods.
We adapted CBT by developing treatment modules targeting skills for relaxation, coping with cancer worries, and activity pacing. Adults with incurable malignancies and elevated anxiety based on the Hamilton Anxiety Rating Scale (HAM-A) were randomly assigned to individual CBT or a waitlist control group. Primary outcomes included the number of completed CBT visits and the change in HAM-A scores from baseline to 8-week follow-up per a treatment-blind evaluator. The feasibility criterion was 75% adherence to the intervention.
Results.
We randomized 40 patients with terminal cancers to CBT (n = 20) or waitlist control (n = 20) groups; 70% completed posttreatment assessments. Most patients who received CBT (80%) participated in at least five of the required six therapy sessions. Analysis of covariance models, adjusted for baseline scores, showed that those assigned to CBT had greater improvements in HAM-A scores compared to the control group, with an adjusted mean difference of –5.41 (95% confidence interval: –10.78 to –0.04) and a large effect size for the intervention (Cohen's d = 0.80).
Conclusion.
Providing brief CBT tailored to the concerns of patients with terminal cancer was not only feasible but also led to significant improvements in anxiety.
Introduction
The diagnosis of terminal cancer typically provokes fear and worry as patients cope with an uncertain future, changes in functioning, and intensive anticancer therapies. Up to 30%–40% of individuals with advanced cancer report anxiety symptoms that are sufficiently severe to reach clinical levels, exacerbating physical symptoms and impairing quality of life [1, 2]. For this population, anxiety may increase over time as anticancer therapies fail to prevent disease progression and patients face an uncertain life expectancy [3]. Moreover, such symptoms may compromise medical treatment because anxiety is associated with challenges in the physician-patient relationship, chemotherapy dose delays and reductions, and more aggressive care at the end of life [4–7]. Therefore, in this report, we examine the utility of a cognitive-behavioral intervention designed to address the concerns of patients with anxiety comorbid with terminal cancer, given the growing recognition of the psychosocial needs of this vulnerable population [8, 9].
Psychotherapy researchers have tested an emerging base of mental health treatments for individuals with cancer, including educational interventions, cognitive-behavioral therapy (CBT), problem-solving therapy, mindfulness-based approaches, and supportive-expressive group therapy, among others [10–13]. Meta-analyses of published clinical trials point to the promise of psychosocial treatments for reducing anxiety symptoms, although poor methodological quality, inadequate statistical power, and predominant focus on nondistressed patients with early-stage cancers limit conclusions regarding the efficacy of such interventions [10]. Many barriers exist in conducting research with patients who have terminal cancers in particular, such as the lack of appropriate measures to screen for anxiety and depression; limited dissemination of empirically supported therapies and practice guidelines; and concerns that disease burden and impaired functioning may interfere with participation [14–16]. Thus, clinical researchers may want to modify the delivery and setting of treatment to reduce the burden of additional clinic visits and increase access to therapy.
For patients with terminal cancer, anxiety is often associated with both medical and psychosocial factors. Psychological interventions need to be tailored to address the specific concerns related to having incurable cancer, such as existential distress over poor prognosis; increased disability and decrements in functioning; perceived burden posed to family caregivers; and difficulty in managing fatigue, pain, and adverse effects resulting from anticancer therapies [2, 17, 18]. Although CBT is a well-established and efficacious treatment for anxiety disorders in the general population [19], the intervention usually targets unrealistic fears and maladaptive avoidance behaviors. Traditional CBT helps individuals reframe irrational thoughts and beliefs that exacerbate anxiety as well as overcome their fear and avoidance through graduated exposure to anxiety-provoking situations [20]. However, aspects of this approach may be less useful for patients with terminal cancer who must continually adjust to very real changes in disease status. We therefore developed a brief CBT intervention tailored to patients with anxiety comorbid with incurable malignancies [21]. Specifically, we not only made efforts to improve access to care by allowing patients to schedule psychotherapy sessions in tandem with other medical appointments and via the telephone, but we also adapted our CBT approach to incorporate skills for managing realistic, cancer-related worries and progressive disability.
Given the paucity of research on psychosocial interventions for individuals with terminal cancer and anxiety, we conducted a pilot randomized controlled trial to determine the feasibility and preliminary efficacy of brief CBT tailored to this patient population. We assessed feasibility by calculating adherence to the intervention and hypothesized that participants who received brief CBT would experience significant reductions in anxiety compared to those in the control group. As secondary aims, we examined whether the study intervention improved depression symptoms and quality of life.
Patients and Methods
Participant eligibility criteria included a diagnosis of an incurable solid tumor, the presence of clinically significant anxiety symptoms (i.e., a Hamilton Anxiety Rating Scale [HAM-A] score ≥14) [22], and age greater than 18 years. We enrolled participants at least 4 weeks after cancer diagnosis to ensure that the anxiety symptoms persisted beyond the initial adjustment to a new cancer diagnosis. Patients prescribed psychotropic medications were eligible to participate because benzodiazepines in particular are a standard component of many chemotherapy regimens for alleviation of symptoms, such as nausea. Study referrals came from oncology clinicians, palliative care specialists, psychiatrists, or patients themselves through advertisements in the Massachusetts General Hospital (MGH) Cancer Center in Boston, Massachusetts.
Study Measures
Intervention Feasibility
We assessed the feasibility of the intervention by examining the enrollment and attrition rates as well as the number of psychotherapy sessions that patients in the intervention group completed. Recognizing the high symptom burden and limited life expectancy of the participants, we established 75% adherence to the CBT intervention as the a priori feasibility criterion (equivalent to completing at least five of the six sessions). Also, we recorded the number of psychotherapy sessions that occurred on the same day as other medical appointments at the hospital, including chemotherapy infusions, and via telephone.
Clinician-Administered (Blinded) Assessments
The primary outcome measure for the study was the total score on the HAM-A [23]. Widely used in psychiatry research to evaluate anxiety symptoms, the HAM-A consists of 14 items that are each scored on a scale from 0 (not present) to 4 (very severe) and summed to a total value ranging from 0 to 56. Higher scores reflect greater anxiety. We used the Structured Interview Guide for the HAM-A [24], which possesses strong psychometric properties (test-retest reliability = 0.89; α = 0.82). We also assessed patients' anxiety using the single-item, Clinical Global Impression Scale (CGI) [25], which measures overall severity and impairment from 1 (not ill) to 7 (extremely ill).
The secondary outcome measure was the total score on the Montgomery Asberg Depression Rating Scale (MADRS) [26], an empirically derived 10-item interview that measures depression symptoms during the past week. The total score ranges from 0 to 60, with higher scores indicating worse depression. The scale has excellent psychometric properties and relies less on somatic symptoms, making the instrument better suited for medical populations.
Self-Report Measures
Each participant completed a questionnaire regarding demographic characteristics including sex, age, race, ethnicity, marital status, and education level. We measured self-reported anxiety and mood symptoms with the Hospital Anxiety and Depression Scale (HADS) [27] and the Impact of Events Scale (IES) [28]. Designed for medical patients, the HADS demonstrates adequate psychometric properties for use in samples of individuals with cancer [29]. The instrument contains 14 items that comprise two subscales for anxiety and depression symptoms in the past week, with scores ranging from 0 (no distress) to 21 (maximum distress). The IES is a validated, 15-item self-report questionnaire that assesses intrusive and avoidant thoughts related to a stressor, in this case cancer. The total score ranges from 0 to 75, with higher scores indicating worse symptoms.
Finally, to assess quality of life, we used the Functional Assessment of Cancer Therapy-General (FACT-G) Questionnaire [30], which has been administered in multiple medical settings to patients with diverse tumor types. Consisting of 28 items, the FACT-G has four subscales assessing physical, functional, emotional, and social well-being during the past 7 days. Higher scores on the total score and each subscale indicate better quality of life.
Chart Review
We reviewed participants' electronic medical records to confirm demographic information, type and stage of cancer, date of diagnosis of incurable malignancy, type of anticancer therapy, and use of psychotropic medications.
Study Procedures and Intervention
From October 2007 to June 2010, we enrolled adult patients presenting to the MGH Cancer Center who were at least 4 weeks postdiagnosis of incurable cancer. As patients were referred to the study, the research assistant or one of the interventionists contacted potential participants via telephone to explain the purpose of the trial, to screen for eligibility, and to schedule the baseline assessment. Prior to randomization, all participants met with a licensed clinical psychologist or postdoctoral psychology fellow for a baseline assessment that included (a) a clinical interview using the HAM-A and MADRS and (b) completion of the self-report questionnaires. Upon request, participants could finish the self-report questionnaires at home and return them by mail. Study patients who had a HAM-A score ≥14 were then randomly assigned to individual CBT or a waitlist control group. We block randomized participants in groups of two and stratified randomization by tumor type, given the variable life expectancies across cancers. Although the CBT intervention was not delivered as part of palliative care, we did record the numbers of participants who also received outpatient palliative care services.
Approximately 1 week after the baseline assessment, participants assigned to the intervention began meeting with a study therapist for six or seven sessions of CBT (the seventh session was optional) tailored to the needs and concerns of patients with anxiety and terminal cancer. The sessions occurred weekly whenever possible. The principal investigator (a licensed clinical psychologist) and four clinical psychology fellows with at least 4 years supervised experience in delivering CBT served as study therapists. The duration of treatment was brief (approximately 2 months) by design, considering the progressive morbidity of the study population.
Described in detail elsewhere [21], the CBT intervention was developed to help patients with terminal cancer learn coping strategies for reducing anxiety and worry, while also reinforcing skills for managing cancer-related symptoms and treatment side effects. The intervention was comprised of four modules that targeted the following: (a) one session on goal setting and education about anxiety; (b) one session on relaxation training; (c) three sessions on coping with cancer fears; and (d) one or two sessions on activity planning and pacing. Each module included skills practice and homework assignments for between sessions. For example, in the final activity pacing module, participants learned to modify daily tasks by alternating strenuous activity with periods of rest to allow for engagement with meaningful life events in a manner consistent with changes in functional status. To increase flexibility and generalizability, we allowed study therapists to apply the CBT modules in the order that was most relevant for the problem areas of each patient.
Participants then underwent a posttreatment assessment with an independent evaluator after completing the intervention or at approximately 8 weeks, depending on study group assignment. The independent evaluator was either a trained master's level psychology graduate student or postdoctoral psychology fellow who was blinded to group assignment so as not to bias the outcome data collection. Once the posttreatment assessment was finished, participants in the waitlist control group could cross over to receive the CBT intervention if they desired. Participants received $50 for their time in completing study assessments. The MGH institutional review board approved the study procedures, and all participants provided written informed consent prior to enrollment.
Protocol Training, Integrity, and Adherence
All study therapists and independent evaluators received extensive training from the principal investigator (PI), a licensed clinical psychologist, regarding the intervention and evaluation procedures prior to meeting with participants. Specifically, study therapists were required to complete a didactic overview of the intervention, listen to audio recordings of the PI conducting CBT sessions with two or three study patients using the protocol, and meet with the PI weekly throughout the entire clinical trial for supervision of participant progress and protocol adherence. Additionally, the PI reviewed audio recordings of CBT sessions on an as-needed basis for the purpose of training and supervision.
Independent evaluators did not attend the supervision meetings with study therapists and had no knowledge of participant group assignment. Rather, their training involved first receiving didactics on the instruments and then listening to and scoring at least five clinician-administered assessments until perfect interrater agreement was achieved. To prevent rater drift, the independent evaluators also reviewed approximately 20% of audio recordings of their study assessments with a clinical psychologist who possessed expertise in administering the interviews.
Statistical Analyses
We conducted statistical analyses using SPSS version 20.0 (IBM SPSS Statistics, Redmond, WA). We examined differences in baseline sample characteristics between the CBT and waitlist control participants using two-sided χ2 and Fisher's exact tests for categorical data, independent sample t tests for continuous data, and the Mann-Whitney U test for median time since diagnosis of incurable cancer. Analysis of covariance (ANCOVA) models were calculated to assess the effect of the CBT intervention on the primary and secondary outcome measures (adjusted for baseline scores), yielding parameter estimates of between-group differences with 95% confidence intervals (CI). We also computed between-group effect sizes using Cohen's d ([mean change score for CBT – mean change score for waitlist control]/SD pooled).
Although a primary goal of this study was to estimate the effect size of the intervention to reduce anxiety, we conducted hypothesis testing to examine differences between the intervention and control groups in the outcome variables. As a pilot clinical trial, we aimed to achieve 30 study completers (15 in each study group). All statistical tests were two-sided with α = .05. In the next section, we first present the complete case analyses and then report the findings from multiple imputation analyses to account for any missing data in the outcome variables.
Results
Between October 2007 and June 2010, 123 individuals were referred to the study. Of these, 49 (39.8%) patients enrolled by first providing written informed consent (Fig. 1). We randomly assigned 40 participants to either individual CBT (n = 20) or a waitlist control group (n = 20). Demographic and clinical characteristics did not differ significantly between study groups at baseline (Table 1). The majority of patients in the study sample was white (95%, n = 38) and female (70%, n = 28), with an average age of 55.90 years (SD = 10.89; range = 31–81 years). Malignancies included metastatic lung (30%), pancreatic (17%), and colorectal (15%) cancers, among others.
Figure 1.
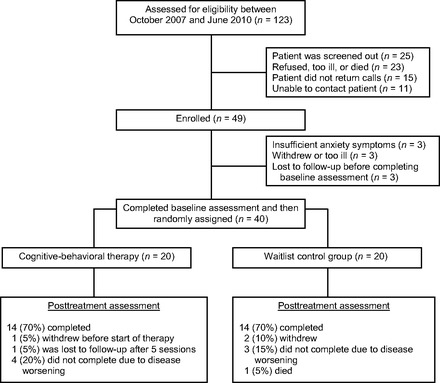
Study flow diagram.
Table 1.
Participant baseline demographic and clinical characteristics (n = 40)

p values were derived from two-sided χ2 and Fisher's exact tests for categorical variables, the independent sample t test for continuous variables, and the Mann-Whitney U test for median time since diagnosis of incurable cancer.
aFisher's exact test comparing rates of married/partner versus other (i.e., single, divorced, widowed) by study group.
bPearson χ2 test comparing rates of high school or less, some college/college graduate, and master/doctoral degree by study group.
cPearson χ2 test comparing rates of lung cancer, pancreatic/colorectal cancer, and other cancer by study group.
dBecause of missing data in baseline self-report assessments, sample sizes were as follows: Hospital Anxiety and Depression Scale, n = 36; Impact of Events Scale, n = 35; Functional Assessment of Cancer Therapy–General, n = 35.
The majority of patients in the study was prescribed psychotropic medications, with no significant differences between groups in rates for antidepressants, anxiolytics, antipsychotics, or stimulants. Also, most participants were undergoing chemotherapy while enrolled in the CBT study, and approximately one-third received outpatient palliative care services in the oncology clinic. Time since diagnosis of incurable cancer, which was similar between groups, did not correlate with anxiety and depression scores at baseline (HAM-A p = .65; MADRS p = .92) or posttreatment assessment (HAM-A p = .88; MADRS p = .98).
Feasibility of Completing CBT Intervention and Study Assessments
Figure 1 details the number of participants who were unable to complete the intervention by study group and reasons for attrition. As shown in Figure 2, 80% of patients in the intervention group completed five or more CBT sessions, exceeding our pre-established criterion for feasibility (i.e., 75% adherence to the intervention). Only one patient did not participate in any sessions after the baseline assessment, noting that he did not want to think about anxiety. Additionally, of the total 109 completed CBT sessions, 45 (41.3%) occurred either on the same day as other hospital appointments (n = 25; 22.9%) or within the Cancer Center during chemotherapy infusions (n = 20; 18.3%). Thirteen (11.9%) CBT sessions took place via the telephone per the request of participants. Finally, the majority of participants in the intervention group (n = 14; 73.7%) completed the CBT modules in the order outlined by the treatment manual, although five patients deviated by addressing activity planning and pacing earlier in the therapy.
Figure 2.
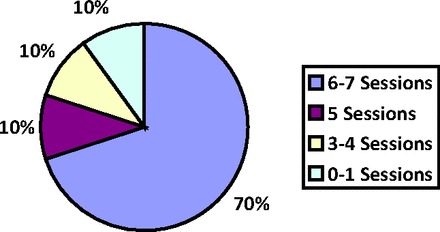
Percentage of cognitive-behavioral therapy sessions completed in the intervention arm (n = 20).
In evaluating the feasibility of administering the study assessments, we observed that 28 (70%) patients completed the posttreatment clinician interviews (i.e., HAM-A, CGI, MADRS). Some participants elected to finish the self-report instruments at home, and rates of completion for those posttreatment assessment measures were lower, ranging from 25 (62.5%) for the IES and FACT-G to 27 (67.5%) for the HADS.
Effect of CBT on (Blinded) Clinician-Rated Outcomes
The primary outcome of the study was change in HAM-A scores from baseline to posttreatment assessment between the study groups. As shown in Figure 3, patients assigned to the CBT group had an 8.57-point (SD = 8.46) decrease in mean HAM-A scores, which represents a 35% reduction in symptoms and lowering of average symptom severity from the moderate to mild range. By contrast, the waitlist control group had only an 11% reduction or 2.79-point (SD = 5.73) decrease in HAM-A scores on average (between-group mean difference = –5.79, SE = 2.73, 95% CI = –11.40 to –0.18; p = .04). Participants assigned to CBT also had greater reductions in mean CGI ratings from baseline to posttreatment assessment compared to the control group (between-group mean difference = –1.00, SE = 0.40, 95% CI = –1.83 to –0.17; p = .02).
Figure 3.

Mean changes in clinician-rated and self-reported anxiety from baseline to posttreatment assessment by study group.
Abbreviation: CBT, cognitive-behavioral therapy.
Using ANCOVA to adjust for baseline values (Table 2), study group assignment remained a significant predictor of posttreatment HAM-A and CGI scores, with large corresponding effect sizes for the intervention (HAM-A Cohen's d = 0.80; CGI Cohen's d = 0.94). Although clinician ratings on the HAM-A and MADRS correlated significantly at baseline (r = .67, p < .001), the average decrease in MADRS scores of 6.07 points (SD = 12.89) for the CBT group did not differ significantly from the 0.69-point (SD = 6.55) reduction for the control group (between-group mean difference = –5.38, SE = 3.90, 95% CI = –13.52 to 2.76; p = .18).
Table 2.
Results of analysis of covariance models with group assignment predicting clinician-rated and patient-reported outcome measures, adjusted for baseline scores

Abbreviations: CGI, Clinical Global Impression Scale; FACT-G, Functional Assessment of Cancer Therapy–General; HADS, Hospital Anxiety and Depression Scale; HAM-A, Hamilton Anxiety Rating Scale; IES, Impact of Events Scale; MADRS, Montgomery Asberg Depression Rating Scale.
Effect of CBT on Participant Self-Reported Outcomes
Consistent with the blind clinician-rated outcomes, patients assigned to CBT reported significantly greater reductions in anxiety symptoms on the HADS and IES compared to those in the waitlist control group (Fig. 3, Table 2). The effect sizes of the intervention for these measures were large and in excess of 0.80. Self-reported depression symptoms on the HADS-Depression subscale decreased from baseline to posttreatment assessment in the entire sample (mean difference = –1.91, SE = 0.83, 95% CI = –3.62 to –0.19; p = .03), but this improvement in mood did not differ significantly between study groups.
As shown in Table 2, the change in overall quality of life was similar between participants receiving CBT versus those in the waitlist control group. However, an examination of the subscales of the FACT-G revealed that, compared to those assigned to the control group, participants who received CBT reported worse physical well-being (p =.07; Cohen's d = 0.73) over time but improved emotional (p = .07; Cohen's d = 0.93) and functional well-being (p = .08; Cohen's d = 0.76). These findings did not quite meet the threshold for statistical significance, even though the effect sizes for the quality-of-life domains were large.
Missing Data Analyses
To account for missing data for patients who did not complete the study for any reason or failed to return any self-report questionnaires, we conducted multiple imputation analyses. Using this method, the CBT intervention still had significant effects on clinician-rated HAM-A (adjusted mean difference = –4.98, SE = 2.34, 95% CI = –9.60 to –0.37; p = .03) and CGI scores (adjusted mean difference = –1.09, SE = 0.44, 95% CI = –2.00 to –0.18; p = .02), as well as self-reported anxiety on the HADS-Anxiety subscale (adjusted mean difference = –2.05, SE = 0.84, 95% CI = –3.70 to –0.39; p = .02) and the IES (adjusted mean difference = –9.24, SE = 3.72, 95% CI = –16.62 to –1.86; p = .02). Study groups did not differ significantly in depression scores on either the MADRS (p = .22) or HADS-Depression subscale (p = .90).
Multiple imputation analyses for quality of life showed that, compared to those assigned to the waitlist control group, participants who received CBT had a marginally significant reduction in physical well-being (adjusted mean difference = –4.02, SE = 2.13, 95% CI = –8.35 to 0.32; p = .07) versus improvements in emotional well-being (adjusted mean difference = 3.15, SE = 1.38, 95% CI = 0.45–5.86; p = .02) and functional well-being (adjusted mean difference = 2.73, SE = 1.36, 95% CI = 0.04–5.41; p = .05), as measured by the FACT-G. Social well-being did not differ between groups (p = .39).
Discussion
In this pilot feasibility and randomized controlled trial of brief CBT tailored to patients with anxiety comorbid with terminal cancer, we found that the majority of participants in the sample was able to complete the intervention despite expected declines in physical health status associated with disease progression and toxicities from medical treatment. Moreover, we observed beneficial effects of the therapy for reducing anxiety symptoms and improving certain aspects of quality of life over time.
Patients with poor-prognosis cancers are at heightened risk for psychiatric morbidity and suffer considerable disease burden that may limit their ability to engage in psychosocial services [31]. Considering the disability associated with terminal cancers, we modified the delivery of our CBT intervention in several ways to accommodate patients' needs. Specifically, we reduced the number of sessions to 6–7 from the standard 12–15 visits often reported in psychotherapy trials for anxiety because of the constraints imposed by patients' shortened life expectancies. Notably, of the 20 participants assigned to the waitlist control group, only 9 completed the CBT intervention after the 2-month time point, primarily because of worsening disease that limited further participation, underscoring the importance of timely access to care.
To enhance retention, more than half of all CBT visits took place on the same day as other medical appointments, during chemotherapy infusions, or via telephone. These adaptations to standard clinical practice are often necessary when working with seriously ill patients. Investigators have begun examining alternate approaches to the delivery of CBT for individuals with cancer, such as through home-based visits, videoconferencing, and the telephone [32–34]. The results of the present study suggest that such accommodations may contribute to intervention effectiveness.
Despite the obstacles associated with treating a medically complex population, our study demonstrated statistically significant effects of brief CBT for reducing anxiety symptoms per blinded-clinician and patient reports. Across anxiety measures, effect sizes of change were large, highlighting the clinical utility of the brief treatment approach for helping patients cope with realistic cancer-related worries. However, we observed no significant differences between groups in depression from baseline to posttreatment assessment, in part because the variability in MADRS ratings was larger in the CBT group compared to the control group. Self-reported depression symptoms on the HADS appeared to lessen over time in the entire sample overall.
Our findings are consistent with the work of Moorey and colleagues, who conducted a cluster-randomized controlled trial demonstrating the benefit of CBT for reducing anxiety symptoms but not depression in home care patients with advanced cancer receiving palliative care [32]. We believe the targeted approach we have employed to treat anxiety in patients with terminal cancer would require specification for alleviating depression, perhaps by addressing demoralization and hopelessness, which strongly correlate with mood symptoms in this population [35, 36].
Without medical intervention, patients with terminal cancer experience declines in physical health status over time, which impair quality of life. In the present trial, the two study groups did not differ with respect to change in overall quality of life, but a finer analysis of the subscales revealed a more nuanced picture of this outcome. Despite decrements in physical well-being over time, we observed marginally significant improvements in emotional and functional well-being among those assigned to CBT compared to the waitlist control group. The large effect sizes and 2- to 3-point changes on these respective subscales of the FACT-G are clinically meaningful [37]. Although we urge caution in interpreting these results given the small sample, perhaps the acceptance-based and activity-pacing components of our tailored intervention enhanced coping with cancer [21], allowing patients to experience less emotional distress and improved functioning even while the physical disease worsened. Using mindfulness interventions to increase acceptance requires further study and may have particular benefit for patients diagnosed with cancer, not simply for alleviating anxiety [13] but also for buffering the expected declines in health-related quality of life.
Several limitations of the methods warrant attention. Because the study was a combined pilot feasibility and randomized controlled trial, we planned to enroll a small sample, aiming to achieve 30 study completers. Yet, the rate of accrual was low during the 3-year study period and attrition was high due to medical factors. Rather than relying on clinician referral, future studies would benefit from using routine screening procedures to identify patients with elevated anxiety symptoms in the oncology care setting. Additionally, the sample lacked racial and ethnic diversity, limiting the generalizability of intervention to minority patients. Moreover, we were able to meet patients in the chemotherapy infusion suite at the cancer center—a convenience that might be challenging to replicate in the community.
Although the results of our investigation are promising, a large-scale follow-up randomized controlled trial is necessary to confirm the efficacy and generalizability of the brief CBT intervention for patients with terminal cancers. Investigators of future psychosocial trials for anxiety in this population ought to consider the use of an attention-matched control group to ensure that CBT is the reason for the anxiety reduction, as well as alternative methods for delivering the intervention, such as web-based approaches, to increase access and dissemination. Finally, further work is needed to discern the optimal number of CBT sessions and whether the benefits of this intervention persist over the long term.
Despite the challenges of delivering a psychosocial intervention in this medically complex patient population, our study shows that CBT can be modified and tailored to address salient psychological needs of individuals with anxiety comorbid with terminal cancers. Although ongoing research is needed to ascertain the most effective methods for treating depression, brief CBT appears feasible and clinically beneficial for reducing suffering related to anxiety in patients with cancer who are coping with a terminal diagnosis.
Acknowledgments
This research was supported by a grant from the National Cancer Institute (R03CA128478).
Footnotes
- (C/A)
- Consulting/advisory relationship
- (RF)
- Research funding
- (E)
- Employment
- (H)
- Honoraria received
- (OI)
- Ownership interests
- (IP)
- Intellectual property rights/inventor/patent holder
- (SAB)
- Scientific advisory board
Author Contributions
Conception/Design: Joseph A. Greer, Elyse R. Park, William F. Pirl, Holly G. Prigerson, Steven A. Safren
Provision of study material or patients: Joseph A. Greer, Lara Traeger, Ellen S. Hendriksen, Jennifer S. Temel, Steven A. Safren
Collection and/or assembly of data: Joseph A. Greer, Lara Traeger, Heather Bemis, Jessica Solis, Ellen S. Hendriksen
Data analysis and interpretation: Joseph A. Greer, Lara Traeger, Heather Bemis, Jessica Solis, Ellen S. Hendriksen, Elyse R. Park, William F. Pirl, Jennifer S. Temel, Holly G. Prigerson, Steven A. Safren
Manuscript writing: Joseph A. Greer, Lara Traeger, Heather Bemis, Jessica Solis, Ellen S. Hendriksen, Elyse R. Park, William F. Pirl, Jennifer S. Temel, Holly G. Prigerson, Steven A. Safren
Final approval of manuscript: Joseph A. Greer, Lara Traeger, Heather Bemis, Jessica Solis, Ellen S. Hendriksen, Elyse R. Park, William F. Pirl, Jennifer S. Temel, Holly G. Prigerson, Steven A. Safren
References
- 1.Hopwood P, Stephens RJ. Depression in patients with lung cancer: Prevalence and risk factors derived from quality-of-life data. J Clin Oncol. 2000;18:893–903. doi: 10.1200/JCO.2000.18.4.893. [DOI] [PubMed] [Google Scholar]
- 2.Delgado-Guay M, Parsons HA, Li Z, et al. Symptom distress in advanced cancer patients with anxiety and depression in the palliative care setting. Support Care Cancer. 2009;17:573–579. doi: 10.1007/s00520-008-0529-7. [DOI] [PubMed] [Google Scholar]
- 3.Roth AJ, Massie MJ. Anxiety and its management in advanced cancer. Curr Opin Support Palliat Care. 2007;1:50–56. doi: 10.1097/SPC.0b013e32813aeb23. [DOI] [PubMed] [Google Scholar]
- 4.Spencer R, Nilsson M, Wright A, et al. Anxiety disorders in advanced cancer patients: Correlates and predictors of end-of-life outcomes. Cancer. 2010;116:1810–1819. doi: 10.1002/cncr.24954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greer JA, Pirl WF, Park ER, et al. Behavioral and psychological predictors of chemotherapy adherence in patients with advanced non-small cell lung cancer. J Psychosom Res. 2008;65:549–552. doi: 10.1016/j.jpsychores.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Temel JS, McCannon J, Greer JA, et al. Aggressiveness of care in a prospective cohort of patients with advanced NSCLC. Cancer. 2008;113:826–833. doi: 10.1002/cncr.23620. [DOI] [PubMed] [Google Scholar]
- 7.Mack JW, Block SD, Nilsson M, et al. Measuring therapeutic alliance between oncologists and patients with advanced cancer: The Human Connection Scale. Cancer. 2009;115:3302–3311. doi: 10.1002/cncr.24360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Institute of Medicine. Washington, DC: National Academic Press; 2007. Cancer Care for the Whole Patient: Meeting Psychosocial Health Needs. [Google Scholar]
- 9.Holland JC, Alici Y. Management of distress in cancer patients. J Support Oncol. 2010;8:4–12. [PubMed] [Google Scholar]
- 10.Jacobsen PB, Jim HS. Psychosocial interventions for anxiety and depression in adult cancer patients: Achievements and challenges. CA Cancer J Clin. 2008;58:214–230. doi: 10.3322/CA.2008.0003. [DOI] [PubMed] [Google Scholar]
- 11.Uitterhoeve R, Vernooy M, Litjens M, et al. Psychosocial interventions for patients with advanced cancer: A systematic review of the literature. Br J Cancer. 2004;91:1050–1062. doi: 10.1038/sj.bjc.6602103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Newell SA, Sanson-Fisher RW, Savolainen NJ. Systematic review of psychological therapies for cancer patients: Overview and recommendations for future research. J Natl Cancer Inst. 2002;94:558–584. doi: 10.1093/jnci/94.8.558. [DOI] [PubMed] [Google Scholar]
- 13.Shennan C, Payne S, Fenlon D. What is the evidence for the use of mindfulness-based interventions in cancer care? A review. Psychooncology. 2011;20:681–697. doi: 10.1002/pon.1819. [DOI] [PubMed] [Google Scholar]
- 14.Luckett T, Butow PN, King MT, et al. A review and recommendations for optimal outcome measures of anxiety, depression and general distress in studies evaluating psychosocial interventions for English-speaking adults with heterogeneous cancer diagnoses. Support Care Cancer. 2010;18:1241–1262. doi: 10.1007/s00520-010-0932-8. [DOI] [PubMed] [Google Scholar]
- 15.Jacobsen PB. Promoting evidence-based psychosocial care for cancer patients. Psychooncology. 2009;18:6–13. doi: 10.1002/pon.1468. [DOI] [PubMed] [Google Scholar]
- 16.Pessin H, Galietta M, Nelson CJ, et al. Burden and benefit of psychosocial research at the end of life. J Palliat Med. 2008;11:627–632. doi: 10.1089/jpm.2007.9923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thompson GN, Chochinov HM, Wilson KG, et al. Prognostic acceptance and the well-being of patients receiving palliative care for cancer. J Clin Oncol. 2009;27:5757–5762. doi: 10.1200/JCO.2009.22.9799. [DOI] [PubMed] [Google Scholar]
- 18.Chochinov HM, Kristjanson LJ, Hack TF, et al. Burden to others and the terminally ill. J Pain Symptom Manage. 2007;34:463–471. doi: 10.1016/j.jpainsymman.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 19.Hofmann SG, Smits JA. Cognitive-behavioral therapy for adult anxiety disorders: A meta-analysis of randomized placebo-controlled trials. J Clin Psychiatry. 2008;69:621–632. doi: 10.4088/jcp.v69n0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barlow D. Anxiety and Its Disorders: The Nature and Treatment of Anxiety and Panic. 2nd ed. New York: Guilford Press; 2002. [Google Scholar]
- 21.Greer JA, Park ER, Prigerson HG, et al. Tailoring cognitive-behavioral therapy to treat anxiety comorbid with advanced cancer. J Cogn Psychother. 2010;24:294–313. doi: 10.1891/0889-8391.24.4.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kobak KA, Reynolds WM, Greist JH. Development and validation of a computer-administered version of the Hamilton Rating Scale. Psychol Assessment. 1993;5:487–492. [Google Scholar]
- 23.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 24.Shear MK, Vander Bilt J, Rucci P, et al. Reliability and validity of a structured interview guide for the Hamilton Anxiety Rating Scale. Depress Anxiety. 2001;13:166–178. [PubMed] [Google Scholar]
- 25.National Institute of Mental Health. CGI (Clinical Global Impression) Scale. Psychopharmacol Bull. 1985;21:839–843. [Google Scholar]
- 26.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 27.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 28.Horowitz M, Wilner N, Alvarez W. Impact of Event Scale: A measure of subjective stress. Psychosom Med. 1979;41:209–218. doi: 10.1097/00006842-197905000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Vodermaier A, Linden W, Siu C. Screening for emotional distress in cancer patients: A systematic review of assessment instruments. J Natl Cancer Inst. 2009;101:1464–1488. doi: 10.1093/jnci/djp336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: Development and validation of the general measure. J Clin Oncol. 1993;11:570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 31.Fallowfield L, Ratcliffe D, Jenkins V, et al. Psychiatric morbidity and its recognition by doctors in patients with cancer. Br J Cancer. 2001;84:1011–1015. doi: 10.1054/bjoc.2001.1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moorey S, Cort E, Kapari M, et al. A cluster randomized controlled trial of cognitive behaviour therapy for common mental disorders in patients with advanced cancer. Psychol Med. 2009;39:713–723. doi: 10.1017/S0033291708004169. [DOI] [PubMed] [Google Scholar]
- 33.DuHamel KN, Mosher CE, Winkel G, et al. Randomized clinical trial of telephone-administered cognitive-behavioral therapy to reduce post-traumatic stress disorder and distress symptoms after hematopoietic stem-cell transplantation. J Clin Oncol. 2010;28:3754–3761. doi: 10.1200/JCO.2009.26.8722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shepherd L, Goldstein D, Whitford H, et al. The utility of videoconferencing to provide innovative delivery of psychological treatment for rural cancer patients: Results of a pilot study. J Pain Symptom Manage. 2006;32:453–461. doi: 10.1016/j.jpainsymman.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 35.Lo C, Zimmermann C, Rydall A, et al. Longitudinal study of depressive symptoms in patients with metastatic gastrointestinal and lung cancer. J Clin Oncol. 2010;28:3084–3089. doi: 10.1200/JCO.2009.26.9712. [DOI] [PubMed] [Google Scholar]
- 36.Mehnert A, Vehling S, Hocker A, et al. Demoralization and depression in patients with advanced cancer: Validation of the German version of the Demoralization Scale. J Pain Symptom Manage. 2011;42:768–776. doi: 10.1016/j.jpainsymman.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 37.Cella D, Hahn EA, Dineen K. Meaningful change in cancer-specific quality of life scores: Differences between improvement and worsening. Qual Life Res. 2002;11:207–221. doi: 10.1023/a:1015276414526. [DOI] [PubMed] [Google Scholar]


