Abstract
Objective
In this study, we measured the cortical bone thickness in the mandibular buccal and lingual areas using computed tomography in order to evaluate the suitability of these areas for application of temporary anchorage devices (TADs) and to suggest a clinical guide for TADs.
Methods
The buccal and lingual cortical bone thickness was measured in 15 men and 15 women. Bone thickness was measured 4 mm apical to the interdental cementoenamel junction between the mandibular canine and the 2nd molar using the transaxial slices in computed tomography images.
Results
The cortical bone in the mandibular buccal and lingual areas was thicker in men than in women. In men, the mandibular lingual cortical bone was thicker than the buccal cortical bone, except between the 1st and 2nd molars on both sides. In women, the mandibular lingual cortical bone was thicker in all regions when compared to the buccal cortical bone. The mandibular buccal cortical bone thickness increased from the canine to the molars. The mandibular lingual cortical bone was thickest between the 1st and 2nd premolars, followed by the areas between the canine and 1st premolar, between the 2nd premolar and 1st molar, and between the 1st molar and 2nd molar.
Conclusions
There is sufficient cortical bone for TAD applications in the mandibular buccal and lingual areas. This provides the basis and guidelines for the clinical use of TADs in the mandibular buccal and lingual areas.
Keywords: Computed tomography, Temporary anchorage device, Mandibular cortical bone thickness
INTRODUCTION
Recently, temporary anchorage devices (TADs) were developed to achieve maximum anchorage using a simple method. Unlike general implants, TADs can be loaded immediately and have the advantages of low cost, short chair time and small size. The small size allows them to be placed in inter-dental spaces. Therefore, TADs are used widely in orthodontic treatment.
When placing an TAD, site selection is important. Important factors that affect site selection are stability and safety.1,2 Stable implants are retained and do not loosen when an orthodontic force is applied. The safety of a site refers to the ability of a clinician to place an implant at that site without damage to the root or other important anatomical structures.
Many studies have reported that the stability of TADs is affected by the initial site stability. Implant stability depends on the condition of the cortical bone and operator's technique.3-6 Therefore, it is important to know the cortical bone thickness, which is critical for the initial stability of an TAD.
In the mandible, the space between the 2nd premolar and 1st molar is the preferred site for anterior tooth retraction, and the space between the canine and 1st premolar or between the 1st premolar and 2nd premolar is often used for the mesial movement of molars. In addition, the mandibular buccal and lingual molar areas can be used for an TAD to intrude the molars. The mandibular molar area is often preferred for placement of TADs, along with the maxillary midpalatal suture,7,8 palatal, and maxillary buccal areas.9 Therefore, determination of the cortical bone thickness of the mandibular molar region will be helpful for the selection of TAD placement sites.
In this study, we evaluated the suitability of the mandibular molar area for TAD placement site by comparing the buccal and lingual cortical bone thicknesses using computed tomography (CT) images.
MATERIALS AND METHODS
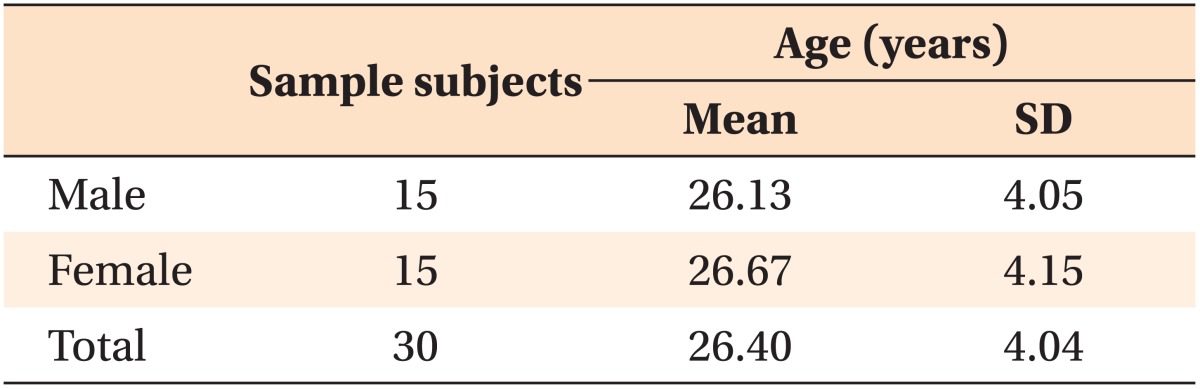
We examined CT images of 30 adults (15 men with a mean age of 26.13 years and 15 women with a mean age of 26.67 years; Table 1). Images were selected from a larger sample of adults who received CT scans prior to extraction of the lower third molars at the Department of Oral and Maxillofacial Surgery, Yonsei University, Seoul, South Korea. The inclusion criteria were as follows: completion of growth, no severe crowding, no periodontal disease, no missing teeth, and no intrabony lesions in the mandible.
Table 1.
Mean age and standard deviation of each group
SD, Standard deviation.
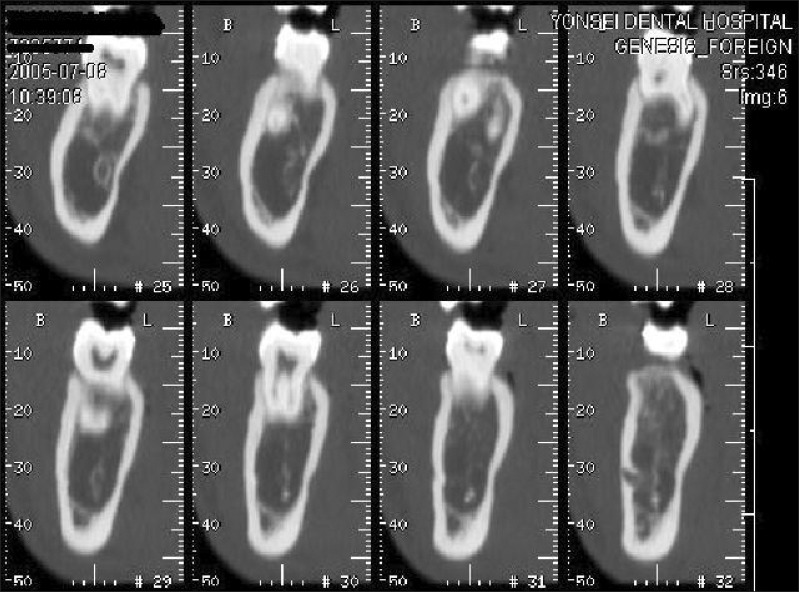
Images were taken using a CT Hispeed advantage (GE Medical System, Milwaukee, WI, USA) using a high-resolution bone algorithm at the following settings; 9.6 cm Display Field of View (DFOV), 200 mA, 120 kV, 1 second scanning time, and 1-mm slice thickness. When taking the CT images, the occlusal plane of each subject was perpendicular to the floor and each subject bit a tongue blade placed in the premolar region. The scanning range was from the mandibular occlusal plane to the mandibular border. The images were saved in the Picture Archiving Communication System (PACS) at Yonsei University Dental Hospital and the interdental bone thicknesses 4 mm apical to the cementoenamel junction (CEJ) between the canine and 1st premolar, 1st premolar and 2nd premolar, 2nd premolar and 1st molar, and 1st molar and 2nd molar on both sides of the mandible, were measured on the transaxial slices of CT images using the PiView STAR (INFINITT, Seoul, Korea) program (Figure 1).
Figure 1.
Representative computed tomography (CT) image of the transaxial slices of the mandibular body, as displayed by the PiView STAR program (INFINITT, Seoul, Korea).
The transaxial images of the teeth were divided into 1-mm slices. The most distal transaxial image of the tooth that displayed the largest amount of the pulp chamber most clearly was measured. This image was chosen because it revealed the CEJ clearly.
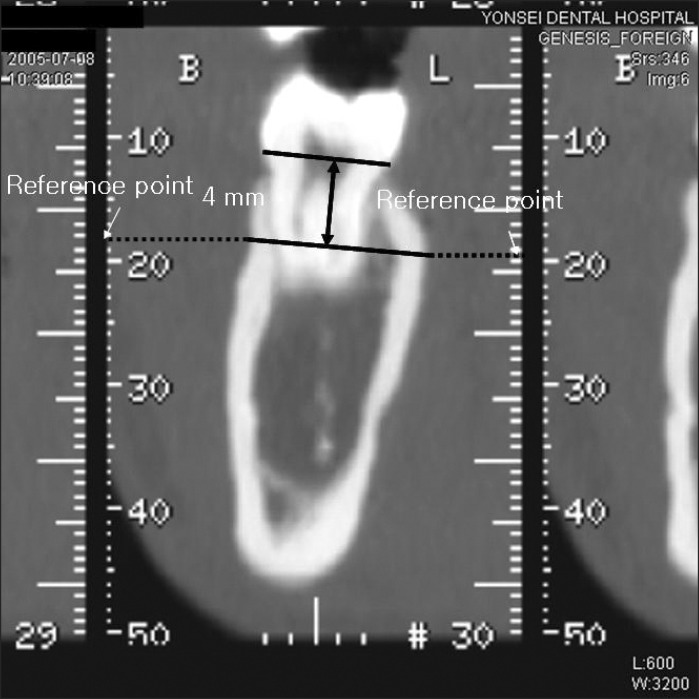
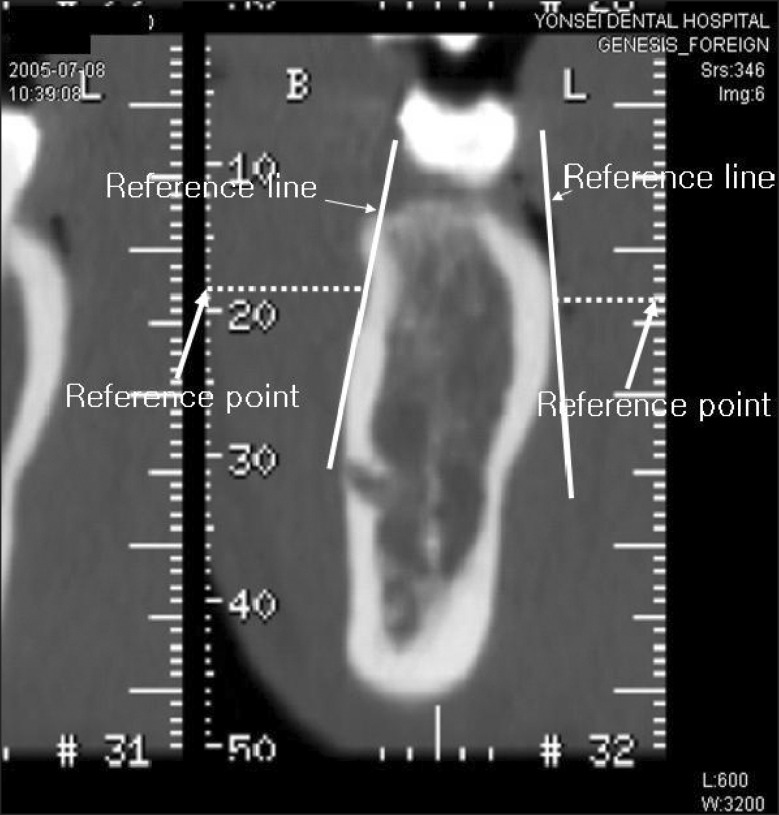
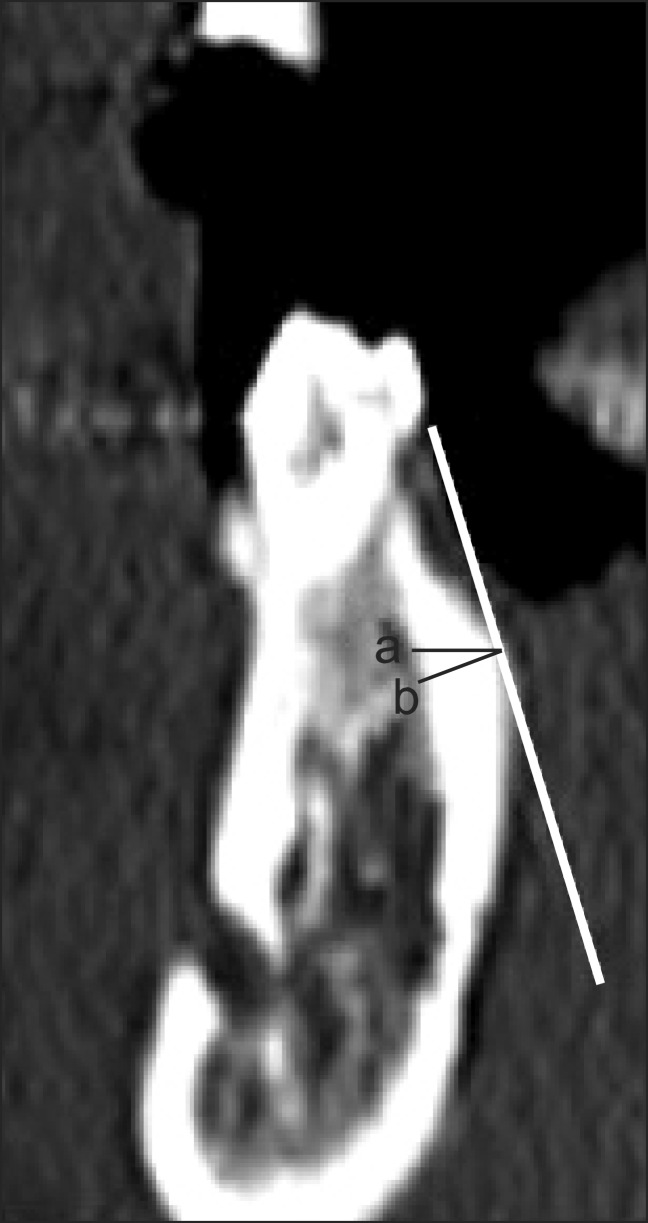
In this image, a line was drawn on the image that was parallel to and 4 mm below the CEJ. Two points were placed on this line that corresponded to where the tooth met with the buccal and lingual cortical bone. A horizontal line was drawn from each of these points to the right and left ruler on the computer screen, and the ruler measurements were marked as reference points (Figure 2). The reference points were then moved to the selected interdental transaxial image, based on the axial CT image, and a horizontal line was drawn from these reference points to the two points that corresponded to where the tooth met with the buccal and lingual cortical bone. Two more reference lines were set tangential to these two points and the buccal and lingual surfaces (Figure 3).
Figure 2.
Reference points placed on an image of the reference site.
Figure 3.
Reference lines placed on the measurement site.
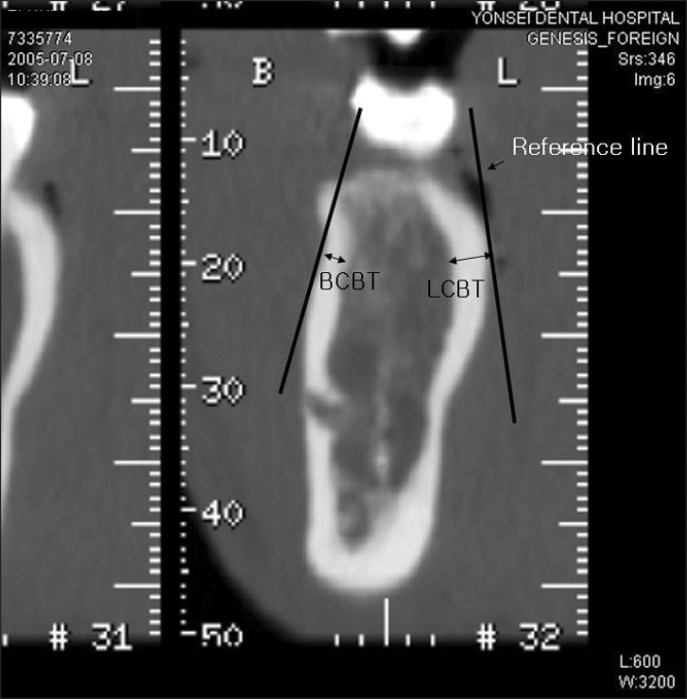
Buccal cortical bone thickness (BCBT) and lingual cortical bone thickness (LCBT) were measured on the line perpendicular to the reference line using PiView STAR (INFINITT) in mm (Figure 4).
Figure 4.
Measurement of buccal cortical bone thickness (BCBT) and lingual cortical bone thickness (LCBT).
Statistical analysis
We calculated the means and standard deviations of the buccal and lingual cortical bone thicknesses. Shapiro-Wilk's test revealed a normal distribution of the data. We used independent t-tests to compare the cortical bone thickness of men and women and between the buccal and lingual sides. A one-way repeated-measures ANOVA test was used to compare the buccal and lingual cortical bone thicknesses of the interdental areas between the canine and 2nd molar in men and women. All statistical analyses were carried out by the SPSS software program (version 19.0; SPSS Inc., Chicago, IL, USA).
RESULTS
Buccal cortical bone thickness
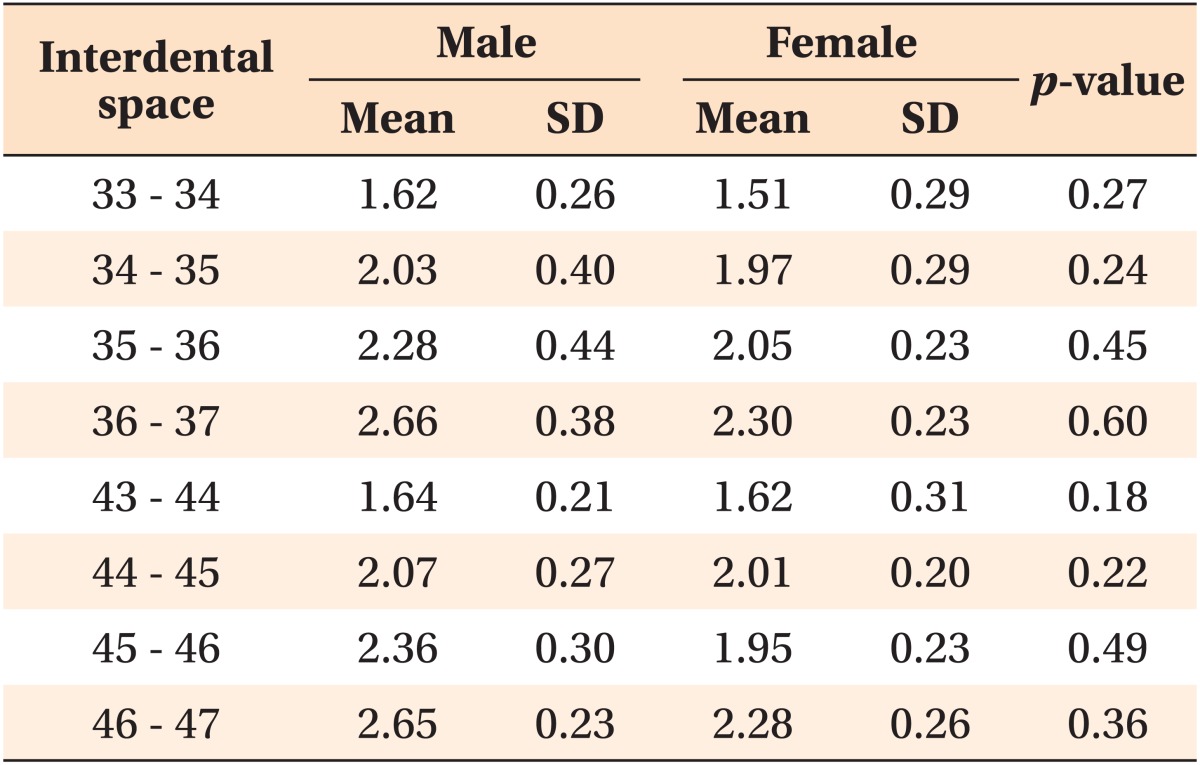
The buccal cortical bone was thicker in men than in women, but the difference was not statistically significant (Table 2).
Table 2.
Buccal cortical bone thickness (mm) by region
Independent t-test was performed to compare the buccal cortical bone thickness between males and females.
SD, Standard deviation.
Lingual cortical bone thickness
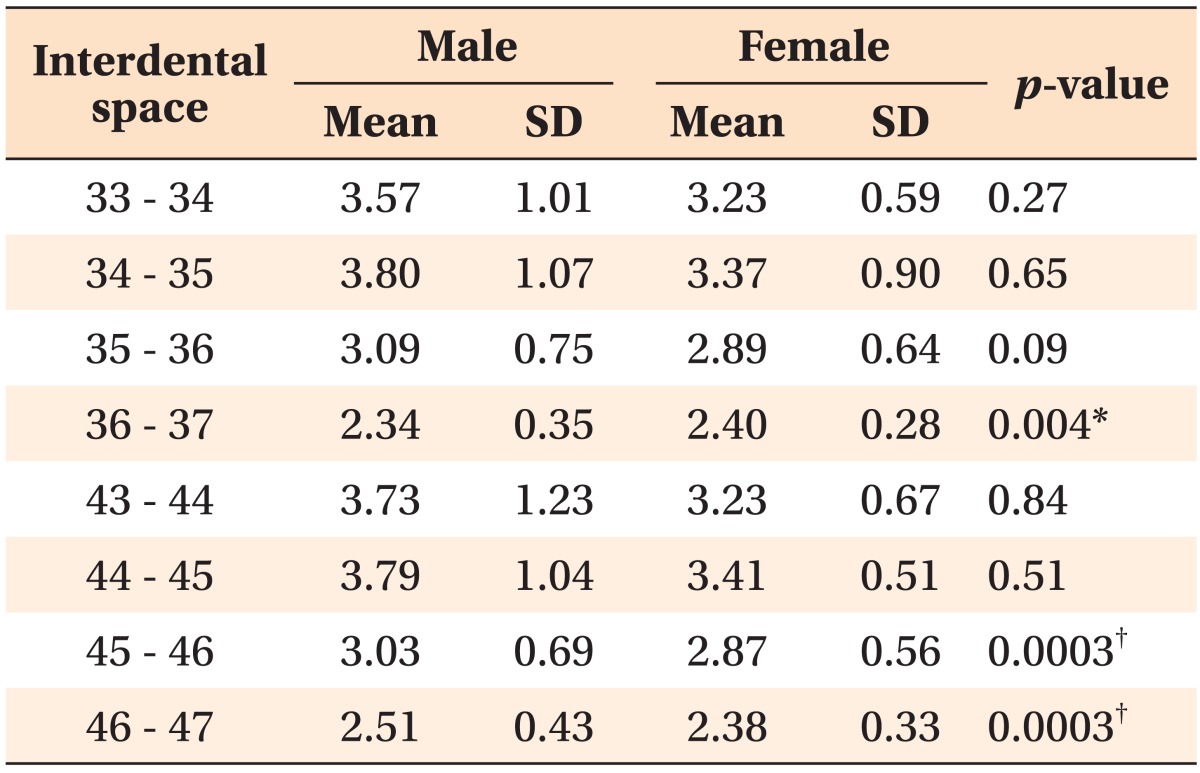
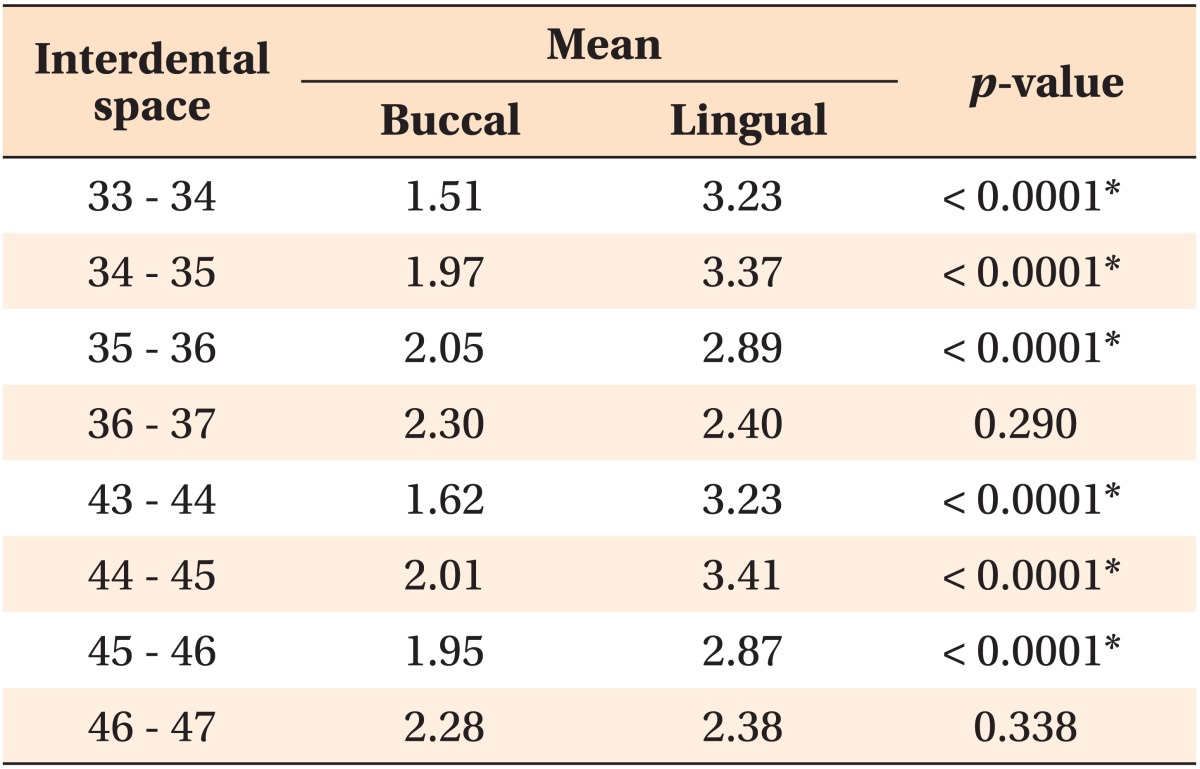
The lingual cortical bone was thicker in men than in women, except between the left 1st and 2nd molars (Table 3).
Table 3.
Lingual cortical bone thickness (mm) by region
Independent t-test was performed to compare the lingual cortical bone thickness between males and females.
SD, Standard deviation.
*p < 0.01, †p < 0.001.
Comparison of buccal and lingual cortical bone thickness
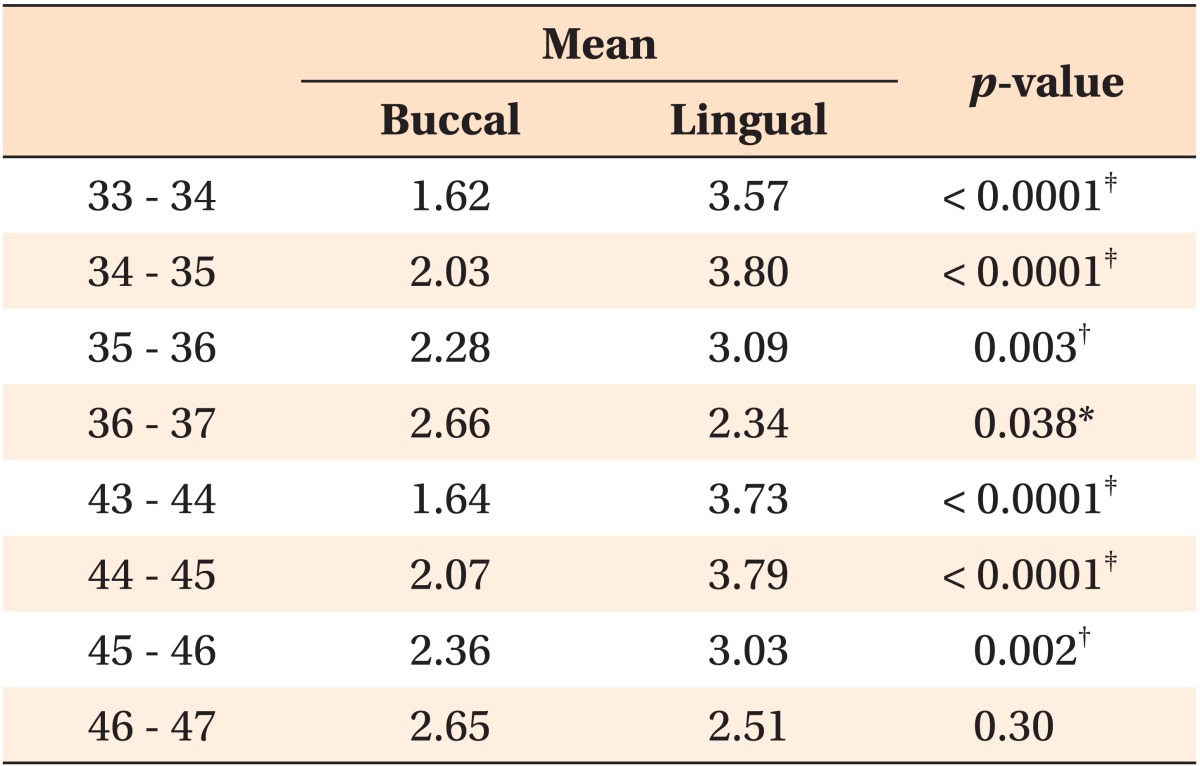
In men, the buccal cortical bone in the molar region was thicker than the lingual cortical bone, but this measurement was not statistically significant on the right side (Table 4). In women, the lingual cortical bone was thicker than the buccal cortical bone in all regions. This difference was statistically significant for all regions, except between the 1st and 2nd molars (Table 5).
Table 4.
Differences between the buccal and lingual cortical bone thickness (mm) in males
Paired t-test was performed.
*p < 0.05, †p < 0.01, ‡p < 0.001.
Table 5.
Differences between the buccal and lingual cortical bone thickness (mm) in females
Paired t-test was performed.
*p < 0.001.
Comparison of interdental cortical bone thickness
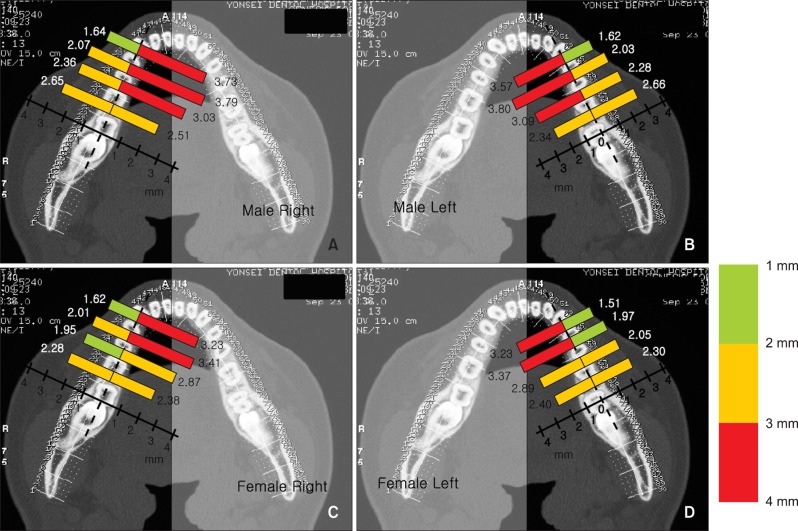
Mandibular lingual cortical bone thickness was the thickest between the 1st and 2nd premolar region and the thickness gradually decreased posterior to this region. On the other hand, the mandibular buccal cortical bone thickness increased between the anterior and posterior regions (Tables 4 and 5, Figure 5). One-way repeated-measures ANOVA tests indicated significant differences in cortical bone thickness between interdental regions (p < 0.01, Table 6).
Figure 5.
Average cortical bone thickness for (A) male right; (B) male left; (C) female right; (D) female left.
Table 6.
Differences in cortical bone thickness between interdental regions
One-way repeated-measures ANOVA test was performed.
Rt, Right; Lt, left.
*p < 0.001.
DISCUSSION
We used CT images to measure cortical bone thickness. In general, measurements using a dry skull make it difficult to ascertain the precise age of the skull. Even if the age of the donor is known, most samples show advanced bone resorption. Unlike general radiography, CT images can be used to provide accurate measurements of bone thickness without any expansion, transformation, or superimposition of anatomical structures.10-12 In addition, measurements from CT images are almost identical to those using dry skull specimens.10 Masumoto et al.13 reported that CT images were accurate and could be used to measure tooth angulation and cortical bone thickness with a margin of error less than 0.13° and 0.1 mm, respectively.
In previous studies, Kim et al.14 and Park15 used the axial plane as a reference plane in CT images. In this study, however, we used the transaxial plane as a reference plane in order to minimize errors due to the anatomical form of the mandible. Use of the axial plane as a reference plane results in an overestimation of thickness due to the mandibular buccolingual inclination (Figure 6). Park15 reported that the mandibular buccal cortical bone thickness of the 2nd premolar, 1st molar, and 2nd molar areas to be 1.71 ± 0.05 mm, 2.48 ± 0.71 mm, and 3.17 ± 0.93 mm, respectively. These measurements are larger than those in the present study. Therefore, the transaxial plane must be used as a reference plane for precise measurements.
Figure 6.
Cortical bone thickness from (a) the axial plane and (b) the transaxial plane as reference planes.
TADs should be placed in an area with attached gingiva to increase their stability. Kim16 reported that healthy keratinized attached gingiva was located 3.5 - 5.3 mm below the gingival margin in the buccal area. Voigt et al.17 and Linde et al.18 reported that the lingual attached gingiva was located 1 - 9 mm below the gingival margin, but was 2 - 6 mm below the gingival margin in the premolar and molar area. On the basis of these studies, we estimated the location of the stable vertical position of TADs to be 4 mm below the CEJ in the buccal and lingual areas.
Cortical bone on both the buccal and lingual sides was thicker in men than in women. In this study, the buccal cortical bone thickness increased gradually from the anterior to posterior regions. This is in contrast to the lingual cortical bone thickness, which decreased gradually from the anterior to posterior regions. Similarly, Kim19 and Silvestrini Biavati20 found that the buccal cortical bone thickness increased from the anterior areas to the ramus and the lingual cortical bone was thicker in the anterior areas than posterior areas. On the other hand, we found that in both men and women, the lingual cortical bone was thickest between the 1st premolar and 2nd premolar, followed by the area between the canine and 1st premolar, the area between the 2nd premolar and 1st molar, and the area between the 1st molar and 2nd molar. Our measurements were smaller than those reported by Kim19 for the buccal area, and larger than those Kim reported for the lingual area. We attribute this difference to the difference in measurement methods. Kim19 measured the thickest and thinnest areas of cortical bone for each region, and calculated the average. However, we measured cortical bone thickness at the same distance from the CEJ in each region. In general, the cortical bone between the 1st premolar and 2nd premolar area is thickest in the lingual area because the lingual torus can be located 4 mm below the CEJ, although there are some individual differences. In some samples, the cortical bone thickness was > 6 mm in this area.
The buccal cortical bone thickness increased gradually from the anterior to posterior regions, but the lingual cortical bone thickness decreased from the anterior to posterior regions. This was related to buccolingual molar inclination. The mandibular molars are inclined lingually due to masticatory muscle activity and function.13,21 The buccolingual inclination of the mandibular molars is related to the force direction of the muscles acting on the molars. The axis of all molars is parallel to the direction of the pull of the medial pterygoid muscles. When the axis of mandibular molars and the direction of a muscle contraction coincide, mandibular molars can effectively resist masticatory forces, forming the curve of Wilson.
The lingual inclination of the mandibular molars helps the tongue and buccinator muscles to easily place food on the occlusal table, thus improving masticatory function. Ichim et al.22 and Hirabayashi23 reported that at the occlusal terminal phase, the load is directed buccally on the posterior teeth in the mandible. Because mandibular molars are inclined lingually, the mandibular molar buccal and lingual structures, particularly the cortical bone thickness, are affected by masticatory functions.13,21 When chewing and swallowing, the area around the mandibular posterior teeth also receives buccal loads from actions of the tongue. Therefore, the area surrounding the mandibular posterior teeth are supported by strong structures, such as the thick buccal cortical bone. This is especially true in the area of the 2nd molar where the masticatory muscle attachment is located. The buccal cortical bone in the 2nd molar area is thickest. In contrast, there is less need for strong support on the lingual side, so the lingual cortical bone appears to be thinner in the 2nd molar area. The forces acting on the mandibular cortical bone can also explain gender differences in cortical bone thickness in the area of the 2nd molar. The buccal cortical bone is thicker than the lingual cortical bone at the 2nd molar in men because men have a stronger masticatory forces than women. On the other hand, although buccal cortical bone thickness increases gradually posteriorly in women, it is thinner than the lingual cortical bone thickness at the 2nd molar because the masticatory forces of women tend to be weaker than that of men.
The gradual increase in the thickness of the buccal cortical bone from the anterior regions to the posterior regions implies that the stability of TADs would be greater if implanted in the molar region than in the premolar region. However, because the lingual cortical bone is thicker in the premolar regions than the molar regions, TADs implanted in the premolar region on the lingual side would be more stable than those implanted in the lingual molar region. This is because TADs are more stable when inserted into sites with thicker cortical bone.24,25 Although the lingual molar area has lower stability than the buccal molar area, the thinnest lingual cortical bone in this area was > 2 mm. This measurement was not significantly different from that of the buccal side. Therefore, the lingual cortical bone is as stable as the buccal cortical bone for TAD implantation.
CONCLUSION
There was more than 2 mm of cortical bone in all areas, except between the canine and 1st premolar on the buccal side of the mandible. The cortical bone on both mandibular buccal and lingual sides is thick enough for TAD applications. This provides that mandibular buccal and lingual areas are proper site for stable placement of TADs.
Footnotes
The authors report no commercial, proprietary, or financial interest in the products or companies described in this article.
References
- 1.Poggio PM, Incorvati C, Velo S, Carano A. "Safe zones": a guide for miniscrew positioning in the maxillary and mandibular arch. Angle Orthod. 2006;76:191–197. doi: 10.1043/0003-3219(2006)076[0191:SZAGFM]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 2.Hu KS, Kang MK, Kim TW, Kim KH, Kim HJ. Relationships between dental roots and surrounding tissues for orthodontic miniscrew installation. Angle Orthod. 2009;79:37–45. doi: 10.2319/083107-405.1. [DOI] [PubMed] [Google Scholar]
- 3.Park HS, Jeong SH, Kwon OW. Factors affecting the clinical success of screw implants used as orthodontic anchorage. Am J Orthod Dentofacial Orthop. 2006;130:18–25. doi: 10.1016/j.ajodo.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 4.Santiago RC, de Paula FO, Fraga MR, Picorelli Assis NM, Vitral RW. Correlation between miniscrew stability and bone mineral density in orthodontic patients. Am J Orthod Dentofacial Orthop. 2009;136:243–250. doi: 10.1016/j.ajodo.2007.08.031. [DOI] [PubMed] [Google Scholar]
- 5.Miyamoto I, Tsuboi Y, Wada E, Suwa H, Iizuka T. Influence of cortical bone thickness and implant length on implant stability at the time of surgery--clinical, prospective, biomechanical, and imaging study. Bone. 2005;37:776–780. doi: 10.1016/j.bone.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 6.Wilmes B, Rademacher C, Olthoff G, Drescher D. Parameters affecting primary stability of orthodontic mini-implants. J Orofac Orthop. 2006;67:162–174. doi: 10.1007/s00056-006-0611-z. [DOI] [PubMed] [Google Scholar]
- 7.Kim DH. Anatomical characteristics of the midpalatal suture area for miniscrew implantation using CT image [master's thesis] Seoul: Yonsei University; 2003. [Google Scholar]
- 8.Lee JS, Kim DH, Park YC, Kyung SH, Kim TK. The efficient use of midpalatal miniscrew implants. Angle Orthod. 2004;74:711–714. doi: 10.1043/0003-3219(2004)074<0711:TEUOMM>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 9.Kim HJ, Yun HS, Park HD, Kim DH, Park YC. Soft-tissue and cortical-bone thickness at orthodontic implant sites. Am J Orthod Dentofacial Orthop. 2006;130:177–182. doi: 10.1016/j.ajodo.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 10.Waitzman AA, Posnick JC, Armstrong DC, Pron GE. Craniofacial skeletal measurements based on computed tomography: Part I. Accuracy and reproducibility. Cleft Palate Craniofac J. 1992;29:112–117. doi: 10.1597/1545-1569_1992_029_0112_csmboc_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- 11.Hanazawa T, Sano T, Seki K, Okano T. Radiologic measurements of the mandible: a comparison between CT-reformatted and conventional tomographic images. Clin Oral Implants Res. 2004;15:226–232. doi: 10.1111/j.1600-0501.2004.00991.x. [DOI] [PubMed] [Google Scholar]
- 12.Spoor CF, Zonneveld FW, Macho GA. Linear measurements of cortical bone and dental enamel by computed tomography: applications and problems. Am J Phys Anthropol. 1993;91:469–484. doi: 10.1002/ajpa.1330910405. [DOI] [PubMed] [Google Scholar]
- 13.Masumoto T, Hayashi I, Kawamura A, Tanaka K, Kasai K. Relationships among facial type, buccolingual molar inclination, and cortical bone thickness of the mandible. Eur J Orthod. 2001;23:15–23. doi: 10.1093/ejo/23.1.15. [DOI] [PubMed] [Google Scholar]
- 14.Kim JH, Joo JY, Park YW, Cha BK, Kim SM. Study of maxillary cortical bone thickness for skeletal anchorage system in Korean. J Korean Assoc Oral Maxillofac Surg. 2002;28:249–255. [Google Scholar]
- 15.Park HS. An anatomical study using CT images for the implantation of micro-implants. Korean J Orthod. 2002;32:435–441. [Google Scholar]
- 16.Kim JS. Clinical study on the width of attached gingiva in the subjects with healthy gingiva, or early stage of gingivitis [master's thesis] Seoul: Yonsei University; 1997. [Google Scholar]
- 17.Voigt JP, Goran ML, Flesher RM. The width of lingual mandibular attached gingiva. J Periodontol. 1978;49:77–80. doi: 10.1902/jop.1978.49.2.77. [DOI] [PubMed] [Google Scholar]
- 18.Linde J, Lang NP, Karring T. Clinical periodontology and implant dentistry. 5th ed. Oxford: Blackwell Munksgaard; 2008. pp. 6–8. [Google Scholar]
- 19.Kim HJ. The morphology of the mandibular canal and the structure of the compact and sponge bone in Korean adult mandibles [master's thesis] Seoul: Yonsei University; 1993. [Google Scholar]
- 20.Silvestrini Biavati A, Tecco S, Migliorati M, Festa F, Marzo G, Gherlone E, et al. Three-dimensional tomographic mapping related to primary stability and structural miniscrew characteristics. Orthod Craniofac Res. 2011;14:88–99. doi: 10.1111/j.1601-6343.2011.01512.x. [DOI] [PubMed] [Google Scholar]
- 21.Tsunori M, Mashita M, Kasai K. Relationship between facial types and tooth and bone characteristics of the mandible obtained by CT scanning. Angle Orthod. 1998;68:557–562. doi: 10.1043/0003-3219(1998)068<0557:RBFTAT>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 22.Ichim I, Kieser JA, Swain MV. Functional significance of strain distribution in the human mandible under masticatory load: numerical predictions. Arch Oral Biol. 2007;52:465–473. doi: 10.1016/j.archoralbio.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 23.Hirabayashi M, Motoyoshi M, Ishimaru T, Kasai K, Namura S. Stresses in mandibular cortical bone during mastication: biomechanical considerations using a three-dimensional finite element method. J Oral Sci. 2002;44:1–6. doi: 10.2334/josnusd.44.1. [DOI] [PubMed] [Google Scholar]
- 24.Wei X, Zhao L, Xu Z, Tang T, Zhao Z. Effects of cortical bone thickness at different healing times on microscrew stability. Angle Orthod. 2011;81:760–766. doi: 10.2319/111610-667.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Motoyoshi M, Inaba M, Ono A, Ueno S, Shimizu N. The effect of cortical bone thickness on the stability of orthodontic mini-implants and on the stress distribution in surrounding bone. Int J Oral Maxillofac Surg. 2009;38:13–18. doi: 10.1016/j.ijom.2008.09.006. [DOI] [PubMed] [Google Scholar]