Abstract
Alginate-based materials have received considerable attention for biomedical applications because of their hydrophilic nature, biocompatibility, and physical architecture. Applications include cell encapsulation, drug delivery, stem cell culture, and tissue engineering scaffolds. In fact, clinical trials are currently being performed in which islets are encapsulated in PLO coated alginate microbeads as a treatment of type I diabetes. However, large numbers of islets are required for efficacy due to poor survival following transplantation. The ability to locally stimulate microvascular network formation around the encapsulated cells may increase their viability through improved transport of oxygen, glucose and other vital nutrients. Fibroblast growth factor-1 (FGF-1) is a naturally occurring growth factor that is able to stimulate blood vessel formation and improve oxygen levels in ischemic tissues. The efficacy of FGF-1 is enhanced when it is delivered in a sustained fashion rather than a single large-bolus administration. The local long-term release of growth factors from islet encapsulation systems could stimulate the growth of blood vessels directly towards the transplanted cells, potentially improving functional graft outcomes. In this article, we outline procedures for the preparation of alginate microspheres for use in biomedical applications. In addition, we describe a method we developed for generating multilayered alginate microbeads. Cells can be encapsulated in the inner alginate core, and angiogenic proteins in the outer alginate layer. The release of proteins from this outer layer would stimulate the formation of local microvascular networks directly towards the transplanted islets.
Keywords: Medicine, Issue 66, Biomedical Engineering, Bioengineering, Chemical Engineering, Molecular Biology, Alginate, angiogenesis, FGF-1, encapsulation
Protocol
The protocol here describes a three-step procedure for generating multilayered alginate microbeads (Figure 1). First, alginate microbeads are formed (Figure 2A). This procedure is described in section 1 below. Cells or proteins can be added to the microbeads in this step in order to act as a delivery system. The next step involves the formation of a permselective layer on the microbeads and is described in section 2. The final step involves the formation of an additional alginate layer and is described in section 3. This layer forms on the outside of the surface of the beads (Figure 2B) can be used to encapsulate and deliver therapeutic molecules (Figure 2C) to direct cellular response to the system following transplantation.
1. Alginate Microbead Preparation
Prepare a 1.5% (w/v) LVM alginate solution by dissolving 15 grams of LVM alginate in 1 mL of inner layer alginate solution (25 mM HEPES buffer, 118 mM NaCl, 5.6 mM KCl, and 2.5 mM MgCl2 in DI water, adjusted to pH 7.4). Mix on a Vortexer until the alginate power has fully dissolved to form a clear, viscous solution. Note: This protocol describes conditions optimal for islet encapsulation within alginate microcapsules. 1 The concentration and composition of the alginate microspheres can be altered to tune properties for other applications (e.g. drug delivery, tissue engineering, etc.) Note: For islet encapsulation the islets can be added to the alginate solution at this step prior to loading in the microencapsulator.
Prepare the crosslinking solution (22 mM CaCl2 in DI water) by dissolving 100 mM CaCl2 and 10 mM HEPES buffer in DI water and adjusting to pH 7.4. Note: other divalent cations such as Br2+, Sr2+ etc can be used instead of Ca2+, depending on the nature of alginate gelation desired.
Set up the two-channel air jacket alginate microencapsulator by adding a 25-gauge needle to a syringe, and adjust the valves on each side to make sure the needle is in the center of the air jacket. Different gauge needles can be used for this step, depending on the size of alginate microbeads targeted. This step can also be performed with a syringe, should a microencapsulator not be available. Add the alginate solution to the syringe, and select a needle gauge based on the size of microbeads desired.
Place a flask containing 10 mL of crosslinking solution directly underneath the needle. Place a stir bar in the solution.
Inject droplets directly into the CaCl2 solution in order to crosslink the alginate to form microbeads. Incubate the beads in the crosslinking solution to cure for at least fifteen minutes, with continuous stirring.
Transfer the beads to a 15 mL centrifuge tube. Remove the residual solution, and perform three washes with a 0.2% CaCl2 in normal saline solution for two minutes each.
2. Coating the Microbeads with Poly-L-ornithine
Prepare 3 mL of a 0.1% (w/v) solution of poly-L-ornithine (PLO) in normal saline. Place the solution on a vortex until it is completely dissolved, forming a clear solution. Poly-L-lysine (PLL) can be used instead of PLO for this step, which results in similar levels of permselectivity.
Transfer the alginate microbeads into the PLO solution. Place on a Vortex for 30 minutes to allow the PLO sufficient time to interact with the alginate, forming a polycation-polyanion complex. At the end of 30 minutes, there should be a distinctly visible white coating around the alginate microbeads.
Remove the PLO solution, and perform three washes with 0.2% CaCl2 in normal saline for two minutes each.
3. Creating the Outer Alginate Layer
Prepare an alginate solution of desired concentration that is to be used to create the outer layer. The solution is prepared as described in (1.1) above. The size of the outer layer is influenced by the composition and concentration of alginate used (Figure 3).
Transfer the alginate microbeads to a cell-strainer. Use a Kimwipe to absorb excess solution in order to dry the microbeads as much as possible.
Transfer the microbeads to a Parafilm surface. Other smooth surfaces such as glass or even a plastic Petri dish can be substituted for Parafilm, if desired.
Transfer the alginate solution prepared in (3.1) onto the alginate microbeads. The volume of alginate solution be should completely cover the alginate microbeads. Let the microbeads remain in the alginate solution for 45 minutes.
Use a pipette to remove the excess alginate solution not bound to the microbeads.
Transfer the microbeads into a solution of 22mM CaCl2. This crosslinks the alginate solution around the microbeads, resulting in the formation of the distinct outer alginate layer.
Perform three washes with 22 mM CaCl2 in normal saline for two minutes each. Viewing the microbeads under a microscope should allow for a distinct outer alginate layer to be seen formed around the core microbead.
4. Representative Results
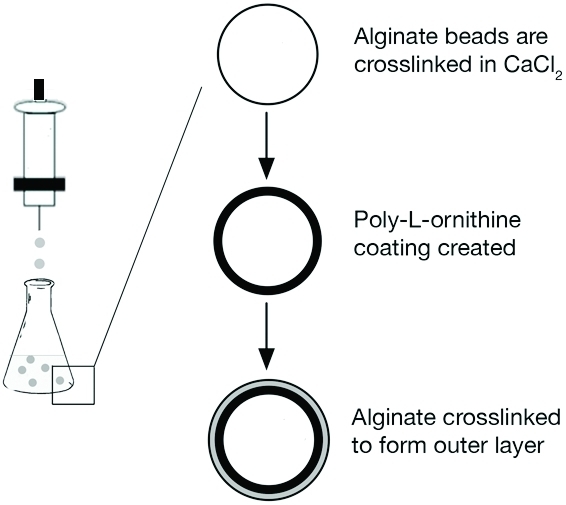 Figure 1. A schematic of the procedure for creating multilayered alginate microbeads. Reproduced with permission from Khanna et al. J Biomed Mater Res A. Nov; 95(2), 632-40 (2010).
Figure 1. A schematic of the procedure for creating multilayered alginate microbeads. Reproduced with permission from Khanna et al. J Biomed Mater Res A. Nov; 95(2), 632-40 (2010).
 Figure 2. (A) and (B) are phase contrast images of alginate microbeads. (A) shows a microbead after synthesis step (1.7), while (B) displays a distinct outer alginate layer present after completion of step (3.7). (C) is a FITC image of fluorescently-labeled BSA protein encapsulated in the outer layer. Reproduced with permission from Khanna et al. J Biomed Mater Res A. Nov;95(2), 632-40 (2010).
Figure 2. (A) and (B) are phase contrast images of alginate microbeads. (A) shows a microbead after synthesis step (1.7), while (B) displays a distinct outer alginate layer present after completion of step (3.7). (C) is a FITC image of fluorescently-labeled BSA protein encapsulated in the outer layer. Reproduced with permission from Khanna et al. J Biomed Mater Res A. Nov;95(2), 632-40 (2010).
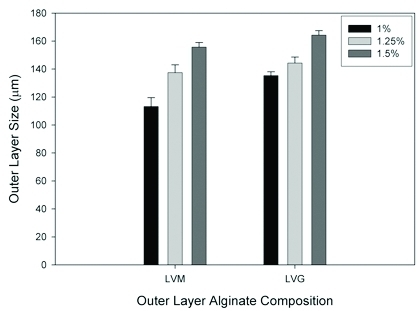 Figure 3. The size of the outer alginate layer can be varied based on the composition and concentration of alginate used. Our results show that, for both LVM and LVG alginate, the outer layer size increases with increasing alginate concentration, and that LVG alginate yields thicker outer layers than LVM alginate at equal concentrations. Reproduced with permission from Khanna et al. J Biomed Mater Res A. Nov; 95(2), 632-40 (2010).
Figure 3. The size of the outer alginate layer can be varied based on the composition and concentration of alginate used. Our results show that, for both LVM and LVG alginate, the outer layer size increases with increasing alginate concentration, and that LVG alginate yields thicker outer layers than LVM alginate at equal concentrations. Reproduced with permission from Khanna et al. J Biomed Mater Res A. Nov; 95(2), 632-40 (2010).
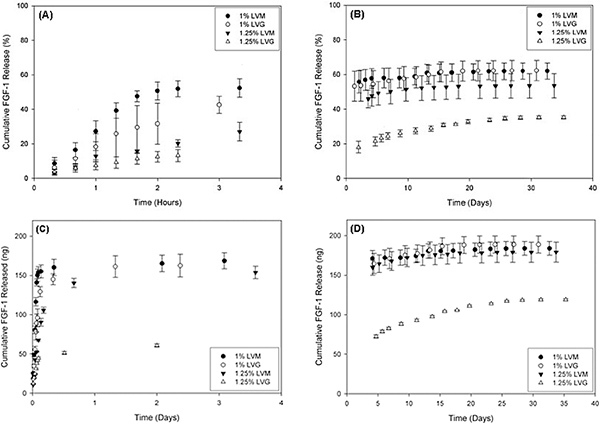 Figure 4. Release of FGF-1, an angiogenic growth factor protein, from the outer alginate layer varied based on the concentration of LVM and LVG alginate used. (A) and (B) show percent release, and (C) and (D) denote the corresponding mass release of FGF-1 versus time for different outer layer formulations. There is a burst release exhibited for all conditions within the initial 5 h (A and C), and low-dose continuous release for up to 30 days (B and D). Reproduced with permission from Khanna et al. J Biomed Mater Res A. Nov; 95(2), 632-40 (2010). Click here to view larger figure.
Figure 4. Release of FGF-1, an angiogenic growth factor protein, from the outer alginate layer varied based on the concentration of LVM and LVG alginate used. (A) and (B) show percent release, and (C) and (D) denote the corresponding mass release of FGF-1 versus time for different outer layer formulations. There is a burst release exhibited for all conditions within the initial 5 h (A and C), and low-dose continuous release for up to 30 days (B and D). Reproduced with permission from Khanna et al. J Biomed Mater Res A. Nov; 95(2), 632-40 (2010). Click here to view larger figure.
Discussion
Alginate is a natural, acidic polysaccharide extracted from algae and is composed of units of 1,4'-β-D-mannuronic acid (M) and α-L-guluronic acid (G) 2,3. Simple gelation occurs when divalent cations, such as Ca2+, Sr2+, or Ba2+ interact with G-monomers forming ionic bridges between adjacent alginate chains. Microbeads of alginate have been utilized to deliver a variety of proteins, including fibroblast growth factor-1 (FGF-1), nerve growth factor, leukemia inhibiting factor, vascular endothelial growth factor (VEGF), and for encapsulation of cells including chondrocytes, hepatocytes and islets. Advantages of using such a microparticle polymer system include the protection of the proteins and cells from degradation and reaction in the body, small particle volume allowing for easy administration through injection, and control of solute diffusion by the manipulation of the physical properties of the material.
Alginate microspheres coated with a permselective polymer layer are currently in clinical trials for the treatment of type I diabetes. However, the long-term viability of transplanted encapsulated islets is dependent, in part, on its ability to acquire oxygen and nutrients from a vascular blood supply. A biomaterial system that can serve simultaneously as an encapsulation system for islets as well as a sustained delivery system for angiogenic proteins may stimulate vascular growth around the transplanted cells, resulting in improved graft viability. Persistent neovascularization requires the sustained release of FGF-1 rather than a single bolus administration. In this JoVE article, we present an approach for generating multilayered alginate microbeads (Figure 1). The outer layer can be used for the encapsulation and sustained release of FGF-1 while the inner layer can be used for the immunoisolation of islet transplants (Figure 2).
Our laboratory has previously shown that a sustained delivery of FGF-1 rather than a high-bolus administration of the protein, results in a persistent microvascular network response 4-6. The system described here can be used to generate an outer layer for delivery of angiogenic proteins, such as FGF-1 (Figure 2). The size of the outer layer can be varied based on the composition and concentration of alginate used (Figure 3). The size of this outer layer could play an important role in the success of the systems. Properties of the outer layer could influence both the release of therapeutic molecules into the surrounding tissue and the transport of nutrients and signaling molecules to the cells in the inner alginate. The outer layer properties will need to be optimized for a given application. For the delivery of FGF-1, sustained release could be achieved for up to 30 days depending on the alginate formulation (Figure 4)7, 8.
Clinical trials using islets encapsulated in alginate microcapsules have shown some promise as a treatment for type I diabetes. However, large numbers of islets are required for efficacy because of poor survival following transplantation. The ability to encapsulate islets in multilayer microcapsules in which an angiogenic protein is released from the outer layer may enhance the viability of transplanted islets. This improved viability could reduce the number of islets required for treatment, increasing the potential clinical impact and availability of these therapies.
Disclosures
No conflicts of interest declared.
Acknowledgments
This study was supported by the US Department of Veterans Affairs (Washington D.C.), grants 0852048, 0731201, and 0854430 from the National Science Foundation (Arlington, VA), , and grant RO1 DK080897 from the National Institutes of Health (Bethesda, MD). Mr. Khanna received support from a generous donation by Mr. Edward Ross and Dr. Monica Moya from the Bill & Melinda Gates Foundation (Seattle, WA).
References
- Darrabie MD, Kendall WF, Opara EC. Characteristics of Poly-L- Ornithine-coated alginate microcapsules. Biomaterials. 2005;26:6846–6852. doi: 10.1016/j.biomaterials.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Yamagiwa K, Kozawa T, Ohkawa A. Effects of alginate composition and gelling conditions on diffusional and mechanical properties of calcium-alginate gel beads. J. Chem. Eng. Jpn. 1995;28:462–467. [Google Scholar]
- Amsden B, Turner N. Diffusion characteristics of calcium alginate gels. Biotechnol. Bioeng. 1999;65:605–610. doi: 10.1002/(sici)1097-0290(19991205)65:5<605::aid-bit14>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Uriel S, Brey EM, Greisler HP. Sustained low levels of fibroblast growth factor-1 promote persistent microvascular network formation. Am. J. Surg. 2006;192:604–609. doi: 10.1016/j.amjsurg.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Moya ML, Lucas S, Francis-Sedlak M, Liu X, Garfinkel MR, Huang JJ, Cheng MH, Opara EC, Brey EM. Sustained delivery of FGF-1 increases vascular density in comparison to bolus administration. Microvasc. Res. 2009;78:142–147. doi: 10.1016/j.mvr.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Moya ML, Cheng MH, Huang JJ, Francis-Sedlak ME, Kao SW, Opara EC, Brey EM. The effect of FGF-1 loaded alginate microbeads on neovascularization and adipogenesis in a vascular pedicle model of adipose tissue engineering. Biomaterials. 2010;31:2816–2826. doi: 10.1016/j.biomaterials.2009.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna O, Moya ML, Greisler HP, Opara EC, Brey EM. Multilayered Microcapsules for the Sustained-Release of Angiogenic Proteins From Encapsulated Cells. Am. J. Surg. 2010;200:655–658. doi: 10.1016/j.amjsurg.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna O, Moya ML, Opara EC, Brey EM. Synthesis of multilayered alginate microcapsules for the sustained-release of fibroblast growth factor-1. J. Biomed. Mater. Res. A. 2010;95:632–640. doi: 10.1002/jbm.a.32883. [DOI] [PMC free article] [PubMed] [Google Scholar]


