Abstract
Objective
This study aimed to evaluate the effect of an intentionally created socket on bone remodeling with orthodontic tooth movement in rabbits.
Methods
Eighteen male rabbits weighing 3.8 - 4.25 kg were used. An 8-mm deep and 2-mm wide socket was drilled in the bone 1 mm mesial to the right mandibular first premolar. The left first premolar was extracted to serve as an extraction socket. A traction force of 100 cN was applied to the right first premolar and left second premolar. Sections were obtained at the middle third of the moving tooth for both the drilled and extraction sockets and evaluated with hematoxylin and eosin staining and immunohistochemical analyses. The amount of tooth movement and tartrate-resistant acid phosphatase (TRAP)-positive cell count were compared between the 2 groups using the Mann-Whitney U test.
Results
At week 2, the distance of tooth movement was significantly higher in the intentional socket group (p < 0.05) than in the extraction socket group. The number of TRAP-positive cells decreased in week 2 but increased in week 3 (p < 0.05). However, there were no significant differences between the groups. Furthermore, results of transforming growth factor (TGF)-β staining revealed no significant differences.
Conclusions
The intentional socket group showed greater distance of tooth movement than did the extraction socket group at week 2. Osteoclast counts and results of immunohistochemical analyses suggested elevated bone remodeling in both the groups. Thus, osteotomy may be an effective modality for enhancing tooth movement in orthodontic treatment.
Keywords: Intentional socket, Accelerated tooth movement, Bone remodeling
INTRODUCTION
Conventional orthodontic treatment is a time-consuming process, due to which many patients refrain from seeking treatment. Tooth movement occurs by application of the mechanical force to teeth, which triggers a series of biological reactions such as bone remodeling and changes in the dental and periodontal tissues.1
Previously, various methods such as local administration of chemicals, physical or mechanical stimulation of the alveolar bone, and surgery have been used to assist tooth movement.2-9 However, chemicals are difficult to deliver and contain within the target area, whereas it is difficult to adequately sustain physical stimulation to the dentoalveolar bone. On the other hand, recent case reports have indicated that minor surgeries such as corticotomy or osteotomy may offer some degree of success.10-12
Wilcko et al.8,9 implied that accelerated tooth movement was due to the regional acceleratory phenomenon induced by bony tissue damage and not by movement of independent bone blocks. In addition, Sebaoun et al.13 reported that selective alveolar decortications induced a rapid and increased localized turnover of alveolar spongiosa, which are the prerequisites for rapid tooth movement.
Several studies have been carried out to evaluate the tissue response to alveolar surgery-assisted tooth movement.14-17 Ren et al.16 performed interseptal osteotomy and reported a higher rate of tooth movement on the osteotomy site than on the extraction socket on the contralateral side. Several corticotomy designs have been reported to achieve different tooth movements.8,9,18 However, no studies have compared the histological effects of intentional sockets with those of extraction sockets.
Therefore, the null hypothesis of this study was that there are no significant differences in tooth movement and bone remodeling between intentional and extraction sockets. This study aimed to histologically evaluate the effects of intentional sockets on bone remodeling using biological markers and to compare the tooth movement between intentional and extraction sockets in rabbits.
MATERIALS AND METHODS
Eighteen male New Zealand white rabbits (2 for undecalcified specimens and 16 for decalcified specimens) weighing 3.8 - 4.25 kg were housed in individual cages under controlled light and temperature. The rabbits were fed soft food to prevent damage to the experimental device. Approval for this study was obtained from the institutional review board of the Catholic University of Korea, Catholic Clinical Research Coordinating Center.
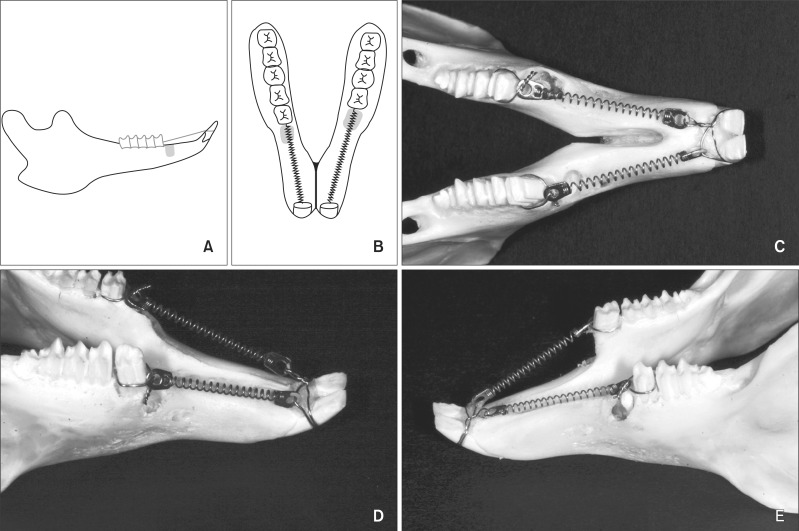
The left first premolars (P1s) were extracted. Then, 2-mm wide and 8-mm deep sockets, which was comparable to the dimensions of the extraction sockets, were created using a 1-mm round bur in the bone mesial to the right P1s on the other side (Figure 1).
Figure 1.
Study design and device. A, Buccal view; B, occlusal view of a schematic drawing. Right, drilled socket; left, extraction socket. The drilled socket was created mesial to the first premolar on the right side while the left first premolar was extracted. Clinical photos of rabbit mandible showing the occlusal view and the buccal views of the intentional and extraction sockets (C, D, and E, respectively).
A ligature wire was tied around each of the right P1s, the left second premolars (P2), and anterior teeth. Traction force of 100 cN was applied by connecting the anterior teeth to the premolars with nickel-titanium (NiTi) closed coil springs (Jin Sung Corp., Seoul, Korea) on each side, following the method of a previous study.19
Tissue preparation
For undecalcified specimens
Two rabbits received intramuscular injections of 60 mg/kg of intense fluorescent tetracycline (Sigma, St. Louis, MO, USA) after the apparatus was placed. Calcein (10 mg/kg; Sigma) and Alizarin red (10 mg/kg; Sigma) for green and red fluorescence, respectively, and 60 mg/kg tetracycline were injected at 1, 2, and 3 weeks respectively. At the fourth week, the rabbits were sacrificed by injection with an overdose of sodium phenobarbital. Then, specimens consisting of the socket area and posterior teeth were obtained, fixed in 10% formaldehyde, washed, and embedded in Osteo-Bed resin. The embedded blocks were trimmed and sectioned using an Exakt machine (BS-3000N; Exakt, Norderstedt, Germany) and ground with sandpaper to achieve 40- to 50-µm-thick sections to reveal the specimens. They were examined by fluorescence microscopy.
Decalcified specimens
Four rabbits were sacrificed every week by an overdose of sodium phenobarbital. Specimens were fixed in 10% formaldehyde for 24 hours at 4℃, decalcified in 10% ethylenediamine tetraacetic acid (EDTA-2Na, pH 7.4), embedded in paraffin, and sectioned to mesiodistally 4-µm-thick slices. The sections were then stained with hematoxylin and eosin (H&E) staining.
The sites between the mesial root of P1 and the intentional socket and between the P2 and the extraction socket were examined.
Measuring the distance of tooth movement
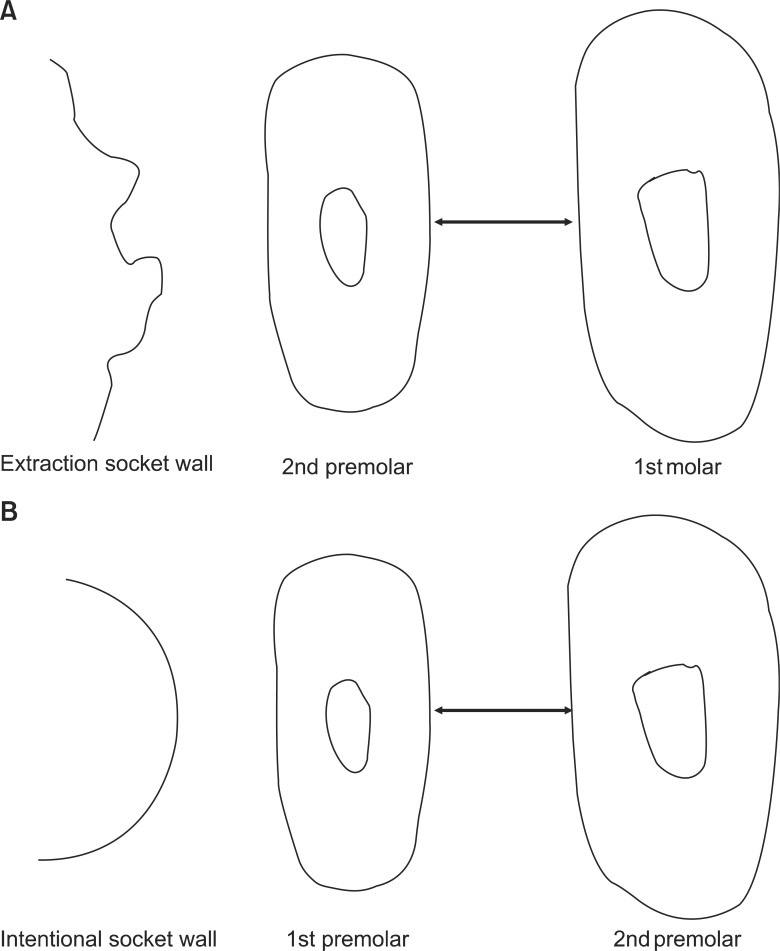
The amount of tooth movement was measured at the level of the alveolar crest of the interdental bone. The measurements were obtained using images of the decalcified transverse sections (TS) at 40× magnification using Adobe Photoshop CS2 (Adobe Systems Inc., San Jose, CA, USA). The distances between the distal surface of P1 and the mesial surface of P2 in the intentional socket group and between the distal surface of P2 and the mesial surface of the first molar (M1) in the extraction socket group were measured (Figure 2). However, the initial measurements of the distance between the roots were not reported. These missing measurements cast some doubts on the amount of tooth movement at week 1. Moreover, the records at each week were obtained from different samples.
Figure 2.
Schematic drawing shows the method of measuring the amount of tooth movement. A, In the extraction socket group, the distance between the distal surface of 2nd premolar and the mesial surface of the 1st molar was measured. B, In the intentional socket group, the distance between the distal surface of 1st premolar and the mesial surface of 2nd premolar was measured.
TRAP-positive cell count
Tartrate-resistant acidic phosphatase (TRAP) staining was performed to verify the presence of osteoclasts, according to the protocols described in a previous report.20 Tissue sections were treated with a mixture of acetate buffer and 24 mg of red violet salt (Sigma) at 37℃ for 15 minutes. Specimens were stained with hematoxylin contrast staining. TRAP-positive cells were counted on light microscope images of the region of interest, which extended vertically along the root and horizontally from the mesial root of the moving tooth to the wall of the intentional or extraction socket.
Immunohistochemistry
The sections were deparaffinized with xylene, hydrated, and the antigen was retrieved using pepsin at 37℃ for 10 minutes. After bench-cooling, the sections were washed with tris-buffered saline-tween buffer (TBST 0.1% Tween-20) and rinsed with 3% H2O2 for 10 minutes. Rat monoclonal antibodies against proliferating cell nuclear antigen (PCNA) and transforming growth factor-β (TGF-β) were added to the sections at 1:200 and 1:700 dilutions, respectively, and incubated for 90 minutes at room temperature. After washing with TBST, sections were treated by enhancer reagent (HK 518-50K; BioGenex, Fremont, CA, USA) for 20 minutes at room temperature, and washed with TBST buffer. Then they were treated by polymer-horseradish peroxidase (HRP) (BioGenex) for 30 minutes at room temperature to allow direct binding of the secondary antigens to HRP. After washing in TBST buffer, the specimens were stained with 3,3-diamine-benzidine (DAB) chromogen (BioGenex) at room temperature. The slides were counterstained with Mayer's hematoxylin and mounted on Canada balsam for microscopic examination.
The immunostained sections were semi-quantitatively evaluated on the alveolar bone surface and neighboring marrow spaces by using methods previously described with modifications.21 The specimens were scored on the basis of the number of positive cells as 0% (0), < 10% (1), 10 - 39% (2), 40 - 70% (3), or > 70% (4). The intensity of the staining was scored as weak (1), moderate (2), or strong (3). For each sample, the values of these 2 parameters (percentage of positive cells and staining intensity) were multiplied and then averaged, resulting in scores ranging from 0 to 12. Then the samples were classified into 4 categories: - (score 0 - 1), + (score 2 - 3), ++ (score 4 - 8), and +++ (score 9 - 12).
Statistical analysis
Statistical analysis was performed using SPSS for Windows version 14.0 (SPSS Inc., Chicago, IL, USA). Data were found to follow a normal distribution, when assessed by the Shapiro-Wilk test. The Mann-Whitney U test was used to assess differences in the tooth movement and the TRAP-positive cell count between the intentional and extraction socket groups and between different time points within each group. Statistical significance was determined at p < 0.05.
RESULTS
Evaluation of undecalcified sections
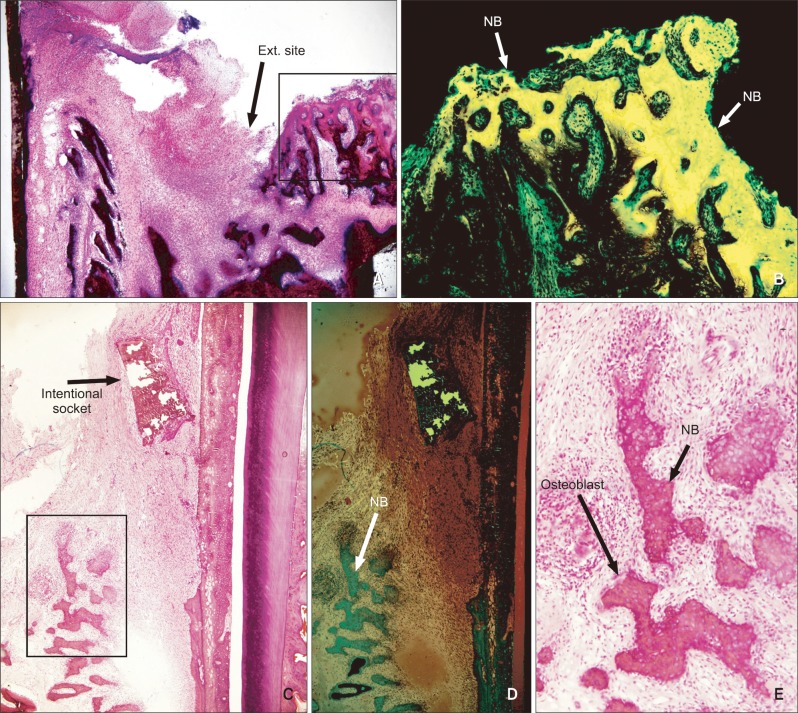
New bone formation was seldom found in the extraction socket group at week 1. At week 2, the inflammatory response had subsided and active new bone formation was detected around the extraction socket with high osteoblastic activity (Figure 3A and 3B). At weeks 3 and 4, a considerable amount of new bone had formed in the extraction socket, which was not replaced by lamellar bone.
Figure 3.
Representative histological features of the intentional socket site in an undecalcified section. The extraction (Ext.) socket group (A, ×40) shows newly formed bone (NB) and host bone after 2 weeks. B, Fluorescent microscope photo of A. At week 2, the intentional socket group (C, ×40) shows new bone under fluorescence microscopy (D, ×100) and active osteoblasts (E, ×100).
In the intentional socket group, bone remodeling had already begun at week 1. New bone formation was more pronounced at week 2, but no significant difference was found between the 2 groups. Bone remodeling continued at weeks 3 and 4. The intentional socket was replaced by new bone and condensed fibrous tissue. Osteoblastic activity decreased compared with that in week 2 (Figure 3C, 3D and 3E).
Evaluation of decalcified sections
Tooth movement
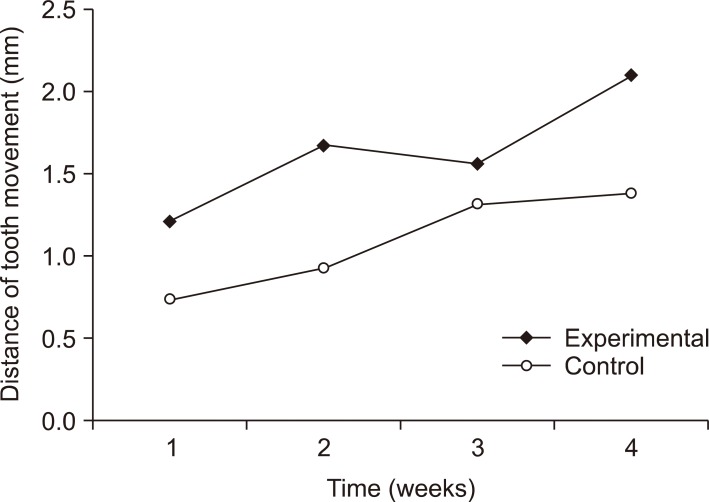
The intentional socket group showed more tooth movement than the extraction socket group at weeks 1 (1.2 ± 0.5 mm vs. 0.8 ± 0.2 mm), 2 (1.7 ± 0.7 mm vs. 0.9 ± 0.7 mm), 3 (1.6 ± 0.9 mm vs. 1.3 ± 0.8 mm), and 4 (2.1 ± 0.4 mm vs. 1.4 ± 0.4 mm). However, the difference was significant only at week 2 (p < 0.05; Figure 4).
Figure 4.
Comparison of tooth movement between the extraction and intentional socket groups. The intentional socket group showed significantly higher amount of movement in week 2 (p < 0.05).
H&E staining
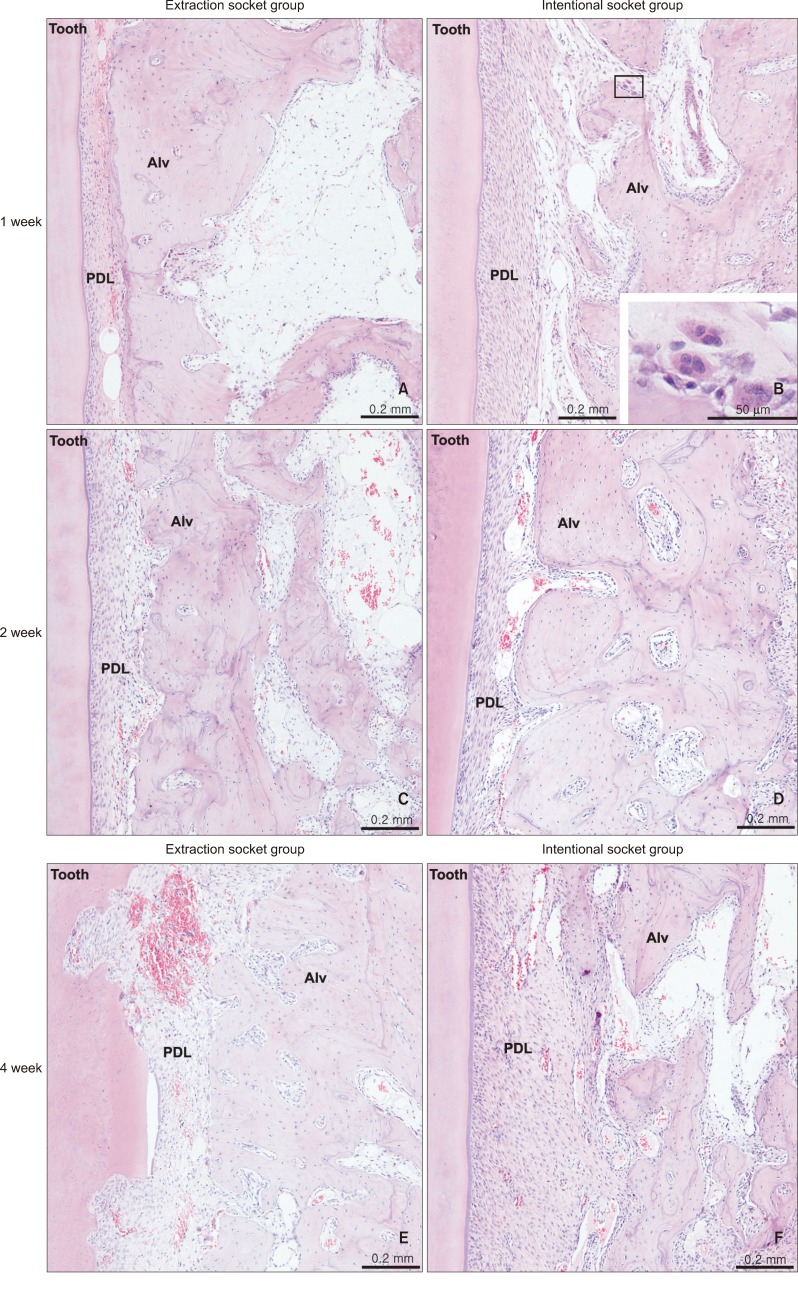
Microscopic examination of H&E-stained specimens revealed more pronounced alveolar bone resorption in the intentional socket group from weeks 1 - 4. Periodontal ligament space was enlarged (Figure 5B, 5D, and 5F). The number of osteoclasts increased on some surfaces where alveolar bone was resorbed (Figure 5B). At week 4, root resorption was noticed in the extraction socket group (Figure 5E).
Figure 5.
Microphotograph of the periodontium with H&E staining. A, C, E, Extraction socket at weeks 1, 2, and 4, respectively; B, D, F, intentional socket at weeks 1, 2, and 4. PDL, Periodontal ligament; Alv, alveolar bone. Note the enlarged PDL space in B, D, and F, and the increased number of osteoclasts on some resorbed bone surfaces, especially at week 1 in the intentional socket (B), which might have caused the enlarged PDL space by its increased activity. Root resorption can be noticed in E. The figure suggests elevated alveolar bone resorption in both groups from week 1 - 4, particularly in the intentional socket group.
TRAP-positive cell count
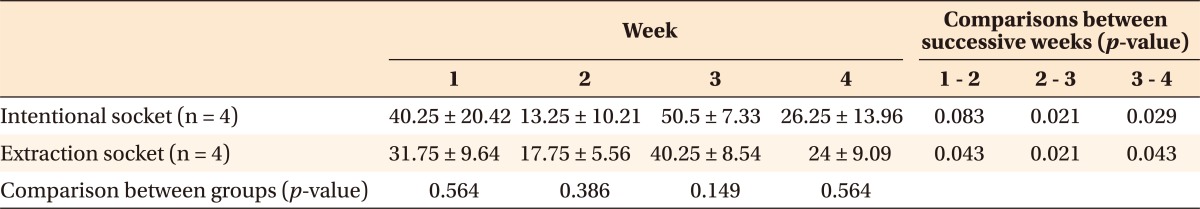
TRAP-positive osteoclasts and preosteoclasts were found on resorbed alveolar bone surface on the compression side, as a straight band in the extraction socket group (Figure 6A, 6C, and 6E) or grouped together in the intentional socket group (Figure 6B, 6D, and 6F). There were no significant differences in the number of TRAP-positive cells/area between groups. However, significant intra-group differences existed between successive measurements in both the groups (Table 1).
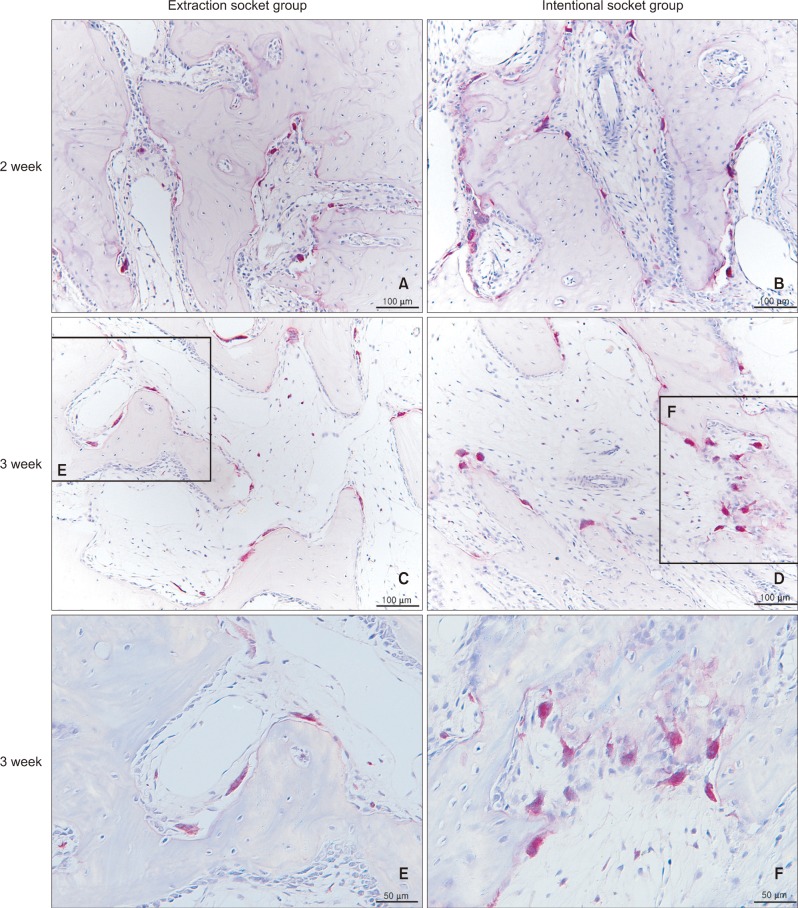
Figure 6.
Microphotograph of periodontal tissues with tartrate-resistant acid phosphatase (TRAP) staining. A, C, and E, Extraction socket group; B, D, and F, intentional socket group. A and B are week 2 specimens, while C, D, E, and F are week 3 specimens. TRAP-positive cells can be observed on the compression side along the resorbed surface of the alveolar bone. The intentional socket group shows several clusters of TRAP-positive cells, while the extraction socket group forms a straight band. There were no significant differences in the TRAP-positive cells/area between the 2 groups.
Table 1.
Comparison of the TRAP-positive cell count between the intentional and extraction socket groups and between successive weeks
Values are presented as mean ± standard deviation.
Analyzed by Mann-Whitney U test, unit: × 106/µm2.
TRAP, tartrate-resistant acid phosphatase.
Immunohistochemistry
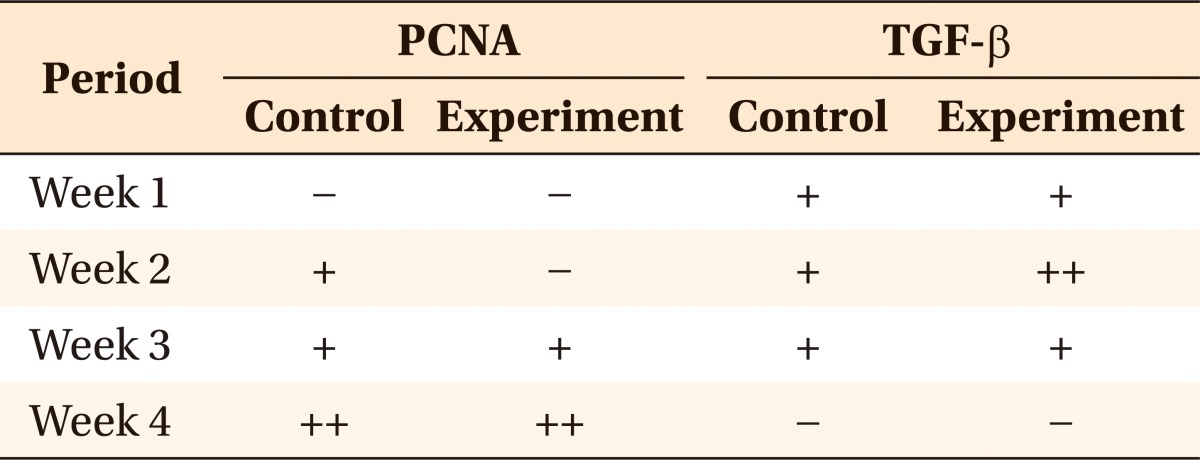
PCNA staining demonstrated no change in both the groups at week 1. At week 3, the extraction socket group showed a linear pattern on the resorbed root surface, but the intentional socket group showed few stained cells coronal to the alveolar bone. At week 4, the stained cells were slightly more distinctive where the root surface was in contact with the alveolar crest. Staining was slightly more intense along the resorbed areas. TGF-β staining revealed no difference between the 2 groups (Table 2).
Table 2.
Comparison of PCNA and TGF-β expressions after tooth movement between the intentional and extraction socket groups
-, Score 0 - 1; +, score 2 - 3; ++, score 4 - 8.
PCNA, proliferating cell nuclear antigen; TGF, transforming growth factor.
DISCUSSION
In order to achieve efficient tooth movement, it is important to understand the sequence of biological responses by periodontal ligament (PDL) and alveolar bone when induced by a stimulus from application of the mechanical force. Therefore, our histological study aimed to examine the underlying cellular response to an intentional and extraction sockets in rabbits.
Rabbits were selected in this study due to the similarity between the edentulous space posterior to their anterior teeth and the atrophied alveolar ridge. Park et al.22 performed corticotomy on the occlusal surface of the edentulous alveolar ridge region to avoid root damage. A force of 100 cN was applied to the premolars, similar to previous studies where 100 - 120 cN was applied to rabbit molars.19,23,24 The force was applied for 4 weeks because rabbits' bone metabolism is 3 times faster than that of human beings. Thus, in terms of the bone turnover rates in rabbits, 4 weeks would correspond to 3 months in humans.25
In accordance with the results of previous studies, microscopic examination demonstrated more early alveolar bone resorption in the intentional socket group than in the extraction socket group.4,13,16,17 In addition, previous radiographic studies have shown an increased amount of tooth movement assisted by alveolar surgery in dogs.4,16 However, the position of the film and identification of the reference points might contribute to higher measurement errors; therefore, we measured the amount of tooth displacement by analyzing tissue specimens under a microscope. In our study, we found higher tooth displacement in the intentional socket group than in the extraction socket group, but this was found only at week 2. This result seemed unusual, as there was more tooth movement in week 2 than in week 3; however, this might be because each measurement was obtained from a different sample. Moreover, since there were no initial records of the distance between the roots, it was not possible to determine the amount of tooth movement at week 1. Considering these limitations, it might be stated that the intentional socket was as effective as the natural socket regardless of the lack of precursor cells.
Generally, apart from lamellar bone, extraction sites exhibit a trabecular bone pattern with large marrow spaces filled with large numbers of adipocytes with scarce inflammatory cells.26 Fibroblasts and osteoblasts in the PDL initially form bone and connective tissue within the area of the socket. Then, they are replaced by cells from adjacent tissue.27 Similarly, after extraction, the socket is filled by bone formed by fibroblasts and osteoblasts from the remaining PDL. However, the intentional socket follows bone defect healing, in which the defect is highly vascularized and filled with bone marrow and few newly formed single bone trabeculae. Cells responsible for bone remodeling are derived from adjacent bone tissue. This is a plausible explanation for the relatively slower bone healing process observed in extraction sockets. It may suggest intense and prolonged RAP in the intentional socket group, which may have influenced the rate of tooth movement.
Iino et al.4 displayed an increase in the TRAP-positive cells 1 week after the corticotomy, and then the count decreased in the second and fourth weeks. In that study, the control group showed an increased number of TRAP-positive cells in the second week, while Sebaoun et al.13 demonstrated an increase in the number of TRAP-positive cells 3 weeks after cortical injury. The inconsistency in these TRAP staining results across studies could be due to differences in the specimen or duration of the experiments.
In contrast, our results showed that TRAP-positive osteoclasts and preosteoclasts increased in both groups by the end of the first week, followed by a decrease in the TRAP-positive cells in both groups in week 2. This may be because the P1 had moved into the area of the intentional socket by week 2 (1.7 mm). Similar time-dependent osteoclast formation was noted in the surgery group of Iglesias-Linares et al.28 study. They linked this change to the level of receptor activator of the nuclear factor kappa-B ligand (RANKL).
At week 3, TRAP-positive cells showed an increase, which may have occurred due to the presence of newly formed bone around the intentional socket. This might, in turn, explain the decrease in tooth movement at week 3. The number of TRAP-positive cells decreased again at week 4, indicating considerable resorption of the newly formed bone, facilitating increased tooth movement (Figure 3, Table 1).
Since TGF-β may act as a bone-coupling factor linking bone resorption to bone formation,29 the insignificant difference in the increased TGF-β staining between both groups in our study confirms the occurrence of bone remodeling in both groups. Moreover, osteoclast activity as measured by PCNA staining increased gradually in both groups. Furthermore, Wang et al.17 reported that at the third week of their study period, the corticotomy-assisted tooth movement group showed replacement of the missing alveolar bone with multicellular tissue, and that there was an abundance of PCNA- and TGF-β1-positive cells.
Our results showed that there were no significant differences between the intentional and extraction socket groups. In both groups, alveolar bone resorption was induced and osteoclasts were recruited with no significant differences in the number of TRAP-positive cells or TGF-β staining between the groups at each time point.
Evaluation of tooth movement without extraction as a control group might show significant differences. Therefore, in this study, the comparison was performed between the intentional and extraction socket groups. Further studies including a non-extraction group might provide more insights in this area, and additional studies on the long-term evaluation of corticotomy-assisted tooth movement are recommended.
CONCLUSION
The intentional socket group showed a larger amount of tooth movement than that of the extraction socket group only at week 2. The osteoclast count and the immunolocalization of bone markers suggest increased alveolar bone turnover in the region surrounding the roots in both groups. Thus, the null hypothesis was not rejected and it was suggested that osteotomy could be as effective as extraction socket in terms of enhancing tooth movement.
Footnotes
The authors report no commercial, proprietary, or financial interest in the products or companies described in this article.
References
- 1.Krishnan V, Davidovitch Z. Cellular, molecular, and tissue-level reactions to orthodontic force. Am J Orthod Dentofacial Orthop. 2006;129:469. doi: 10.1016/j.ajodo.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 2.Chumbley AB, Tuncay OC. The effect of indomethacin (an aspirin-like drug) on the rate of orthodontic tooth movement. Am J Orthod. 1986;89:312–314. doi: 10.1016/0002-9416(86)90053-9. [DOI] [PubMed] [Google Scholar]
- 3.Chung KR, Oh MY, Ko SJ. Corticotomy-assisted orthodontics. J Clin Orthod. 2001;35:331–339. [PubMed] [Google Scholar]
- 4.Iino S, Sakoda S, Ito G, Nishimori T, Ikeda T, Miyawaki S. Acceleration of orthodontic tooth movement by alveolar corticotomy in the dog. Am J Orthod Dentofacial Orthop. 2007;131:448. doi: 10.1016/j.ajodo.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 5.Kole H. Surgical operations on the alveolar ridge to correct occlusal abnormalities. Oral Surg Oral Med Oral Pathol. 1959;12:515–529. doi: 10.1016/0030-4220(59)90153-7. [DOI] [PubMed] [Google Scholar]
- 6.Lee WC. Experimental study of the effect of prostaglandin administration on tooth movement--with particular emphasis on the relationship to the method of PGE1 administration. Am J Orthod Dentofacial Orthop. 1990;98:231–241. doi: 10.1016/s0889-5406(05)81600-2. [DOI] [PubMed] [Google Scholar]
- 7.Stark TM, Sinclair PM. Effect of pulsed electromagnetic fields on orthodontic tooth movement. Am J Orthod Dentofacial Orthop. 1987;91:91–104. doi: 10.1016/0889-5406(87)90465-3. [DOI] [PubMed] [Google Scholar]
- 8.Wilcko WM, Ferguson DJ, Bouquot JE, Wilcko MT. Rapid orthodontic decrowding with alveolar augmentation: case report. World J Orthod. 2003;4:197–205. [Google Scholar]
- 9.Wilcko WM, Wilcko T, Bouquot JE, Ferguson DJ. Rapid orthodontics with alveolar reshaping: two case reports of decrowding. Int J Periodontics Restorative Dent. 2001;21:9–19. [PubMed] [Google Scholar]
- 10.Converse JM, Horowitz SL. The surgical-orthodontic approach to the treatment of dentofacial deformities. Am J Orthod. 1969;55:217–243. doi: 10.1016/0002-9416(69)90104-3. [DOI] [PubMed] [Google Scholar]
- 11.Işeri H, Kişnişci R, Bzizi N, Tüz H. Rapid canine retraction and orthodontic treatment with dentoalveolar distraction osteogenesis. Am J Orthod Dentofacial Orthop. 2005;127:533–541. doi: 10.1016/j.ajodo.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 12.Sukurica Y, Karaman A, Gürel HG, Dolanmaz D. Rapid canine distalization through segmental alveolar distraction osteogenesis. Angle Orthod. 2007;77:226–236. doi: 10.2319/0003-3219(2007)077[0226:RCDTSA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 13.Sebaoun JD, Kantarci A, Turner JW, Carvalho RS, Van Dyke TE, Ferguson DJ. Modeling of trabecular bone and lamina dura following selective alveolar decortication in rats. J Periodontol. 2008;79:1679–1688. doi: 10.1902/jop.2008.080024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baloul SS, Gerstenfeld LC, Morgan EF, Carvalho RS, Van Dyke TE, Kantarci A. Mechanism of action and morphologic changes in the alveolar bone in response to selective alveolar decortication-facilitated tooth movement. Am J Orthod Dentofacial Orthop. 2011;139(4 Suppl):S83–S101. doi: 10.1016/j.ajodo.2010.09.026. [DOI] [PubMed] [Google Scholar]
- 15.Mostafa YA, Mohamed Salah Fayed M, Mehanni S, ElBokle NN, Heider AM. Comparison of corticotomy-facilitated vs standard tooth-movement techniques in dogs with miniscrews as anchor units. Am J Orthod Dentofacial Orthop. 2009;136:570–577. doi: 10.1016/j.ajodo.2007.10.052. [DOI] [PubMed] [Google Scholar]
- 16.Ren A, Lv T, Kang N, Zhao B, Chen Y, Bai D. Rapid orthodontic tooth movement aided by alveolar surgery in beagles. Am J Orthod Dentofacial Orthop. 2007;131:160. doi: 10.1016/j.ajodo.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 17.Wang L, Lee W, Lei DL, Liu YP, Yamashita DD, Yen SL. Tisssue responses in corticotomy- and osteotomy-assisted tooth movements in rats: histology and immunostaining. Am J Orthod Dentofacial Orthop. 2009;136:770. doi: 10.1016/j.ajodo.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 18.Chung KR, Mitsugi M, Lee BS, Kanno T, Lee W, Kim SH. Speedy surgical orthodontic treatment with skeletal anchorage in adults--sagittal correction and open bite correction. J Oral Maxillofac Surg. 2009;67:2130–2148. doi: 10.1016/j.joms.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 19.Seifi M, Shafeei HA, Daneshdoost S, Mir M. Effects of two types of low-level laser wave lengths (850 and 630 nm) on the orthodontic tooth movements in rabbits. Lasers Med Sci. 2007;22:261–264. doi: 10.1007/s10103-007-0447-9. [DOI] [PubMed] [Google Scholar]
- 20.Kim T, Handa A, Iida J, Yoshida S. RANKL expression in rat periodontal ligament subjected to a continuous orthodontic force. Arch Oral Biol. 2007;52:244–250. doi: 10.1016/j.archoralbio.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 21.Verna C, Zaffe D, Siciliani G. Histomorphometric study of bone reactions during orthodontic tooth movement in rats. Bone. 1999;24:371–379. doi: 10.1016/s8756-3282(99)00009-5. [DOI] [PubMed] [Google Scholar]
- 22.Park WK, Kim SS, Park SB, Son WS, Kim YD, Jun ES, et al. The effect of cortical punching on the expression of OPG, RANK, and RANKL in the periodontal tissue during tooth movement in rats. Korean J Orthod. 2008;38:159–174. [Google Scholar]
- 23.Kiliç N, Oktay H, Ersöz M. Effects of force magnitude on tooth movement: an experimental study in rabbits. Eur J Orthod. 2010;32:154–158. doi: 10.1093/ejo/cjp083. [DOI] [PubMed] [Google Scholar]
- 24.Kilic N, Oktay H, Ersoz M. Effects of force magnitude on relapse: An experimental study in rabbits. Am J Orthod Dentofacial Orthop. 2011;140:44–50. doi: 10.1016/j.ajodo.2009.12.035. [DOI] [PubMed] [Google Scholar]
- 25.Roberts WE. Bone tissue interface. Int J Oral Implantol. 1988;5:71–74. [PubMed] [Google Scholar]
- 26.Barone A, Aldini NN, Fini M, Giardino R, Calvo Guirado JL, Covani U. Xenograft versus extraction alone for ridge preservation after tooth removal: a clinical and histomorphometric study. J Periodontol. 2008;79:1370–1377. doi: 10.1902/jop.2008.070628. [DOI] [PubMed] [Google Scholar]
- 27.Herr Y, Matsuura M, Lin WL, Genco RJ, Cho MI. The origin of fibroblasts and their role in the early stages of horizontal furcation defect healing in the beagle dog. J Periodontol. 1995;66:716–730. doi: 10.1902/jop.1995.66.8.716. [DOI] [PubMed] [Google Scholar]
- 28.Iglesias-Linares A, Moreno-Fernandez AM, Yañez-Vico R, Mendoza-Mendoza A, Gonzalez-Moles M, Solano-Reina E. The use of gene therapy vs. corticotomy surgery in accelerating orthodontic tooth movement. Orthod Craniofac Res. 2011;14:138–148. doi: 10.1111/j.1601-6343.2011.01519.x. [DOI] [PubMed] [Google Scholar]
- 29.Bonewald LF, Mundy GR. Role of transforming growth factor-beta in bone remodeling. Clin Orthop Relat Res. 1990;(250):261–276. [PubMed] [Google Scholar]