Abstract
We investigated breathing patterns and the occurrence of arrhythmias and ST-segment changes during sleep in patients with Brugada Syndrome. Patients with Brugada Syndrome are more likely to die from ventricular arrhythmias during sleep. ST-segment changes have been correlated with risk of SCD. Whether sleep disturbances may contribute to arrhythmogenesis is unknown. Patients with Brugada Syndrome underwent an overnight polysomnography (PSG) with simultaneous 12-lead ECG recording. A control group matched by age, gender and BMI also underwent PSG. Twenty patients were included (50 ± 15 yo, 75% male). Despite their normal BMI (24.7 ± 2.7 kg/m2), 45% had sleep disordered breathing (SDB), with a mean AHI of 17.2 ± 14 events/hour. Amongst patients with a high risk of arrhythmias, 5 (63%) had SDB. In the control group, 27% had SDB. Atrial or ventricular arrhythmias were not observed. Spontaneous ST-segment changes occurred in 2 patients over 45 different time-points. The majority of the ST changes was observed either during REM sleep (31%) or within one minute of arousals (44%). Regarding the respiratory events, 25 (56%) ST changes were related to the occurrence of apnea or hypopnea. In conclusion, patients with Brugada Syndrome have a high prevalence of SDB even in the setting of normal BMI. The higher incidence of nocturnal death in patients with Brugada Syndrome may be conceivably related to comorbid SDB. Moreover, autonomic instability encountered in REM sleep and arousals could potentiate the risk of arrhythmias.
Keywords: Brugada syndrome, sleep apnea, sudden cardiac death, ST-segment elevation
Introduction
The Brugada syndrome was described in 1992, in patients presenting with recurrent aborted sudden cardiac death (SCD) and a typical electrocardiography sign of ST-elevation in leads V1 to V3 1. It was later shown that patients die of ventricular tachycardia or fibrillation, often during sleep 2,3. The arrhythmogenic role of sleep is being increasingly recognized. The nocturnal occurrence of apnea and hypopneas has been related to SCD and atrial fibrillation 4. Apneic events elicit significant autonomic responses, which have been linked to ST elevation in patients with the Brugada syndrome 5,6. Besides the respiratory events, changes from non-rapid-eye-movement (non-REM) sleep to REM sleep are also associated with autonomic instability, which can play a role in arrhythmogenesis 7,8. Therefore, this study evaluated breathing patterns during sleep in patients with the Brugada syndrome and investigated the occurrence of arrhythmias and ST-segment changes during different sleep stages.
Methods
Consecutive patients being evaluated at the Hospital Clinic, University of Barcelona, were invited to participate in the present study. Patients were recruited from the Sudden Death and ICD outpatient clinics, and about 10% of those invited declined participation. The inclusion criteria were: age above 21 and a definite diagnosis of the Brugada syndrome based on a spontaneous or drug-induced type 1 Brugada ECG. Patients underwent overnight polysomnography (PSG), with simultaneous recording of 12 lead ECG throughout the night. The control group (recruited in Rochester MN) consisted of healthy subjects, of similar age, gender and BMI as the patients. This study was approved by the Institutional Review Boards of University of Barcelona and the Mayo Clinic (Rochester MN) and all patients signed an informed consent. Patients were classified as high risk for fatal arrhythmias according to the following criteria: (1) previous aborted SCD associated with type 1 ECG (spontaneous or induced) or (2) unexplained syncope with spontaneous type 1 ECG.
All subjects underwent a full-night PSG, acquired on Compumedics Siesta802 wireless amplifier/recorder (Compumedics, Abbotsford, VIC, Australia). Airflow was monitored by oro-nasal thermal airflow sensor and respiratory effort was monitored by calibrated respiratory impedance plethysmography. During all PSGs, the electroencephalogram, electrooculogram and sub-mental electromyogram were recorded according to the American Academy of Sleep Medicine standards. Oxyhemoglobin saturation was recorded by finger pulse oximetry. The PSGs were scored by an experienced polysomnography technologist who was blind to the subject’s status and arrhythmic risk. Apneas were defined as a ≥ 90% drop in peak sensor excursion from baseline for at least 10 seconds. Hypopneas were defined by a ≥ 50 % decline in sensor excursion for at least 10 seconds accompanied by an oxyhemoglobin desaturation of ≥ 4 %. Disordered breathing events were quantified by the apnea-hypopnea index (AHI). An AHI ≥ 5 events/hour established the diagnosis of sleep disordered breathing (SDB). The 12-lead ECG was simultaneously recorded in Labview (National Instruments) and processed using ScopeWin software (ISI, Brno, Czech Republic). The ECG was analyzed by a cardiac electrophysiologist blinded to the patient’s arrhythmic risk and to the sleep stages. A spontaneous change in the ST segment was considered if there was a modification in the type of Brugada ECG or a visible change in the T wave in the right precordial leads, according to previously published method 9. The time-points where these changes began were correlated to the sleep stages and respiratory events in the PSG tracings.
Data are summarized as frequencies for categorical variables and means with standard deviations for continuous variables. Group differences were evaluated by Wilcoxon rank sum test. Differences in proportions were tested by Fisher’s exact test. Analyses were performed with JMP version 7 (SAS Institute, Cary, NC). For all comparisons p < 0.05, for a 2-tailed test, was considered significant.
Results
Twenty patients were included in the study. Mean age was 50 ± 15 yo, BMI was 24.7 ± 2.7 kg/m2 and the majority was male (75%). Six patients (30%) had unexplained syncope and 3 (15%) had aborted SCD. A family history of SCD was reported by 7 patients (35%) (Table 1). Twelve patients (60%) had an implantable cardiac defibrillator (ICD), 2 of whom were also on medication, one on quinidine and the other on a beta-blocker. The control group consisted of 11 subjects, mean age was 43 ± 16 yo, mean BMI was 25 ± 3 kg/m2 and 82% was male.
Table 1.
Clinical and polysomnographic characteristics in patients with the Brugada Syndrome, according to the presence or absence of sleep disordered breathing (listed by ascending AHI)
| Patients without SDB | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pt | Age (y) | Gender | BMI (Kg/cm3) | Familial history of SCD | VF/SCD | Syncope | High arrhythmic risk | HTN | DM | Sleep Efficiency (%) | REM (%) | Lowest SpO2 (%) | AHI (events/hour) |
| 1 | 32 | F | 24 | + | 0 | 0 | 0 | 0 | 0 | 93 | 19 | 97 | 0 |
| 2 | 66 | F | 21 | 0 | 0 | + | 0 | + | 0 | 89 | 15 | 94 | 0 |
| 3 | 31 | M | 26 | 0 | + | + | + | 0 | 0 | 90 | 16 | 86 | 0.1 |
| 4 | 31 | M | 23 | + | 0 | 0 | 0 | 0 | 0 | 75 | 12 | 93 | 0.4 |
| 5 | 60 | M | 27 | 0 | + | + | + | 0 | 0 | 78 | 28 | 90 | 0.5 |
| 6 | 29 | M | 24 | 0 | 0 | 0 | 0 | 0 | 0 | 80 | 24 | 93 | 0.6 |
| 7 | 22 | M | 25 | 0 | 0 | + | + | 0 | 0 | 87 | 19 | 94 | 0.8 |
| 8 | 50 | M | 23 | 0 | 0 | 0 | 0 | 0 | 0 | 86 | 28 | 87 | 1.5 |
| 9 | 56 | M | 28 | + | 0 | 0 | 0 | + | 0 | 69 | 9 | 89 | 1.5 |
| 10 | 39 | M | 24 | 0 | 0 | 0 | 0 | 0 | 0 | 81 | 21 | 91 | 1.7 |
| 11 | 43 | M | 24 | 0 | 0 | 0 | 0 | 0 | 0 | 75 | 18 | 86 | 4.4 |
| Patients with SDB | |||||||||||||
| Pt | Age (y) | Gender | BMI (Kg/cm3) | Familial history of SCD | VF/SCD | Syncope | High arrhythmic risk | HTN | DM | Sleep Efficiency (%) | REM (%) | Lowest SpO2 (%) | AHI (events/hour) |
| 12 | 65 | F | 26 | 0 | + | + | + | + | 0 | 72 | 10 | 85 | 5.3 |
| 13 | 71 | F | 22 | 0 | 0 | + | + | + | 0 | 57 | 7 | 87 | 6.5 |
| 14 | 56 | M | 28 | + | 0 | 0 | 0 | 0 | 0 | 94 | 8 | 84 | 10.4 |
| 15 | 62 | F | 21 | + | 0 | 0 | 0 | 0 | 0 | 91 | 14 | 79 | 10.7 |
| 16 | 64 | M | 26 | 0 | 0 | + | + | 0 | 0 | 61 | 11 | 89 | 10.7 |
| 17 | 52 | M | 22 | + | 0 | 0 | 0 | 0 | 0 | 79 | 11 | 78 | 14.5 |
| 18 | 51 | M | 24 | 0 | 0 | + | + | 0 | 0 | 69 | 13 | 84 | 16.5 |
| 19 | 58 | M | 23 | 0 | 0 | + | + | 0 | 0 | 62 | 4 | 78 | 32.4 |
| 20 | 61 | M | 28 | + | 0 | 0 | 0 | + | + | 86 | 29 | 72 | 48.1 |
AHI = apnea-hypopnea index, BMI = body mass index, BQ= Berlin questionnaire, DM= diabetes mellitus, HTN= arterial hypertension, Pt= patient, REM = rapid eye movement, SCD/VF= Sudden cardiac death/ventricular fibrillation, SDB = sleep disordered breathing, SpO2= arterial oxygen saturation.
Nine patients (45%) had SDB on PSG, with a mean AHI of 17.2 ± 14 events/hour. Six patients had a diagnosis of obstructive sleep apnea (OSA), 2 of central sleep apnea and 1 patient had mixed sleep apnea. In the control group, 3 (27%) had SDB and all were diagnosed as OSA. Regarding the clinical characteristics, the groups with or without SDB were very similar except that patients with SDB were older (mean 60 ± 6 yo vs. 42 ± 14 yo, p=0.008). There was no difference in the mean BMI between the SDB and the non-SDB group (25 vs. 24.4 kg/m2, p=0.85). Clinically-defined high risk patients were more frequently observed in the SDB group (56%) than in the non-SDB group (27%). During sleep, patients with SDB spent less time in REM (12 vs. 19%, p= 0.02), had a lower arterial oxygen saturation nadir (82 vs. 91%, p< 0.001) and a higher AHI (17.2 vs. 1.0 events/hour, p< 0.001) (Table 1). Eight (40%) patients (75% male, mean age 53 ± 17 yo) were classified as having a high risk for fatal arrhythmias, as described above. In spite of their relatively normal BMI (25 ± 1.6 kg/m2), 5 patients (63%) had sleep apnea in this subgroup, with a mean AHI of 14.3 ± 11 events/hour. There was no significant difference in the mean age or BMI between patients with or without SDB (62 yo vs. 38 yo, p= 0.10; 24.2 vs. 25.9 kg/m2, p= 0.29, respectively).
Atrial or ventricular arrhythmias were not observed in this study population. The analysis of bradyarrhythmias was limited because 60% of patients had an implanted ICD with back-up pace setting. In 2 patients without an ICD, 6 episodes of significant bradycardia were observed: 5 sinus pauses of a maximal duration of 3.1 seconds and 1 episode of Mobitz I second-degree atrioventricular block (pause of 2.5 seconds). None of them had SDB or high arrhythmic risk.
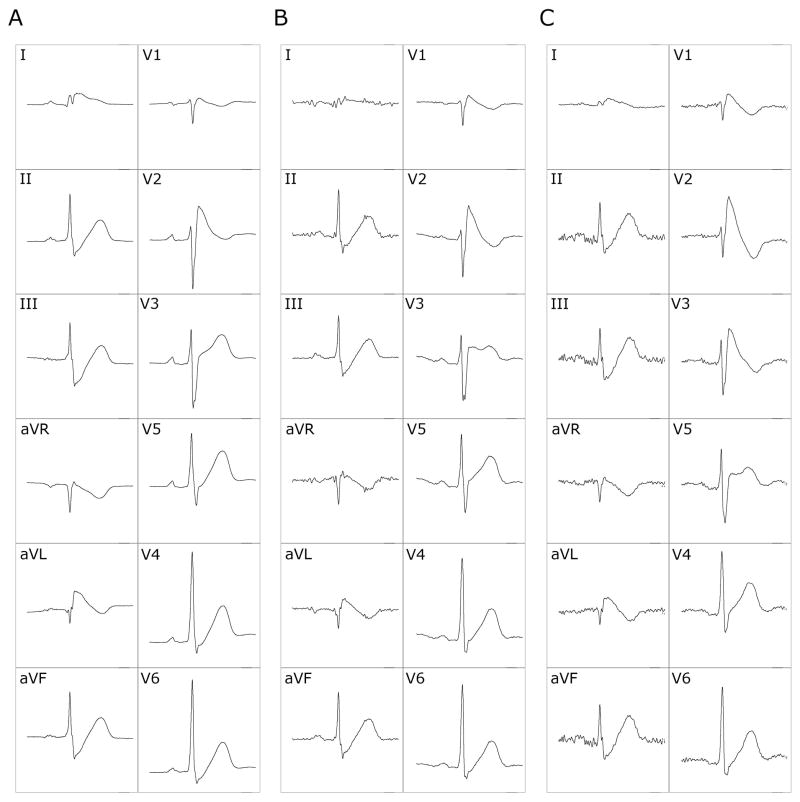
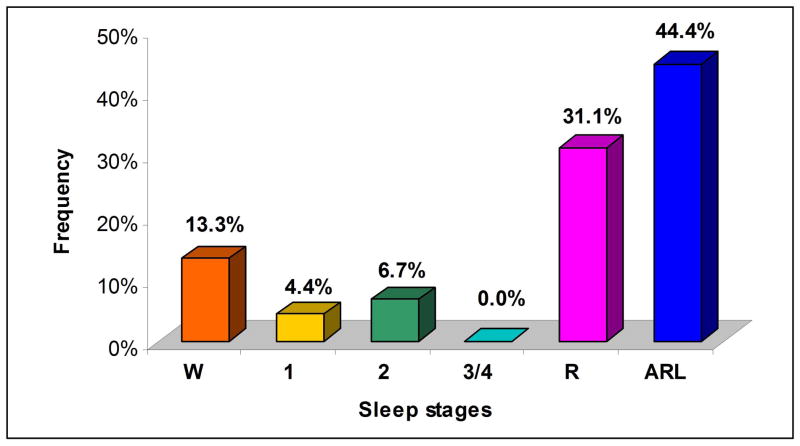
Spontaneous ST-segment changes were found in 2 patients (10%). Both were previously asymptomatic, had low arrhythmic risk and were not on medications. However, these 2 patients were in their third decade of life and had a history of SCD in a first-degree relative. One had an AHI of 14.5 events/hour and the other of 1.5 events/hour. The changes observed in the first patient were a spontaneous augmentation of the ST-segment elevation in V1 and V2 and a shift in the V3 pattern, from type 3 to type 2 or to type 1 (Figure 1). In the second patient, there was a visible change in the T wave morphology and amplitude in lead V1. The ST-segment changes occurred over 45 different time-points. The majority of the ST-segment changes were observed either during REM sleep (31%) or within one minute of arousals (44%). There were no changes in ST-segments during sleep stages 3 or 4 (Figure 2). In relation to the respiratory events, 25 (56%) ST-changes occurred during or within one minute after an episode of apnea or hypopnea.
Figure 1.
ST-segment changes in leads V1 to V3 in one patient with Brugada syndrome during REM sleep. Twelve-lead electrocardiogram (ECG) showing progressive augmentation of ST-segment elevation in V1 and V2 and changes in the ST pattern in lead V3 from type 3 Brugada ECG (A) to type 2 (B) and type 1 (C). These tracings were recorded during the same REM period within a 13-second time frame.
Figure 2.
ST-segment changes in 2 patients, according to sleep stages - Distribution of ST-segment changes in sleep stages. W= wakefulness, 1= stage 1, 2= stage 2, 3/4= stage 3 and 4, R= REM, ARL= arousals.
Discussion
In the present study we found that SDB is relatively common in patients with the Brugada syndrome, especially in patients at a high risk for fatal arrhythmic events. Our data also suggest that spontaneous changes in the ST segment, a risk factor for SCD, may be predominantly present either during REM sleep or after arousals. Recently, a very large prospective study analyzed predictive risk factors for SCD in patients with the Brugada syndrome, but the presence of SDB was not included in the analysis 10. To the best of our knowledge, this is the first study to analyze polysomnographic and electrocardiographic characteristics in this patient population.
The reported prevalence of SDB in the general population is around 25% 11,12. Similarly, in the present study, 27% of the control subjects had a diagnosis of SDB. However, we found that SDB is present in 45% of patients with the Brugada syndrome, and in 63% of Brugada patients who fulfill clinical criteria of high arrhythmic risk. These values are surprisingly high, especially considering that these patients had a normal BMI. Peppard et al found a mean AHI of 1.2 events/hour in a lean population, while we observed a mean AHI of 8 events/hour 13.
The Brugada Syndrome is a rare disease, and is associated with SCD in seemingly healthy patients. Approximately 10% of patients die annually, usually in their fourth decade of life 14. Matsuo et al showed that patients with the Brugada syndrome are more likely to die while sleeping 3. They observed that 87% of the ventricular fibrillation episodes occur between midnight and 6 am. Another disease previously called Sudden Unexplained Nocturnal Death Syndrome (SUNDS), more common in the Southeastern Asian countries, is now recognized as probably the same disorder as the Brugada syndrome 15.
Sleep apnea is also associated with SCD 7,16. Patients with SDB are also more likely to die during sleep, and it is believed that oscillations in autonomic nervous activation and cardiopulmonary dynamics may play a key role. Obstructive apneas are associated with surges in arterial blood pressure, vascular sympathetic-nerve activation and increased cardiac vagal drive 17,18. Furthermore, in patients with OSA, hypoxemia is associated with an increased incidence of complex ventricular ectopy, tachycardia and surges in blood pressure 4,19. In contrast, bradyarrhythmias with long pauses may occur due to vagal activation triggered by the diving reflex 18,20,21. Low oxygen and increased myocardial demand may facilitate the occurrence of myocardial ischemia and related arrhythmias. Indeed, nocturnal myocardial infarction occurs more frequently in patients with OSA 22.
The presence of spontaneous coved-type ST-segment elevation (type 1 ECG pattern) has been associated with a higher incidence of appropriate ICD shocks 23. Moreover, repeated ECGs in patients with the Brugada Syndrome show very frequent spontaneous fluctuations between diagnostic and non-diagnostic ECG patterns, which have been linked to ventricular arrhythmias 9,23,24. A prospective study, including 124 patients, found that spontaneous changes in the ST segment were associated with the highest risk for ventricular tachyarrhythmias and SCD, with a relative hazard ratio of 9.2.9. It has been suggested that acute modifications in transmembrane ionic currents participating in early phases of repolarization may underlie ST-segment changes in patients with the Brugada syndrome25. Oscillations in the autonomic system, through direct effects on ionic currents, seem to play an important role in ST-segment fluctuations 5,25. Therefore, we analyzed the ST-segment characteristics during polysomnographic monitoring of sleep stages, when significant changes in autonomic status are known to occur 26.
We observed changes in the ST segment in 2 patients, most frequently during REM sleep and after arousals, when 34 (75.5%) of the 45 recorded events occurred. Although less significant, those changes were also more common during the apneic/hypopneic events (56%). No changes were observed during sleep stages 3 or 4, and only 5 (11%) were detected in stages 1 and 2. In normal subjects, REM has been associated with surges in sympathetic activity as well as abrupt vagal discharges, explaining the higher incidence of tachycardias and bradyarrhythmias in this period 8,26,27. Arousals are also accompanied by transient bursts of sympathetic discharges 28,29. It has been hypothesized that the vivid and emotional dreams experienced during REM sleep may also contribute to nocturnal arrhythmogenesis 7. Therefore, autonomic instability during REM sleep and after arousals could play an important role in triggering arrhythmias. This may be especially true in Brugada patients, in whom an intrinsic autonomic impairment has been described 30–32. Studies using 123I-MIBG-SPECT have shown reduced tracer uptake in the left ventricle of patients with the Brugada syndrome, which is compatible with a dysfunction in myocardial sympathetic innervation. Those patients may also have cardiac autonomic neuropathy, based on abnormalities evident in tests of cardiovascular sympathetic function 30. Accordingly, the dynamic changes in the ST segment were more frequent during REM sleep and after arousals. Spontaneous ST-segment changes may provide a marker of increased arrhythmic risk for nocturnal SCD in these patients.
The strengths of our study were the use of overnight PSG in all patients, which is a gold standard for the diagnosis of SDB, the use of 12-lead ECG monitoring throughout PSG and the blinded analysis of the data. However, this was a cross-sectional study with a relatively small sample size and, therefore, the high frequency of SDB encountered in these patients with the Brugada syndrome should be interpreted with caution. Nevertheless, it is notable that SDB was evident in a large proportion of patients, despite the normal BMI. Finally, while these data do not support a causal relationship between SDB and ST-segment changes, they provide a compelling basis for further investigation. Prospective controlled studies are needed to address the role of SDB in triggering ventricular arrhythmias in patients with the Brugada syndrome.
Acknowledgments
Funding Support:
Virend K. Somers is supported by NIH Grants HL65176 and 1 UL1 RR 024150.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brugada P, Brugada J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: a distinct clinical and electrocardiographic syndrome. A multicenter report. J Am Coll Cardiol. 1992;20:1391–1396. doi: 10.1016/0735-1097(92)90253-j. [DOI] [PubMed] [Google Scholar]
- 2.Brugada J, Brugada P, Brugada R. The syndrome of right bundle branch block ST segment elevation in V1 to V3 and sudden death--the Brugada syndrome. Europace. 1999;1:156–166. doi: 10.1053/eupc.1999.0033. [DOI] [PubMed] [Google Scholar]
- 3.Matsuo K, Kurita T, Inagaki M, Kakishita M, Aihara N, Shimizu W, Taguchi A, Suyama K, Kamakura S, Shimomura K. The circadian pattern of the development of ventricular fibrillation in patients with Brugada syndrome. Eur Heart J. 1999;20:465–470. doi: 10.1053/euhj.1998.1332. [DOI] [PubMed] [Google Scholar]
- 4.Gami AS, Somers VK. Implications of obstructive sleep apnea for atrial fibrillation and sudden cardiac death. J Cardiovasc Electrophysiol. 2008;19:997–1003. doi: 10.1111/j.1540-8167.2008.01136.x. [DOI] [PubMed] [Google Scholar]
- 5.Mizumaki K, Fujiki A, Tsuneda T, Sakabe M, Nishida K, Sugao M, Inoue H. Vagal activity modulates spontaneous augmentation of ST elevation in the daily life of patients with Brugada syndrome. J Cardiovasc Electrophysiol. 2004;15:667–673. doi: 10.1046/j.1540-8167.2004.03601.x. [DOI] [PubMed] [Google Scholar]
- 6.Noda T, Shimizu W, Taguchi A, Satomi K, Suyama K, Kurita T, Aihara N, Kamakura S. STsegment elevation and ventricular fibrillation without coronary spasm by intracoronary injection of acetylcholine and/or ergonovine maleate in patients with Brugada syndrome. J Am Coll Cardiol. 2002;40:1841–1847. doi: 10.1016/s0735-1097(02)02494-4. [DOI] [PubMed] [Google Scholar]
- 7.Verrier RL, Josephson ME. Impact of sleep on arrhythmogenesis. Circ Arrhythm Electrophysiol. 2009;2:450–459. doi: 10.1161/CIRCEP.109.867028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guilleminault C, Pool P, Motta J, Gillis AM. Sinus arrest during REM sleep in young adults. N Engl J Med. 1984;311:1006–1010. doi: 10.1056/NEJM198410183111602. [DOI] [PubMed] [Google Scholar]
- 9.Ikeda T, Takami M, Sugi K, Mizusawa Y, Sakurada H, Yoshino H. Noninvasive risk stratification of subjects with a Brugada-type electrocardiogram and no history of cardiac arrest. Ann Noninvasive Electrocardiol. 2005;10:396–403. doi: 10.1111/j.1542-474X.2005.00055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Probst V, Veltmann C, Eckardt L, Meregalli PG, Gaita F, Tan HL, Babuty D, Sacher F, Giustetto C, Schulze-Bahr E, Borggrefe M, Haissaguerre M, Mabo P, Le Marec H, Wolpert C, Wilde AA. Long-term prognosis of patients diagnosed with Brugada syndrome: Results from the FINGER Brugada Syndrome Registry. Circulation. 121:635–643. doi: 10.1161/CIRCULATIONAHA.109.887026. [DOI] [PubMed] [Google Scholar]
- 11.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleepdisordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 12.Duran J, Esnaola S, Rubio R, Iztueta A. Obstructive sleep apnea-hypopnea and related clinical features in a population-based sample of subjects aged 30 to 70 yr. Am J Respir Crit Care Med. 2001;163:685–689. doi: 10.1164/ajrccm.163.3.2005065. [DOI] [PubMed] [Google Scholar]
- 13.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 14.Naccarelli GV, Antzelevitch C. The Brugada syndrome: clinical, genetic, cellular, and molecular abnormalities. Am J Med. 2001;110:573–581. doi: 10.1016/s0002-9343(01)00625-8. [DOI] [PubMed] [Google Scholar]
- 15.Fowler SJ, Priori SG. Clinical spectrum of patients with a Brugada ECG. Curr Opin Cardiol. 2009;24:74–81. doi: 10.1097/hco.0b013e32831cb920. [DOI] [PubMed] [Google Scholar]
- 16.Gami AS, Howard DE, Olson EJ, Somers VK. Day-night pattern of sudden death in obstructive sleep apnea. N Engl J Med. 2005;352:1206–1214. doi: 10.1056/NEJMoa041832. [DOI] [PubMed] [Google Scholar]
- 17.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96:1897–1904. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Somers VK, Dyken ME, Mark AL, Abboud FM. Parasympathetic hyperresponsiveness and bradyarrhythmias during apnoea in hypertension. Clin Auton Res. 1992;2:171–176. doi: 10.1007/BF01818958. [DOI] [PubMed] [Google Scholar]
- 19.Mehra R, Stone KL, Varosy PD, Hoffman AR, Marcus GM, Blackwell T, Ibrahim OA, Salem R, Redline S. Nocturnal Arrhythmias across a spectrum of obstructive and central sleepdisordered breathing in older men: outcomes of sleep disorders in older men (MrOS sleep) study. Arch Intern Med. 2009;169:1147–1155. doi: 10.1001/archinternmed.2009.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grimm W, Hoffmann J, Menz V, Kohler U, Heitmann J, Peter JH, Maisch B. Electrophysiologic evaluation of sinus node function and atrioventricular conduction in patients with prolonged ventricular asystole during obstructive sleep apnea. Am J Cardiol. 1996;77:1310–1314. doi: 10.1016/s0002-9149(96)00197-x. [DOI] [PubMed] [Google Scholar]
- 21.Caples SM, Gami AS, Somers VK. Obstructive sleep apnea. Ann Intern Med. 2005;142:187–197. doi: 10.7326/0003-4819-142-3-200502010-00010. [DOI] [PubMed] [Google Scholar]
- 22.Kuniyoshi FH, Garcia-Touchard A, Gami AS, Romero-Corral A, van der Walt C, Pusalavidyasagar S, Kara T, Caples SM, Pressman GS, Vasquez EC, Lopez-Jimenez F, Somers VK. Day-night variation of acute myocardial infarction in obstructive sleep apnea. J Am Coll Cardiol. 2008;52:343–346. doi: 10.1016/j.jacc.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richter S, Sarkozy A, Veltmann C, Chierchia GB, Boussy T, Wolpert C, Schimpf R, Brugada J, Brugada R, Borggrefe M, Brugada P. Variability of the diagnostic ECG pattern in an ICD patient population with Brugada syndrome. J Cardiovasc Electrophysiol. 2009;20:69–75. doi: 10.1111/j.1540-8167.2008.01282.x. [DOI] [PubMed] [Google Scholar]
- 24.Veltmann C, Schimpf R, Echternach C, Eckardt L, Kuschyk J, Streitner F, Spehl S, Borggrefe M, Wolpert C. A prospective study on spontaneous fluctuations between diagnostic and non-diagnostic ECGs in Brugada syndrome: implications for correct phenotyping and risk stratification. Eur Heart J. 2006;27:2544–2552. doi: 10.1093/eurheartj/ehl205. [DOI] [PubMed] [Google Scholar]
- 25.Antzelevitch C. Brugada syndrome. Pacing Clin Electrophysiol. 2006;29:1130–1159. doi: 10.1111/j.1540-8159.2006.00507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Somers VK, Dyken ME, Mark AL, Abboud FM. Sympathetic-nerve activity during sleep in normal subjects. N Engl J Med. 1993;328:303–307. doi: 10.1056/NEJM199302043280502. [DOI] [PubMed] [Google Scholar]
- 27.Verrier RL, Lau TR, Wallooppillai U, Quattrochi J, Nearing BD, Moreno R, Hobson JA. Primary vagally mediated decelerations in heart rate during tonic rapid eye movement sleep in cats. Am J Physiol. 1998;274:R1136–1141. doi: 10.1152/ajpregu.1998.274.4.R1136. [DOI] [PubMed] [Google Scholar]
- 28.Morgan BJ, Crabtree DC, Puleo DS, Badr MS, Toiber F, Skatrud JB. Neurocirculatory consequences of abrupt change in sleep state in humans. J Appl Physiol. 1996;80:1627–1636. doi: 10.1152/jappl.1996.80.5.1627. [DOI] [PubMed] [Google Scholar]
- 29.Blasi A, Jo J, Valladares E, Morgan BJ, Skatrud JB, Khoo MC. Cardiovascular variability after arousal from sleep: time-varying spectral analysis. J Appl Physiol. 2003;95:1394–1404. doi: 10.1152/japplphysiol.01095.2002. [DOI] [PubMed] [Google Scholar]
- 30.Bigi MA, Aslani A, Aslani A. Significance of cardiac autonomic neuropathy in risk stratification of Brugada syndrome. Europace. 2008;10:821–824. doi: 10.1093/europace/eum272. [DOI] [PubMed] [Google Scholar]
- 31.Nomura M, Nada T, Endo J, Kondo Y, Yukinaka M, Saito K, Ito S, Mori H, Nakaya Y, Shinomiya H. Brugada syndrome associated with an autonomic disorder. Heart. 1998;80:194–196. doi: 10.1136/hrt.80.2.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wichter T, Matheja P, Eckardt L, Kies P, Schafers K, Schulze-Bahr E, Haverkamp W, Borggrefe M, Schober O, Breithardt G, Schafers M. Cardiac autonomic dysfunction in Brugada syndrome. Circulation. 2002;105:702–706. doi: 10.1161/hc0602.103677. [DOI] [PubMed] [Google Scholar]