Abstract
Waxy wheat (Triticum aestivum L.) lacks the waxy protein, which is also known as granule-bound starch synthase I (GBSSI). The starch granules of waxy wheat endosperm and pollen do not contain amylose and therefore stain red-brown with iodine. However, we observed that starch from pericarp tissue of waxy wheat stained blue-black and contained amylose. Significantly higher starch synthase activity was detected in pericarp starch granules than in endosperm starch granules. A granule-bound protein that differed from GBSSI in molecular mass and isoelectric point was detected in the pericarp starch granules but not in granules from endosperm. This protein was designated GBSSII. The N-terminal amino acid sequence of GBSSII, although not identical to wheat GBSSI, showed strong homology to waxy proteins or GBSSIs of cereals and potato, and contained the motif KTGGL, which is the putative substrate-binding site of GBSSI of plants and of glycogen synthase of Escherichia coli. GBSSII cross-reacted specifically with antisera raised against potato and maize GBSSI. This study indicates that GBSSI and GBSSII are expressed in a tissue-specific manner in different organs, with GBSSII having an important function in amylose synthesis in the pericarp.
Starch is composed of two distinct Glc polymers, amylose and amylopectin. Amylose consists of linear molecules of (1–4)-linked α-d-glucopyranosyl units, whereas amylopectin is made up of highly branched molecules of α-d-glucopyranosyl units linked primarily by (1–4) bonds with branches resulting from (1–6) linkages (Whistler and Daniel, 1984). The two polymers differ in their ability to form complexes with fatty acids, low-Mr alcohols, and iodine: amylose easily forms complexes with these compounds, whereas amylopectin does not (Banks and Muir, 1980).
These differences in complex formation with iodine have been useful in determining amylose content in starch (Hollo and Szeitli, 1968), as well as in distinguishing waxy (or glutinous) mutants from the wild type (Hixon and Brimhall, 1968). Starch of non-waxy lines, which contains amylose, forms blue-black complexes with iodine; starch of waxy mutants, which lacks amylose, stains red-brown. Waxy mutants have been identified in maize, rice, barley, sorghum (Eriksson, 1963), and amaranth (Okuno and Sakaguchi, 1982). Recently, amylose-free (amf) mutants of potato (Hovenkamp-Hermelink et al., 1987) and waxy wheats (Nakamura et al., 1995) have been produced. In waxy and amf mutants, the absence of waxy protein was coincidental with a lack of amylose in storage starch. The waxy protein was thus shown to be GBSSI, and is considered to be the only SS involved in amylose synthesis in storage organs (Preiss, 1991; Smith and Martin, 1993).
Starch is deposited not only in storage organs but in other tissues as well (Badenhuizen, 1969; Greenwood, 1970), and it appears that GBSSI plays little or no role in amylose synthesis in nonstorage tissues. In a review of starch formation in the vegetative organs of rice, starches were classified as either permanent (storage) or temporary (Sato, 1984). Temporary starch was further divided into assimilation starch, located in the chloroplast; waiting starch, located in the young cells near meristems; and transitory starch, located in the parenchyma cells. All temporary starches in waxy mutants stained blue-black with iodine. Amylose contents of leaf and stem tissues of waxy rice were around 25% to 35%, comparable to levels in nonwaxy types (Igaue, 1964), suggesting the existence of a GBSS isoform(s) other than GBSSI.
We also observed blue-black-staining starch in the pericarp tissue of immature waxy wheat (Triticum aestivum), although starch in the endosperm and pollen stained red-brown, indicating that starch synthesis, in particular that of amylose, differs between pericarp and endosperm tissues. Wheat pericarp tissue is a good material with which to study the mechanism of starch synthesis in nonstorage organs, because young pericarp tissue has a higher starch content than leaf or stem tissue, and therefore pure starch is easier to obtain. We present the characterization of a GBSS isoform, designated GBSSII, which is found in the starch granules of wheat pericarp.
MATERIALS AND METHODS
Plant Material
The common wheat (Triticum aestivum L.) cvs Morikei CD-1479 (CD-1479) and Chinese Spring (CS) were planted in a greenhouse under 22°C (day)/15°C (night) conditions. CD-1479 is a double-haploid cultivar of waxy wheat (Hoshino et al., 1996), and CS is a nonwaxy wheat cultivar. CS has three waxy proteins, whereas CD-1479 lacks all waxy proteins from endosperm starch.
Immature seeds were collected from spikes at 5-d intervals until 35 DPA. The immature seeds were frozen in liquid nitrogen and stored at −70°C until starch granules were extracted.
Preparation of Starch Granules
Pericarp and endosperm were dissected from immature seeds. Endosperm tissue could not be observed by a light microscope at 5 DPA, so at this stage only ovaries were removed from the seeds. Separated pericarp and endosperm were briefly washed three times with an excess amount of 0.25 m Tris-HCl, pH 7.5. The tissues were homogenized with a mortar and pestle in three times their fresh weight of SDS buffer containing 0.1 m Tris-HCl, pH 7.5, 3% (w/v) SDS, 10% (v/v) glycerol, and 0.05% (v/v) 2-mercaptoethanol. The homogenates were centrifuged at 15,000g for 5 min. The supernatant was stored at −20°C until it was used as the soluble fraction in immunoblot analysis. The pellet was resuspended in SDS buffer and left overnight. The suspension was then filtered through a 40-μm nylon mesh and centrifuged at 15,000g for 2 min. The pellet was washed twice with SDS buffer, three times with deionized distilled water, and three times with cold acetone. The pellet was then vacuum dried and stored at −20°C.
Starch Staining and Microscopic Analysis
Cross-sections of immature seeds and starch granules were stained with an iodine solution (0.74 g of resublimated iodine and 1.48 g of KI dissolved in 400 mL of distilled water) and observed under a light microscope. For scanning electron microscopy, starch granules were mounted on aluminum stubs with double-stick tape and then coated with platinum using an ion coater (Eiko Engineering Co., Ibaragi, Japan). Examination was conducted with an electron microscope (model S-2700, Hitachi, Tokyo, Japan) at an accelerating potential of 10 kV.
Amylose Content
Amylose content was measured by the method described by Yamamori et al. (1992). Starch granules (20 mg) were suspended in 5 mL of 0.75 n NaOH solution with 25% (v/v) ethanol, and left at room temperature for 12 h. The sample was made up to 50 mL with distilled water, and amylose content was determined with an analyzer (Tecnicon, Bran-Lubbe Co., Tokyo, Japan) using KI-I2 solution (0.005% [w/v] KI and 0.003% [w/v] I2). Potato amylose and amylopectin (Sigma) were used as standards for regression curves.
The λmax of the iodine-starch complexes was determined according to the method of Konishi et al. (1985).
SDS-PAGE and Two-Dimensional PAGE
Starch granule-bound proteins were separated by SDS-PAGE using 10% polyacrylamide gels (Laemmli, 1970). To extract starch granule-bound proteins the purified starch was suspended in SDS buffer and heated in boiling water. The starch solution was cooled on ice and centrifuged at 15,000g for 10 min, and the supernatant was subjected to SDS-PAGE. The soluble fractions were also heated in boiling water for 5 min and cooled on ice prior to SDS-PAGE. After electrophoresis, proteins were visualized using a silver-staining kit (Wako Chemicals, Osaka, Japan).
Starch granule-bound proteins were analyzed by two-dimensional PAGE according to the method of Nakamura et al. (1993). Starch granules were suspended in lysis buffer (8 m urea, 2% [v/v] Nonidet-P40, 2% ampholine, pH 3.5–10, 5% [v/v] 2-mercaptoethanol, and 5% [w/v] PVP), and the suspension was heated, cooled on ice, and centrifuged at 15,000g for 10 min. The supernatant was subjected to IEF for 14 h at 400 V. The focused gel was applied to a 15% low-Bis acrylamide gel to conduct SDS-PAGE (Kagawa et al., 1988).
Starch Synthase Activity
To measure starch synthase activity, 500 mg of 5-DPA immature seeds and 500 mg of endosperm tissues from 20-DPA seeds were homogenized in a mortar and pestle with 3 mL of ice-cold buffer (50 mm Hepes-NaOH, pH 7.5, 5 mm MgCl2, 2 mm DTT, and 1 mg/mL BSA). The homogenate was centrifuged at 15,000g for 10 min at 4°C, and the supernatant was assayed for soluble enzyme activity. The pellet was resuspended in buffer and filtered through a 40-μm nylon mesh. The suspension was left at 4°C for 15 min to allow the starch granules to settle, and then the supernatant was removed. The starch granules were washed by two cycles of resuspension and centrifugation with buffer and one cycle with ice-cold acetone. The starch granules were air dried at 4°C and stored at −20°C.
Starch synthase activities of the granule-bound and soluble fractions were assayed by the incorporation of [14C]ADP-Glc according to the method of Singletary et al. (1997), with minor modifications. For measurement of GBSS activity, 1 mg of starch granules was incubated in 200 μL of reaction buffer (100 mm Bicine, pH 8.3, 25 mm KCl, 10 mm GSH, 4.5 mm EDTA, 3.5 mm [14C]ADP-Glc [120 dpm/nmol]) at 25°C with continuous gentle shaking. After 1 h the reaction was terminated with methanol (70%, v/v) containing 1% KCl (Vos-Scheperkeuter et al., 1986) and incubated on ice for 10 min.
Soluble starch synthase activity was measured using a primer-dependent assay with 1 mg of rabbit-liver glycogen and 50 μL of soluble extract in 200 μL of the above reaction buffer containing 1 mm [14C]ADP-Glc (222 dpm/nmol). The sample was incubated for 30 min at 25°C, and the reaction was stopped by adding 100 μL of 0.2 n NaOH. After adding 1 mL of ice-cold ethanol, the reaction was incubated on ice for 10 min. In both assays the control reaction was stopped immediately after the addition of labeled ADP-Glc.
After incubation on ice, reactions in both assays were centrifuged at 15,000g for 10 min and supernatants were removed. Pellets were washed twice by suspension and ethanol precipitation. The pellets were then resuspended in 500 μL of 1 n HCl, boiled for 5 min, cooled, and mixed with 7 mL of scintillation cocktail. Radioactivity was measured with a liquid-scintillation counter (Aloka Co. Ltd., Tokyo).
Protein Sequencing
After SDS-PAGE, proteins were transferred from gels onto a PVDF membrane by electroblotting (Hirano and Watanabe, 1990), and stained with Coomassie brilliant blue R. The band of interest was excised and applied to a gas-phase protein sequencer (Perkin-Elmer-ABI). Comparison of the amino acid sequence with other sequences deposited in the database was performed using the Basic Local Alignment Search Tool (BLAST).
Immunoblot Analysis
Proteins from starch granules and soluble fractions were separated by SDS-PAGE using a 10% acrylamide gel and electroblotted onto PVDF membrane. Blots were incubated in TBST (20 mm Tris-HCl, pH 7.5, 150 mm NaCl, and 0.05% [v/v] Tween 20) solution containing 1% (w/v) BSA for 60 min, and then incubated for 60 min with anti-GBSSI rabbit serum diluted with TBST solution. After a 60-min incubation with rabbit anti-IgG alkaline phosphatase conjugate (Promega), immunoreactive bands were detected with Western Blue stabilized substrate for alkaline phosphatase (Promega).
RESULTS
PSGs
The pericarp is the wall of the mature ovary and surrounds the entire seed (Sakri and Shannon, 1975). Investigations of wheat pericarp morphology showed that blue-black-staining starch granules appear in this tissue at about the time of anthesis (Eckerson, 1917; Sandstedt, 1946; Jenkins et al., 1975). We observed a thick pericarp layer with an abundance of starch at 5 DPA (Fig. 1), although endosperm tissue was not yet detectable at this stage. Starch granules were still present in pericarp tissue at 20 DPA, at which time starch had accumulated in the developing endosperm (Fig. 1). The gradual decrease in pericarp starch during endosperm development has been observed previously in both wheat (Sandstedt, 1946; Chevalier and Lingle, 1983) and rice (Sato, 1984). This starch may be stored temporarily in the pericarp and later converted to sugar and transferred to the endosperm as a substrate for starch synthesis (Chevalier and Lingle, 1983).
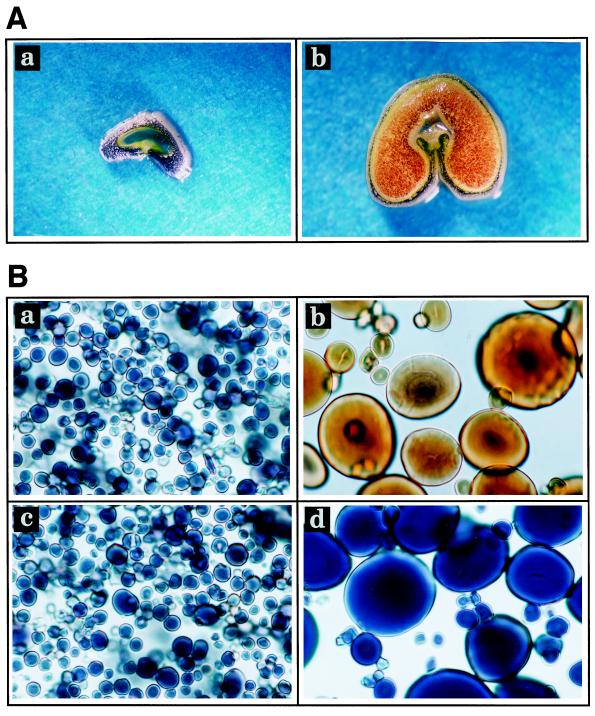
Figure 1.
Iodine staining of wheat starches. Samples were viewed under a light microscope. A, Cross-sections of immature waxy wheat seeds harvested at 5 DPA (a) and 20 DPA (b). B, Starch granules isolated from waxy pericarp (a), waxy endosperm (b), nonwaxy pericarp (c), and nonwaxy endosperm (d).
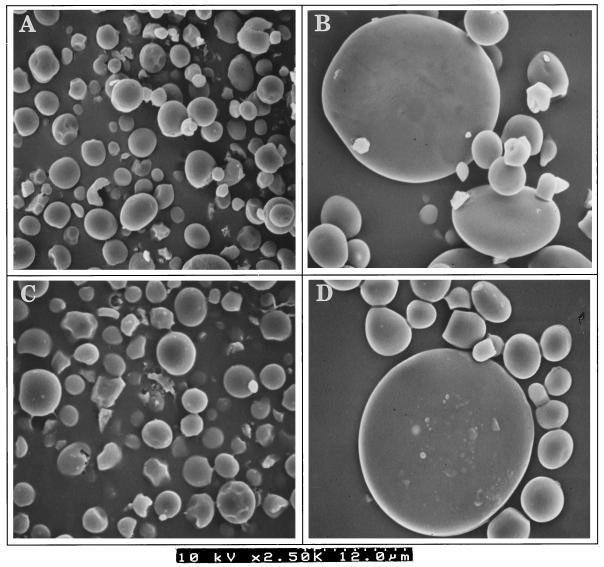
PSGs and ESGs in waxy wheat differed in starch composition and granule morphology. PSGs of waxy wheat stained blue-black, indicating the presence of amylose, whereas ESGs stained red-brown (Fig. 1). ESGs consisted of large (A-type) and small (B-type) granules (Figs. 1 and 2), as previously observed by Parker (1985). However, PSGs were relatively uniform in size, and slightly smaller in diameter than B-type granules. PSGs were round and flat, and their average diameter was around 3 μm (Fig. 2).
Figure 2.
Scanning electron micrographs of PSGs and ESGs. Starch granules were extracted from waxy wheat pericarp (A), waxy wheat endosperm (B), nonwaxy wheat pericarp (C), and nonwaxy wheat endosperm (D). The ruled area below the micrographs represents 12.0 μm.
The λmax of starch-iodine complexes of ESGs from waxy and nonwaxy wheat were 538 and 595 nm, respectively. This difference was due to the absence of amylose in starch of waxy wheat. In contrast to ESGs, λmax of PSGs of both waxy and nonwaxy wheat was around 600 nm, indicating that both starches contained amylose. In waxy wheat the amylose content of PSGs was 18%, whereas that of ESGs was less than 1% (Table I). However, the amylose content of PSGs from both waxy and nonwaxy wheat was lower than that of nonwaxy ESGs.
Table I.
Amylose content of starch granules and maximum absorbance of iodine-starch complexes
| Starch Granules | Amylose Content | λmax |
|---|---|---|
| % | nm | |
| Waxy wheat | ||
| ESG | 0.8 | 538 |
| PSG | 19.4 | 598 |
| CS (nonwaxy) | ||
| ESG | 26.3 | 595 |
| PSG | 18.5 | 599 |
| Waxy maize | ||
| ESG | 0.8 | 541 |
For measurement of the λmax of starch-iodine complexes, starch granules were dissolved in 1 n NaOH and neutralized with acetic acid. Absorbances were examined using 1 mg of starch granules with 0.2 mL of iodine solution in a total volume of 25 mL.
Electrophoresis of Starch Granule-Bound Proteins
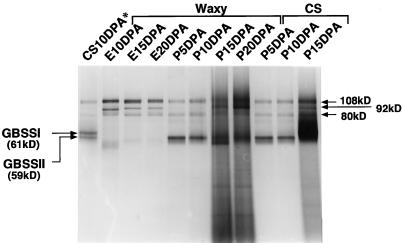
Starch granules from 10-DPA whole, immature seeds of nonwaxy wheat contained two major proteins with relative molecular masses of 61 and 59 kD (Fig. 3). The 61-kD protein was waxy protein (Yamamori et al., 1992), and was therefore not detected in ESGs of waxy wheat. The 59-kD protein was detected in PSGs of waxy and nonwaxy wheat (Fig. 3). Furthermore, the 59-kD protein seemed to bind tightly to the starch granules, because it remained in or on them after washing with SDS solution, and was only released after heat-induced swelling occurred. The same characteristics have been reported for the waxy protein of maize (Echt and Schwartz, 1981; Iman, 1989).
Figure 3.
SDS-PAGE patterns of starch granule-bound proteins. ESGs (E) and PSGs (P) tissues were extracted at 5-d intervals starting at 5 DPA. Starch granule-bound proteins were released by heating 5 mg of starch granules in 80 μL of SDS buffer at 100°C for 5 min, cooling on ice for 10 min, and centrifuging at 15,000g. Equal volumes (20 μL) of the supernatant containing starch granule-bound proteins were loaded in each lane. Electrophoresis was conducted at room temperature. CS10DPA*, Starch granules extracted from 10-DPA whole, immature seeds of CS wheat.
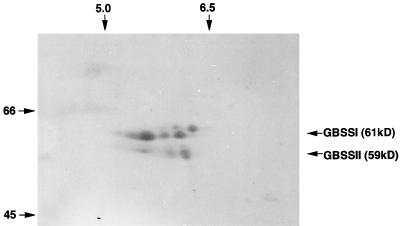
The two-dimensional-PAGE analysis showed that GBSSI (waxy protein) and GBSSII differ in pI range and in molecular mass (Fig. 4). The two-dimensional PAGE pattern of GBSSII (59 kD) seemed to be composed of three overlapping proteins, similar to GBSSI (61 kD), which separates into three proteins encoded by three homologous waxy genes, Wx-A1, Wx-B1, and Wx-D1 (Nakamura et al., 1993).
Figure 4.
Two-dimensional-PAGE analysis of pericarp starch granule-bound proteins. Equal amounts (10 mg) of PSGs from waxy wheat and nonwaxy wheat were mixed in lysis buffer and heated in boiling water for 2 min. The solution was cooled on ice for 10 min and centrifuged at 15,000g for 10 min. The supernatant (500 μL) was applied to an isofocusing gel in a 20-cm glass capillary with a 5-mm i.d. The focused gel was equilibrated in sample buffer (0.06 m Tris-HCl, pH 6.8, 10% [v/v] glycerol, 2.5% [w/v] SDS, and 5% [v/v] β-mercaptoethanol) for 30 min, and then subjected to SDS-PAGE using a 15% low-Bis acrylamide gel. Electrophoresis was conducted at 4°C, and the gel was stained with Coomassie brilliant blue R. The pH range for the first dimension (top) and the molecular mass range (kD) for the second dimension (left) are indicated.
Three minor SGPs of approximately 80, 92, and 108 kD were found in both pericarp and endosperm starch by SDS-PAGE (Fig. 3). The relative amounts of these proteins, particularly of the 92-kD protein, were lower in pericarp than in endosperm. These SGPs have been referred to as SGP1 (108 kD), SGP2 (92 kD), and SGP3 (80 kD) (Yamamori and Endo, 1996). SGP1 and SGP3 are soluble starch synthase, and SGP2 is a branching enzyme (Takaoka et al., 1997).
Starch Synthase Activity
Starch synthase activity of waxy PSGs was similar to that of nonwaxy PSGs, although there was a significant difference between waxy and nonwaxy ESGs (Table II). Both waxy and nonwaxy PSGs had starch synthase activities 12-fold higher than waxy ESGs. Starch synthase activities of PSGs were approximately 1.5-fold higher than that of nonwaxy ESGs. However, this does not necessarily indicate that GBSS activity is higher in pericarp than in endosperm tissue in nonwaxy wheat, since PSGs were extracted from 5-DPA seeds and ESGs were extracted at 20 DPA. Starch sythase activities of the soluble fractions of waxy and nonwaxy pericarp tissue were also similar.
Table II.
Starch synthase activity of pericarp and endosperm tissues
| Sample | GBSS | Soluble SS |
|---|---|---|
| nmol min−1 mg−1 | ||
| Pericarp | ||
| Waxy | 0.324 ± 0.045 | 1.22 ± 0.54 |
| CS (nonwaxy) | 0.327 ± 0.009 | 1.11 ± 0.45 |
| Endosperm | ||
| Waxy | 0.026 ± 0.004 | |
| CS (nonwaxy) | 0.208 ± 0.036 | |
Values represent the means ± se of three replications, and are based on milligrams of starch granules for the granule-bound activity and on milligrams of fresh tissue weight for soluble activity.
N-Terminal Amino Acid Sequence
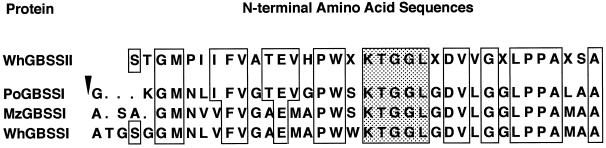
To further investigate the similarities between GBSSII and GBSSI, the N-terminal sequence was determined from SDS-PAGE blots of GBSSII by a gas-phase sequencer. A 35-amino acid sequence was obtained and a homology search was conducted. The GBSSII sequence showed homology to GBSSIs or waxy proteins of potato, maize, sorghum, barley, rice, and wheat, and to the glycogen synthase of Escherichia coli (Fig. 5). The homology started at the N-terminal end of the mature proteins, which is the cleavage site of the transit peptides. The 14th to 18th residues (Lys-Thr-Gly-Gly-Leu) constitute a conserved motif found in GBSSI and in soluble starch synthases (Ainsworth et al., 1993; Baba et al., 1993). The motif is thought to be the binding site for the substrate ADP-Glc (Furukawa et al., 1990).
Figure 5.
Comparison of the N-terminal sequence of wheat GBSSII with the deduced amino acid sequences of potato (Po) (van der Leij et al., 1991), maize (Mz) (Klosgen et al., 1986), and wheat (Wh) (Ainsworth et al., 1993) GBSSI or waxy cDNAs. Open boxes denote residues with identity to the GBSSI sequence and filled boxes denote the conserved motif KTGGL. The putative cleavage site of the transit peptides is indicated by an arrowhead. X, Undetermined residues.
Immunoblot Analysis of GBSSII
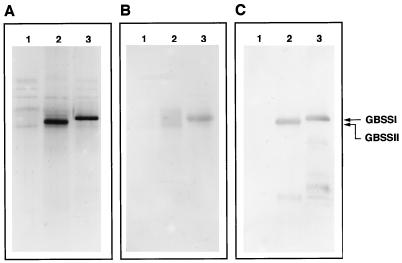
Antibodies against potato and maize GBSSI were used in immunoblot analyses. Wheat GBSSII and GBSSI cross-reacted with both maize and potato GBSSI antiserum (Fig. 6). Although GBSSII reacted strongly with potato GBSSI antibody, a sharp band was not obtained with the maize antibody. The structure of the epitope(s) of maize GBSSI may be similar but not identical to that of wheat GBSSII. Alternatively, the weaker reactivity of the maize antibody may have been due to a titer difference between the potato and maize antibodies used in this experiment. The potato antiserum reacted with GBSSI after a 1:2000 dilution, whereas the maize antiserum could only be diluted 100-fold. The western analysis also showed that GBSSII was not associated with endosperm starch of waxy or nonwaxy wheat (Fig. 6).
Figure 6.
SDS-PAGE of starch granule-bound proteins (A) and SDS-PAGE immunoblots using anti-maize waxy protein antibody (B) and anti-potato GBSSI antibody (C). To extract SGPs, 5 mg of ESGs from waxy wheat (lanes 1), PSGs from waxy wheat (lanes 2), and ESGs from nonwaxy wheat (lanes 3) were heated in 80 μL of SDS buffer. For SDS-PAGE, 20 mL of the supernatant was applied to each track of 10% polyacrylamide gels. Gels were stained with a silver-staining kit (A) or electroblotted onto PVDF membranes (B and C). Blotted membranes were incubated with antiserum to maize waxy protein at a dilution of 1:100 and to potato GBSSI at a dilution of 1:2000. For secondary antibody binding, the membranes were incubated with anti-rabbit IgG alkaline phosphatase conjugate (1:5000 dilution). Sites of antigen localization were visualized by a substrate solution for alkaline phosphatase containing nitroblue tetrazolium and 5-bromo-4-chloro-3-indolyl-phosphate.
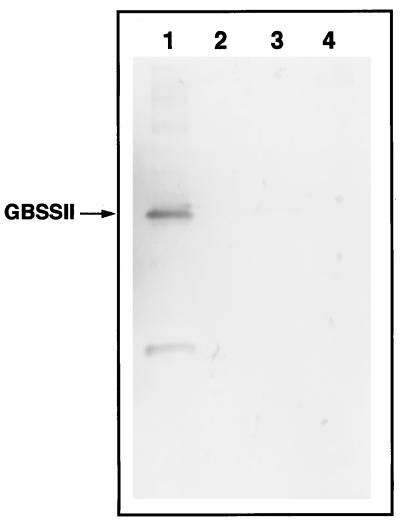
Due to the large number of proteins in the soluble fraction, it was not possible to determine if GBSSII was present in this fraction by staining of polyacrylamide gels. For this reason, soluble fractions from pericarp and endosperm tissues were subjected to immunoblot analysis. Potato GBSSI antibody did not cross-react with blots of the soluble fraction from pericarp tissue (Fig. 7), even when four times the amount of soluble fraction was blotted (data not shown), nor was GBSSII detected in the soluble fraction from endosperm of waxy wheat. These results indicate that GBSSII is specific to the granule-bound fraction from pericarp tissue, as is GBSSI to the granule-bound fraction from endosperm.
Figure 7.
Immunoblots of granule-bound and soluble fractions of waxy wheat pericarp and endosperm. Lane 1, Granule-bound fraction of pericarp; lane 2, soluble fraction of pericarp; lane 3, granule-bound fraction of endosperm; lane 4, soluble fraction of endosperm. To prepare granule-bound fractions, 5 mg of starch granules was heated in 80 μL of SDS buffer. The soluble fractions, prepared as described in “Materials and Methods,” were also heated before loading. The granule-bound and soluble fractions (20 μL of each) were applied to a 10% polyacrylamide gel for SDS-PAGE. Separated proteins were electroblotted onto PVDF membrane, and blots were probed with antiserum to potato GBSSI diluted 1:2000.
DISCUSSION
The basic process of starch synthesis is similar in all plant tissues (Badenhuizen, 1969; Shannon and Garwood, 1984; Preiss, 1988) and consists of three main events in the chloroplast and amyloplast (Muller-Rober and Kossmann, 1994): (a) the supply of Glc-1-P into the plastid; (b) the synthesis of ADP-Glc from Glc-1-P; and (c) the synthesis of starch (amylose and amylopectin) from ADP-Glc. However, starch characteristics, especially granule shape and amylose:amylopectin ratios, differ among tissues in the same plant (Sato, 1984; Tomlinson et al., 1997). The third step in starch synthesis, the formation of starch from ADP-Glc, must therefore involve different enzyme isoforms and/or processes in different tissues.
Wheat pericarp tissue accumulates starch granules that are different in appearance (Fig. 1) and in the amylose:amylopectin ratio (Table I) from ESGs. The differences between the two tissues were particularly obvious in waxy wheat, since waxy PSGs contained amylose and ESGs had a negligibly low amount (Fig. 1; Table I). The presence of amylose in PSGs was confirmed by a high-performance size-exclusion chromatography analysis, in which PSGs showed an amylose molecule peak that could not be detected in ESGs of waxy wheat (data not shown).
The presence of amylose in the PSGs of waxy wheat implied the existence of either GBSSI or a GBSSI isoform in the pericarp tissue since this enzyme is required for amylose synthesis from ADP-Glc (for review, see Nelson and Pan, 1995). If GBSSI produced amylose in pericarp tissue, a 61-kD protein would be present in PSGs, and no such protein was found in PSGs from waxy or nonwaxy wheats (Fig. 3). This result is consistent with the endosperm- and pollen-specific expression of the waxy genes encoding GBSSI as reported in wheat (Ainsworth et al., 1993) and other cereals (Klosgen et al., 1986; Hirano and Sano, 1991; Baba et al., 1993). However, another granule-bound protein, GBSSII, which had characteristics similar to those reported for GBSSI (or waxy protein) in maize (Echt and Schwartz, 1981), rice (Sano, 1984), wheat (Yamamori et al., 1992), potato (Vos-Scheperkeuter et al., 1986), and pea (Smith, 1990), was detected in pericarp tissue. GBSSII was strongly bound to the starch granules, could not be detected in the soluble fraction, and its relative molecular mass was close to those reported for GBSSIs. Three observations strongly suggest that this new protein is a GBSS: (a) significant SS activity was detected in PSGs; (b) the N-terminal sequence of GBSSII showed that this protein was not a wheat GBSSI or a GBSSI degradation product, yet it had homology to waxy proteins and GBSSIs (Fig. 5); and (c) GBSSII was antigenically related to GBSSI of potato and maize (Fig. 6).
Starch syntase isoforms referred to as GBSSII have been reported in pea (Dry et al., 1992) and potato (Edwards et al., 1995). However, these enzymes, which are likely to be involved in amylopectin synthesis, are present in both the soluble and granule-bound phases, and probably become entrapped in the granules during starch formation (Martin and Smith, 1995). Recently, GBSSII from pea and potato has been referred to simply as starch synthase II (SSII) (Smith et al., 1997). Therefore, we feel that the designation “GBSS” should be reserved for starch synthase isoforms that are largely limited to and active in the granule-bound fraction and are involved in the synthesis of amylose. GBSSII of wheat fits all of these criteria.
Although the presence of additional GBSS isoforms has not been reported in other cereals, several studies present evidence that such isoforms occur. In waxy maize, starch in the pollen, embryo sac, and endosperm lacks amylose, but starch in other tissues, including pericarp, stains blue-black (Hixon and Brimhall, 1968; Badenhuizen, 1969). Two rice waxy mutants that produced inactive waxy proteins in endosperm starch had blue-black-staining starch in leaf tissue, clearly indicating that separate waxy genes are active in these two tissues (Sano, 1985). In barley, Ono and Suzuki (1957) reported that reddish-brown-staining starch was detected not only in endosperm and pollen, but also in leaves of several waxy cultivars. However, re-examination of the same waxy barley lines showed only blue-staining starch in leaves (N. Ishikawa, personal communication). The reason for this discrepancy is unclear but it should be noted that the waxy barley lines used in these experiments were not complete waxy mutants, since they contained 2% to 10% amylose and detectable levels of waxy protein in ESGs (Ishikawa et al., 1994). Recently, a barley waxy mutant lacking both amylose and waxy protein in the endosperm starch was produced (Ishikawa et al., 1994), and blue-black-staining starch was found in the young pericarp tissue (T. Nakamura, P. Vrinten, K. Hayakawa, and J. Ikeda, unpublished data). These results provide evidence that there is more than one gene encoding GBSS isoforms in monocot plants and that the expression of these genes is regulated in a tissue-specific manner.
In dicot plants the situation may be somewhat more variable. For example, low-amylose (lam) mutants of pea (Denyer et al., 1995) had a major SGP in starch from leaf (Tomlinson et al., 1998) and pod (Denyer et al., 1997) tissues. This protein was reported to be a GBSSI isoform (Denyer et al., 1997; Tomlinson et al., 1998), suggesting that pea also has more than one gene encoding GBSS. In contrast, a single GBSS may be responsible for the synthesis of amylose in potato. An amf mutant (Vos-Scheperkeuter et al., 1987) that carries a point deletion in the GBSS gene (van der Leij et al., 1991) displayed red-brown-staining starch not only in the tuber but also in the roots, leaves, and pollen (Jacobsen et al., 1989).
The level of homology between the waxy (GBSSI) and GBSSII genes is apparently not high enough to allow cross-hybridization. When a waxy cDNA was used as a probe in Southern hybridizations, no extra genomic fragments with strong homology to the waxy gene were identified (T. Nakamura, P. Vriten, K. Hayakawa, and J. Ikeda, unpublished data). Similar experiments in other cereals have also identified only a single gene with homology to waxy cDNA (Shure et al., 1983; Hirano and Sano, 1991). Cloning of the gene for GBSSII will provide more detail about the differences between GBSSI and GBSSII, and will increase our understanding of amylose synthesis in plants.
ACKNOWLEDGMENTS
We thank Drs. T. Baba and K. Takaha for antisera of maize and potato, and Dr. R. Giroux for comments on an earlier version of this manuscript.
Abbreviations:
- λmax
maximum absorbance
- DPA
days post- anthesis
- ESG
endosperm starch granule
- GBSS
granule-bound starch synthase
- PSG
pericarp starch granule
- SGP
starch granule protein
Footnotes
This research was supported by the Ministry of Agriculture, Forestry, and Fisheries and the Science Technology Agency of Japan.
LITERATURE CITED
- Ainsworth C, Clark J, Balsdon J. Expression, organisation and structure of the genes encoding the waxy protein (granule-bound starch synthase) in wheat. Plant Mol Biol. 1993;22:67–82. doi: 10.1007/BF00038996. [DOI] [PubMed] [Google Scholar]
- Baba T, Nishihara Mizuno K, Kawasaki T, Shimada T, Kobayashi E, Ohnishi S, Tanaka K, Arai Y. Identification, cDNA cloning, and gene expression of soluble starch synthase in rice (Oriza sativa L.) immature seeds. Plant Physiol. 1993;103:565–573. doi: 10.1104/pp.103.2.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badenhuizen NP (1969) The Biosynthesis of Starch Granules in Higher Plants. Appleton-Century-Crofts, New York, pp 1–115
- Banks WD, Muir DD (1980) Structure and chemistry of the starch granule. In J Press, ed, Biochemistry of Plants, Vol 3. Academic Press, New York, pp 321–369
- Chevalier P, Lingle SE. Sugar metabolism in developing kernels of wheat and barley. Crop Sci. 1983;23:272–277. [Google Scholar]
- Denyer K, Barber LM, Burton R, Hedley CL, Hylton CM, Johnson S, Jones DA, Marshall J, Smith AM, Tatge H and others. The isolation and characterization of novel low-amylose mutants of Pisum sativum L. Plant Cell Environ. 1995;18:1019–1026. [Google Scholar]
- Denyer K, Barber LM, Edward EA, Smith AM, Wang TL. Two isoforms of the GBSSI class of granule-bound starch synthase are differentially expressed in the pea plant (Pisum sativum L.) Plant Cell Environ. 1997;20:1566–1572. [Google Scholar]
- Dry I, Smith A, Edward A, Bhattacharyya M, Dunn P, Martin C. Characterization of cDNAs encoding two isoforms of granule-bound starch synthase which show differential expression in developing storage organs of pea and potato. Plant J. 1992;2:193–202. [PubMed] [Google Scholar]
- Echt CS, Schwartz D. Evidence for the inclusion of controlling elements within the structural gene at the waxy locus in maize. Genetics. 1981;99:275–284. doi: 10.1093/genetics/99.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckerson SH. Microchemical studies in the progressive development of the wheat plant. State College of Washington Agricultural Experiment Station Bulletin. 1917;139:3–21. [Google Scholar]
- Edwards A, Marshall J, Sidebottom C, Visser RGF, Smith AM, Martin C. Biochemical and molecular characterization of a novel starch synthase from potato tubers. Plant J. 1995;8:283–294. doi: 10.1046/j.1365-313x.1995.08020283.x. [DOI] [PubMed] [Google Scholar]
- Eriksson G. The waxy character. Heredity. 1963;63:180–204. [Google Scholar]
- Furukawa K, Tagaya M, Inouye M, Preiss J, Fukui T. Identification of lysine 15 at the active site in Escherichia coli glycogen synthase. J Biol Chem. 1990;265:2086–2090. [PubMed] [Google Scholar]
- Greenwood C (1970) Starch and glycogen. In W Pigman, D Horton, eds, The Carbohydrates: Chemistry and Biochemistry, Ed 2. Academic Press, New York, pp 471–513
- Hirano H, Watanabe T. Microsequencing of proteins electrotransferred onto immobilizing matrices from polyacrylamide gel electrophoresis: application to an insoluble protein. Electrophoresis. 1990;11:573–580. doi: 10.1002/elps.1150110708. [DOI] [PubMed] [Google Scholar]
- Hirano H-Y, Sano Y. Molecular Characterization of the waxy locus of rice (Oriza sativa) Plant Cell Physiol. 1991;32:989–997. [Google Scholar]
- Hixon RM, Brimhall B. Waxy cereals and red iodine starches. In: Radley JA, editor. Starch and Its Derivatives, Ed 4. London: Chapman and Hall; 1968. pp. 247–281. [Google Scholar]
- Hollo J, Szeitli J. The reaction of starch with iodine. In: Radley JA, editor. Starch and Its Derivatives, Ed 4. London: Chapman and Hall; 1968. pp. 203–246. [Google Scholar]
- Hoshino T, Ito S, Hatta K, Nakamura T, Yamamori M. Development of waxy common wheat by haploid breeding. Breeding Sci. 1996;46:185–188. [Google Scholar]
- Hovenkamp-Hermelink JHM, Jacobsen E, Ponstein AS, Visser RGF, Vos-Scheperkeuter GH, Bijmolt EW, de Vries JN, Witholt B, Feenstra WJ. Isolation of an amylose-free starch mutant of the potato (Solanum tuberosum L.) Theor Appl Genet. 1987;75:217–221. [Google Scholar]
- Igaue I. Studies on Q-enzyme of rice plant. Mem Fac Agr Niigata Univ. 1964;4:1–54. [Google Scholar]
- Imam SH. A tightly bound Mr 55000 polypeptide in cornstarch associated with the amylose portion of the granule. J Cereal Sci. 1989;9:231–236. [Google Scholar]
- Ishikawa N, Ishihara J, Itoh M. Artificial induction and characterization of amylose-free mutants of barley. Barley Genet News. 1994;24:49–53. [Google Scholar]
- Jacobsen E, Hovenkamp-Hermelink JHM, Krijgsheld HT, Nijdam H, Pijnacker LP, Witholt B, Feenstra WJ. Phenotypic and genotypic characterization of an amylose-free starch mutant of the potato. Euphytica. 1989;44:43–48. [Google Scholar]
- Jenkins LD, Meredith P, Loney PD. The developing starch granule. Part I. The starch content of cereal grains during their development. Starch/Starke. 1975;27:105–109. [Google Scholar]
- Kagawa H, Hirano H, Kikuchi F. Variation of glutelin seed storage protein in rice (Oryza sativa L.) Jpn J Breed. 1988;38:327–332. [Google Scholar]
- Klosgen RB, Gierl A, Schwarz-Sommer Z, Saedler H. Mol Gen Genet. 1986;203:237–244. [Google Scholar]
- Konishi Y, Nojima H, Okuno K, Asaoka M, Fuwa H. Characterization of starch granules from waxy, nonwaxy, and hybrid seeds of Amaranthus hypochondriacus L. Agric Biol Chem. 1985;49:1965–1971. [Google Scholar]
- Laemmli VK. Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Martin C, Smith A. Starch biosynthesis. Plant J. 1995;7:971–985. doi: 10.1105/tpc.7.7.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller-Rober B, Kossmann J. Approaches to influence starch quantity and starch quality in transgenic plants. Plant Cell Environ. 1994;17:601–613. [Google Scholar]
- Nakamura T, Yamamori M, Hirano H, Hidaka S. ) Biochem Genet. 1993;248:253–259. doi: 10.1007/BF02399821. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Yamamori M, Hirano H, Hidaka S, Nagamine T. Production of waxy (amylose-free) wheats. Mol Gen Genet. 1995;248:253–259. doi: 10.1007/BF02191591. [DOI] [PubMed] [Google Scholar]
- Nelson O, Pan D. Starch synthesis in maize endosperm. Annu Rev Plant Physiol Plant Mol Biol. 1995;46:475–496. [Google Scholar]
- Okuno K, Sakaguchi S. J Heredity. 1982;73:467. [Google Scholar]
- Ono T, Suzuki H. Endosperm characters in hybrids between barley varieties with starchy and waxy endosperms. Seiken Ziho. 1957;8:11–19. [Google Scholar]
- Parker ML. The relationship between A-type and B-type starch granules in the developing endosperm of wheat. J Cereal Sci. 1985;3:271–278. [Google Scholar]
- Preiss J. Biosynthesis of starch and its regulation. In: Preiss J, editor. The Biochemistry of Plants, Vol 14. San Diego: Academic Press; 1988. pp. 181–254. [Google Scholar]
- Preiss J (1991) Biology and molecular biology of starch synthesis and its regulation. In BJ Miflin, ed, Oxford Surveys of Plant Molecular and Cell Biology, Vol 7. Oxford University Press, New York, pp 59–114
- Sakri FAK, Shannon JC. Movement of 14C-labeled sugars into kernels of wheat (Triticum aestivum L.) Plant Physiol. 1975;55:881–889. doi: 10.1104/pp.55.5.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandstedt RM. Photomicrographic studies of wheat starch. I. Development of the starch granules. Cereal Chem. 1946;23:337–359. [Google Scholar]
- Sano Y. Differential regulation of waxy gene expression in rice endosperm. Theor Appl Genet. 1984;68:467–473. doi: 10.1007/BF00254822. [DOI] [PubMed] [Google Scholar]
- Sano Y. Gene regulation at the waxy locus in rice. Gamma Field Symposia. 1985;24:63–79. [Google Scholar]
- Sato K. Starch granules in tissues of rice plants and their changes in relation to plant growth. JAQR. 1984;18:79–86. [Google Scholar]
- Shannon JC, Garwood DL (1984) Genetics and physiology of starch development. In RL Whistler, JN Bemiller, EF Paschall, eds, Starch: Chemistry and Technology, Ed 2. Academic Press, New York, pp 26–86
- Shure M, Wessler S, Federoff N. Molecular identification and isolation of the waxy locus in maize. Cell. 1983;35:225–233. doi: 10.1016/0092-8674(83)90225-8. [DOI] [PubMed] [Google Scholar]
- Singletary GW, Banisadr R, Keeling PL. Influence of gene dosage on carbohydrate synthesis and enzymatic activities in endosperm of starch-deficient mutants of maize. Plant Physiol. 1997;113:293–304. doi: 10.1104/pp.113.1.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AM. Evidence that the “waxy” protein of pea (Pisum sativum L.) is not the major starch-granule-bound starch synthase. Planta. 1990;182:599–604. doi: 10.1007/BF02341037. [DOI] [PubMed] [Google Scholar]
- Smith AM, Denyer K, Martin C. The synthesis of the starch granule. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:67–87. doi: 10.1146/annurev.arplant.48.1.67. [DOI] [PubMed] [Google Scholar]
- Smith AM, Martin C (1993) Starch biosynthesis and the potential for its manipulation. In D Grierson, ed, Plant Biotechnology, Vol 3. Blackie Academic & Professional, Glasgow, pp 1–54
- Takaoka M, Watanabe S, Sassa H, Yamamori M, Nakamura T, Sasakuma T, Hirano H. ) J Agric Food Chem. 1997;45:2929–2934. [Google Scholar]
- Tomlinson K, Craig J, Smith AM. Major differences in isoform composition of starch synthase between leaves and embryos of pea (Pisum sativum L.) Planta. 1998;204:86–92. [Google Scholar]
- Tomlinson KL, Lloyd JR, Smith AM. Importance of isoforms of starch-branching enzyme in determining the structure of starch in pea leaves. Plant J. 1997;11:31–43. [Google Scholar]
- van der Leij FR, Visser RGF, Ponstein AS, Jacobsen E, Feenstra WJ. Sequence of the structural gene for granule-bound starch synthase of potato (Solanum tuberosum L.) and evidence for a single point deletion in the amf allele. Mol Gen Genet. 1991;228:240–248. doi: 10.1007/BF00282472. [DOI] [PubMed] [Google Scholar]
- Vos-Scheperkeuter GH, de Bore W, Visser RGF, Feenstra WJ, Witholt B. Identification of granule-bound starch synthase in potato tubers. Plant Physiol. 1986;82:411–416. doi: 10.1104/pp.82.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whistler RL, Daniel JR (1984) Molecular structure of starch. In RL Whistler, JN Bemiller, EF Paschall, eds, Starch: Chemistry and Technology, Ed 2. Academic Press, New York, pp 153–182
- Yamamori M, Endo TR. Variation of starch granule proteins and chromosome mapping of their coding genes in common wheat. Theor Appl Genet. 1996;93:275–281. doi: 10.1007/BF00225757. [DOI] [PubMed] [Google Scholar]
- Yamamori M, Nakamura T, Kuroda A. Variations in the content of starch-granule bound protein among several Japanese cultivars of common wheat (Triticum aestivum L.) Euphytica. 1992;64:215–219. [Google Scholar]