Abstract
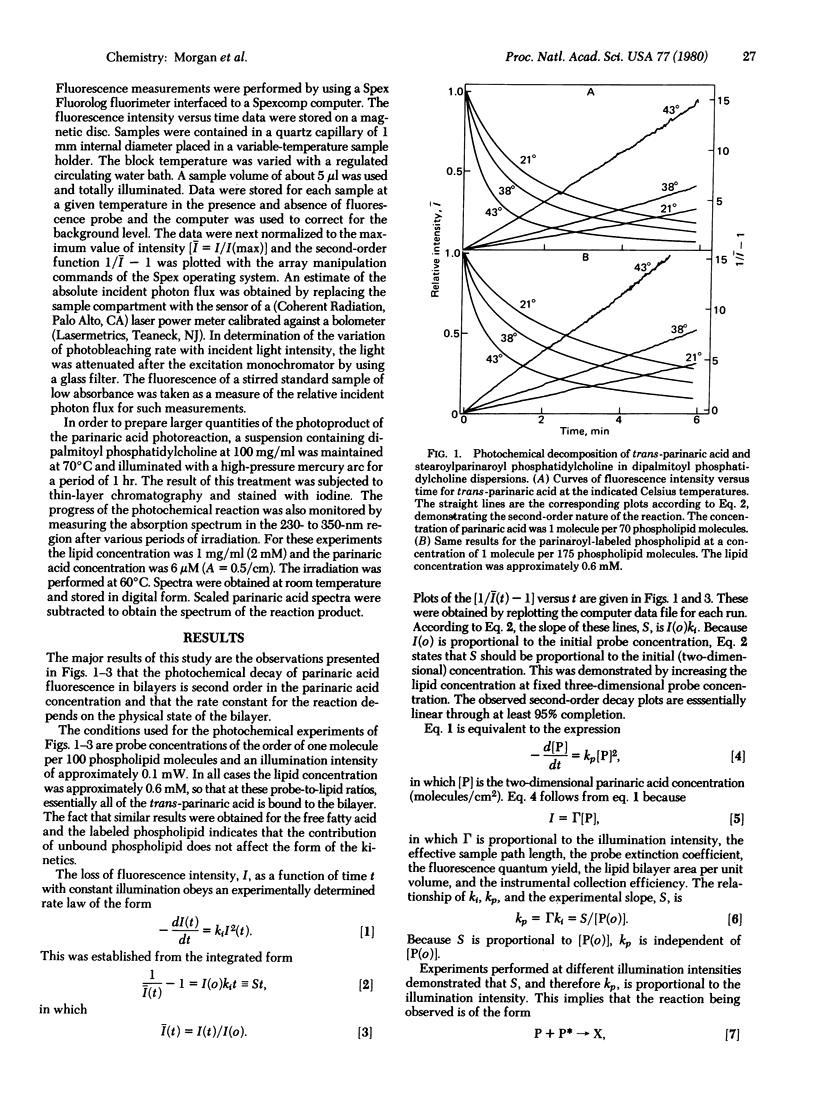
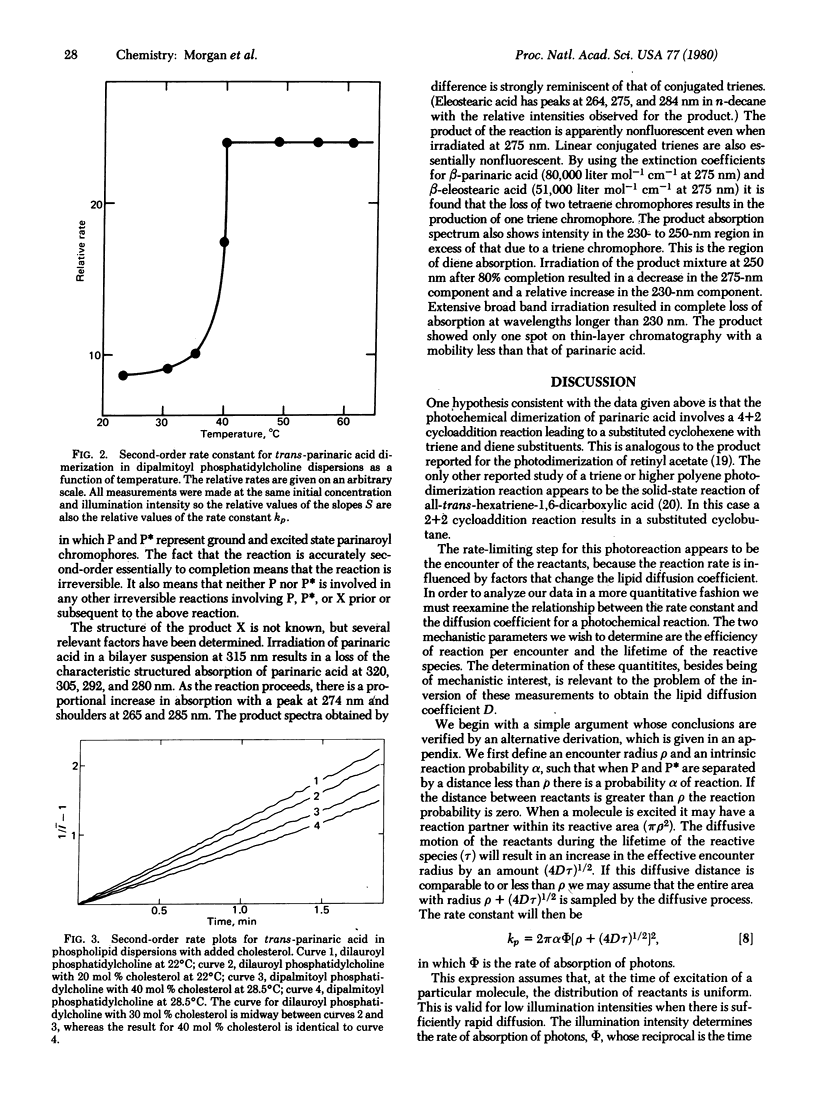
Parinaric acid (9,11,13,15-octadecatetraenoic acid), a conjugated tetraene fatty acid, undergoes a second-order photochemical reaction in phospholipid bilayers. The reaction results in the loss of the characteristic absorption of this chromophore and the development of new absorption demonstrating the presence of a triene chromophore. The progress of this reaction is easily monitored by measurement of the decrease in the fluorescence intensity from a uniformly illuminated sample. The reaction rate measured in this way is sensitive to the thermal phase transition of the bilayer and to the presence of cholesterol. The relationship of the second-order rate constant to the lipid diffusion coefficient is discussed. This relationship differs from that previously used for the analysis of similar photochemical processes.
Keywords: membranes, photochemistry, diffusion
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berde C. B., Hudson B. S., Simoni R. D., Sklar L. A. Human serum albumin. Spectroscopic studies of binding and proximity relationships for fatty acids and bilirubin. J Biol Chem. 1979 Jan 25;254(2):391–400. [PubMed] [Google Scholar]
- Galla H. J., Sackmann E. Lateral diffusion in the hydrophobic region of membranes: use of pyrene excimers as optical probes. Biochim Biophys Acta. 1974 Feb 26;339(1):103–115. doi: 10.1016/0005-2736(74)90336-8. [DOI] [PubMed] [Google Scholar]
- Gupta C. M., Radhakrishnan R., Khorana H. G. Glycerophospholipid synthesis: improved general method and new analogs containing photoactivable groups. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4315–4319. doi: 10.1073/pnas.74.10.4315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbell W. L., McConnell H. M. Molecular motion in spin-labeled phospholipids and membranes. J Am Chem Soc. 1971 Jan 27;93(2):314–326. doi: 10.1021/ja00731a005. [DOI] [PubMed] [Google Scholar]
- Kuo A. L., Wade C. G. Lipid lateral diffusion by pulsed nuclear magnetic resonance. Biochemistry. 1979 May 29;18(11):2300–2308. doi: 10.1021/bi00578a026. [DOI] [PubMed] [Google Scholar]
- McGrath A. E., Morgan C. G., Radda G. K. Photobleaching. A novel fluorescence method for diffusion studies in lipid system. Biochim Biophys Acta. 1976 Mar 5;426(2):173–185. doi: 10.1016/0005-2736(76)90330-8. [DOI] [PubMed] [Google Scholar]
- Mousseron-Canet M., Lerner D., Mani J. C. Structure d'un photodimère de la vitamin A. Bull Soc Chim Fr. 1968 Nov;11:4639–4645. [PubMed] [Google Scholar]
- Rubenstein J. L., Smith B. A., McConnell H. M. Lateral diffusion in binary mixtures of cholesterol and phosphatidylcholines. Proc Natl Acad Sci U S A. 1979 Jan;76(1):15–18. doi: 10.1073/pnas.76.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheats J. R., McConnell H. M. A photochemical technique for measuring lateral diffusion of spin-labeled phospholipids in membranes. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4661–4663. doi: 10.1073/pnas.75.10.4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklar L. A., Hudson B. S. Conjugated polyene fatty acids as fluorescent membrane probes: model system studies. J Supramol Struct. 1976;4(4):449–465. doi: 10.1002/jss.400040404. [DOI] [PubMed] [Google Scholar]
- Sklar L. A., Hudson B. S., Petersen M., Diamond J. Conjugated polyene fatty acids on fluorescent probes: spectroscopic characterization. Biochemistry. 1977 Mar 8;16(5):813–819. doi: 10.1021/bi00624a001. [DOI] [PubMed] [Google Scholar]
- Sklar L. A., Hudson B. S., Simoni R. D. Conjugated polyene fatty acids as fluorescent probes: binding to bovine serum albumin. Biochemistry. 1977 Nov 15;16(23):5100–5108. doi: 10.1021/bi00642a024. [DOI] [PubMed] [Google Scholar]
- Sklar L. A., Hudson B. S., Simoni R. D. Conjugated polyene fatty acids as fluorescent probes: synthetic phospholipid membrane studies. Biochemistry. 1977 Mar 8;16(5):819–828. doi: 10.1021/bi00624a002. [DOI] [PubMed] [Google Scholar]
- Sklar L. A., Hudson B. S., Simoni R. D. Conjugated polyene fatty acids as membrane probes: preliminary characterization. Proc Natl Acad Sci U S A. 1975 May;72(5):1649–1653. doi: 10.1073/pnas.72.5.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklar L. A., Miljanich G. P., Dratz E. A. Phospholipid lateral phase separation and the partition of cis-parinaric acid and trans-parinaric acid among aqueous, solid lipid, and fluid lipid phases. Biochemistry. 1979 May 1;18(9):1707–1716. doi: 10.1021/bi00576a012. [DOI] [PubMed] [Google Scholar]
- Tecoma E. S., Sklar L. A., Simoni R. D., Hudson B. S. Conjugated polyene fatty acids as fluorescent probes: biosynthetic incorporation of parinaric acid by Escherichia coli and studies of phase transitions. Biochemistry. 1977 Mar 8;16(5):829–835. doi: 10.1021/bi00624a003. [DOI] [PubMed] [Google Scholar]
- Truscott T. G., Land E. J., Sykes A. The in vitro photochemistry of biological molecules. 3. Absorption spectra, lifetimes and rates of oxygen quenching of the triplet states of beta-carotene, retinal and related polyenes. Photochem Photobiol. 1973 Jan;17(1):43–51. doi: 10.1111/j.1751-1097.1973.tb06329.x. [DOI] [PubMed] [Google Scholar]
- Vanderkooi J. M., Callis J. B. Pyrene. A probe of lateral diffusion in the hydrophobic region of membranes. Biochemistry. 1974 Sep 10;13(19):4000–4006. doi: 10.1021/bi00716a028. [DOI] [PubMed] [Google Scholar]


