Abstract
Objective
To determine the role of hyperleptinemia on caveolin-1 expression and leptin signaling.
Method
Endothelial cells are critical to atherosclerosis development; therefore we investigated hyperleptinemia in cultured vascular endothelial cells. Dose-dependent effect of leptin on caveolin-1 expression was determined by Western blot analysis. Also, the consequence of increased caveolin-1 expression on leptin signaling was investigated by adenovirus mediated caveolin-1 overexpression. The effect of increased caveolin-1 expression on leptin-dependent activation of ERK1/2 and eNOS was determined by Western blot analysis.
Results
Leptin upregulates caveolin-1 protein expression in a dose dependent manner and increased caveolin-1 expression impairs leptin signaling.
Conclusions
Leptin increases caveolin-1 protein expression which impairs leptin signaling in vascular endothelial cells. Our study identifies an additional leptin mediated proatherogenic mechanism and a novel caveolin-1 dependent leptin feedback mechanism which may have implications for development of peripheral leptin resistance in the endothelium.
Keywords: Atherosclerosis, Obesity, leptin, caveolin-1, cell signaling
Introduction
The contribution of obesity to the development of atherosclerosis is partly explained by hyperleptinemia [1]. Leptin, an adipokine, is also independently associated with increased cardiovascular risk and its elevated expression in most cases of obesity indicates central leptin resistance [1, 2]. However, leptin signaling in peripheral organs is not completely investigated in hyperleptinemic conditions.
Caveolin-1 is a structural protein of caveolae, which plays an important regulatory role in cell signaling, development of atherosclerosis, and obesity [3–5]. Caveolin-1 also functions similar to the family of proteins called Suppressor Of Cytokine Signaling (SOCS) which are a part of the classical negative feedback circuit [6]. They are upregulated by cytokines and in turn they inhibit the cellular cytokine-induced signaling pathways.
Recent studies demonstrate that caveolin-1 expression is increased in human obesity [7]. However, neither the factors leading to increased caveolin-1 expression nor the consequence of increased caveolin-1 expression in human obesity are completely understood. To this end, we investigated the role of leptin in regulation of caveolin-1 expression and its implication on leptin signaling. Considering the importance of vascular endothelial cells in the initiation, development and progression of atherosclerosis we used cultured vascular endothelial cells for our studies.
Methods
In-vitro experiments were done using commercially available human vascular endothelial cells (human coronary artery endothelial cells, HCAEC, and human umbilical vein endothelial cells, HUVEC, Cambrex, Walkersville, MD). Cells were grown in endothelial growth media-2 (EGM2, Cambrex) supplemented with growth factors and 2 % fetal bovine serum. All experiments were performed at 3–5 passages with 70–80 % confluency after overnight incubation in serum and growth factor free media.
To determine the regulatory role of leptin on caveolin-1 protein expression, the cells were incubated with increasing concentration of leptin (0–150 ng/ml) for 24 hours before protein analysis. Buffer [15 mM HCl (500 μl), 7.5 mM NaOH (300 μl)] used to make leptin stock was used as a zero leptin vehicle control. Western blot analysis was done to quantify caveolin-1 protein in experimental cell lysates. The cells were lysed immediately after each experiment using lysis buffer containing 50 mM NaCl, 50 mM NaF, 50 mM Na4O7P2, 5 mM EDTA, 5 mM EGTA, 0.1 mM Na3VO4, 1 % Triton X-100, 10 mM HEPES, pH 7.4. Equal amounts of protein from each sample were loaded and transferred to PVDF membranes. The membranes were blocked with 5 % non fat milk and incubated with specific primary antibody against caveolin-1 (BD Transduction lab, San Diego, CA), and anti-GAPDH antibody (loading control; Abcam Inc, Cambridge, MA). Membranes were then incubated with HRP-conjugated secondary antibodies and developed with enhanced chemiluminescence (Amersham biosciences, U.K.). The optical density of the band was measured using Scion Image software (Scion Corp.). The protein expression levels were normalized to GAPDH and expressed as fold increases as compared to vehicle (zero leptin) control.
To determine the consequence of increased caveolin-1 expression on leptin signaling, a commercially available adenovirus encoding caveolin-1 (Ad-Cav-1) (Vector Biolabs) was used to overexpress caveolin-1. Null adenovirus (Ad-Null) was used as control for these experiments. HUVEC were infected with either Ad-Cav-1 or Ad-Null at an MOI of 30 for 12 hours, followed by 48 hours of growth prior to leptin treatment. Downstream leptin signaling was determined in these cells after 0–30 min of leptin (100 ng/ml) treatment by Western blot analysis as described above.
All experiments were performed at least three times. Data are presented as mean ± SEM. Pairwise analysis and statistical significance was determined using Wilcoxon rank sum test, with level of significance set at p < 0.05. All data were analyzed with JMP software version 7 (SAS Institute, Cary, NC).
Results
Leptin upregulates caveolin-1 protein expression
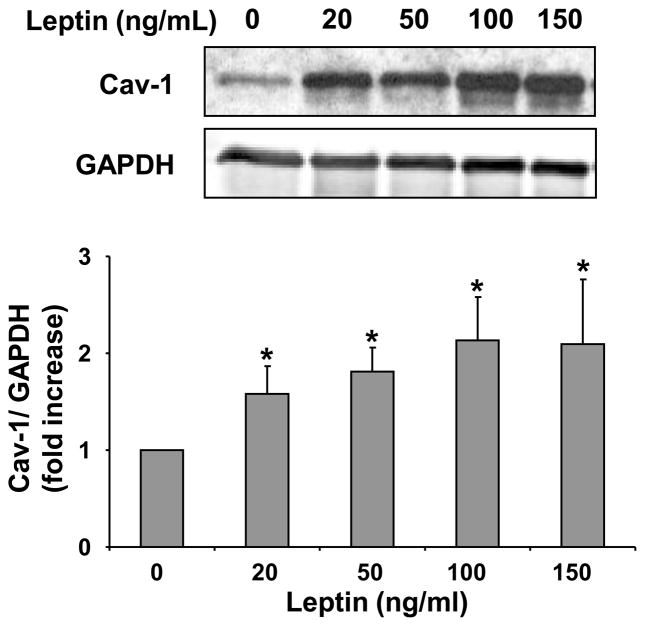
To investigate the role of leptin on caveolin-1 protein expression we conducted in-vitro experiments using HCAEC (figure 1). Leptin induced caveolin-1 protein expression in a dose dependent manner in HCAEC (p=0.004). The leptin-dependent increases in caveolin-1 expression were seen at all experimental concentrations of leptin (>20 ng/ml).
Figure 1.
Leptin upregulates caveolin-1 expression in HCAEC. Representative Western blot and densitometry graph from four independent experiments showing leptin concentration dependent increases in caveolin-1 expression. Data are presented as mean ± SEM. * denotes statistical significance as determined by Wilcoxon rank sum test compared to control (0 leptin) experiment.
Increased caveolin-1 protein expression impairs leptin signaling
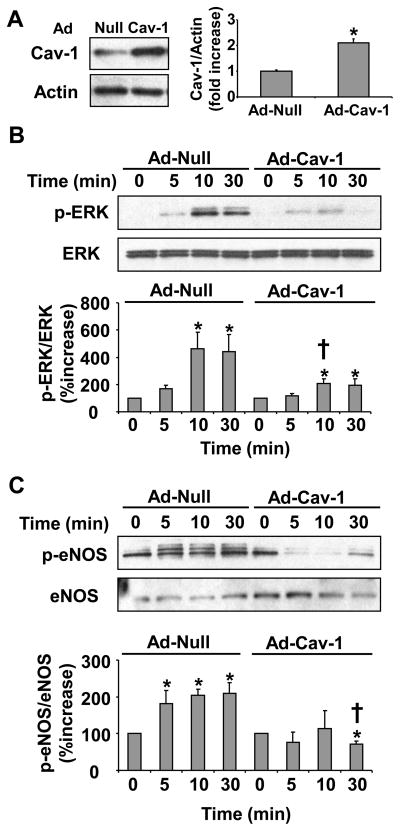
Caveolin-1 is known to act as a negative regulator of endocrine signaling [6]. We therefore sought to determine the effect of increased caveolin-1 expression on leptin signaling in vascular endothelial cells. Increased expression of caveolin-1 impaired downstream leptin signaling (figure 2). Infection of the cells with caveolin-1 encoding adenovirus (Ad-Cav-1) as compared to control virus (Ad-Null) showed an increase in caveolin-1 expression (p=0.03) (figure 2a). Following infection, the cells were treated with leptin (100 ng/ml) and downstream activation of signaling molecules was determined with increasing time (0–30 min) by Western blot analysis. Cells overexpressing caveolin-1 showed impaired leptin-dependent ERK phosphorylation (p=0.0001) (figure 2b) and leptin-dependent endothelial nitric oxide synthase (eNOS) phosphorylation (p=0.03) (figure 2c).
Figure 2.
Increased caveolin-1 expression impairs leptin signaling in HUVEC. Representative Western blot and densitometry graph from three independent experiments showing increased caveolin-1 expression in cells infected with Ad-Cav-1 virus as compared to cells infected with Ad-Null virus (A). Following adenovirus treatment the cells were treated with leptin (100ng/ml) and the downstream leptin-dependent cellular signaling was analyzed with time (0–30 min). Representative Western blot and densitometry graph from three experiments showing impaired leptin-dependent ERK (B) and eNOS (C) phosphorylation in cells with increased caveolin-1 expression. Data are presented as mean ± SEM. Statistical significance was determined by Wilcoxon rank sum test. * denotes p≤0.05 as compared to zero time point in each virus treated group and † denotes p≤0.05 as compared to same time point in the two groups.
Discussion
The important and novel findings reported in this study include first, that leptin induces caveolin-1 protein expression; and second, that increased caveolin-1 expression impairs leptin signaling. Our study thus provides an additional pathway through which leptin may contribute to the development of atherosclerosis. We also propose a novel caveolin-1-dependent leptin feedback mechanism which could have implications in the development of endothelial leptin resistance in obesity.
Caveolin-1 has important cellular functions which include potocytosis and regulation of cell signaling [4]. In vascular endothelial cells, cavelolin-1 promotes the transcytosis of LDL to the sub-endothelial space and inhibits eNOS activity, thereby regulating several processes that play an important role in the initiation and development of atheroma [3, 8]. However, the role of caveolin-1 in obesity is only beginning to be elucidated. In both animal models and humans, caveolin-1 deficiency is associated with a lean phenotype and increased expression with obesity [5, 7, 9, 10]. Nonetheless, the factors responsible for increased caveolin-1 expression in obesity remain largely unknown. To this end, we investigated the role of leptin in regulation of caveolin-1 expression. We report that hyperleptinemia upregulates caveolin-1 expression in HCAEC. Significantly increased caveolin-1 expression was seen at physiologically relevant (≥20 ng/ml) leptin concentrations. Considering the importance of caveolin-1 in the development of atherosclerosis, our study thus provides a novel pathway through which hyperleptinemia may directly cause pro-atherogenic effects on the vascular endothelium.
Caveolin-1 shares a functional similarity to the SOCS proteins which are a part of the classical negative feedback signaling mechanism [6]. Therefore, we sought to determine the consequence of increased caveolin-1 expression on leptin signaling. We show, for the first time, that increased caveolin-1 expression impairs leptin-dependent ERK and eNOS activation. The increases in caveolin-1 expression observed with adenovirus treatment were similar to those observed by leptin treatment, which speaks to the physiological relevance of our study. While we show that increased caveolin-1 expression impairs leptin dependent ERK and eNOS phosphorylation we have not investigated the effect of increased caveolin-1 expression on other leptin signaling pathways including JAK/STAT, and AMPK. Further investigations into the role of caveolin-1 in inhibiting other leptin signaling pathways are necessary to determine the development of partial or complete leptin resistance within vascular endothelial cells in hyperleptinemia.
Leptin is an important energy homeostasis regulator both at the level of the central nervous system and at the periphery [11]. Resistance to leptin signaling in hyperleptinemia at both these levels is partly responsible for metabolic imbalance in obesity. Elevated leptin levels are also linked to increased incidence of atherosclerosis [1]. However, it is not completely understood whether hyperleptinemia or impaired leptin signaling in the vasculature contributes to the development of atherosclerosis [2, 12]. Studies in animal models of obesity and atherosclerosis have shown both pro- and anti-atherogenic effects of leptin. While resistance to atherosclerosis in leptin deficient ob/ob mice demonstrates a proatherogenic role of leptin, the increased incidence of atherosclerosis in apoE−/− and LDLR−/− mice in leptin deficient ob/ob and db/db background indicates an anti-atherogenic role of leptin [1, 13]. Additionally, studies indicate that direct signaling from high levels of leptin in the vascular endothelium cause a proinflammatory, prothrombotic and anti-fibrinolytic state in the endothelium which potentiates the development of atheroma. On the other hand, leptin dependent eNOS activation and endothelial migration are both anti-atherogenic.[14, 15]. However, considering that leptin signaling may be at least partially impaired in vascular endothelium during hyperleptinemia, the molecular mechanism through which leptin contributes to the development of atheroma needs to be reconsidered.
Based on our findings, we hypothesize a potential mechanism which may be responsible for development of leptin resistance in vascular endothelial cells in obesity. However our study is limited by its in-vitro design and in an in-vivo environment where other factors may together contribute to development of atherosclerosis. Further in-vivo and in-vitro studies evaluating the role of vascular endothelial leptin signaling in obesity are required to address the role of leptin in development of atherosclerosis. These data would be pivotal in the development of therapeutics related to leptin resistance, obesity and atherosclerosis.
Conclusion
We show that hyperleptinemia increases caveolin-1 protein expression in the vascular endothelial cells, which may contribute to the development of atherosclerosis and may impair leptin signaling. We also propose a novel leptin feedback mechanism which may be involved in development of peripheral leptin resistance in the endothelium during obesity.
Acknowledgments
PS is supported by AHA grant 0725787Z; FHSK is supported by AHA grant 09-20069G; MDJ is supported by NIH grants DK45343, DK40484 and HL73211; VKS is supported by NIH Grants R01 HL73211 and R21 DK81014.
Footnotes
Conflict of interest: There is no conflict of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Beltowski J. Leptin and atherosclerosis. Atherosclerosis. 2006;189(1):47–60. doi: 10.1016/j.atherosclerosis.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 2.Martin SS, Qasim A, Reilly MP. Leptin resistance: A possible interface of inflammation and metabolism in obesity-related cardiovascular disease. J Am Coll Cardiol. 2008;52(15):1201–1210. doi: 10.1016/j.jacc.2008.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frank PG, Hassan GS, Rodriguez-Feo JA, et al. Caveolae and caveolin-1: Novel potential targets for the treatment of cardiovascular disease. Curr Pharm Des. 2007;13(17):1761–1769. doi: 10.2174/138161207780831202. [DOI] [PubMed] [Google Scholar]
- 4.Gratton JP, Bernatchez P, Sessa WC. Caveolae and caveolins in the cardiovascular system. Circ Res. 2004;94(11):1408–1417. doi: 10.1161/01.RES.0000129178.56294.17. [DOI] [PubMed] [Google Scholar]
- 5.Razani B, Combs TP, Wang XB, et al. Caveolin-1-deficient mice are lean, resistant to diet-induced obesity, and show hypertriglyceridemia with adipocyte abnormalities. J Biol Chem. 2002;277(10):8635–8647. doi: 10.1074/jbc.M110970200. [DOI] [PubMed] [Google Scholar]
- 6.Jasmin JF, Mercier I, Sotgia F, et al. Socs proteins and caveolin-1 as negative regulators of endocrine signaling. Trends Endocrinol Metab. 2006;17(4):150–158. doi: 10.1016/j.tem.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 7.Catalan V, Gomez-Ambrosi J, Rodriguez A, et al. Expression of caveolin-1 in human adipose tissue is upregulated in obesity and obesity-associated type 2 diabetes mellitus and related to inflammation. Clin Endocrinol (Oxf) 2008;68(2):213–219. doi: 10.1111/j.1365-2265.2007.03021.x. [DOI] [PubMed] [Google Scholar]
- 8.Fernandez-Hernando C, Yu J, Suarez Y, et al. Genetic evidence supporting a critical role of endothelial caveolin-1 during the progression of atherosclerosis. Cell Metab. 2009;10(1):48–54. doi: 10.1016/j.cmet.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gomez-Ruiz A, Milagro FI, Campion J, et al. Caveolin expression and activation in retroperitoneal and subcutaneous adipocytes. Influence of a high-fat diet. J Cell Physiol. doi: 10.1002/jcp.22241. [DOI] [PubMed] [Google Scholar]
- 10.Kim CA, Delepine M, Boutet E, et al. Association of a homozygous nonsense caveolin-1 mutation with berardinelli-seip congenital lipodystrophy. J Clin Endocrinol Metab. 2008;93(4):1129–1134. doi: 10.1210/jc.2007-1328. [DOI] [PubMed] [Google Scholar]
- 11.Unger RH. Longevity, lipotoxicity and leptin: The adipocyte defense against feasting and famine. Biochimie. 2005;87(1):57–64. doi: 10.1016/j.biochi.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 12.Mark AL, Correia ML, Rahmouni K, et al. Loss of leptin actions in obesity: Two concepts with cardiovascular implications. Clin Exp Hypertens. 2004;26(7–8):629–636. doi: 10.1081/ceh-200031948. [DOI] [PubMed] [Google Scholar]
- 13.Stein O, Stein Y. Resistance to obesity and resistance to atherosclerosis: Is there a metabolic link? Nutr Metab Cardiovasc Dis. 2007;17(7):554–559. doi: 10.1016/j.numecd.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 14.Goetze S, Bungenstock A, Czupalla C, et al. Leptin induces endothelial cell migration through akt, which is inhibited by ppargamma-ligands. Hypertension. 2002;40(5):748–754. doi: 10.1161/01.hyp.0000035522.63647.d3. [DOI] [PubMed] [Google Scholar]
- 15.Vecchione C, Maffei A, Colella S, et al. Leptin effect on endothelial nitric oxide is mediated through akt-endothelial nitric oxide synthase phosphorylation pathway. Diabetes. 2002;51(1):168–173. doi: 10.2337/diabetes.51.1.168. [DOI] [PubMed] [Google Scholar]