Abstract
Background
Screening of high-risk patients for hepatocellular carcinoma (HCC) may result in early diagnosis and improved outcomes. Our aim was to assess gastroenterologists’ knowledge of HCC management guidelines established by the American Association for the Study of Liver Diseases (AASLD) and usual clinical practice.
Methods
We surveyed gastroenterologists attending two gastroenterology board review courses regarding their knowledge of HCC screening guidelines and usual practice of screening for HCC. Practices were compared and adherence to the 2005 published HCC guidelines was assessed.
Results
The median age of gastroenterology attending physicians (n = 160) was 41 years, and 75% were men with a median of 11.5 years of practice. A total of 79% of respondents correctly identified the high-risk patients who qualify for HCC screening. Most gastroenterologists correctly identified the screening methods (88.5%) and screening interval (98%). Among those who knew guideline recommendations (i.e., correct identification and certainty of guideline recommendations), 100% reported that they followed the guideline recommendation in their own practices. Regarding the management of abnormal test, 31% of gastroenterologists did not identify that referral for liver transplantation is the recommended management strategy for small HCC in a Child B patient with cirrhosis. The number of years in clinical practice (p = 0.30) and involvement in a malpractice suit (p = 0.34) did not affect the practice patterns.
Conclusions
Most gastroenterologists correctly identified the common high-risk scenarios, methods, and interval of HCC screening as recommended by AASLD. Gastroenterologists who knew the HCC guidelines applied them in their own practice. However, approximately one-quarter do not know the appropriate management of a positive result, thereby likely hampering the overall effectiveness of screening.
Keywords: Hepatocellular carcinoma, Survey, Outcomes
Introduction
Hepatocellular carcinoma (HCC) was a relatively rare malignancy in the United States until the 1990s. In the past, HCC was typically diagnosed at an advanced stage in a symptomatic patient, and there were no known effective palliative or therapeutic options. Currently, HCC is the fifth most common cancer in the world [1], with an increasing incidence in both Europe and United States [2, 3].
Cirrhosis secondary to any etiology is the major risk factor for HCC, particularly hepatitis B and hepatitis C [4–6]. The outlook for HCC patients has improved with emerging evidence for efficacy of screening in high-risk patients, liver transplantation as a curative option in selected patients, the ability to make a definitive diagnosis using high-resolution imaging of the liver, less dependency on obtaining a tissue diagnosis, and proven efficacy of palliative therapy with loco-regional therapies and sorafenib as palliative therapy [2, 7–13]. As a result, major societies, including the American Association for Study of Liver Diseases (AASLD), recommend screening for HCC in high-risk patients [14].
In 2005, the AASLD issued evidence-based guidelines on the management of HCC, including screening in high-risk patients (screening strategies and screening interval) in addition to the management of early, intermediate, and late-stage HCC [14]. No prior studies have evaluated the knowledge of these guidelines and whether gastroenterologists agree with and follow these guidelines in their usual practice. We have previously shown that involvement in a malpractice suit results in aggressive surveillance for esophageal adenocarcinoma [15]. Another study evaluating the same gastroenterologists’ knowledge of colorectal screening guidelines concluded that despite the adequate knowledge of colon polyp surveillance guidelines, gastroenterologists are aggressive and perform surveillance colonoscopy sooner than recommended [16]. Based on the results of these studies, we hypothesized that the same gastroenterologists would be aggressive in performing HCC screening, irrespective of their knowledge of HCC guideline recommendations. Therefore, we aimed to assess the knowledge of HCC guidelines and identify the clinical practice for HCC screening and management among practicing gastroenterologists attending two gastroenterology board review courses. Furthermore, we evaluated the impact of prior experience, including the number of years in practice and involvement as a defendant in a malpractice suit, on practice patterns.
Methods
Survey Sample
Subjects were recruited from gastroenterologists attending either the William Steinberg Board Review in Gastroenterology, or the Mayo Clinic Gastroenterology and Hepatology Board Review in September 2006. The survey was administered to the attendees on the first day of the course and was collected at the end of the day. Attendees at these courses included practicing gastroenterologists preparing for mandated re-certification in gastroenterology, practicing gastroenterologists interested in receiving continued medical education credit, as well as gastroenterology fellows preparing for initial certification in gastroenterology. The analyses focused on attending gastroenterologists who had completed fellowship training. Fellows were excluded from analyses as their answers were likely to reflect their attending physicians practice patterns.
Questionnaire
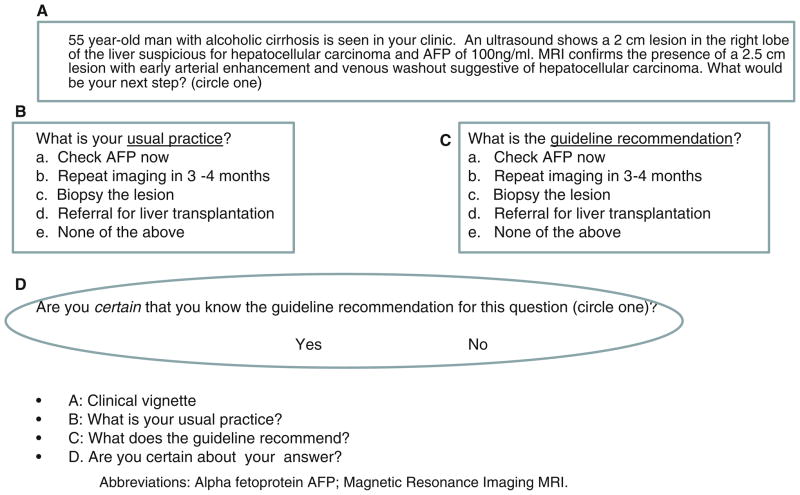
A 12-item, multiple-choice survey about HCC screening was developed based on AASLD practice guidelines [14]. The questionnaire underwent a thorough content validation through review by experts. The questionnaire included 12 brief clinical scenarios regarding the screening population, screening methods and interval, and the management strategy for a positive screening test (Table 1). For each scenario, the respondents were asked to choose among multiple choices regarding their usual practice in each scenario. Respondents were also tested on their knowledge of a published recommendation regarding each scenario, as shown in Fig. 1. After the clinical scenarios, respondents were then requested to estimate “What proportion of deaths from HCC do you believe is currently preventable by using appropriate screening?” by placing an ‘X’ on a visual analog scale ranging from 0 to 100%. This was compared to perceived preventable deaths from colorectal cancer among practicing gastroenterologists.
Table 1.
The 12-item, multiple-choice questionnaire used in the study
| 1) For each scenario below, please indicate (circle) whether or not you perform initial screening for hepatocellular carcinoma (HCC) in your usual practice, and whether or not the AASLD guidelines recommend initial screening for HCC. | ||
| a) 42 year-old Asian man who is a carrier for hepatitis B. | ||
| What is the guideline recommendation? | What is your usual practice? | |
| Screen | Screen | |
| Don’t screen | Don’t Screen | |
| Are you certain that you know the guideline recommendation for this scenario (circle one)? | ||
| Yes | No | |
| b) 45 year-old Asian woman who is a carrier for hepatitis B. | ||
| What is the guideline recommendation? | What is your usual practice? | |
| Screen | Screen | |
| Don’t screen | Don’t Screen | |
| Are you certain that you know the guideline recommendation for this scenario (circle one)? | ||
| Yes | No | |
| c) 51 year-old man with hemochromatosis who has cirrhosis. | ||
| What is the guideline recommendation? | What is your usual practice? | |
| Screen | Screen | |
| Don’t screen | Don’t Screen | |
| Are you certain that you know the guideline recommendation for this scenario (circle one)? | ||
| Yes | No | |
| d) 44 year-old woman with alcoholic cirrhosis. | ||
| What is the guideline recommendation? | What is your usual practice? | |
| Screen | Screen | |
| Don’t screen | Don’t Screen | |
| Are you certain that you know the guideline recommendation for this scenario (circle one)? | ||
| Yes | No | |
| e) A 45 year old man with a history of heavy alcohol use who has been sober for 5 years. He has normal platelets and prothrombin time, and ultrasound shows a normal liver. | ||
| What is the guideline recommendation? | What is your usual practice? | |
| Screen | Screen | |
| Don’t screen | Don’t Screen | |
| Are you certain that you know the guideline recommendation for this scenario (circle one)? | ||
| Yes | No | |
| f) A 50 year-old woman with hepatitis C cirrhosis who has responded to antiviral therapy. | ||
| What is the guideline recommendation? | What is your usual practice? | |
| Screen | Screen | |
| Don’t screen | Don’t Screen | |
| Are you certain that you know the guideline recommendation for this scenario (circle one)? | ||
| Yes | No | |
| 2) Is AFP recommended for screening/surveillance of hepatocellular carcinoma? (circle one) | ||
| What is the guideline recommendation? | What is your usual practice? | |
| a. Use AFP alone | a. Use AFP alone | |
| b. Use AFP and imaging together | b. Use AFP and imaging together | |
| c. Use AFP alternating with imaging | c. Use AFP alternating with imaging | |
| d. Do not use AFP at all | d. Do not use AFP at all | |
| Are you certain that you know the guideline recommendation for this question (circle one)? | ||
| Yes | No | |
| 3) Is imaging recommended for screening for hepatocellular carcinoma? | ||
| What is the guideline recommendation ? | What is your usual practice? | |
| a. Do not use imaging to screen | a. Do not use imaging to screen | |
| b. Screen with CT Scan | b. Screen with CT scan | |
| c. Screen with MRI | c. Screen with MRI | |
| d. Screen with ultrasonography | d. Screen with ultrasonography | |
| Are you certain that you know the guideline recommendation for this question (circle one)? | ||
| Yes | No | |
| 4) How often do you perform some type of screening test for hepatocellular carcinoma in a patient in whom screening is indicated? (circle one) | ||
| What is the guideline recommendation ? | What is your usual practice? | |
| a. Every 3 months | a. Every 3 months | |
| b. Every 6 months | b. Every 6 months | |
| c. Every 1 year | c. Every 1 year | |
| d. Every 2 years | d. Every 2 years | |
| e. Every 3 years | e. Every 3 years | |
| Are you certain that you know the guideline recommendation for this question (circle one)? | ||
| Yes | No | |
| 5) A 55 year-old man with Child B alcoholic cirrhosis is seen in your clinic. An ultrasound shows a 2.5 cm hyperechoic lesion in the right lobe of the liver. AFP is 100ng/ml (normal < 5). MRI confirms the presence of a 2.5 cm lesion with early arterial enhancement and venous washout suggestive of hepatocellular carcinoma. What would be your next step? (circle one) | ||
| What is the guideline recommendation ? | What is your usual practice? | |
| a. Repeat imaging in 3–4 months | a. Repeat imaging in 3–4 months | |
| b. Biopsy the lesion | b. Biopsy the lesion | |
| c. Refer for liver transplantation | c. Refer for liver transplantation | |
| d. None of the above | d. None of the above | |
| Are you certain that you know the guideline recommendation for this question (circle one)? | ||
| Yes | No | |
| 6) What proportion of deaths from hepatocellular carcinoma do you believe is currently preventable by using appropriate screening? (Please place an X anywhere on the line): | ||
|
| ||
Fig. 1.
For each question, respondents reviewed a clinical scenario (a), identified their usual practice (b), identified the appropriate intervention based upon the guideline recommendation (c), and stated if they were certain about the guideline recommendation for this question (d)
Respondents were then queried regarding demographic data and practice characteristics including practice setting, practice structure, compensation structure, number of years in practice, and whether they had ever been identified as a defendant in a malpractice suit. This study was granted a waiver of informed consent by the University of Michigan Institutional Review Board.
Statistical Analysis
For each clinical scenario, respondents’ usual practice was compared to the HCC guidelines established by AASLD to determine if the practice was more aggressive (i.e., shorter interval to repeat screening), more conservative (i.e., not performing initial screening), or identical to the society guidelines. The results are expressed as proportions. The continuous variables are expressed as median and range and the categorical variables are expressed as proportion. The group characteristics were compared using the Chi-square test and Fisher’s exact test.
Results
Demographics of the Respondents
The survey was completed by 160 respondents, out of 481 gastroenterologists at the two board review courses. The response rate was 33%. The median age of respondents was 41 years (inter-quartile 35–48 years), 75% were males with a median of 11.5 years of clinical practice (inter-quartile range 4–17 years). Most gastroenterologists were in single-specialty, private practices with a low volume of hepatology patients (Table 2). Gastroenterologists in private practice were more likely to receive compensation based on productivity without any salary than those in academic practice (34 vs. 0%; p <0.001). Those in academic practice were more likely to be salaried without productivity incentives than those in private practice (74 vs. 27%; p <0.001). Of the respondents, 21% had been identified as a defendant in a malpractice suit in the past.
Table 2.
Practice setting of gastroenterologists
| Practice setting | Percentage |
|---|---|
| Academic | 16% |
| Mixed | 8% |
| Private | 76% |
| Solo practice | 12% |
| Single-specialty | 63% |
| Multi-specialty | 23% |
| Unknown | 2% |
| Number of years in clinical practice | |
| 1st quartile (0–4 years) | 34% |
| 2nd quartile (5–12 years) | 23% |
| 3rd quartile (13–17 years) | 19% |
| 4th quartile (>17 years) | 24% |
| Hepatology practice | |
| 0–10% | 37% |
| 11–25% | 54% |
| >25% | 5% |
A total of 61% of responding gastroenterologists were taking the gastroenterology board for certification or re-certification. Eight percent of gastroenterologists reported that they had never heard of AASLD HCC practice guidelines. Ninety-seven percent of the gastroenterologists reported to ‘sometimes or always’ following these guidelines.
Knowledge of the AASLD HCC Guidelines
Gastroenterologists correctly responded to 79% of the clinical scenarios assessing HCC screening guidelines. Overall, there was no difference between their usual practice patterns and the knowledge of the HCC guidelines (Table 3). However, gastroenterologists’ knowledge of the guidelines was deficient in scenarios involving hepatitis B or treated hepatitis C cirrhosis (Table 3). Sixty-eight percent of respondents thought that the guidelines recommended HCC screening among Asian females with hepatitis B as early as age 45; the guidelines state to begin screening at 50 years of age. Sixteen percent of the respondent gastroenterologists did not think that the guidelines recommended the HCC screening for patients with hepatitis C cirrhosis successfully treated with antiviral therapy.
Table 3.
Comparison between the knowledge of HCC guidelines and usual clinical practice among gastroenterologists
| Questions | Clinical practice c/w guidelines | Correctly identify guidelines (Knowledge) | p value |
|---|---|---|---|
| Identify the high-risk group for HCC screening (answers) | 0.97 | ||
| 42-year-old Asian man, HBV carrier (Screen) | 82% | 76% | |
| 45-year-old Asian female, HBV carrier (Do not screen) | 22% | 32% | |
| 51-year-old cirrhotic male due to hemochromatosis (Screen) | 100% | 98% | |
| 44-year-old female with alcoholic cirrhosis (Screen) | 90% | 86% | |
| 45-year-old ex-alcoholic with normal liver (Do not screen) | 94% | 96% | |
| 50-year-old female with HCV cirrhosis, successfully treated (Screen) | 87% | 84% | |
| Methods and duration of screening | 0.69 | ||
| Use of alpha-fetoprotein and imaging for screening | 94% | 94% | |
| Use of imaging alone for screening | 83% | 83% | |
| Interval of screening | 98% | 98% | |
| Identify recommended treatment strategy | |||
| Child B cirrhotic with a single 2.5-cm mass in the liver with MRI features diagnostic of HCC (liver transplant/resection/ablation) | 73% | 69% | 0.3 |
p values show the difference in the knowledge and usual clinical practice
Although 89% of the respondents correctly identified the recommended methods of surveillance, fewer knew that imaging without AFP is an acceptable screening strategy than those who identified imaging plus AFP as an acceptable strategy (83 vs. 94%, p = 0.09). Nearly all respondents (98%) correctly identified the recommended interval of screening (6–12 months) as their usual practice patterns. Despite generally good knowledge regarding the guidelines for screening for HCC, 31% of gastroenterologists did not know the recommended standard of care for a Child B cirrhotic with a single 2.5-cm liver mass with magnetic resonance imaging features diagnostic of HCC (Table 3).
Clinical Scenarios and Practice Patterns
Overall, there was no difference between gastroenterologists’ usual practice patterns and their knowledge of the HCC guidelines (Table 3). A substantial majority of gastroenterologists (79%) correctly identified the clinical scenarios, screening methods and interval for HCC screening as their usual practice pattern. However, 78% initiated the HCC screening among Asian females with hepatitis B earlier than 50 years of age. Moreover, 27% would not refer a Child B cirrhotic with a single 2.5-cm liver mass consistent with HCC for liver transplantation.
Impact of Experience and Involvement in a Malpractice Suit on Practice Patterns and Knowledge
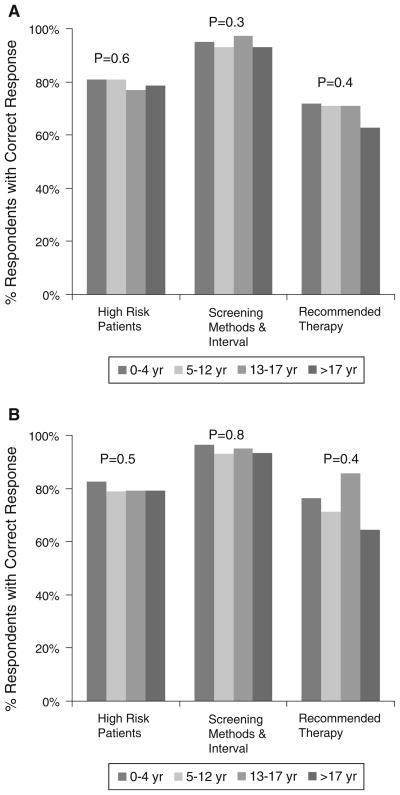
The number of years in practice did not affect practice patterns (Fig. 2a). Moreover, practice patterns were not associated with prior involvement in malpractice suit (p = 0.30). As expected, the number of years in clinical practice (Fig. 2b) as well as involvement in a malpractice suit (p = 0.34) did not affect the knowledge of the HCC guidelines among the gastroenterologists.
Fig. 2.
a Clinical scenarios and usual practice patterns: stratified by the number of years in gastroenterology practice. b Knowledge of AASLD HCC guidelines: stratified by number of years in gastroenterology practice
Belief in Efficacy of HCC Screening
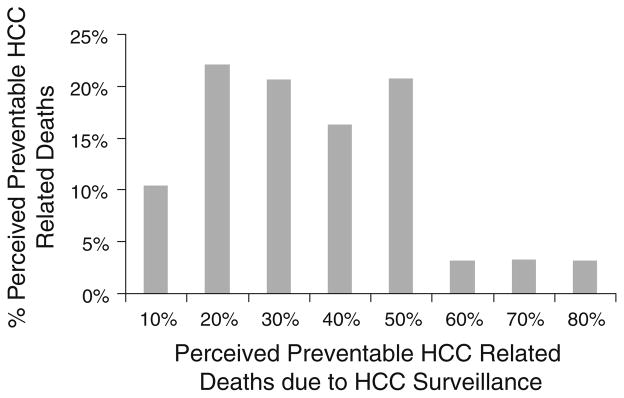
Most gastroenterologists believed that appropriate screening and surveillance would prevent only a minority of HCC deaths (median 30%, interquartile range 20–50%) (Fig. 3). In contrast, most believed that screening would prevent a majority of colorectal cancer deaths (median 75%, inter-quartile range 50–80%).
Fig. 3.
Belief in efficacy of HCC surveillance in preventing HCC-related deaths
Discussion
The AASLD practice guidelines on the management of HCC were published in 2005 in order to provide an evidence-based approach to screening, diagnosis, staging, and management of HCC [14]. This is the first study after the publication of these guidelines to assess the knowledge and belief in the efficacy of AASLD HCC practice guidelines and to evaluate the self-reported clinical practice patterns among practicing gastroenterologists. Our results indicated that the majority of gastroenterologists correctly identified the population at risk for HCC, the screening methods and interval, and followed these recommendations in their usual clinical practices. This is contrary to the screening and surveillance strategies for colorectal cancer and Barrett’s esophagus where gastroenterologists were more aggressive in their screening practices [15, 16].
Although the median perceived efficacy of HCC screening strategies in preventing HCC-related deaths was similar to the results from the largest randomized controlled trial of HCC screening [8], this was much lower than the median perceived efficacy of colorectal screening (75%) in preventing colorectal cancer related mortality. These observations suggest that gastroenterologists believe that screening for colorectal cancer is more efficacious than HCC screening in preventing cancer-related mortality and explain their aggressive screening practices than published guidelines for colorectal cancer surveillance [16].
Despite similar median perceived efficacy for surveillance for Barrett’s esophagus and HCC screening, gastroenterologists are more aggressive in performing surveillance for Barrett’s esophagus than for HCC relative to published guidelines [15]. The reason for these discrepant behaviors could be the different patient populations. Patients with HCC usually have underlying cirrhosis that increase their competing risk of death, so providers may not be overly enthusiastic about the prospects of HCC screening for saving lives. In contrast, patients at risk for esophageal adenocarcinoma may be very healthy with the exception of symptoms of gastroesophageal reflux, which may be easily controlled with medication. Another plausible reason could be the financial rewards from esophagoduodenoscopy, but we were not able to detect an association between compensation structure and esophageal adenocarcinoma screening aggressiveness [15]. This hypothesis needs to be explored in future studies.
The screening for any disease is a process that involves decision-making regarding whether and when to perform screening, which screening methods to use, the interval between screening, and management of abnormal results. Although our study showed that the majority of the respondents generally correctly identified the common high-risk scenarios, methods, and interval of HCC screening and this knowledge appeared to translate to their usual clinical practice, this may be an overestimate because HCC is frequently diagnosed late in its course due to the absence of symptoms and the liver’s large functional reserve. In reality, the screening rates may be lower in the actual practice. Moreover, respondents displayed deficient knowledge of the guidelines pertaining to patients with hepatitis B and treated hepatitis C patients with cirrhosis.
Screening will not improve patient outcomes if treatment options are not available for the disease. The management of HCC involves a multidisciplinary approach and is based upon the stage of the disease, underlying liver synthetic function, and the patient’s performance status. Liver transplantation is one of the curative options for small HCC meeting Milan criteria (one lesion 2–5 cm, up to three lesions all less than 3 cm without extrahepatic spread) with excellent post-transplant survival [7, 17]. Although our study showed that the majority of gastroenterologists knew and adhered to AASLD HCC practice guidelines in their usual practice in terms of whom, how, and when to perform screening, 27% did not know that the appropriate management of patient with a small HCC and 31% did not refer them for liver transplantation in their usual clinical practice. These figures may reflect the relative infrequency with which the gastroenterologists encounter such patients. However, screening without appropriate management of an abnormal result is not only a lost opportunity to save a life, but also wasteful of healthcare resources.
Serum alpha fetoprotein (AFP) and ultrasound of the liver are the most widely used tools for HCC screening [14, 18, 19]. The recommended screening interval is 6 months to 1 year based upon the estimated HCC doubling time. The sensitivity and specificity of ultrasound in detecting HCC is greater than 60 and 90%, respectively [20]. The serum AFP level of 20 ng/ml, commonly used as the upper limit of normal, has low sensitivity (25–65%) for detecting HCC and is therefore considered inadequate as the sole screening test [14, 21, 22]. Almost all of the gastroenterologists in the survey study acknowledged that the use of AFP alone is an inadequate screening tool and used both ultrasound and AFP or ultrasound alone as screening strategies in their usual practice.
Prior to the establishment of AASLD practice guidelines, HCC screening was performed by gastroenterologists in high-risk patients despite the lack of evidence for benefit [23, 24]. A previous survey of 483 AASLD members, predominantly hepatologists, showed that 84% routinely performed HCC screening [23]. Independent predictors of HCC screening in this study were (a) less than 10 years in practice, (b) seeing one new patient with cirrhosis per week, (c) an opinion that screening is cost-effective, prolongs survival and (d) failure to screen could result in a malpractice suit [23]. Another survey of gastroenterologists and other primary care physicians showed that the fear of malpractice and quality control concerns affects the physicians’ screening behavior [25]. In our study, neither the number of years in practice nor a history of involvement in a malpractice suit affected the practice of HCC screening.
One of the main limitations of our study is that our survey sample consists of gastroenterologists who were taking a board review course and may not be generalizable, as sampling of gastroenterologists attending a board review course may have biased the results by overestimating knowledge of the general population of gastroenterologists. While sampling/surveying the practice patterns of a more generalized segment of the gastroenterology community may have been beyond the scope of the study, it would have been a more valid assessment. Moreover, we did not measure actual practice, but relied on self-reported practice. However, we did not find any independent association between the number of years in practice and usual practice patterns. Despite these limitations, this is the first study to measure gastroenterologists’ knowledge of HCC guidelines in a broader way. A prior study surveyed AASLD members (the majority of whom were hepatologists) in 1999 regarding their practice, and not regarding their knowledge of any guidelines [23]. The current study surveyed gastroenterologists with a varying volume of hepatology practice.
In conclusion, although the majority of practicing gastroenterologists knew the AASLD practice guidelines for HCC and followed them in their usual clinical practices, the screening rates might be lower than reported. Knowledge regarding screening in the setting of treated hepatitis C cirrhosis or in hepatitis B was relatively deficient. Alarmingly, despite apparent high awareness of HCC screening guidelines among study participants, the identification of early stage HCC via screening did not correctly lead to transplant referral, potentially curative therapy for early stage HCC, in 31% of participants. This gap in the knowledge can be potentially corrected by effectively educating gastroenterologists to further improve their knowledge, adherence, and associated clinical outcomes.
Acknowledgments
This research was presented, in part, as a free communication at the American College of Gastroenterology, 2007, held in Philadelphia, PA. Pratima Sharma is supported by an American Society of Transplantation/Roche clinical science faculty development grant (2008–2010) and Michigan Institute for Clinical and Health Research NIH-CTSA, UL1RR024986, Collaborative Type 2 (bedside to community) grant (2009–2010).
Abbreviations
- HCC
Hepatocellular carcinoma
- AASLD
American Association for the Study of Liver Diseases
- AFP
Alpha fetoprotein
Contributor Information
Pratima Sharma, Email: pratimas@med.umich.edu, Division of Gastroenterology, Department of Internal Medicine, University of Michigan Health System, 3912, Taubman Center, SPC 5362, Ann Arbor, MI 48109, USA.
Sameer D. Saini, Division of Gastroenterology, Department of Internal Medicine, University of Michigan Health System, 3912, Taubman Center, SPC 5362, Ann Arbor, MI 48109, USA. Veterans Affairs Center of Excellence for Clinical Management Research, Ann Arbor, MI, USA
Latoya B. Kuhn, Division of Gastroenterology, Department of Internal Medicine, University of Michigan Health System, 3912, Taubman Center, SPC 5362, Ann Arbor, MI 48109, USA. Veterans Affairs Center of Excellence for Clinical Management Research, Ann Arbor, MI, USA
Joel H. Rubenstein, Division of Gastroenterology, Department of Internal Medicine, University of Michigan Health System, 3912, Taubman Center, SPC 5362, Ann Arbor, MI 48109, USA. Veterans Affairs Center of Excellence for Clinical Management Research, Ann Arbor, MI, USA
Darrell S. Pardi, Division of Gastroenterology and Hepatology, Mayo Clinic, Rochester, MN, USA
Jorge A. Marrero, Division of Gastroenterology, Department of Internal Medicine, University of Michigan Health System, 3912, Taubman Center, SPC 5362, Ann Arbor, MI 48109, USA
Philip S. Schoenfeld, Division of Gastroenterology, Department of Internal Medicine, University of Michigan Health System, 3912, Taubman Center, SPC 5362, Ann Arbor, MI 48109, USA. Veterans Affairs Center of Excellence for Clinical Management Research, Ann Arbor, MI, USA
References
- 1.Espey DK, Wu XC, Swan J, et al. Annual report to the nation on the status of cancer, 1975–2004, featuring cancer in American Indians and Alaska natives. Cancer. 2007;110:2119–2152. doi: 10.1002/cncr.23044. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB, Davila JA, Petersen NJ, McGlynn KA. The continuing increase in the incidence of hepatocellular carcinoma in the united states: an update. Ann Intern Med. 2003;139:817–823. doi: 10.7326/0003-4819-139-10-200311180-00009. [DOI] [PubMed] [Google Scholar]
- 3.El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745–750. doi: 10.1056/NEJM199903113401001. [DOI] [PubMed] [Google Scholar]
- 4.Velazquez RF, Rodriguez M, Navascues CA, et al. Prospective analysis of risk factors for hepatocellular carcinoma in patients with liver cirrhosis. Hepatology. 2003;37:520–527. doi: 10.1053/jhep.2003.50093. [DOI] [PubMed] [Google Scholar]
- 5.Tsukuma H, Hiyama T, Tanaka S, et al. Risk factors for hepatocellular carcinoma among patients with chronic liver disease. N Engl J Med. 1993;328:1797–1801. doi: 10.1056/NEJM199306243282501. [DOI] [PubMed] [Google Scholar]
- 6.Marrero JA, Fontana RJ, Su GL, Conjeevaram HS, Emick DM, Lok AS. Nafld may be a common underlying liver disease in patients with hepatocellular carcinoma in the United States. Hepatology. 2002;36:1349–1354. doi: 10.1053/jhep.2002.36939. [DOI] [PubMed] [Google Scholar]
- 7.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 8.Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004;130:417–422. doi: 10.1007/s00432-004-0552-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shiina S, Teratani T, Obi S, et al. A randomized controlled trial of radiofrequency ablation with ethanol injection for small hepatocellular carcinoma. Gastroenterology. 2005;129:122–130. doi: 10.1053/j.gastro.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 10.Lencioni RA, Allgaier HP, Cioni D, et al. Small hepatocellular carcinoma in cirrhosis: randomized comparison of radio-frequency thermal ablation versus percutaneous ethanol injection. Radiology. 2003;228:235–240. doi: 10.1148/radiol.2281020718. [DOI] [PubMed] [Google Scholar]
- 11.Bruix J, Llovet JM, Castells A, et al. Transarterial embolization versus symptomatic treatment in patients with advanced hepatocellular carcinoma: results of a randomized, controlled trial in a single institution. Hepatology. 1998;27:1578–1583. doi: 10.1002/hep.510270617. [DOI] [PubMed] [Google Scholar]
- 12.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 13.Lopez PM, Villanueva A, Llovet JM. Systematic review: evidence-based management of hepatocellular carcinoma–an updated analysis of randomized controlled trials. Aliment Pharmacol Ther. 2006;23:1535–1547. doi: 10.1111/j.1365-2036.2006.02932.x. [DOI] [PubMed] [Google Scholar]
- 14.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 15.Rubenstein JH, Saini SD, Kuhn L, et al. Influence of malpractice history on the practice of screening and surveillance for Barrett’s esophagus. Am J Gastroenterol. 2008;103:842–849. doi: 10.1111/j.1572-0241.2007.01689.x. [DOI] [PubMed] [Google Scholar]
- 16.Saini SD, Nayak RS, Kuhn L, Schoenfeld P. Why don’t gastroenterologists follow colon polyp surveillance guidelines? Results of a national survey. J Clin Gastroenterol. 2009;43:554–558. doi: 10.1097/MCG.0b013e31818242ad. [DOI] [PubMed] [Google Scholar]
- 17.Sharma P, Balan V, Hernandez JL, et al. Liver transplantation for hepatocellular carcinoma: the MELD impact. Liver Transpl. 2004;10:36–41. doi: 10.1002/lt.20012. [DOI] [PubMed] [Google Scholar]
- 18.Bolondi L, Sofia S, Siringo S, et al. Surveillance programme of cirrhotic patients for early diagnosis and treatment of hepatocellular carcinoma: a cost-effectiveness analysis. Gut. 2001;48:251–259. doi: 10.1136/gut.48.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang B, Yang B. Combined alpha fetoprotein testing and ultrasonography as a screening test for primary liver cancer. J Med Screen. 1999;6:108–110. doi: 10.1136/jms.6.2.108. [DOI] [PubMed] [Google Scholar]
- 20.Singal A, Volk ML, Waljee A, et al. Meta-analysis: surveillance with ultrasound for early stage hepatocellular carcinoma in patients with cirrhosis. Aliment Pharmacol Ther. 2009;30:37–47. doi: 10.1111/j.1365-2036.2009.04014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sherman M. Alphafetoprotein: an obituary. J Hepatol. 2001;34:603–605. doi: 10.1016/s0168-8278(01)00025-3. [DOI] [PubMed] [Google Scholar]
- 22.Marrero JA, Feng Z, Wang Y, et al. Alpha-fetoprotein, des-gamma carboxyprothrombin, and lectin-bound alpha-fetoprotein in early hepatocellular carcinoma. Gastroenterology. 2009;137:110–118. doi: 10.1053/j.gastro.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chalasani N, Said A, Ness R, Hoen H, Lumeng L. Screening for hepatocellular carcinoma in patients with cirrhosis in the United States: results of a national survey. Am J Gastroenterol. 1999;94:2224–2229. doi: 10.1111/j.1572-0241.1999.01297.x. [DOI] [PubMed] [Google Scholar]
- 24.Chen JG, Parkin DM, Chen QG, et al. Screening for liver cancer: results of a randomised controlled trial in Qidong, China. J Med Screen. 2003;10:204–209. doi: 10.1258/096914103771773320. [DOI] [PubMed] [Google Scholar]
- 25.Nguyen TT, Gildengorin G, Truong A, McPhee SJ. Factors influencing physicians’ screening behavior for liver cancer among high-risk patients. J Gen Intern Med. 2007;22:523–526. doi: 10.1007/s11606-007-0128-1. [DOI] [PMC free article] [PubMed] [Google Scholar]