Abstract
Introduction:
To facilitate translational work in medications development for smoking cessation, we have developed a human laboratory analogue of smoking lapse behavior. Our paradigm models 2 critical features of smoking lapse: the ability to resist the first cigarette and subsequent ad libitum smoking. In this paper we present the results of 2 studies designed to develop and validate the effect of nicotine deprivation on smoking lapse behavior.
Methods:
Study 1 (n = 30) was designed to develop the model parameters by examining varying levels of nicotine deprivation (1, 6, and 18 hr; within-subject) and identifying optimum levels of monetary reinforcement to provide while modeling the ability to resist smoking. Study 2 was designed to validate the model by screening smoking cessation medications with known clinical efficacy. Subjects (n = 62) were randomized to either varenicline 2 mg/day, bupropion 300 mg/day, or placebo, and we then modeled their ability to resist smoking and subsequent ad libitum smoking.
Results:
In Study 1, increasing levels of nicotine deprivation and decreasing levels of monetary reinforcement decreased the ability to resist smoking. In Study 2, the lapse model was found to be sensitive to medication effects among smokers who demonstrated a pattern of heavy, uninterrupted, and automated smoking (i.e., smoked within 5 min of waking). Ratings of craving, mood, withdrawal, and subjective cigarette effects are presented as secondary outcomes with results mirroring clinical findings.
Conclusions:
Our smoking lapse model demonstrates promise as a translational tool to screen novel smoking cessation medications. Next steps in this line of research will focus on evaluating predictive validity.
Introduction
The use of human laboratory analogues of smoking behavior can provide efficient, cost-effective, mechanistic evaluations of a medication signal on smoking behavior, with the result of facilitating translational work in medications development. A number of available human laboratory models have been utilized to investigate medication effects on various aspects of smoking behavior and nicotine dependence phenomena (see Lerman et al., 2007 for review), including nicotine discrimination (Perkins, Fonte, Sanders, White, & Wilson, 1999; Perkins, Saners, D’Amico, & Wilson, 1997), nicotine reinforcement and tolerance (Perkins, Broge, Gerlach, Cherry, & Wilson, 2002; Perkins, Fonte, Meeker, White, & Wilson, 2001), deprivation effects (Hatsukami, Hughes, Pickens, & Svikis, 1984), self-administration behavior (Hatsukami et al., 1998; Perkins et al., 1997), cue reactivity (Niaura, Abrams, Demuth, Pinto, & Monti, 1989), and reinstatement (Chornock, Stitzer, Gross, & Leischow, 1992; Juliano et al., 2006). However, medication effects demonstrated within these models often fail to mirror clinical findings highlighting the need to identify models which demonstrate medication effects on markers or predictors of clinical response.
We have been involved in a program of research developing a laboratory analogue of smoking lapse behavior. The first occurrence of smoking during a cessation attempt (i.e., lapse) is highly predictive of relapse (Brandon, Tiffany, Obremski, & Baker, 1990; Shiffman, Paty, Gnys, Kassel, & Hickcox, 1996) and represents a critical transition during a quit attempt and an important target for medication development. Our smoking lapse analogue models two critical features of lapse behavior: (a) the ability to resist the first cigarette and (b) subsequent smoking if a subject decides to “give in” and starts to smoke. Focusing on an abstinence outcome is critical for medication screening as Food and Drug Administration approval for cessation medications is contingent on demonstrating effects on smoking abstinence. The general procedure is that smokers are first exposed to known precipitants of smoking relapse behavior, and then their preferred brand of cigarettes is placed in front of them with a lighter and an ashtray. Smokers are then instructed that they have the option to initiate a tobacco self-administration session or to delay initiation for up to 50 min in exchange for monetary reinforcement. A fixed level of monetary reinforcement is provided for each 5-min increment that they can resist smoking during the 50-min delay period. This delay period models their ability to resist smoking. Once subjects “give in” and decide to smoke, they then participate in a 60-min tobacco self-administration session, during which they can choose to smoke their preferred brand of cigarettes or receive monetary reinforcement for cigarettes not smoked. To date, we have developed models examining stress and alcohol use as predictors of relapse, with results mirroring clinical findings (McKee, Krishnan-Sarin, Shi, Mase, & O’Malley, 2006; McKee, 2009, 2011).
For the current investigation, we describe two studies designed to develop and validate a smoking lapse model examining the effect of nicotine deprivation as a predictor for lapse behavior for the purpose of medication screening. Our aim in the first study is to investigate the effect of two periods of nicotine deprivation, 6-hr and 18-hr, on smoking lapse behavior compared with 1 hr of deprivation which corresponds to the typical inter-cigarette interval for a pack-a-day smoker. The 6-hr nicotine deprivation condition represented acute deprivation, targeting increases in tobacco craving (Drobes & Tiffany, 1997), whereas the 18-hr deprivation condition represented a period of more prolonged deprivation, designed to target increases in craving as well as additional tobacco withdrawal symptoms (Hatsukami et al., 1984). Our goal in the first study was to identify the level of monetary reinforcement needed for each level of nicotine deprivation so that smokers, on average, delayed smoking for approximately half of the delay window (i.e., ∼25 min of the 50 min window). In subsequent investigations, this “target model behavior” will limit potential floor or ceiling effects when examining whether a medication increases or decreases the ability to resist smoking.
The second study was designed to validate the smoking lapse model by examining medications with proven efficacy for counteracting effects of nicotine deprivation and increasing rates of smoking cessation. To this end, we examined whether varenicline and bupropion (Gonzales et al., 2006; Jorenby et al., 2006) increased the ability to resist smoking and reduced subsequent smoking. Importantly, we also examined the sensitivity of our model across factors which might impact on its ability to detect medication effects, including gender, income, motivation to quit, and nicotine dependence. Medication development for alcohol use often examines drinkers who are at the heavier end of the dependence spectrum (Litten et al., 2012). Using a similar approach, we examined a subset of our sample that reported smoking within 5 min of waking. Smoking within the first 5 min of waking is fairly prevalent in smokers (∼20%; Fagan et al., 2010; Fu et al., 2011; Luo et al., 2008), is found in higher rates in treatment seeking populations (Baker et al., 2007; Steinberg et al., 2011), and is associated with a pattern of heavy, uninterrupted, and automatic smoking (i.e., relatively insensitive to introceptive or exteroceptive cues) which is strongly predictive of poorer treatment outcomes, including shorter latency to lapse and relapse and faster progression from a lapse to relapse (Baker et al., 2007; Borland, Yong, O’Connor, Hyland, & Thompson, 2010; Fidler, Shahab, & West, 2011).
Study 1 Methods: Developing the Smoking Lapse Model
Subjects
Eligible subjects were 18–60 years of age, smoked 10–30 cigarettes/day for the past year, had positive urine cotinine levels (>150 ng/ml), were not using illicit drugs (cocaine, opiate, benzodiazepine, barbiturate, or amphetamine), were not currently seeking treatment for smoking, did not present with current Axis I disorders (except nicotine dependence), were not pregnant or nursing mothers, had no 30-day past use of psychoactive substances, nor had medical conditions that would contraindicate smoking. Thirty-five subjects were found to be eligible, and 30 subjects completed the study. Demographic and smoking variables are presented in Table 1.
Table 1.
Demographic and Smoking Variables for all Subjects for Study 1 (n = 30) and by Medication Group for Study 2 (n = 62)
| M (SD) or n (%) | Study 1 | Study 2 | ||
| All subjects (n = 30) | Placebo (n = 21) | Varenicline (n = 20) | Bupropion (n = 21) | |
| Age | 31.93 (10.29) | 36.47 (10.95) | 34.95 (11.36) | 34.95 (9.70) |
| Female | 15 (50) | 9 (42.9) | 9 (45) | 8 (38) |
| Race/ethnicitya | 16C, 11AA, 3H | 13C, 5AA, 0H | 10C, 6AA, 1H | 15C, 4AA, 1H |
| Education (% high school) | 16 (53) | 10 (47.6) | 12 (60) | 8 (38.1) |
| Yearly family income | $25,689 ($24,765) | $34,078 ($30,586) | $26,617 ($18,894) | $39,047 ($25,068) |
| Cigarettes per day | 16.67 (6.38) | 19.16 (7.61) | 16.62 (5.85) | 16.86 (6.06) |
| FTNDb | 5.13 (1.70) | 5.76 (2.70) | 5.05 (1.79) | 5.76 (2.10) |
| Smoke within 5 min of wakingc | 2 (6.7) | 10 (46.7) | 8 (40) | 9 (42.9) |
| Baseline breath CO (ppm) | 25.03 (13.32) | 25.76 (10.60) | 22.95 (13.24) | 21.38 (8.99) |
| Contemplation ladderd | 3.76 (2.50) | 5.00 (2.81) | 4.59 (2.62) | 5.45 (2.21) |
Note. aRace: (C) Caucasian, (AA) African American, (H) Hispanic.
FTND: Fagerström Test of Nicotine Dependence (range 1–10; Heatherton et al., 1991).
FTND-item 1.
Range 1–10.
Design
This study was a mixed design that examined varying levels of nicotine deprivation on smoking lapse behavior modeled in the laboratory. Nicotine deprivation (1, 6, or 18 hr) was a within-subject factor, and monetary reinforcement ($0.25, $0.50, $1.00) was a fully crossed between-subjects variable (n = 10 per cell). The study consisted of an intake session and three laboratory sessions (order of sessions was randomly determined). Subjects were paid $349 for completing the study. Payments were provided by check 2–3 weeks following study completion.
Procedures
Intake Sessions
The study was approved by the Yale University Human Investigation Committee, and written informed consent was obtained at the start of the intake session. The Structured Clinical Interview for DSM-IV (First, Spitzer, Gibbon, & Williams, 1997) was used to evaluate Axis I disorders. The Timeline Followback (Toll, Cooney, McKee, & O’Malley, 2005) was used to assess past 30-day smoking behavior. Expired breath carbon monoxide (CO) levels were assessed using a CO-meter (MCO2 Monitor, MicroDirect, Auburn, ME). The absence of recent cocaine, opiate, benzodiazepine, barbiturate, or amphetamine use was determined by urine drug test (JANT Pharmaceuticals, Encino, CA).
Nicotine Deprivation Conditions
For the 18-hr deprivation condition, subjects were instructed to have their last cigarette at 10 p.m., the prior evening. Compliance was biochemically confirmed initially with CO readings (less than 50% of their CO level at intake) and later with serum nicotine levels (all <4 ng/ml). Volunteers who failed to comply with instructions were rescheduled. For the 6-hr deprivation condition, subjects were able to smoke as they normally would before the laboratory session and had their last cigarette at 10 a.m. Subjects in the 1-hr deprivation condition were able to smoke whenever they wished until 3 p.m. when they smoked a final cigarette. Across the three laboratory sessions, there were no differences in baseline serum cotinine indicating similar recent nicotine exposure (overall mean = 233.16 ng/ml, SE = 18.58).
Laboratory Sessions
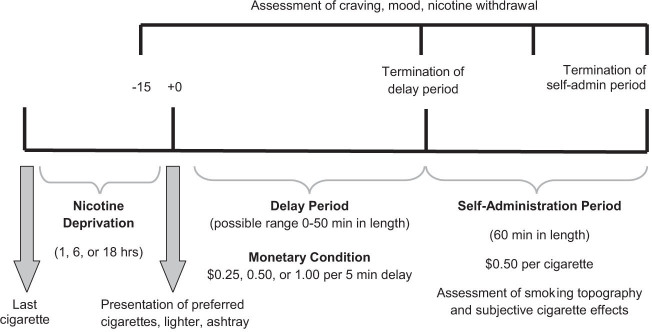
Each subject completed three 9-hr laboratory sessions which took place at the Yale Center for Clinical Investigation. Laboratory sessions were scheduled within 14 days of each other. Time between sessions was not a significant covariate of the primary outcomes. See Figure 1 for study design and timeline for laboratory sessions.
Figure 1.
Design and timeline of STUDY 1 procedures. Nicotine deprivation was a within-subject condition and monetary condition was a between-subject variable. The delay period commenced at 4 p.m. Study 2 was of identical design but subjects were randomized to placebo, bupropion, or varenicline; only completed one laboratory session following 18 hr of nicotine deprivation; and were reinforced $1.00 for every 5 min they could resist smoking.
Baseline assessment period
Laboratory sessions began at 9 a.m. and started with the collection of baseline assessments including breath CO; breath alcohol, levels; serum cotinine and nicotine levels; urine drug screens; urine pregnancy screen; and measures of positive and negative affect, tobacco craving, and nicotine withdrawal. Subjects were provided with a standardized lunch at 12 p.m. to control the time since last food consumption. Subjects completed cognitive testing from 3 p.m. to 3:45 p.m. (see Harrison, Coppola, & McKee, 2009 for details and results).
Delay period
At 4 p.m., subjects were presented with a tray containing cigarettes of their preferred brand, a lighter, and an ashtray. Subjects were instructed that they could commence smoking at any point over the next 50 min. However, for each 5-min block of time that they delayed or “resisted” smoking they would earn $0.25, $0.50, or $1.00 depending on their assigned monetary condition. We recorded the time (in minutes and seconds) when subjects announced that they wanted to smoke (range 0–50 min).
Smoking self-administration period
The ad-lib smoking session was 60 min in length and started once subjects decided to end the delay period (or delayed for the full 50 min). Subjects were provided with eight cigarettes of their preferred brand. Subjects were also provided with a $4 “smoking tab” and were instructed to “smoke as little or as much as you wish” using the smoking topography equipment. Subjects were further instructed that for each cigarette they lit, it would cost them $0.50 of their tab. Money earned for delaying smoking and any unused portion of the “smoking tab” was paid to the subjects at the end of each laboratory session. All subjects were discharged at 6:15 p.m.
Timing of assessments
Tobacco craving, emotion ratings, and nicotine withdrawal were assessed before the delay period, at the end of the delay period, and during the self-administration period following the first cigarette and at +30 min and +60 min. Smoking topography and subjective cigarette effects were assessed during the ad-lib period.
Measures
The primary measures were length of the delay period (i.e., latency to start smoking presented in minutes) and the number of cigarettes smoked during the ad-lib period.
Subjective Measures
Tobacco craving was assessed with the Tiffany Questionnaire of Smoking Urges-Brief (QSU-Brief; Cox, Tiffany, & Christen, 2001), a 10-item measure used to evaluate urges to smoke in response to positive (Factor 1) or negative (Factor 2) reinforcement [Visual Analogue Scale (VAS) scale, range 1–100]. The Differential Emotion Scale (DES), a 30-item self-report questionnaire, was used to assess current emotional state for positive (e.g., happy, joy) and negative (e.g., sadness, anger) emotion states (VAS scale, range 1–100; Izard, 1972). DSM-IV symptoms of nicotine withdrawal were assessed with the 8-item Minnesota Nicotine Withdrawal Scale (MNWS; range 0–32; Hughes & Hatsukami, 1986). Instructions were worded to assess current symptoms of withdrawal. The Cigarette Effects Scale is a 10-item self-report questionnaire which assesses “satisfaction,” “psychological reward,” “nausea/dizziness,” “craving relief,” and “enjoyment of airway sensations” associated with smoking (VAS scale, range 1–100; West, Levin, & Rose, 1992).
Smoking Topography
A hand-held Clinical Research Support System (CreSS from Plowshare Technologies) was used to assess smoking topography. Measures included puff frequency, puff volume, puff duration, inter-puff interval, depth of inhalation, and inter-cigarette interval.
Biochemical Measures
Serum nicotine and cotinine were collected at the start of the laboratory session to biochemically confirm current nicotine exposure. Cotinine and nicotine levels were measured by reversed-phase high-performance liquid chromatography with UV detection, modified from the literature (Hariharan, Van Noord, & Greden, 1988) to include a micro acid back extraction clean up step which allows for cleaner chromatograms. The lower limit of quantitation for nicotine was set to 4 ng/ml and cotinine was set to 25 ng/ml. Assays were conducted by Peter Jatlow, M.D., Laboratory Medicine, Yale-New Haven Hospital.
Statistical Analysis
Multivariate analyses of variance were used to examine the within-subject effect of nicotine deprivation condition (1, 6, 18 hr) by monetary condition ($0.25, $0.50, $1.00) on the primary outcomes of the length of the delay period and number of cigarettes smoked during the ad-lib period. We evaluated gender, nicotine dependence (Fagerström Test of Nicotine Dependence [FTND] scores; Heatherton et al., 1991), motivation to quit (Contemplation Ladder; Biener & Abrams, 1991), and income and other basic demographic variables as potential covariates for our primary outcomes. According to our predefined analysis plan, covariates were retained if they reduced residual variance. Multivariate analyses of variance were used to examine secondary outcomes of tobacco craving, emotion ratings, and nicotine withdrawal within nicotine deprivation condition, within time (predelay, postdelay), and by monetary reinforcement. These analyses were repeated for the self-administration period in subjects who smoked. To examine smoking topography measures for the first cigarette smoked during the 60-min ad-lib period, we conducted multivariate analyses of variance. Given a significant omnibus test (which controls for experiment-wise Type I error), contrasts were used to examine puff number, puff volume (ml), puff duration (s), inter-puff interval (s), and peak puff velocity (ml/s) within nicotine deprivation conditions. Similar to smoking topography, contrasts (within a multivariate analysis) were used to examine subjective cigarette effects following the first cigarette smoked. These analyses were confined to those who smoked during the self-administration period.
Study 1 Results
Smoking Lapse Behavior
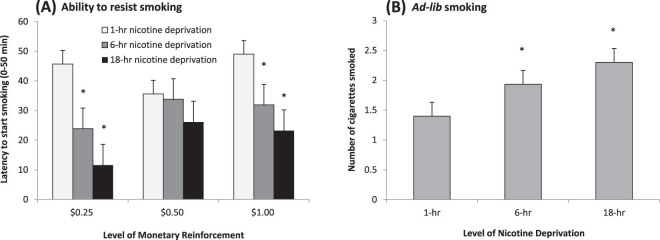
Subjects were less able to resist smoking and terminated their delay period sooner across increasing levels of nicotine deprivation (F(1, 27) = 38.24, p < .01; see Figure 2A). Additionally, monetary condition interacted with nicotine deprivation (F(2, 27) = 3.73, p < .04). Smaller reinforcement and longer periods of nicotine deprivation decreased the ability to resist smoking. Gender, FTND scores, Contemplation Ladder scores, and income were not significant covariates of latency to start smoking.
Figure 2.
(A) Mean latency to start smoking (+SE) across monetary reinforcement levels and levels of nicotine deprivation (interaction of monetary condition × nicotine deprivation, p < .04). (B) Mean number of cigarettes smoked (+SE) across levels of nicotine deprivation (main effect of nicotine deprivation, p < .01). *p < .05 for paired comparisons of 1-hr deprivation versus 6-hr or 18-hr (within monetary condition for A).
Ad-L ib Smoking, Subjective Cigarette Effects, and Smoking Topography
Subjects smoked more cigarettes following increasing periods of nicotine deprivation (F(1, 27) = 9.00, p < .01; see Figure 2B). Monetary condition did not influence the number of cigarettes smoked (p = .78). There were no effects of nicotine deprivation or monetary condition on measures of smoking topography. Nicotine deprivation, but not monetary condition, influenced reactivity to the first cigarette (see Figure 3). Significant effects of nicotine deprivation were demonstrated for “satisfaction” (p < .05), “reward” (p < .01), “aversion” (p < .0005), “relief of craving” (p < .01), but not “respiratory sensations.”
Figure 3.
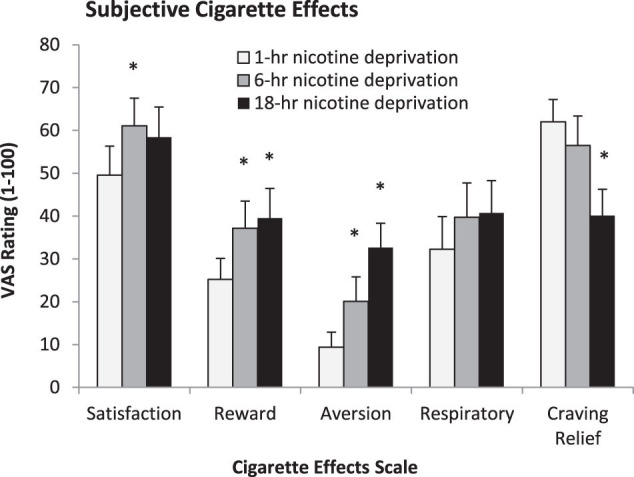
Mean cigarette effect scale scores (+SE) following the first ad libitum cigarette (n = 19). All scales but “respiratory sensations” demonstrated main effects of nicotine deprivation (all p < .05). *p < .05 for paired comparisons of 1-hr nicotine deprivation versus 6-hr or 18-hr.
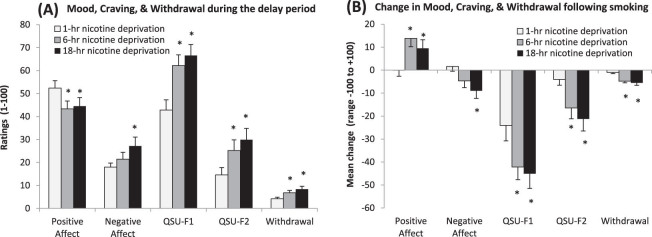
Mood, Craving, and Nicotine Withdrawal During the Delay Period
Positive affect (p < .01), negative affect (p < .05), craving for positive reinforcement (QSU-F1; p < .0005), craving for negative reinforcement (QSU-F2; p < .0005), and nicotine withdrawal (p < .0005) demonstrated significant main effects of nicotine deprivation but not monetary condition (see Figure 4A). Only craving for positive reinforcement (QSU-F1) demonstrated an effect of time and significantly increased from the start to the end of the delay period (p < .005), with the 1-hr deprivation condition demonstrating the largest increase (mean change = 17.12), compared with the 6-hr (mean change = 7.06) and the 18-hr (mean change = 0.17) deprivation conditions.
Figure 4.
(A) Mean mood, craving, and withdrawal scale scores (+SE) averaged from the start to the end of the delay period. All scales demonstrated significant main effects of nicotine deprivation (all p < .05). Ratings for mood and craving can range from 0 to 100, and withdrawal scores can range from 0 to 32. (B) Mean change in mood, craving, and withdrawal scale scores (+SE) from the start to the end of the self-administration period. All scales demonstrated significant nicotine deprivation × time interactions (all p < .05). Change scores for mood and craving can range from −100 to +100, and withdrawal scores can range from −32 to +32. *p < .05 for paired comparisons of 1-hr nicotine deprivation versus 6-hr or 18-hr.
Mood, Craving, and Nicotine Withdrawal During the Self-administration Period
Greater levels of nicotine deprivation resulted in greater increases in positive affect (p < .05) and decreases in negative affect (p < .05), craving for positive reinforcement (p < .001), craving for negative reinforcement (p < .002), and nicotine withdrawal (p < .002) following smoking (see Figure 4B). No effects of the delay period monetary condition were demonstrated.
Study 1 Discussion
Smoking lapse behavior varied as a function of nicotine deprivation and monetary condition. Increasing levels of nicotine deprivation and decreasing levels of monetary reinforcement reduced the ability to resist smoking. For the 6-hr deprivation window, designed to target increases in craving responses, the $0.25 condition (per 5-min delay) demonstrated target model behavior (i.e., delaying for approximately ∼25 min of the 50 min period). The 18-hr deprivation window, designed to target increases in other tobacco withdrawal symptoms, including craving, demonstrated target model behavior with a $1.00 level of reinforcement (per 5-min delay). Importantly, gender, motivation to quit (within our sample of nontreatment seekers), income, and level of nicotine dependence (within our restricted smoking range of 10–30 cigarettes/day) did not impact on the ability to resist smoking, suggesting that results are generalizable across these factors. As expected, nicotine deprivation increased craving, negative mood, and nicotine withdrawal and decreased positive mood during the delay period, and smoking alleviated these effects. Further, nicotine deprivation increased satisfaction, reward, aversion (i.e., dizziness), and decreased craving relief following smoking. For Study 2, we decided to use the 18-hr deprivation window to target increases in craving and other withdrawal symptoms. On the basis of Study 1 results, we paired the 18-hr deprivation window with the $1.00 reinforcement level.
Study 2 Methods: Validating the Smoking Lapse Model
All participant criteria, methods, and procedures for Study 2 were identical to Study 1, except where otherwise noted.
Subjects
Subjects were eligible to enroll in this study if they smoked at least 10 cigarettes/day for the past year and were excluded if they had medical conditions that would contraindicate the use of varenicline or bupropion. Seventy subjects were randomized to medication, and 62 subjects completed the study. Demographic and smoking variables did not differ by medication group (see Table 1).
Design
This study examined the effect of smoking cessation medications on smoking lapse behavior modeled in the laboratory. Subjects were randomized to receive either placebo, varenicline (Chantix®) 2 mg/day, or sustained-release bupropion (Zyban®) 300 mg/day for a 7-day period and then completed one laboratory session. Subjects were paid a total of $506 for completing the study.
Procedures
Medication Pretreatment Period
One week of pretreatment for both bupropion and varenicline is the typical pretreatment period used in smoking cessation trials (prior to the quit day), as steady-state levels are achieved within this timeframe (Jorenby et al., 1999, 2006). Varenicline was titrated to steady-state levels over 7 days (0.5 mg daily for Days 1–2, 0.5 mg twice daily for Days 3–5, and 1.0 mg twice daily on Days 6–7). Bupropion was titrated to steady-state levels over 7 days (150 mg daily for Days 1–3, 300 mg daily on Days 4–7). At each administration, subjects consumed two capsules (consisting of active and/or placebo capsules dependent on medication condition and titration schedule). Side effects were monitored throughout the study, and all medications were well tolerated. No subjects discontinued due to adverse side effects, and none of the side effects ratings exceeded a threshold of minimal or mild. Medication compliance (which was 100%) was monitored with pill counts and riboflavin markers (Del Boca, Kranzler, Brown, & Korner, 1996) with urine florescence evaluated on Days 1 and 4.
Laboratory Session
Sessions occurred on Day 8, with the final dose of the medication provided at 10 a.m. All sessions were completed following 18 hr of nicotine deprivation and subjects were reinforced $1.00 for every 5 min they could resist smoking, otherwise details are identical to Study 1 (see Figure 1). All subjects maintained overnight abstinence. All serum nicotine levels were <4 ng/ml, and serum cotinine levels did not differ across medication groups (overall mean = 190.44, SD = 118.66). Further, cigarettes per day during the titration week did not significantly differ across the medication groups. Subjects completed cognitive testing from 3 p.m. to 3:45 p.m. (see Ashare & McKee, 2012 for details and results).
Statistical Analysis
The analysis plan for Study 2 was similar in all respects to Study 1, except models were simplified to examine a single between-subjects factor of medication condition, with planned contrasts of varenicline or bupropion versus placebo. Additionally, all outcomes were evaluated for the subsample meeting “high nicotine dependence” criteria based on FTND-Item 1 (smoked within 5 min of waking).
Study 2 Results
Smoking Lapse Behavior
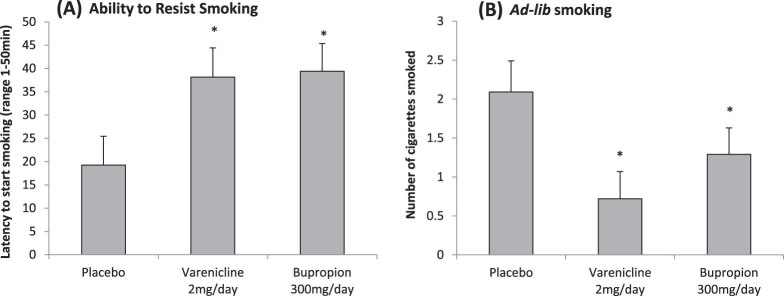
Overall, the effect of medication on time to resist smoking was not significant (F(2, 58) = 2.38, p = .10). However, FTND scores; but not gender, income, or motivation to quit; significantly reduced residual variance in latency to smoke. We then examined the subsample who smoked within 5 min of waking. Simple effects analysis demonstrated that varenicline [t(df = 16) = 2.30, p < .03; see Figure 5A] and bupropion [t(df = 17) = 2.53, p < .03] increased the ability to resist smoking relative to placebo.
Figure 5.
(A) Mean latency to start smoking (+SE) across medication (n = 27) in high nicotine dependent subjects (i.e., smoke within 5 min of waking; main effect of medication, p < .02). (B) Mean number of cigarettes smoked (+SE) across medication in high nicotine dependent subjects (main effect of medication, p < .03). *p < .05 for paired comparisons of varenicline or bupropion versus placebo.
Ad-Lib Smoking, Subjective Cigarette Effects, and Smoking Topography
Within the subsample who smoked within 5 min of waking, but not the entire sample, there was an effect of medication condition on the number of cigarettes smoked. Simple effects analysis demonstrated that varenicline [t(df = 16) = 3.00, p < .005; see Figure 5B] and bupropion-treated subjects [t(df = 17) = 1.81, p < .05] smoked less than placebo-treated subjects. There were no medication differences in smoking behavior among less dependent subjects. There were no effects of gender, motivation to quit, or income on amount smoked. There were no effects of medication on measures of smoking topography. In the entire sample, significant medication effects were demonstrated for “satisfaction” (p < .05) and “respiratory sensations” (p < .01). Varenicline (mean = 51.14, SE = 7.03) but not bupropion-treated subjects (mean = 67.36, SE = 7.03) had significantly lower satisfaction ratings following smoking than placebo-treated subjects (mean = 77.71, SE = 7.60). Varenicline (mean = 24.36, SE = 8.27) and bupropion-treated subjects (mean = 53.94, SE = 8.55) had lower pleasurable respiratory sensations following smoking, compared with placebo-treated subjects (mean = 61.55, SE = 9.29). There were no significant medication effects for ratings of “reward,” “aversion,” or “craving relief.” This pattern of results was similar for the high nicotine dependence group.
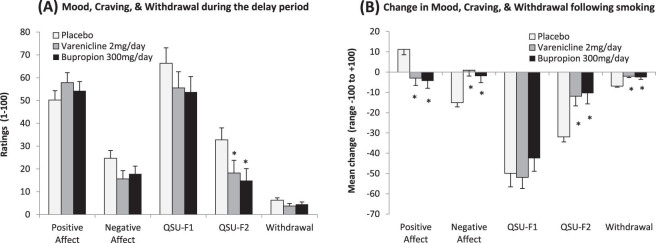
Mood, Craving, and Nicotine Withdrawal During the Delay Period
Craving ratings for negative reinforcement demonstrated a significant main effect of medication averaged from the start to the end of the delay period (F(1, 58) = 3.22, p < .05; see Figure 6A). Craving ratings for negative reinforcement at the end of the delay period were significantly associated with the ability to resist smoking (r = 0.61, p < .0005). There were no significant medication effects found for mood, craving for positive reinforcement, or withdrawal, nor were there any significant effects of time. This pattern of results was similar for the high nicotine dependence group.
Figure 6.
(A) Mean mood, craving, and withdrawal scale scores (+SE) averaged from the start to the end of the delay period. Only craving for negative reinforcement (Questionnaire of Smoking Urges-Brief [QSU-F2]) demonstrated an effect of medication (p < .05). Ratings for mood and craving can range from 0 to 100, and withdrawal scores can range from 0 to 32. (B) Mean change in mood, craving, and withdrawal scale scores (+SE) from the start to the end of the self-administration period. All scales but craving for positive reinforcement (QSU-F1) demonstrated significant medication × time interactions (all p < .05). Change scores for mood and craving can range from −100 to +100, and withdrawal scores can range from −32 to +32. *p < .05 for paired comparisons of placebo versus varenicline or bupropion.
Mood, Craving, and Nicotine Withdrawal During the Self-Administration Period
Positive mood (p < .05), negative mood (p < .05), craving for negative reinforcement (p < .005), and withdrawal (p < .01) demonstrated significant interactions of medication and time (see Figure 6B). Following smoking, the placebo group had the greatest increases in positive mood and decreases in negative mood, craving for negative reinforcement, and withdrawal. Craving for positive reinforcement only demonstrated a significant effect of time (p < .005). This pattern of results was similar for the high nicotine dependence group.
Study 2 Discussion
Both varenicline and bupropion increased the ability to resist smoking and decreased subsequent smoking behavior, but only among smokers displaying heavy and automatic smoking (i.e., smoking within the first 5 min of waking). This finding is consistent with clinical studies demonstrating stronger medication effects for varenicline or bupropion among smokers with greater indices of nicotine dependence (Dale et al., 2001), including time to the first cigarette (Nides et al., 2008). While focusing on a population of smokers likely to demonstrate the strongest medication signal (i.e., highly dependent smokers) makes sense from a screening perspective, ideally a screening tool would have sufficient sensitivity to detect effects in a general sample of smokers. We acknowledge that our model may be limited in this regard, although further testing is needed. Findings identify that our model’s sensitivity to medication effects is influenced by the degree of nicotine dependence, but not gender, income, or motivation to quit among smokers not currently seeking treatment for smoking. Other screening models have identified that intentions to quit in the near future modulated medication effects (Perkins et al., 2008, 2010), however, we have manipulated treatment seeking status in other investigations and have found no effects on our laboratory analogue of smoking lapse behavior (McKee, 2011).
Clinical efficacy studies for smoking cessation demonstrate that varenicline is more effective than bupropion (Jorenby et al., 2006). However, within our model the effects of varenicline and bupropion on the ability to resist smoking were similar, suggesting that our paradigm operates as a threshold model in that it identifies medications with clinical efficacy but is not sensitive to gradations of efficacy. This issue appears to be a factor in other screening models demonstrating clinical efficacy. Using a brief quit period, Perkins et al. (2008, 2010) demonstrate a similar magnitude of effects on number of days abstinent in separate studies examining nicotine replacement therapy and varenicline.
To our knowledge, this is the first laboratory study to examine both varenicline and bupropion on mood, craving, withdrawal, and smoking-related reinforcement. Overall, our laboratory results were highly consistent with clinical results demonstrating that both varenicline and bupropion reduced craving and negative affect but that varenicline was more effective than bupropion at attenuating smoking-related reward (West, Baker, Cappelleri, & Bushmakin, 2008). Also similar to the West et al. (2008) findings, neither drug was effective in reducing other nicotine withdrawal symptoms. We found that nicotine withdrawal scores for varenicline and bupropion-treated subjects after 18 hr of nicotine deprivation did not differ from placebo-treated subjects. Following smoking, we also found that varenicline and bupropion were equally effective at attenuating increases in positive affect and decreases in negative affect, craving, and withdrawal relative to placebo.
Our goal in developing the smoking lapse models is to facilitate translational work in medication development by providing a tool to cost-effectively evaluate whether promising Phase II candidates demonstrate a signal for smoking cessation. As detailed in McKee (2009), use of our smoking lapse models may also be extended to evaluate mechanisms underlying relapse (Ashare et al., 2011) as well as to provide a screening tool for nonpharmacological smoking cessation interventions. Our next step in this line of research will be to further validate our nicotine deprivation-smoking lapse model by evaluating whether the ability to resist smoking is predictive of actual quit behavior. The current findings demonstrate that our smoking lapse model is sensitive to the effect of medications with known clinical efficacy for smoking cessation, among smokers who display a pattern of smoking (i.e., smoke within the first 5 min of waking) which is highly predictive of poorer treatment outcome (Baker et al., 2007). However, it will be critical to demonstrate predictive validity. We have found that medication effects on stress-precipitated smoking lapse behavior were highly predictive of behavior during an actual quit attempt (McKee, 2011). Upon further validation, positive medication findings within our smoking lapse models could then be translated to Phase II or III clinical trial testing in more general groups of smokers.
Funding
The authors declare that this work was supported by the following National Institutes of Health grants: RL1DA024857, R21DA017234, P50AA015632, and UL1RR024139.
Declaration of Interests
SAM has investigator-initiated grants from Pfizer to investigate varenicline–alcohol interactions. The other authors have no interests to declare.
References
- Ashare RL. McKee SA. Effects of varenicline and bupropion on cognitive processes among nicotine-deprived smokers. Experimental and Clinical Psychopharmacology. 2012;20:63–70. doi: 10.1037/a0025594. doi:10.1037/ a0025594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashare RL, Sinha R, Lampert R, Weinberger AH, Anderson GM, Lavery ME, et al. Blunted vagal reactivity predicts stress-precipitated tobacco smoking. Psychopharmacology. 2011 doi: 10.1007/s00213-011-2473-3. Advance online publication. doi:10.1007/s00213-011-2473-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Bolt DM, Smith SS, Kim SY, et al. Time to first cigarette in the morning as an index of ability to quit smoking: Implications for nicotine dependence. Nicotine & Tobacco Research. 2007;9(Suppl. 4):S555–S570. doi: 10.1080/14622200701673480. doi:10.1080/14622200701673480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biener L, Abrams DB. The contemplation ladder: Validation of a measure of readiness to consider smoking cessation. Health Psychology. 1991;10:360–365. doi: 10.1037//0278-6133.10.5.360. [DOI] [PubMed] [Google Scholar]
- Borland R, Yong H.-H., O’Connor RJ, Hyland A, Thompson ME. The reliability and predictive validity of the Heaviness of Smoking Index and its two components: Findings from the International Tobacco Control Four Country study. Nicotine & Tobacco Research. 2010;12:S45–S50. doi: 10.1093/ntr/ntq038. doi:10.1093/ntr/ntq038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon TH, Tiffany ST, Obremski KM, Baker TB. Post cessation cigarette use: The process of relapse. Addictive Behaviors. 1990;15:105–114. doi: 10.1016/0306-4603(90)90013-n. [DOI] [PubMed] [Google Scholar]
- Chornock WM, Stitzer ML, Gross J, Leischow S. Experimental model of smoking re-exposure: Effects on relapse. Psychopharmacology. 1992;108:495–500. doi: 10.1007/BF02247427. doi:10.1007/BF02247427. [DOI] [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine & Tobacco Research. 2001;3:7–16. doi: 10.1080/14622200020032051. doi:10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- Dale LC, Glover ED, Sachs DP, Schroeder DR, Offord KP, Croghan IT, et al. Bupropion for smoking cessation: Predictors of successful outcome. Chest. 2001;119:1357–1364. doi: 10.1378/chest.119.5.1357. doi:10.1378/chest.119.5.1357. [DOI] [PubMed] [Google Scholar]
- Del Boca FK, Kranzler HR, Brown J, Korner PF. Assessment of medication compliance in alcoholics through UV light detection of a riboflavin tracer. Alcohol Clinical & Experimental Research. 1996;20:1412–1417. doi: 10.1111/j.1530-0277.1996.tb01142.x. doi:10.1111/j.1530-0277.1996.tb01142.x. [DOI] [PubMed] [Google Scholar]
- Drobes DJ, Tiffany ST. Induction of smoking urges through imaginal and in vivo procedures: Physiological and self-report manifestations. Journal of Abnormal Psychology. 1997;106:15–25. doi: 10.1037//0021-843x.106.1.15. [DOI] [PubMed] [Google Scholar]
- Fagan P, Moolchan ET, Hart A, Jr, Rose A, Lawrence D, Shavers VL, et al. Nicotine dependence and quitting behaviors among menthol and non-menthol smokers with similar consumptive patterns. Addiction. 2010;105:55–74. doi: 10.1111/j.1360-0443.2010.03190.x. doi:10.1111/j.1360-0443.2010.03190.x. [DOI] [PubMed] [Google Scholar]
- Fidler JA, Shahab L, West R. Strength of urges to smoke as a measure of severity of cigarette dependence: Comparison with the Fagerström Test for Nicotine Dependence and its components. Addiction. 2011;106:631–638. doi: 10.1111/j.1360-0443.2010.03226.x. doi:10.1111/j.1360-0443.2010.03226.x. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV, Patient Edition. Washington, DC: American Psychiatric Press; 1997. [Google Scholar]
- Fu M, Fernández E, Pascual JA, Martínez-Sánchez JM, Agudo A, Moncada A, et al. Stages of change, smoking characteristics, and cotinine concentrations in smokers: Setting priorities for smoking cessation. Preventive Medicine. 2011;52:139–145. doi: 10.1016/j.ypmed.2010.12.003. doi:10.1016/j.ypmed.2010.12.003. [DOI] [PubMed] [Google Scholar]
- Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, et al. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: A randomized controlled trial. Journal of the American Medical Association. 2006;296:47–55. doi: 10.1001/jama.296.1.47. doi:10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- Hariharan M, Van Noord T, Greden JF. A high performance liquid chromatographic method for routine simultaneous determination of nicotine and cotinine in plasma. Clinical Chemistry. 1988;34:724–729. [PubMed] [Google Scholar]
- Harrison EL, Coppola S, McKee SA. Nicotine deprivation and trait impulsivity affect smokers’ performance on cognitive tasks of inhibition and attention. Experimental and Clinical Psychopharmacology. 2009;17:91–98. doi: 10.1037/a0015657. doi:10.1037/a0015657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami D, Lexau G, Nelson D, Pentel PR, Sofuoglu M, Goldman A. Effects of cotinine on cigarette self-administration. Psychopharmacology. 1998;138:184–189. doi: 10.1007/s002130050661. [DOI] [PubMed] [Google Scholar]
- Hatsukami DK, Hughes JF, Pickens RW, Svikis D. Tobacco withdrawal symptoms: An experimental analysis. Psychopharmacology. 1984;84:231–236. doi: 10.1007/BF00427451. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LK, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hughes JF, Hatsukami D. Signs and symptoms of tobacco withdrawal. Archives of General Psychiatry. 1986;43:289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Izard CE. Patterns of emotions: A new analysis of anxiety and depression. London: Academic Press; 1972. [Google Scholar]
- Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, et al. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: A randomized controlled trial. Journal of the American Medical Association. 2006;396:56–63. doi: 10.1001/jama.296.1.56. doi:10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- Jorenby DE, Leischow SJ, Nides MA, Rennard SI, Johnston JA, Hughes AR, et al. A controlled trial of sustained-release bupropion, a nicotine patch, or both for smoking cessation. New England Journal of Medicine. 1999;340:685–691. doi: 10.1056/NEJM199903043400903. [DOI] [PubMed] [Google Scholar]
- Juliano LM, Donny EC, Houtsmuller EJ, Stitzer ML. Experimental evidence for a causal relationship between smoking lapse and relapse. Journal of Abnormal Psychology. 2006;115:166–173. doi: 10.1037/0021-843X.115.1.166. doi:10.1037/0021-843X.115.1.166. [DOI] [PubMed] [Google Scholar]
- Lerman C, LeSage MG, Perkins KA, O’Malley SS, Siegel SJ, Benowitz NL, et al. Translational research in medication development for nicotine dependence. Nature Reviews: Drug Discovery. 2007;6:746–762. doi: 10.1038/nrd2361. doi:10.1038/nrd2361. [DOI] [PubMed] [Google Scholar]
- Litten RZ, Fertig JB, Falk DE, Ryan ML, Mattson ME, Collins JF, et al. A double-blind, placebo-controlled trial to assess the efficacy of quetiapine fumarate XR in very heavy-drinking alcohol-dependent patients. Alcoholism: Clinical & Experimental Research. 2012;36:406–416. doi: 10.1111/j.1530-0277.2011.01649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Z, Alvarado GF, Hatsukami DK, Johnson EO, Bierut LJ, Breslau N. Race differences in nicotine dependence in the Collaborative Genetic study of Nicotine Dependence (COGEND) Nicotine & Tobacco Research. 2008;10:1223–1230. doi: 10.1080/14622200802163266. doi:10.1080/14622200802163266. [DOI] [PubMed] [Google Scholar]
- McKee SA. Developing human laboratory models of smoking lapse behavior for medication screening. Addiction Biology. 2009;14:99–107. doi: 10.1111/j.1369-1600.2008.00135.x. doi:10.1111/j.1369-1600.2008.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA. Guanfacine attenuates the effect of stress on smoking lapse behavior and improves smoking cessation outcomes. 2011, February. Annual Meeting for the Society for Research on Nicotine and Tobacco, Toronto, Canada. [Google Scholar]
- McKee SA, Krishnan-Sarin S, Shi J, Mase T, O’Malley SS. Modeling the effect of alcohol on smoking lapse behavior. Psychopharmacology. 2006;189:201–210. doi: 10.1007/s00213-006-0551-8. doi:10.1007/s00213-006-0551-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Sinha R, Weinberger AH, Sofuoglu M, Harrison ELR, Lavery M, et al. Stress decreases the ability to resist smoking and potentiates smoking reward. Journal of Psychopharmacology. 2011;25:490–502. doi: 10.1177/0269881110376694. doi:10.1177/0269881110376694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niaura R, Abrams D, Demuth B, Pinto R, Monti P. Responses to smoking-related stimuli and early relapse to smoking. Addictive Behaviors. 1989;14:419–428. doi: 10.1016/0306-4603(89)90029-4. doi:10.1016/0306-4603(89)90029-4. [DOI] [PubMed] [Google Scholar]
- Nides M, Glover ED, Reus VI, Christen AG, Make BJ, Billing CB, Jr, et al. Varenicline versus bupropion SR or placebo for smoking cessation: A pooled analysis. American Journal of Health Behavior. 2008;32:664–675. doi: 10.5555/ajhb.2008.32.6.664. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Broge M, Gerlach D, Cherry C, Wilson A. Acute nicotine reinforcement not chronic tolerance predicts withdrawal and relapse after quitting smoking. Health Psychology. 2002;21:332–339. doi: 10.1037//0278-6133.21.4.332. doi:10.1037/0278-6133.21.4.332. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Fonte C, Meeker J, White W, Wilson W. The discriminative stimulus and reinforcing effects of nicotine in humans following nicotine pretreatment. Behavioural Pharmacology. 2001;12:35–44. doi: 10.1097/00008877-200102000-00004. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Fonte C, Sanders M, White W, Wilson A. Effects of training dose and two- versus three-choice testing procedure on nicotine discrimination in humans. Psychopharmacology. 1999;145:418–425. doi: 10.1007/s002130051076. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Lerman C, Fonte CA, Mercincavage M, Stitzer ML, Chengappa KN, et al. Cross-validation of a new procedure for early screening of smoking cessation medications in humans. Clinical Pharmacology & Therapeutics. 2010;88:109–114. doi: 10.1038/clpt.2010.65. doi:10.1038/clpt.2010.65. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Lerman C, Stitzer M, Fonte CA, Briski JL, Scott JA, et al. Development of procedures for early screening of smoking cessation medications in humans. Clinical Pharmacology & Therapeutics. 2008;84:216–221. doi: 10.1038/clpt.2008.30. doi:10.1038/clpt.2008.30. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Saners M, D’Amico D, Wilson A. Nicotine discrimination and self-administration in humans as a function of smoking status. Psychopharmacology. 1997;131:361–370. doi: 10.1007/s002130050304. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paty JA, Gnys M, Kassel JA, Hickcox M. First lapses to smoking: Within-subjects analysis of real-time reports. Journal of Consulting & Clinical Psychology. 1996;64:366–379. doi: 10.1037//0022-006x.64.2.366. [DOI] [PubMed] [Google Scholar]
- Steinberg MB, Bover MT, Richardson DL, Schmelzer AC, Williams JM, Foulds J. Abstinence and psychological distress in co-morbid smokers using various pharmacotherapies. Drug and Alcohol Dependence. 2011;114:77–81. doi: 10.1016/j.drugalcdep.2010.06.022. doi:10.1016/j.drugalcdep.2010.06.022. [DOI] [PubMed] [Google Scholar]
- Toll BA, Cooney NL, McKee SA, O’Malley SS. Do daily interactive voice response reports of smoking behavior correspond with retrospective reports? Psychology of Addictive Behaviors. 2005;19:291–295. doi: 10.1037/0893-164X.19.3.291. doi:10.1037/0893-164X.19.3.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West EC, Levin ED, Rose JD. Smoking while wearing the nicotine patch: Is smoking satisfying or harmful? Journal of Clinical Research. 1992;40:871A. [Google Scholar]
- West R, Baker CL, Cappelleri JC, Bushmakin AG. Effect of varenicline and bupropion SR on craving, nicotine withdrawal symptoms, and rewarding effects of smoking during a quit attempt. Psychopharmacology. 2008;197:371–377. doi: 10.1007/s00213-007-1041-3. doi:10.1007/s00213-007-1041-3. [DOI] [PubMed] [Google Scholar]