Abstract
Rationale:
The role of β4-containing nicotinic acetylcholine receptors (nAChRs) in cognition, anxiety, depression, and analgesia in the absence of nicotine is unclear.
Methods:
Wild-type (β4+/+) and knockout (β4−/−) mice for the nAChR β4 subunit were tested in behavioral tests assessing cognitive function, affective behaviors, and nociception.
Results:
There were no learning and memory deficits in β4−/− mice compared with β4+/+ mice during the acquisition of the Barnes maze, contextual fear conditioning, and Y maze tasks. In the Barnes maze memory retention test, male β4−/− mice showed reduced use of the spatial search strategy, indicating small spatial memory deficits compared with β4+/+ mice. In the cue-induced fear conditioning memory retention test, β4−/− mice exhibited reduced freezing time compared with β4+/+ mice. Compared with β4+/+ mice, β4−/− mice exhibited decreased anxiety-like behavior in the light–dark box. Depression-like behavior in β4−/− mice was decreased in the tail suspension test and increased in the forced swim test compared with β4+/+ mice. β4−/− mice did not differ from β4+/+ mice in basal nociception but were less sensitive to the antinociceptive effect of nicotine in 2 tests of acute thermal pain.
Conclusions:
Lack of β4-containing nAChRs resulted in small deficits in hippocampus- and amygdala-dependent memory retention functions. β4-containing nAChRs are involved in anxiety- and depression-like behaviors and contribute to the analgesic effects of nicotine.
Introduction
Nicotine contained in tobacco is considered to be the main ingredient that leads to addiction in human smokers (Mineur & Picciotto, 2008; Stolerman & Jarvis, 1995). In addition, nicotine induces diverse effects, including improvement of cognitive function (Herman & Sofuoglu, 2010; Levin, 2002; Levin et al., 2009; Sarter, Parikh, & Howe, 2009; Sofuoglu, 2010), amelioration of anxiety-like behavior (File, Cheeta, & Kenny, 2000), and analgesia (Marubio et al., 1999). Nicotine binds to nicotinic acetylcholine receptors (nAChRs) throughout the brain. To date, 12 subunits of nAChRs have been identified, nine α-type (α2–α10) and three β-type (β2–β4) that form structurally and functionally distinct hetero- and homopentametric receptors (Changeux, 2010; Collins, Salminen, Marks, Whiteaker, & Grady, 2009). Genetically modified mice with altered expression of various nAChR subunits have been a useful tool for determining the specific role of nAChR subtypes in neurobehavioral function (Fowler, Arends, & Kenny, 2008; Mineur & Picciotto, 2008). The focus of the present studies was to evaluate the specific role of β4-containing nAChRs in cognitive function and nociception using mutant mice null for the β4 nAChR subunit (β4−/−) and wild-type mice (WT, β4+/+).
β4-containing nAChRs are widely expressed in the peripheral nervous system (Xu et al., 1999). In the brain, high concentrations of β4-containing nAChRs are found in the olfactory bulb, medial habenula, pineal gland, interpenduncular nucleus, and inferior colliculus (Gahring, Persiyanov, & Rogers, 2004; Klink, de Kerchove d’Exaerde, Zoli, & Changeux, 2001; Quick, Ceballos, Kasten, McIntosh, & Lester, 1999; Salas, Pieri, Fung, Dani, & De Biasi, 2003). Considering that the habenulo–peduncular pathway is implicated in cognition (Klemm, 2004; Lecourtier & Kelly, 2007; Lecourtier, Neijt, & Kelly, 2004) and that β4 nAChRs are also detectable in the hippocampus, amygdala, and cortex (Dineley-Miller & Patrick, 1992; Picciotto, Caldarone, King, & Zachariou, 2000), β4-containing nAChRs may be involved in cognitive function. However, the specific role of β4-containing nAChRs in cognition has not been studied extensively. It has been shown that β4−/− mice exhibited unaltered contextual learning compared with WT mice (Wehner et al., 2004). Other behavioral characteristics of β4 KO mice known to date include decreased anxiety-like behavior, reflected in increased exploratory behavior in the elevated plus-maze and increased climbing activity in the staircase maze (Salas et al., 2003). In addition, β4−/− mice were insensitive to nicotine-induced seizures and hypolocomotion, had an attenuated nicotine-induced hypothermic response, and did not show somatic signs of nicotine withdrawal (Kedmi, Beaudet, & Orr-Urtreger, 2004; Sack et al., 2005; Salas et al., 2003; Salas, Cook, Bassetto, & De Biasi, 2004a; Salas, Pieri, & De Biasi, 2004b). Furthermore, β4 null mutation resulted in reduced hyperalgesia during nicotine withdrawal (Salas et al., 2004b), suggesting a role for β4-containing nAChRs in modulating pain sensitivity.
The current study explored the role of β4-containing nAChRs in regulating learning and memory processes by comparing the performance of β4+/+ and β4−/− mice in a variety of cognitive tasks, such as the Barnes circular maze, contextual and cued fear conditioning, and spontaneous alternations in the Y maze. In addition, we evaluated anxiety-like behavior using the light–dark transfer test, compulsive-like behavior using marble burying, and depression-like behavior using the forced swim and tail suspension tests to further characterize the behavioral phenotype of β4−/− mice. Furthermore, locomotor activity and exploratory behavior were also investigated because many of the cognitive tasks used in this study are based on exploration. Finally, the potential role of β4-containing nAChRs in mediating nicotine-induced analgesia was investigated by subjecting β4 knockout mice to two standard assays of noxious heat sensitivity: the tail immersion and hot plate tests.
Materials and Methods
Animals
β4 nAChR subunit knockout breeder mice were obtained from Drs. Al Collins and Michael Marks, University of Colorado at Boulder, CO, and these mice were backcrossed to C57BL/6J for at least 10 generations. Male and female WT and knockout littermates used in our study were generated by mating male and female heterozygous parents. Genotypes were determined by polymerase chain reaction. Mice were housed in groups of 2–4, with food and water available ad libitum except during testing. The animal holding room was maintained on a 12-h light/dark cycle (lights off at 07:00 hr), and behavioral testing occurred during the dark (active) phase. All experiments were conducted in accordance with the guidelines of the American Association for the Accreditation of Laboratory Animal Care and National Research Council's Guide for Care and Use of Laboratory Animals and were approved by the University of California San Diego and The Scripps Research Institute Institutional Animal Care and Use Committees.
Drugs
(−)-Nicotine hydrogen bitartrate salt (Sigma-Aldrich, St. Louis, MO) was dissolved in physiological saline (0.9%; Hospira, Lake Forest, IL) at a concentration of 0.3 mg/ml. The pH was adjusted to 7.2 ± 0.2 using sodium hydroxide, and the solution was sterilized through a 0.22 μm syringe filter. All nicotine doses are expressed as free base concentrations. Saline or nicotine was injected subcutaneously (s.c., 0.05 ml/10 g body weight) 5 min before the test.
Experimental Design
Cognitive, Affective, and Locomotor Behaviors
All behavioral procedures are described in detail in the Supplementary material online. Two cohorts of mice were tested, one (15 β4+/+ and 14 β4−/− females, 10 β4+/+ and 11 β4−/− males) in a battery of cognitive tasks and the other (9 β4+/+ and 9 β4−/− females, 8 β4+/+ and 11 β4−/− males) to replicate the cognitive tests and examine locomotor activity and affective-like behaviors. For behavioral testing, a within-subjects design was used so that mice of each genotype were subjected to testing in the following order: Y maze, Barnes maze training, Barnes maze probe test, cue- and context-induced conditioned fear, Barnes maze retention, Barnes maze reversal, conditioned fear retention. After the completion of the cognitive test battery, the second mouse cohort was tested in additional procedures assessing locomotor activity and affective-like behavior. Again, a within-subjects design was employed such that mice of each genotype were subjected to testing in the following order: light–dark transfer test, marble burying test, locomotor activity test, tail suspension test, and forced swim test.
Nicotine-Induced Analgesia
Two additional cohorts of naïve β4+/+ and β4−/− mice were tested for nicotine-induced analgesia using the tail immersion and hot plate tests (see Supplementary material online for details). The first cohort contained 21 β4+/+ and 21 β4−/− females, and the second cohort contained 12 β4+/+ and 15 β4−/− females, as well as 17 β4+/+ and 28 β4−/− males. In each cohort, mice were first tested in the tail immersion test. Nicotine (0, 0.5, 1, 2, and 3 mg/kg, s.c., 5 min before test) was administered according to a within-subjects Latin square design during 5 consecutive days. One week later, the same mice were tested in the hot plate test. For each sex and genotype, mice were split into three subgroups, and nicotine (0, 1, and 3 mg/kg, s.c., 5 min before test) was administered according to a between-subjects design. Each mouse was only tested once in the hot plate test.
Statistical Analyses
Statistical analyses were performed using the Biomedical Computer Programs for Personal Computers Statistical Package (BMDP, Los Angeles, CA), the GraphPad Prism statistical package (GraphPad, San Diego, CA), and Statview v. 5.0 (SAS Institute Inc., Cary, NC). Two-way repeated-measures analysis of variance (ANOVA) was used to analyze the data collected from the cognitive test battery. The between-subject factors were Genotype and Sex, and the within-subject factors were Time Intervals (cued and contextual conditioning) and Trials/Blocks (the Barnes maze test). Data from the nicotine dose-response functions in the tail immersion and hot plate tests were analyzed using three-way ANOVAs, with Nicotine Dose as a within-subjects factor (tail immersion test) or between-subjects factor (hot plate test) and Genotype and Sex as the between-subjects factors. Data were then analyzed separately for each sex using two-way ANOVAs. When appropriate, post-hoc comparisons were carried out using the Newman–Keuls test. Nonparametric tests (exact Fisher’s test) were used to compare the percentage of mice using the spatial strategy. All values are expressed as mean ± SEM. Differences were considered statistically significant at p < .05.
Results
Cognitive Function in β4+/+ and β4−/− mice
Y maze
Spontaneous alternation behavior did not differ between β4+/+ and β4−/− mice (data not shown). Analyses of the percentage of spontaneous alternations and total number of arm entries revealed no effect of Genotype or Genotype by Sex interaction.
Barnes Maze
Task acquisition.
Both β4+/+ and β4−/− mice acquired the Barnes maze task during 12 daily training trials (Supplementary Figure 1). The ANOVA revealed that the number of errors (Supplementary Figure 1a; main effect of Trials: F(11, 913) = 21.15, p < .00001) and time to find the escape tunnel (Supplementary Figure 1B; main effect of Trials; F(11, 913) = 19.80, p < .0001) were significantly decreased across training trials. There was a significant interaction between the factors Trial and Sex for the number of errors (F(11, 913) = 3.42, p < .01) and time to find the escape tunnel (F(11, 913) = 2.77, p < .01), with females learning faster than males (data not shown). There was no significant main effect of the factors Sex or Genotype and no interaction effects.
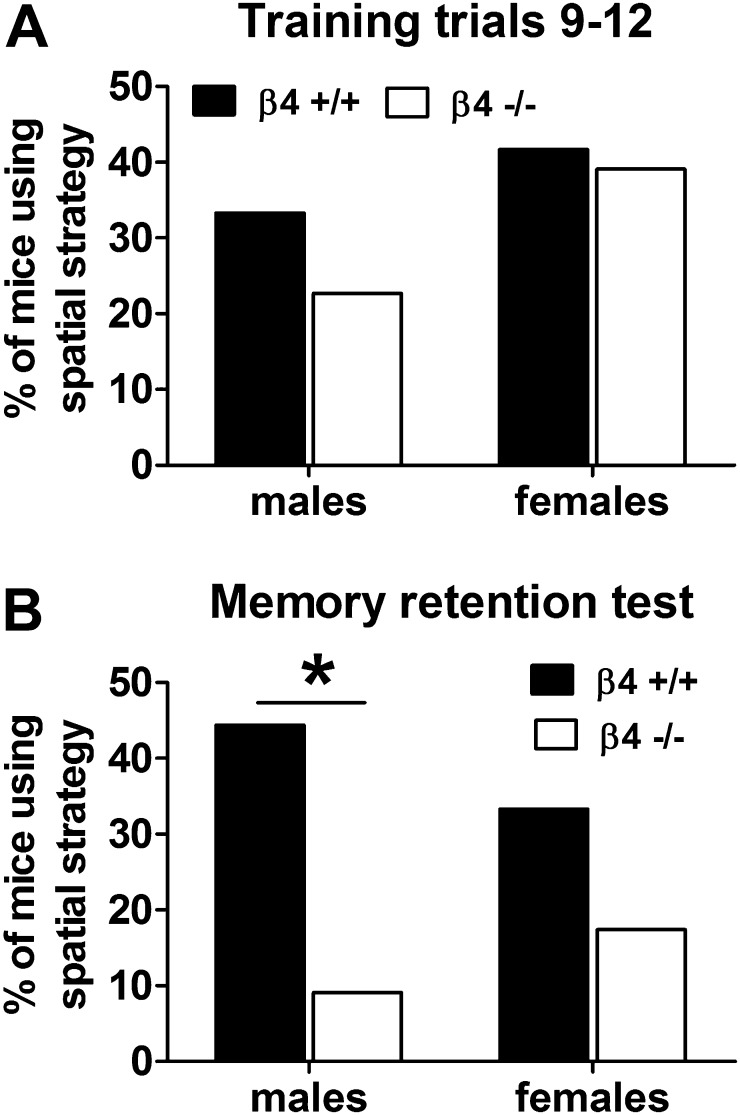
A comparison of strategies used by β4+/+ and β4−/− mice during task acquisition revealed no Genotype differences (Supplementary Figure 2 in Supplementary material online). Across three 4-trial blocks, all mice progressively increased the use of spatial (main effect of Block; F(2, 166) = 34.7, p < .0001; Supplementary Figure 1C) and serial (main effect of Block; F(2, 166) = 7.3, p < .001; Supplementary Figure 1B) strategies and decreased the use of the random strategy (main effect of Block; F(2, 166) = 62.17, p < .0001; Supplementary Figure 1a). By the end of task acquisition (Block 3), independent of genotype, female mice were using the random search strategy significantly less (main effect of Sex; F(3, 83) = 5.8, p < .05) and the serial strategy significantly more (Block by Sex interaction; F(2, 166) = 4.72, p < .01) compared with male mice (data not shown). Additional analyses were performed to compare the use of a spatial strategy to find the target defined as the percentage of mice using spatial memory in at least three of the four last training trials in Block 3. Male β4−/− mice tended to use the spatial strategy less than β4+/+ males or females of either genotype (Figure 1A).
Figure 1.
Use of spatial strategy to find the escape tunnel in the Barnes maze. Data (mean ± SEM) are presented as the percentage of mice using a spatial strategy in at least three of the four trials during the final training trials 9–12 (A) and the memory retention test (B). *Significant difference between genotypes (Fisher’s exact test).
Probe test.
The percentage of time spent in the quadrant associated with the escape tunnel did not differ between genotypes and sexes (Supplementary Table 1A in Supplementary material online).
Memory retention test.
The Memory retention test was performed on Day 73 of the experiment. The number of errors or the latency to find the escape tunnel did not differ between genotypes and sexes (Supplementary Table 1B). However, both male and female β4−/− mice were using the spatial strategy less than β4+/+ mice, but the difference between genotypes was significant only in males (Figure 1B; Fisher’s exact test, p < .05).
Reversal learning test.
The reversal learning test was performed on Day 74 of the experiment. The number of errors did not differ between genotypes and sexes (Supplementary Table 1C). In the reversal test, female mice were faster than males to find the escape tunnel, independent of genotype (Supplementary Table 1C; Sex [F(1, 83) = 9.52, p < .01]).
Contextual Fear Conditioning
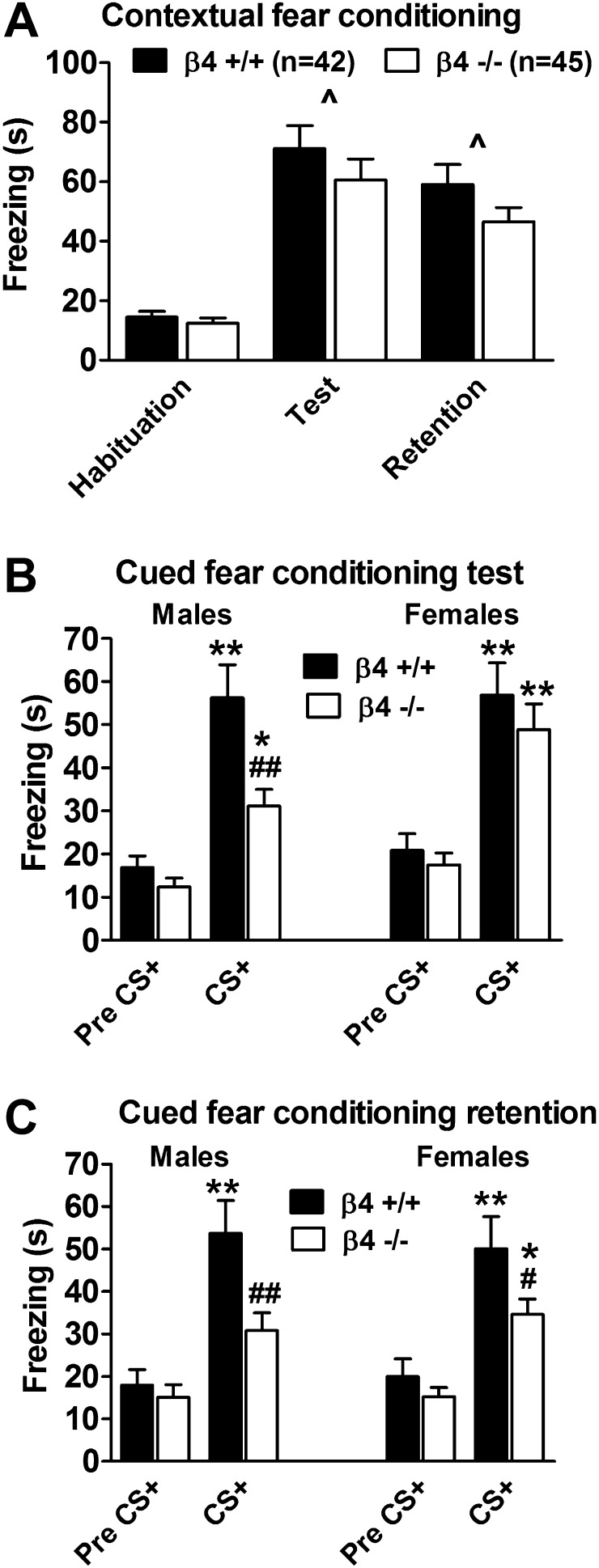
In a context previously associated with footshocks, both β4+/+ and β4−/− mice, independent of sex, increased their freezing behavior during the test and retest of memory retention compared with freezing exhibited during the habituation period (Figure 2A). ANOVAs indicated a significant effect of Exposure to the context previously associated with footshocks during the test (F(1, 83) = 80.55, p < .00001) and memory retention retest (F(1, 81) = 67.57, p < .00001) but no effect of Genotype or Sex and no Genotype × Sex × Exposure interaction.
Figure 2.
Context-induced fear conditioning during the test and retest (A) and cue-induced fear conditioning during test (B) and retest (C). Data are expressed as the number of seconds of freezing (mean ± SEM). ^p < .05, significant effect of exposure to the context previously associated with footshocks indicated in the ANOVA. *p < .05, **p < .01, significant difference between habituation (pre-CS+) and CS+ presentation conditions (Newman–Keuls test). #p < .05, ##p < .01, significant difference between β4+/+ and β4−/− mice (Newman–Keuls test).
Cued Fear Conditioning
Initial presentation of a cue (CS+) previously associated with foot shocks increased freezing in all mice (Figure 2B). ANOVAs revealed significant main effects of Exposure (F(1, 83) = 115.15, p < .00001) and Genotype (F(1, 83) = 6.95, p < .01), and a significant Genotype × Exposure interaction (F(1, 83) = 4.64, p < .05) but no three-way interaction. Post-hoc comparisons showed that both β4+/+ and β4−/− female and male mice exhibited increased freezing during the initial cue-induced fear test (Figure 2B). Interestingly, however, the cue-induced conditioned fear response in β4−/− male mice was significantly attenuated compared with β4+/+ male mice (p < .01, Newman–Keuls test; Figure 2B).
During the cue-induced fear conditioning retest, there were significant main effects of Exposure (F(1, 81) = 73.81, p < .00001) and Genotype (F(1, 81) = 9.59, p < .01) and a significant Genotype × Exposure interaction (F(1, 81) = 6.88, p < .05) but no effect of Sex or three-way interaction. Post-hoc comparisons showed that both β4+/+ and β4−/− female mice exhibited increased freezing during the retest of cue-induced fear (Figure 2C). The cue-induced conditioned fear response in β4−/− female mice was significantly attenuated compared with β4+/+ female mice (p < .05, Newman–Keuls test; Figure 2C). During the retest, β4+/+ male mice exhibited significantly increased freezing (Figure 2C). By contrast, freezing in β4−/− male mice was not significantly different from the pre-CS+ level and was significantly lower than the CS+ level in β4+/+ male mice (Figure 2C).
Activity and Affective-Like Behavior in β4+/+ and β4−/− Mice
Locomotor Activity Test
Overall, there were no significant differences between mouse genotypes and sexes in total ambulation, total activity in the center, and total horizontal activity (Supplementary Table 3 in Supplementary material online). From the total number of rearings, the ANOVA detected a significant effect of Sex (F(1, 35) = 17,24, p < .0001), with males rearing more than females, independent of genotype (data not shown).
Light–Dark Box
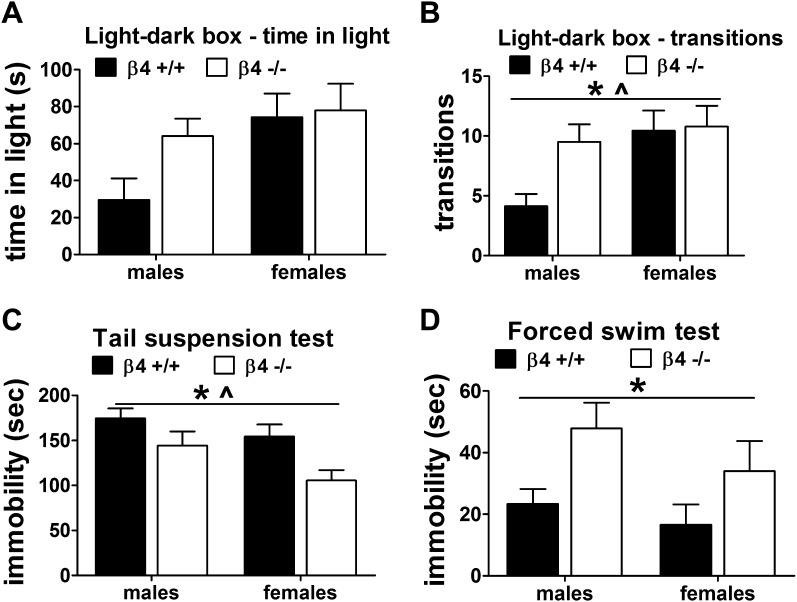
There was a significant main effect of Genotype (F(1, 35) = 4.1, p < .05) and Sex (F(1, 35) = 4.3, p < .05) but no Sex × Genotype interaction for the number of transitions. Decreased anxiety-like behavior was observed in β4−/− mice compared with β4+/+ mice, and this effect was attributable to β4−/− male mice (Figure 3A and B). For the time spent in the light compartment, ANOVAs revealed no effect of Sex or Genotype and no Sex × Genotype interaction, although the direction of the effects was the same as the number of transitions.
Figure 3.
Anxiety-like behavior measured as time spent in the light compartment (A) and number of transitions (B) in the light–dark box test. Immobility measured in the tail suspension test (C) and forced swim test (D). All data are expressed as mean ± SEM. *p < .05, significant main effect of Genotype in the ANOVA. ^p < .05, significant main effect of Sex in the ANOVA.
Marble Burying Test
Both β4+/+ and β4−/− mice exhibited similar compulsive-like behavior in the marble burying test (data not shown).
Tail Suspension Test
In this experiment, six mice (one β4+/+ male, one β4−/− male, one β4+/+ female, and three β4−/− females) were excluded from the analyses because they exhibited excessive tail climbing behavior or fell off. There were significant effects of Genotype (F(1, 24) = 4.8, p < .05) and Sex (F(1, 24) = 6.8, p < .05) but no interaction between the two factors for immobility time. Visual inspection of the data indicated that β4−/− mice spent less time immobile than β4+/+ mice, and this effect was attributable to β4−/− females (Figure 3C).
Forced Swim Test
There was a significant effect of Genotype (F(1, 35) = 7.1, p < .01) but no effect of Sex and no interaction for immobility time. β4−/− mice exhibited increased depression-like behavior compared with β4+/+ mice, reflected by increased immobility time (Figure 3D). In addition, there was a significant effect of Genotype on swimming time (F(1, 35) = 5.4, p < .05) but no effect of Sex and no interaction (data not shown). Climbing time did not differ between genotypes and sexes (data not shown).
Nicotine-Induced Analgesia
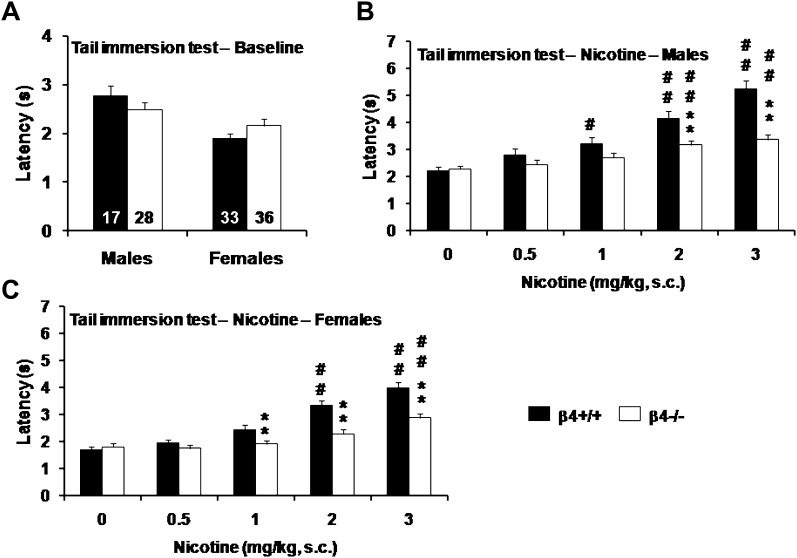
In the tail immersion test, baseline measures revealed an effect of Sex (F(1, 110) = 17.0, p < .001), with females having lower nociceptive thresholds than males, independent of genotype (Figure 4A). Deletion of the β4 subunit, however, did not affect basal thermal pain sensitivity, with no main effect of Genotype and no interaction between Sex and Genotype. Nicotine had a dose-dependent antinociceptive effect in both males (Figure 4B) and females (Figure 4C). A three-way repeated-measures ANOVA revealed a significant effect of Sex (F(1, 110) = 40.9, p < .001), consistent with the sex difference in basal nociception. None of the two- or three-way interactions between Sex and the other factors were significant. Data from each sex were analyzed separately using two-way repeated-measures ANOVAs. There was a significant main effect of Nicotine in both sexes (males: F(1, 43) = 17.3, p < .001, Figure 4B; females: F(1, 67) = 13.5, p < .001, Figure 4C). A main effect of Genotype (males: F(4, 172) = 48.9, p < .001; females: F(4, 268) = 86.0, p < .001) and a significant interaction between Nicotine and Genotype (males: F(4, 172) = 11.8, p < .001; females: F(4, 268) =10.0, p < .001) were also detected in males and females, indicating that nicotine-induced analgesia was reduced in β4−/− mice (Figure 4B–C).
Figure 4.
(A) Basal nociceptive thresholds in the tail immersion test. Tail withdrawal latencies (mean ± S E M) were measured in naïve male and female β4+/+ (black bars) and β4−/− (white bars) mice. The numbers of mice used in each group are indicated in the bars. Data were subjected to two-way ANOVA, followed by post-hoc comparisons (**p < .01, male vs. female, Newman–Keuls test). (B and C) Antinociceptive effect of nicotine in the tail immersion test. Tail withdrawal latencies (mean ± SEM) were measured in male (B) and female (C) β4+/+ (black bars) and β4−/− (white bars) mice 5 min after s.c. injection of nicotine. The numbers of mice used in each group are identical to (A). Data were subjected to repeated-measures ANOVA, followed by post-hoc comparisons (**p < .01, wild-type vs. knockout; #p < .05, ##p < .01, nicotine vs. saline, Newman–Keuls test).
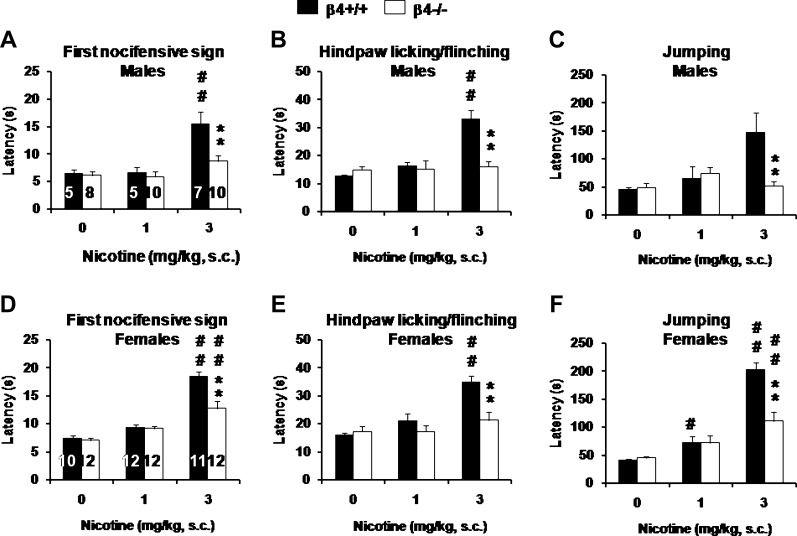
A similar pattern of results emerged in the hot plate test (Figure 5). Nicotine increased the latencies to display the first nocifensive response to noxious heat (males, Figure 5A; females, Figure 5D), hindpaw licking or flinching (males, Figure 5B; females, Figure 5E), and jumping (males, Figure 5C; females, Figure 5F). Three-way ANOVAs revealed a significant effect of Sex for all responses (first sign: F(1, 102) = 16.5, p < .001; hindpaw sign: F(1, 102) = 5.2, p < .05; jumping: F(1, 102) = 4.7, p < .05), with females having longer latencies than males. None of the two- or three-way interactions between Sex and the other factors were significant, with the exception of the Sex × Nicotine interaction for jumping (F(2, 102) = 5.7, p < .05), which reflected the higher sensitivity of females to the antinociceptive effect of nicotine for that sign. Data from each sex were then analyzed separately using two-way ANOVAs. There was a significant effect of Nicotine on the latency to display first sign (males: F(2, 39) = 15.3, p < .001, Figure 5A; females: F(2, 63) = 52.9, p < .001, Figure 5D), hindpaw sign (males: F(2, 39) = 10.7, p < .001, Figure 5B; females: F(2, 63) = 13.8, p < .001, Figure 5E), and jumping (males: F(2, 39) = 3.9, p = .056, Figure 5C; females: F(2, 63) = 48.0, p < .001, Figure 5F). A significant effect of Genotype was detected for all responses, with β4−/− mice displaying shorter latencies than their WT counterparts overall (first sign: males, F(1, 39) = 6.2, p < .05; females, F(1, 63) = 9.1, p < .05; first hindpaw sign: males, F(1, 39) = 6.3, p < .05; females, F(1, 63) = 8.7, p < .01; jumping: males, F(1, 39) = 4.8, p < .05; females, F(1, 63) = 8.9, p < .01). Finally, the interaction between Nicotine and Genotype was also significant for the three measures (first sign: males, F(2, 39) = 4.4, p < .05; females, F(2, 63) = 6.9, p < .01; first hindpaw sign: males, F(2, 39) = 8.6, p < .001; females, F(2, 63) = 5.3, p < .01; jumping: males, F(2, 39) = 6.3, p < .01; females, F(2, 63) = 10.3, p < .001).
Figure 5.
Antinociceptive effect of nicotine in the hot plate test. Latencies to show the first nocifensive sign (A and D), hindpaw licking or flinching (B and E), and jumping (C and F) were measured in male (A, B, and C) and female (D, E, and F) β4+/+ (black bars) and β4−/− (white bars) mice 5 min after s.c. injection of nicotine. The numbers of mice used in each group are indicated in the bars in (A) and (D). Data were subjected to repeated-measures ANOVA, followed by post-hoc comparisons (**p < .01, wild-type vs. knockout; #p < .05, ##p < .01, nicotine vs. saline, Newman–Keuls test).
Discussion
The present studies demonstrated that the lack of β4-containing nAChRs induced no pronounced learning and memory deficits in β4−/− mice compared with β4+/+ mice in simple spatial working memory in the Y maze, during the acquisition of the Barnes maze, and contextual fear conditioning. In the Barnes maze memory retention test, male β4−/− mice showed a reduction in the use of the spatial search strategy, indicating small spatial memory deficits compared with β4+/+ mice. In the cue-induced fear conditioning memory retention test, both male and female β4−/− mice exhibited decreased memory retention, while during task acquisition memory deficits were observed only in male β4−/− mice. Compared with β4+/+ mice, β4−/− mice exhibited decreased anxiety-like behavior in the light–dark box. Depression-like behavior in β4−/− mice was decreased in the tail suspension test and increased in the forced swim test compared with β4+/+ mice. Locomotor activity and exploratory behavior in the Y maze was similar in both genotypes. Finally, male and female β4−/− mice did not differ from their β4+/+ counterparts in terms of basal nociception but were less sensitive to the antinociceptive effect of nicotine in two tests of acute thermal pain, indicating that β4-containing nAchRs are involved in the modulation of nicotine-induced analgesia.
β4-Containing nAChRs in Cognitive Function
In the Y maze and locomotor activity tests, β4−/− and β4+/+ male and female mice had similar levels of exploratory behavior and reactivity to novelty. Therefore, the observed changes in cognitive function and affective behavior in nAChR β4 knockout mice discussed below cannot be attributed to the differences in general locomotor or exploratory activity.
In the Barnes maze, there were no performance differences between β4−/− and β4+/+ mice during task acquisition, the probe test, or the reversal learning test. The transition from the use of hippocampus-independent nonspatial escape strategies (random and serial strategies) to the use of the hippocampus-dependent spatial escape strategy, which requires the use of external visuo-spatial cues to locate the escape tunnel (Harrison, Reiserer, Tomarken, & McDonald, 2006; O’Leary & Brown, 2012), was similar in both β4−/− and β4+/+ mice during the acquisition of the Barnes maze task. The most pronounced differences between genotypes were observed during the memory retention test, during which β4−/− male mice showed a significant reduction in the use of the spatial strategy, indicating deficits in spatial memory retention. The medial habenula receives inputs from the septal nuclei, which are themselves targeted by afferents from the hippocampus involved in spatial learning (Bannerman et al., 2003; Moser, Moser, & Andersen, 1993; Paylor, Zhao, Libbey, Westphal, & Crawley, 2001; Richmond et al., 1999). The habenular complex has been hypothesized to integrate spatial memories with outcome incentive value and thereby contribute to the selection of appropriate coping strategies in stressful situations (for review, see Lecourtier & Kelly, 2007). Accordingly, lesions of the habenular complex resulted in marked spatial memory impairment during acquisition and retrieval in the Morris water maze, a spatial memory task that is more anxiogenic and stressful than the Barnes maze (Harrison, Hosseini, & McDonald, 2009; Lecourtier et al., 2004). Although β4-containing nAChRs are expressed in the hippocampus and medial habenula (Dineley-Miller & Patrick, 1992; Gahring, Persiyanov, & Rogers, 2004; Picciotto et al., 2000), the genetic ablation of the β4 nAChR subunit induced only limited deficits in spatial memory retention and did not affect general performance in the Barnes maze. Furthermore, β4−/− mice tended to exhibit decreased anxiety-like behavior in the light–dark box compared with β4+/+ mice, suggesting that the observed small deficit in spatial memory retention in β4−/− may not be anxiety related, although being in a brightly lit, open space in the Barnes maze is considered anxiogenic for mice.
In agreement with previously published findings (Wehner et al., 2004), contextual learning was similar in both β4−/− and β4+/+ mice as measured by freezing during the contextual fear conditioning test and retest of memory retention. Furthermore, both β4−/− and β4+/+ mice acquired the cued fear conditioning task and showed significant increases in freezing in response to the cue previously associated with footshocks. Interestingly, however, β4−/− male but not β4−/− female mice exhibited reduced freezing during the cue-induced fear conditioning test, indicating memory deficits in this task. In contrast, previously published findings reported no deficits in cue-induced fear conditioning in β4−/− mice (Wehner et al., 2004). During memory retention tests, both β4−/− male and female mice exhibited reduced freezing indicating memory deficits in cue-induced fear conditioning. The differential effect of nAChR β4 subunit deletion on context- and cue-induced learning may be explained by the distinct mechanisms underlying context and cue fear conditioning. Both the hippocampus and amygdala are involved in contextual fear conditioning, whereas only the amygdala is involved in cued fear conditioning (Frankland, Cestari, Filipkowski, McDonald, & Silva, 1998; Maren, Aharonov, & Fanselow, 1997; Phillips & LeDoux, 1992; Rudy, Huff, & Matus-Amat, 2004; Wehner & Radcliffe, 2004). Thus, amygdala-dependent learning was likely impaired in β4 knockout mice to a larger extent than hippocampus-dependent learning.
β4-Containing nAChRs in Affective Behaviors
Our results showed that β4−/− mice tended to exhibit less anxiety-like behavior compared with β4+/+ mice in the light–dark box as measured by the number of transitions but not time spent in the light compartment. Similarly, previous findings indicated that β4−/− mice exhibited less anxiety-like behavior compared with β4+/+ mice on the elevated plus and staircase mazes, with no differences between genotypes in the light–dark box, open field, or mirrored chamber (Salas et al., 2003). Compulsive-like behavior measured in the marble burying test did not differ between β4−/− and β4+/+ mice.
Depression-like behavior was measured in the tail suspension and forced swim tests, and conflicting results were obtained in these two tests. In the forced swim test, β4−/− mice exhibited increased immobility compared with β4+/+ mice, indicating a tendency for increased depression-like behavior. In contrast, in the tail suspension test, antidepressant-like behavior was evident in β4−/− mice. It is postulated that the biological substrates that underlie the depression-like behavior measured in these tests are different (Bai, Li, Clay, Lindstrom, & Skolnick, 2001). In the forced swim test, climbing behavior is indicative of norepinephrine neurotransmitter function (Cryan, Markou, & Lucki, 2002), whereas swimming behavior is indicative of serotonin neurotransmitter function (Cryan et al., 2002). Swimming was significantly decreased in β4−/− mice compared with β4+/+ mice. This finding suggests that the lack of the nAChR β4 subunit alters serotonin but not norepinephrine transmission. On the other hand, β4-containing nAChRs in the medial habenula and interpeduncular nucleus pathway may regulate glutamate release (Girod & Role, 2001; McGehee & Role, 1995) involved in both depression and anxiety (Krystal et al., 2002; Skolnick, 2002; Stewart & Reid, 2002). Therefore, the lack of the β4 subunit may alter glutamatergic tone in the habenulo–raphe pathway and mediate affective behavior in mice lacking β4-containing nAChRs. Finally, potential developmental compensatory changes in β4 nAChR knockout mice cannot be ruled out in the expression of depression-like behavior. Specifically, the β2 subunit of nAChRs, which is involved in depression-like behavior, may partially substitute for the loss of the β4 subunit as it has been shown in the autonomic ganglia (Xu et al., 1999). It should be emphasized, however, that both the forced swim test and tail suspension test were developed and validated as animal models predictive of antidepressant activity (Cryan et al., 2002). Thus, it may not necessarily be the case that the opposite behavior to that induced by antidepressant treatments (i.e., decreased immobility) necessarily reflects a depression-like state.
β4-Containing nAChRs in Nociception
Our data show a role for β4-containing nAChRs in the analgesic effects of nicotine. β4 subunits contributing to nicotine-induced analgesia may be located in the brain and spinal cord because deletion of β4 subunits in the knockout mice affected both reflex and integrated responses to noxious heat. β4 subunits have a restricted pattern of expression in the brain, with notable enrichment in the habenulo–peduncular tract (Gahring et al., 2004). Interestingly, nicotine microinjection into the interpeduncular nucleus produces analgesia in a rat model of acute thermal pain (Hamann & Martin, 1992). At the spinal level, β4 subunits are expressed presynaptically on primary afferents in the superficial layer of the dorsal horn (Khan et al., 2003), but nAChRs expressed on primary afferent terminals have been implicated in the pronociceptive, rather than antinociceptive, effect of nicotinic agonists (Khan et al., 2004).
The β4 subunit belongs to a genomic cluster together with the α3 and α5 subunits, and these three subunits co-assemble into functional receptors (Boulter et al., 1990; Wang et al., 1996). Interestingly, deletion of α5 subunits also decreases the antinociceptive effects of nicotine in models of acute thermal pain, and the α3 and α5 subunits also display strikingly high expression in the habenulo–peduncular pathway (Boulter et al., 1990; Jackson et al., 2010; Salas et al., 2004a; Wada et al., 1989). It can be hypothesized that activation of α3α5β4 nAChRs expressed in the habenula or interpeduncular nucleus produces analgesia because some habenular neurons respond to noxious input (Dafny & Qiao, 1990), and habenular stimulation has analgesic effects (Cohen & Melzack, 1986).
While acute administration of nicotine produces analgesia, spontaneous or antagonist-precipitated withdrawal from chronic nicotine exposure induces hyperalgesia (Damaj, Kao, & Martin, 2003). Interestingly, nicotine withdrawal-induced hyperalgesia is also abolished in β4−/− mice (Salas et al., 2004b). The β4 subunit therefore plays a central role in the modulation of nociceptive thresholds by nicotine, contributing to both nicotine-induced analgesia and the counter adaptive increase in pain sensitivity during nicotine withdrawal.
Conclusions
Overall, our findings provide insights into the role of β4-containing nAChRs in cognitive function, affective behaviors, and nociception. The results indicate that: (a) lack of the β4-containing nAChRs resulted in deficits in hippocampus- and amygdala-dependent memory function; (b) β4-containing nAChRs are involved in anxiety- and perhaps depression-like behaviors; and (c) β4-containing nAChRs contribute to the analgesic effects of nicotine.
Funding
This work was supported by National Institutes of Health grant DA023209 to AM. Breeders for the β4 nAChR subunit knockout lines were provided to our laboratory with the support of grant P30 DA015663 to Dr. MJM at the University of Colorado, Boulder.
Declaration of Interests
The NIH had no role in the study design, collection, analysis, and interpretation of data, writing of the report, or decision to submit the article for publication. AM has received contract research support from Lundbeck, Bristol-Myers Squibb Co., F. Hoffman-La Roche, Pfizer, and Astra-Zeneca and honoraria/consulting fees from Abbott GmbH and Company, Astra-Zeneca and Pfizer during the past 3 years. The remaining authors report no financial conflicts of interests.
Supplementary Material
Acknowledgments
The authors would like to thank Mr. Edwin Obana, Ms. Holly Jarrell, and Mrs. Kimberly Edwards for their help with the mouse colony maintenance and genotyping of the knockout lines.
References
- Bai F, Li X, Clay M, Lindstrom T, Skolnick P. Intra- and interstrain differences in models of “behavioral despair”. Pharmacology Biochemistry and Behavior. 2001;70:187–192. doi: 10.1016/s0091-3057(01)00599-8. doi:S0091-3057(01)00599-8. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Grubb M, Deacon RM, Yee BK, Feldon J, Rawlins JN. Ventral hippocampal lesions affect anxiety but not spatial learning. Behavioural Brain Research. 2003;139:197–213. doi: 10.1016/s0166-4328(02)00268-1. doi:S0166432802002681. [DOI] [PubMed] [Google Scholar]
- Boulter J, O’Shea-Greenfield A, Duvoisin RM, Connolly JG, Wada E, Jensen A, et al. Alpha 3, alpha 5, and beta 4: Three members of the rat neuronal nicotinic acetylcholine receptor-related gene family form a gene cluster. Journal of Biological Chemistry. 1990;265:4472–4482. [PubMed] [Google Scholar]
- Changeux JP. Nicotine addiction and nicotinic receptors: Lessons from genetically modified mice. Nature Reviews Neuroscience. 2010;11:389–401. doi: 10.1038/nrn2849. doi:nrn2849. [DOI] [PubMed] [Google Scholar]
- Cohen SR, Melzack R. Habenular stimulation produces analgesia in the formalin test. Neuroscience Letters. 1986;70:165–169. doi: 10.1016/0304-3940(86)90457-x. [DOI] [PubMed] [Google Scholar]
- Collins AC, Salminen O, Marks MJ, Whiteaker P, Grady SR. The road to discovery of neuronal nicotinic cholinergic receptor subtypes. Handbook of Experimental Pharmacology. 2009;192:85–112. doi: 10.1007/978-3-540-69248-5_4. doi:10.1007/978-3-540-69248-5_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Markou A, Lucki I. Assessing antidepressant activity in rodents: Recent developments and future needs. Trends in Pharmacological Sciences. 2002;23:238–245. doi: 10.1016/s0165-6147(02)02017-5. [DOI] [PubMed] [Google Scholar]
- Dafny N, Qiao JT. Habenular neuron responses to noxious input are modified by dorsal raphe stimulation. Neurology Research. 1990;12:117–121. doi: 10.1080/01616412.1990.11739929. [DOI] [PubMed] [Google Scholar]
- Damaj MI, Kao W, Martin BR. Characterization of spontaneous and precipitated nicotine withdrawal in the mouse. Journal of Pharmacology and Experimental Therapeutics. 2003;307:526–534. doi: 10.1124/jpet.103.054908. doi:10.1124/jpet.103.054908. [DOI] [PubMed] [Google Scholar]
- Dineley-Miller K, Patrick J. Gene transcripts for the nicotinic acetylcholine receptor subunit, beta4, are distributed in multiple areas of the rat central nervous system. Brain Research. Molecular Brain Research. 1992;16:339–344. doi: 10.1016/0169-328x(92)90244-6. [DOI] [PubMed] [Google Scholar]
- File SE, Cheeta S, Kenny PJ. Neurobiological mechanisms by which nicotine mediates different types of anxiety. European Journal of Pharmacology. 2000;393:231–236. doi: 10.1016/s0014-2999(99)00889-4. doi:S0014-2999(99)00889-4. [DOI] [PubMed] [Google Scholar]
- Fowler CD, Arends MA, Kenny PJ. Subtypes of nicotinic acetylcholine receptors in nicotine reward, dependence, and withdrawal: Evidence from genetically modified mice. Behavioural Pharmacology. 2008;19:461–484. doi: 10.1097/FBP.0b013e32830c360e. doi:10.1097/FBP.0b013e32830c360e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankland PW, Cestari V, Filipkowski RK, McDonald RJ, Silva AJ. The dorsal hippocampus is essential for context discrimination but not for contextual conditioning. Behavioural Neuroscience. 1998;112:863–874. doi: 10.1037//0735-7044.112.4.863. [DOI] [PubMed] [Google Scholar]
- Gahring LC, Persiyanov K, Rogers SW. Neuronal and astrocyte expression of nicotinic receptor subunit β4 in the adult mouse brain. Journal of Comparative Neurology. 2004;468:322–333. doi: 10.1002/cne.10942. doi:10.1002/cne.10942. [DOI] [PubMed] [Google Scholar]
- Girod R, Role LW. Long-lasting enhancement of glutamatergic synaptic transmission by acetylcholine contrasts with response adaptation after exposure to low-level nicotine. Journal of Neuroscience. 2001;21:5182–5190. doi: 10.1523/JNEUROSCI.21-14-05182.2001. doi:21/14/5182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann SR, Martin WR. Opioid and nicotinic analgesic and hyperalgesic loci in the rat brain stem. Journal of Pharmacology and Experimental Therapeutics. 1992;261:707–715. [PubMed] [Google Scholar]
- Harrison FE, Hosseini AH, McDonald MP. Endogenous anxiety and stress responses in water maze and Barnes maze spatial memory tasks. Behavioural Brain Research. 2009;198:247–251. doi: 10.1016/j.bbr.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison FE, Reiserer RS, Tomarken AJ, McDonald MP. Spatial and nonspatial escape strategies in the Barnes maze. Learning & Memory. 2006;13:809–819. doi: 10.1101/lm.334306. doi:lm.334306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman AI, Sofuoglu M. Comparison of available treatments for tobacco addiction. Current Psychiatry Reports. 2010;12:433–440. doi: 10.1007/s11920-010-0134-6. doi:10.1007/s11920-010-0134-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KJ, Marks MJ, Vann RE, Chen X, Gamage TF, Warner JA, et al. Role of α5 nicotinic acetylcholine receptors in pharmacological and behavioral effects of nicotine in mice. Journal of Pharmacology and Experimental Therapeutics. 2010;334:137–146. doi: 10.1124/jpet.110.165738. doi:jpet.110.165738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedmi M, Beaudet AL, Orr-Urtreger A. Mice lacking neuronal nicotinic acetylcholine receptor β4-subunit and mice lacking both α5- and β4-subunits are highly resistant to nicotine-induced seizures. Physiological Genomics. 2004;17:221–229. doi: 10.1152/physiolgenomics.00202.2003. doi:10.1152/physiolgenomics.00202.2003. [DOI] [PubMed] [Google Scholar]
- Khan I, Osaka H, Stanislaus S, Calvo RM, Deerinck T, Yaksh TL, et al. Nicotinic acetylcholine receptor distribution in relation to spinal neurotransmission pathways. Journal of Comparative Neurology. 2003;467:44–59. doi: 10.1002/cne.10913. doi:10.1002/cne.10913. [DOI] [PubMed] [Google Scholar]
- Khan IM, Wennerholm M, Singletary E, Polston K, Zhang L, Deerinck T, et al. Ablation of primary afferent terminals reduces nicotinic receptor expression and the nociceptive responses to nicotinic agonists in the spinal cord. Journal of Neurocytology. 2004;33:543–556. doi: 10.1007/s11068-004-0516-6. doi:10.1007/s11068-004-0516-6. [DOI] [PubMed] [Google Scholar]
- Klemm WR. Habenular and interpeduncularis nuclei: Shared components in multiple-function networks. Medical Science Monitor. 2004;10:RA261–RA273. doi:4775. [PubMed] [Google Scholar]
- Klink R, de Kerchove d’Exaerde A, Zoli M, Changeux JP. Molecular and physiological diversity of nicotinic acetylcholine receptors in the midbrain dopaminergic nuclei. Journal of Neuroscience. 2001;21:1452–1463. doi: 10.1523/JNEUROSCI.21-05-01452.2001. doi:21/5/1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal JH, Sanacora G, Blumberg H, Anand A, Charney DS, Marek G, et al. Glutamate and GABA systems as targets for novel antidepressant and mood-stabilizing treatments. Molecular Psychiatry. 2002;7(Suppl. 1):S71–S80. doi: 10.1038/sj.mp.4001021. [DOI] [PubMed] [Google Scholar]
- Lecourtier L, Kelly PH. A conductor hidden in the orchestra? Role of the habenular complex in monoamine transmission and cognition. Neuroscience & Biobehavioral Reviews. 2007;31:658–672. doi: 10.1016/j.neubiorev.2007.01.004. doi:S0149-7634(07)00010-3. [DOI] [PubMed] [Google Scholar]
- Lecourtier L, Neijt HC, Kelly PH. Habenula lesions cause impaired cognitive performance in rats: Implications for schizophrenia. European Journal of Neuroscience. 2004;19:2551–2560. doi: 10.1111/j.0953-816X.2004.03356.x. doi:10.1111/j.0953-816X.2004.03356.x. [DOI] [PubMed] [Google Scholar]
- Levin ED. Nicotinic receptor subtypes and cognitive function. Journal of Neurobiology. 2002;53:633–640. doi: 10.1002/neu.10151. [DOI] [PubMed] [Google Scholar]
- Levin ED, Petro A, Rezvani AH, Pollard N, Christopher NC, Strauss M, et al. Nicotinic α7- or β2-containing receptor knockout: Effects on radial-arm maze learning and long-term nicotine consumption in mice. Behavioural Brain Research. 2009;196:207–213. doi: 10.1016/j.bbr.2008.08.048. doi:S0166-4328(08)00502-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Aharonov G, Fanselow MS. Neurotoxic lesions of the dorsal hippocampus and Pavlovian fear conditioning in rats. Behavioural Brain Research. 1997;88:261–274. doi: 10.1016/s0166-4328(97)00088-0. doi:S0166432897000880. [DOI] [PubMed] [Google Scholar]
- Marubio LM, del Mar Arroyo-Jimenez M, Cordero-Erausquin M, Lena C, Le Novere N, de Kerchove d’Exaerde A, et al. Reduced antinociception in mice lacking neuronal nicotinic receptor subunits. Nature. 1999;398:805–810. doi: 10.1038/19756. doi:10.1038/19756. [DOI] [PubMed] [Google Scholar]
- McGehee DS, Role LW. Physiological diversity of nicotinic acetylcholine receptors expressed by vertebrate neurons. Annual Reviews of Physiology. 1995;57:521–546. doi: 10.1146/annurev.ph.57.030195.002513. doi:10.1146/annurev.ph.57.030195.002513. [DOI] [PubMed] [Google Scholar]
- Mineur YS, Picciotto MR. Genetics of nicotinic acetylcholine receptors: Relevance to nicotine addiction. Biochemical Pharmacology. 2008;75:323–333. doi: 10.1016/j.bcp.2007.06.010. doi:S0006-2952(07)00371-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser E, Moser MB, Andersen P. Spatial learning impairment parallels the magnitude of dorsal hippocampal lesions, but is hardly present following ventral lesions. Journal of Neuroscience. 1993;13:3916–3925. doi: 10.1523/JNEUROSCI.13-09-03916.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary TP, Brown RE. The effects of apparatus design and test procedure on learning and memory performance of C57BL/6J mice on the Barnes maze. Journal of Neuroscience Methods. 2012;203:315–324. doi: 10.1016/j.jneumeth.2011.09.027. doi:S0165-0270(11)00594-2l. [DOI] [PubMed] [Google Scholar]
- Paylor R, Zhao Y, Libbey M, Westphal H, Crawley JN. Learning impairments and motor dysfunctions in adult Lhx5-deficient mice displaying hippocampal disorganization. Physiology & Behavior. 2001;73:781–792. doi: 10.1016/s0031-9384(01)00515-7. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behavioral Neuroscience. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Caldarone BJ, King SL, Zachariou V. Nicotinic receptors in the brain: Links between molecular biology and behavior. Neuropsychopharmacology. 2000;22:451–465. doi: 10.1016/S0893-133X(99)00146-3. doi:S0893-133X(99)00146-3. [DOI] [PubMed] [Google Scholar]
- Quick MW, Ceballos RM, Kasten M, McIntosh JM, Lester RA. α3β4 subunit-containing nicotinic receptors dominate function in rat medial habenula neurons. Neuropharmacology. 1999;38:769–783. doi: 10.1016/s0028-3908(99)00024-6. doi:S0028390899000246. [DOI] [PubMed] [Google Scholar]
- Richmond MA, Yee BK, Pouzet B, Veenman L, Rawlins JN, Feldon J, et al. Dissociating context and space within the hippocampus: Effects of complete, dorsal, and ventral excitotoxic hippocampal lesions on conditioned freezing and spatial learning. Behavioral Neuroscience. 1999;113:1189–1203. doi: 10.1037/0735-7044.113.6.1189. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Huff NC, Matus-Amat P. Understanding contextual fear conditioning: Insights from a two-process model. Neuroscience & Biobehavioral Reviews. 2004;28:675–685. doi: 10.1016/j.neubiorev.2004.09.004. doi:S0149-7634(04)00093-4. [DOI] [PubMed] [Google Scholar]
- Sack R, Gochberg-Sarver A, Rozovsky U, Kedmi M, Rosner S, Orr-Urtreger A. Lower core body temperature and attenuated nicotine-induced hypothermic response in mice lacking the β4 neuronal nicotinic acetylcholine receptor subunit. Brain Research Bulletin. 2005;66:30–36. doi: 10.1016/j.brainresbull.2005.02.032. doi:S0361-9230(05)00107-3. [DOI] [PubMed] [Google Scholar]
- Salas R, Cook KD, Bassetto L, De Biasi M. The α3 and β4 nicotinic acetylcholine receptor subunits are necessary for nicotine-induced seizures and hypolocomotion in mice. Neuropharmacology. 2004a;47:401–407. doi: 10.1016/j.neuropharm.2004.05.002. doi:10.1016/j.neuropharm.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Salas R, Pieri F, De Biasi M. Decreased signs of nicotine withdrawal in mice null for the β4 nicotinic acetylcholine receptor subunit. Journal of Neuroscience. 2004b;24:10035–10039. doi: 10.1523/JNEUROSCI.1939-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas R, Pieri F, Fung B, Dani JA, De Biasi M. Altered anxiety-related responses in mutant mice lacking the β4 subunit of the nicotinic receptor. Journal of Neuroscience. 2003;23:6255–6263. doi: 10.1523/JNEUROSCI.23-15-06255.2003. doi:23/15/6255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M, Parikh V, Howe WM. nAChR agonist-induced cognition enhancement: Integration of cognitive and neuronal mechanisms. Biochemical Pharmacology. 2009;78:658–667. doi: 10.1016/j.bcp.2009.04.019. doi:S0006-2952(09)00305-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skolnick P. Modulation of glutamate receptors: Strategies for the development of novel antidepressants. Amino Acids. 2002;23:153–159. doi: 10.1007/s00726-001-0121-7. doi:10.1007/s00726-001-0121-7. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M. Cognitive enhancement as a pharmacotherapy target for stimulant addiction. Addiction. 2010;105:38–48. doi: 10.1111/j.1360-0443.2009.02791.x. doi:ADD2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart CA, Reid IC. Antidepressant mechanisms: Functional and molecular correlates of excitatory amino acid neurotransmission. Molecular Psychiatry. 2002;7(Suppl. 1):S15–S22. doi: 10.1038/sj.mp.4001014. doi:10.1038/sj.mp.4001014. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Jarvis MJ. The scientific case that nicotine is addictive. Psychopharmacology (Berlin) 1995;117:2–10. doi: 10.1007/BF02245088. discussion, 14–20. [DOI] [PubMed] [Google Scholar]
- Wada E, Wada K, Boulter J, Deneris E, Heinemann S, Patrick J, et al. Distribution of alpha 2, alpha 3, alpha 4, and beta 2 neuronal nicotinic receptor subunit mRNAs in the central nervous system: A hybridization histochemical study in the rat. Journal of Comparative Neurology. 1989;284:314–335. doi: 10.1002/cne.902840212. doi:10.1002/cne.902840212. [DOI] [PubMed] [Google Scholar]
- Wang F, Gerzanich V, Wells GB, Anand R, Peng X, Keyser K, et al. Assembly of human neuronal nicotinic receptor α5 subunits with α3, β2, and β4 subunits. Journal of Biological Chemistry. 1996;271:17656–17665. doi: 10.1074/jbc.271.30.17656. [DOI] [PubMed] [Google Scholar]
- Wehner JM, Keller JJ, Keller AB, Picciotto MR, Paylor R, Booker TK, et al. Role of neuronal nicotinic receptors in the effects of nicotine and ethanol on contextual fear conditioning. Neuroscience. 2004;129:11–24. doi: 10.1016/j.neuroscience.2004.07.016. doi:S0306-4522(04)00621-9. [DOI] [PubMed] [Google Scholar]
- Wehner JM, Radcliffe RA. Cued and contextual fear conditioning in mice. Current Protocols in Neuroscience. 2004 doi: 10.1002/0471142301.ns0805cs27. Chapter 8, Unit 8.5C. doi:10.1002/0471142301.ns0805cs27. [DOI] [PubMed] [Google Scholar]
- Xu W, Orr-Urtreger A, Nigro F, Gelber S, Sutcliffe CB, Armstrong D, et al. Multiorgan autonomic dysfunction in mice lacking the β2 and the β4 subunits of neuronal nicotinic acetylcholine receptors. Journal of Neuroscience. 1999;19:9298–9305. doi: 10.1523/JNEUROSCI.19-21-09298.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.