Abstract
Objectives. We examined the relationship of age at diagnosis and insurance status with stage among cervical cancer patients aged 21 to 85 years.
Methods. We selected data on women (n = 69 739) diagnosed with invasive cervical cancer between 2000 and 2007 from the National Cancer Database. We evaluated the association between late stage (stage III/IV) and both insurance and age, with adjustment for race/ethnicity and other sociodemographic and clinical factors. We used multivariable log binomial models to estimate risk ratios (RRs) and 95% confidence intervals (CIs).
Results. The proportion of late-stage disease increased with age: from 16.53% (21–34 years) to 42.44% (≥ 70 years). The adjusted relative risk of advanced-stage disease among women aged 50 years and older was 2.2 to 2.5 times that of patients aged 21 to 34 years. Uninsured (RR = 1.44; 95% CI = 1.40, 1.49), Medicaid (RR = 1.37, 95% CI = 1.34, 1.41), younger Medicare (RR = 1.12, 95% CI = 1.06, 1.19), and older Medicare (RR = 1.20, 95% CI = 1.15, 1.26) patients had a higher risk of late-stage disease than did privately insured patients.
Conclusions. Screening should be encouraged for women at high risk for advanced-stage disease.
The American Cancer Society estimates that 12 710 women will be diagnosed with cervical cancer and 4290 women will die from the disease in 2011.1 Although incidence and mortality from cervical cancer have declined since the introduction of the Papanicolaou (Pap) test, approximately 35% of cervical cancer patients are diagnosed with regional disease and 11% with distant-stage disease.2,3 Prognosis is strongly related to stage: the 5-year relative survival rate is 91.2% for patients with localized disease, but only 57.8% for patients with regional disease and 17.0% for those with distant disease.3
Socioeconomic status, race, marital status, and geographic location have been identified as factors related to late stage at diagnosis among cervical cancer patients.4–10 Previous studies also documented older age as a significant predictor of advanced stage, although the effects of insurance and age, which are 2 of the strongest predictors of cervical cancer screening, have not been studied together.11,12 Women without health insurance are less likely to receive cervical cancer and other recommended cancer screening tests, yet few studies have examined the association between insurance status and cervical cancer stage at diagnosis, and the existing studies were limited to elderly (aged ≥ 65 years) Medicare recipients or patients from single-state tumor registries.4,13 We examined the relationship of both age and insurance status with late-stage disease after adjustment for other known risk factors. Ours was the first study to our knowledge to examine this relationship in a large national sample of cancer patients.
METHODS
Our data came from the National Cancer Database (NCDB), a hospital-based cancer registry jointly sponsored by the American Cancer Society and the American College of Surgeons. The NCDB documents approximately 70% of all malignant cancers in the United States, from more than 1400 facilities accredited by the American College of Surgeons’ Commission on Cancer.14 The NCDB contains standardized data elements on patient demographics, insurance status, tumor characteristics, and first course of treatment, as well as census-level socioeconomic factors and facility-level factors.
Patients and Measures
We selected patients with a first primary invasive tumor of the cervix diagnosed between 2000 and 2007 (n = 76 862). We excluded patients younger than 21 years or older than 85 years from analyses (n = 1427). Because of small sample sizes, we excluded patients who listed “other forms” of government insurance (Bureau of Indian Affairs, Public Health Service; n = 284). We also excluded patients with unknown clinical or pathological stage (n = 5097) or geographic region (n = 315). The total analytic cohort comprised 69 739 patients.
Our primary outcome was American Joint Commission on Cancer stage at diagnosis, defined as clinical stage.15 If clinical stage was missing, we used pathological stage as a proxy. Independent variables of special focus were age, categorized into 9 groups (21–34, 35–39, 40–44, 45–49, 50–54, 55–59, 60–64, 65–69 and 70–85 years), and insurance type, categorized as Medicaid, Medicare (Medicare alone or with supplemental insurance), uninsured, private insurance (health maintenance organizations, preferred provider organizations), and other or unknown. Because Medicare is available to essentially all US residents aged 65 years and older, but only for permanently disabled individuals younger than 65 years, we dichotomized Medicare patients into age groups: 21 to 64 years and 65 to 85 years.
We also included race/ethnicity and geographic residence (derived from US census classifications).16 We obtained area-level indicators of education (percentage of adults without a high school diploma) from zip code information in 2000 US Census data and reported them as quartiles of the observed distribution in the general US population.17
Statistical Analyses
We conducted analyses with SAS version 9.2 (SAS Institute, Cary, NC). We used the χ2 test to analyze the relationship between insurance and all other covariates (P < .01). Because of the high prevalence (> 10%) of our outcomes, we used multivariable log binomial models to estimate risk ratios (RRs) and 95% confidence intervals (CIs); we used these to measure statistical significance.18 We made 2 separate comparisons: stage II versus stage I and stage III or IV versus stage I. These separate comparisons allowed us to examine the risk of moderate early stage (stage II), which may be curable, and advanced stage (III and IV), which may not be curable. We also stratified results by insurance to more closely examine the relationship of stage with insurance and age at diagnosis. We plotted stage at diagnosis against continuous age at diagnosis, and we calculated unadjusted slopes to measure the change in late stage at diagnosis for each additional year of age.
The NCDB only collects data from hospitals approved by the Commission on Cancer, which are located primarily in urban areas and offer more cancer services than other hospitals.19 Therefore, we compared the NCDB cervical case counts with those of the North American Association of Central Cancer Registries,20 which covers 98% of incident US cancer cases, and the population-based registries in Surveillance Epidemiology and End Results (SEER 17) data, which cover 28% of incident US cancer cases.21 We also assessed factors related to missing stage at diagnosis in the NCDB to estimate potential selection bias, with multivariable log binomial analyses.
Screening data are not available in the NCDB, so we used data from the National Health Interview Survey (NHIS) to estimate the prevalence of cervical cancer screening (defined as reporting a Papanicolau test in the past 3 years) among US women by age group and health insurance status. NHIS, an annual nationwide cross-sectional household survey of the civilian, noninstitutionalized population, is designed to provide national prevalence estimates on personal, socioeconomic, demographic, and health characteristics of the US population. It is the official federal monitoring instrument for US cancer screening objectives. Hispanics and African Americans are oversampled in NHIS to improve estimate precision for these populations.22 Consistent with the NCDB analysis, we conducted assessment of cervical cancer screening with pooled NHIS data for 2000 and 2005 and used comparable age and insurance categories. We also weighted NHIS prevalence estimates to account for the survey's complex population sampling.
RESULTS
Approximately 10.12% of patients in our sample were uninsured, 17.54% were Medicaid recipients, 2.94% were younger Medicare recipients, 12.74% were older Medicare recipients, 52.24% were privately insured, and 4.42% had missing data for insurance status (Table 1). The average age at diagnosis was 49.68 years (SD = 14.13). A higher percentage of Medicaid, uninsured, and younger Medicare patients than of privately insured patients were African American or Hispanic. Histologic types varied by insurance type. Among privately insured patients, 23.98% were diagnosed with adenocarcinoma, as were 12.27% of the uninsured, 11.37% of Medicaid recipients, and 14.39% of younger Medicare recipients.
TABLE 1—
Characteristics of Cervical Cancer Patients by Insurance Status: National Cancer Database, United States, 2000–2007
| Characteristic | Total, No. (%) | Uninsured, No. (%) | Medicaid, No. (%) | Younger Medicare,a No. (%) | Older Medicare,b No. (%) | Private, No. (%) | Missing, No. (%) |
| Total | 69 739 (100.0) | 7056 (10.12) | 12 234 (17.54) | 2050 (2.94) | 8886 (12.74) | 36 429 (52.24) | 3084 (4.42) |
| Stage | |||||||
| I | 32 666 (46.84) | 2566 (36.37) | 4912 (40.15) | 867 (42.29) | 2744 (30.88) | 20 201 (55.45) | 1376 (44.62) |
| II | 16 282 (23.35) | 2005 (28.42) | 3099 (25.33) | 513 (25.02) | 2473 (27.83) | 7474 (20.52) | 718 (23.28) |
| III | 13 679 (19.61) | 1601 (22.69) | 2845 (23.25) | 416 (20.29) | 2198 (24.74) | 5980 (16.42) | 639 (20.72) |
| IV | 7112 (10.2) | 884 (12.53) | 1378 (11.26) | 254 (12.39) | 1471 (16.55) | 2774 (7.61) | 351 (11.38) |
| Age,c y | |||||||
| 21–34 | 9727 (13.95) | 932 (13.21) | 2180 (17.82) | 126 (6.15) | … | 6055 (16.62) | 434 (14.07) |
| 35–39 | 8847 (12.69) | 910 (12.9) | 1867 (15.26) | 156 (7.61) | … | 5492 (15.08) | 422 (13.68) |
| 40–44 | 10 346 (14.84) | 1237 (17.53) | 2101 (17.17) | 239 (11.66) | … | 6346 (17.42) | 423 (13.72) |
| 45–49 | 9461 (13.57) | 1171 (16.6) | 1731 (14.15) | 296 (14.44) | … | 5815 (15.96) | 448 (14.53) |
| 50–54 | 7828 (11.22) | 968 (13.72) | 1485 (12.14) | 300 (14.63) | … | 4669 (12.82) | 406 (13.16) |
| 55–59 | 6521 (9.35) | 792 (11.22) | 1175 (9.6) | 447 (21.8) | … | 3816 (10.48) | 291 (9.44) |
| 60–64 | 5057 (7.25) | 655 (9.28) | 1005 (8.21) | 486 (23.71) | … | 2662 (7.31) | 249 (8.07) |
| 65–69 | 4120 (5.91) | 184 (2.61) | 309 (2.53) | … | 2769 (31.16) | 711 (1.95) | 147 (4.77) |
| 70–85 | 7832 (11.23) | 207 (2.93) | 381 (3.11) | … | 6117 (68.84) | 863 (2.37) | 264 (8.56) |
| Race/ethnicity | |||||||
| Non-Hispanic White | 40 903 (58.65) | 3088 (43.76) | 5585 (45.65) | 1147 (55.95) | 5475 (61.61) | 24 265 (66.61) | 1343 (43.55) |
| Hispanic | 8571 (12.29) | 1796 (25.45) | 2355 (19.25) | 20510 | 614 (6.91) | 2933 (8.05) | 668 (21.66) |
| African American | 11 042 (15.83) | 1409 (19.97) | 2752 (22.49) | 465 (22.68) | 1571 (17.68) | 4282 (11.75) | 563 (18.26) |
| Other/Asian | 3598 (5.16) | 410 (5.81) | 781 (6.38) | 74 (3.61) | 423 (4.76) | 1696 (4.66) | 214 (6.94) |
| Missing | 5625 (8.07) | 3535 | 761 (6.22) | 159 (7.76) | 803 (9.04) | 3253 (8.93) | 296 (9.6) |
| Histology | |||||||
| Adenocarcinoma | 13 484 (19.33) | 866 (12.27) | 1391 (11.37) | 295 (14.39) | 1640 (18.46) | 8736 (23.98) | 556 (18.03) |
| Adenosquamous/GC | 2798 (4.01) | 265 (3.76) | 441 (3.6) | 81 (3.95) | 254 (2.86) | 1629 (4.47) | 128 (4.15) |
| Other specified carcinoma | 1025 (1.47) | 73 (1.03) | 138 (1.13) | 31 (1.51) | 173 (1.95) | 578 (1.59) | 32 (1.04) |
| SCC | 50 381 (72.24) | 5645 (80) | 9904 (80.95) | 1583 (77.22) | 6438 (72.45) | 24 542 (67.37) | 2269 (73.57) |
| Unspecified carcinoma | 2051 (2.94) | 207 (2.93) | 360 (2.94) | 60 (2.93) | 381 (4.29) | 944 (2.59) | 99 (3.21) |
| Region | |||||||
| Northwest | 13 222 (18.96) | 1009 (14.3) | 2147 (17.55) | 395 (19.27) | 1894 (21.31) | 7290 (20.01) | 487 (15.79) |
| Midwest | 15 915 (22.82) | 1290 (18.28) | 2488 (20.34) | 436 (21.27) | 2225 (25.04) | 8868 (24.34) | 608 (19.71) |
| South | 27 847 (39.93) | 3715 (52.65) | 4494 (36.73) | 844 (41.17) | 3603 (40.55) | 13 897 (38.15) | 1294 (41.96) |
| West | 12 755 (18.29) | 1042 (14.77) | 3105 (25.38) | 375 (18.29) | 1164 (13.1) | 6374 (17.5) | 695 (22.54) |
| Diagnosis year | |||||||
| 2000 | 9811 (14.07) | 1026 (14.54) | 1426 (11.66) | 264 (12.88) | 1270 (14.29) | 5205 (14.29) | 620 (20.1) |
| 2001 | 9547 (13.69) | 1028 (14.57) | 1418 (11.59) | 231 (11.27) | 1255 (14.12) | 5069 (13.91) | 546 (17.7) |
| 2002 | 9223 (13.23) | 91713 | 1523 (12.45) | 268 (13.07) | 1237 (13.92) | 4783 (13.13) | 495 (16.05) |
| 2003 | 8377 (12.01) | 869 (12.32) | 1511 (12.35) | 211 (10.29) | 1057 (11.9) | 4346 (11.93) | 383 (12.42) |
| 2004 | 8129 (11.66) | 840 (11.9) | 1556 (12.72) | 245 (11.95) | 1061 (11.94) | 4110 (11.28) | 317 (10.28) |
| 2005 | 8274 (11.86) | 838 (11.88) | 1561 (12.76) | 261 (12.73) | 1033 (11.63) | 4291 (11.78) | 290 (9.4) |
| 2006 | 8307 (11.91) | 788 (11.17) | 1626 (13.29) | 277 (13.51) | 995 (11.2) | 4376 (12.01) | 245 (7.94) |
| 2007 | 8071 (11.57) | 750 (10.63) | 1613 (13.18) | 293 (14.29) | 978 (11.01) | 4249 (11.66) | 188 (6.1) |
| Median no high school diploma, by zip code, % | |||||||
| ≥ 29 | 18 050 (25.88) | 2612 (37.02) | 4567 (37.33) | 640 (31.22) | 2285 (25.71) | 6804 (18.68) | 1142 (37.03) |
| 20–28.9 | 17 340 (24.86) | 1825 (25.86) | 3392 (27.73) | 568 (27.71) | 2345 (26.39) | 8455 (23.21) | 755 (24.48) |
| 14–19.9 | 13 915 (19.95) | 1126 (15.96) | 2047 (16.73) | 412 (20.1) | 1925 (21.66) | 7907 (21.71) | 498 (16.15) |
| < 14 | 16 397 (23.51) | 1125 (15.94) | 159113 | 329 (16.05) | 1852 (20.84) | 10 954 (30.07) | 546 (17.7) |
| Missing | 4037 (5.79) | 368 (5.22) | 637 (5.21) | 101 (4.93) | 479 (5.39) | 2309 (6.34) | 143 (4.64) |
| Residence type | |||||||
| Urban | 63 351 (90.84) | 6350 (89.99) | 11 172 (91.32) | 1879 (91.66) | 8091 (91.05) | 33 014 (90.63) | 2845 (92.25) |
| Rural | 1267 (1.82) | 96 (1.36) | 264 (2.16) | 55 (2.68) | 220 (2.48) | 571 (1.57) | 61 (1.98) |
| Missing | 5121 (7.34) | 610 (8.65) | 798 (6.52) | 116 (5.66) | 575 (6.47) | 2844 (7.81) | 178 (5.77) |
Note. GC = glassy cell carcinoma; SCC = squamous cell carcinoma. Ellipses indicate not applicable. All comparisons were significant (P < .001).
Aged 18–64 years.
Aged ≥ 65 years.
Mean age (SD) by insurance: uninsured, 47.17 (11.17); Medicaid recipient, 45.69 (11.79); younger Medicare recipient, 51.26 (9.52); older Medicare recipient, 73.43 (5.70); privately insured, 45.71 (11.10); missing data, 48.81 (13.32).
Approximately 55.45% of privately insured patients, 36.37% of uninsured patients, 40.15% of Medicaid recipients, 42.29% of younger Medicare recipients, and 30.88% of older Medicare recipients were diagnosed with stage I disease (Table 1). Stage varied greatly by age: the proportion of patients diagnosed with stage I tumors declined by 0.77% with each additional year, and stage IV tumors increased by 0.27% for each additional year (Figure A, available as a supplement to the online version of this article at http://www.ajph.org).
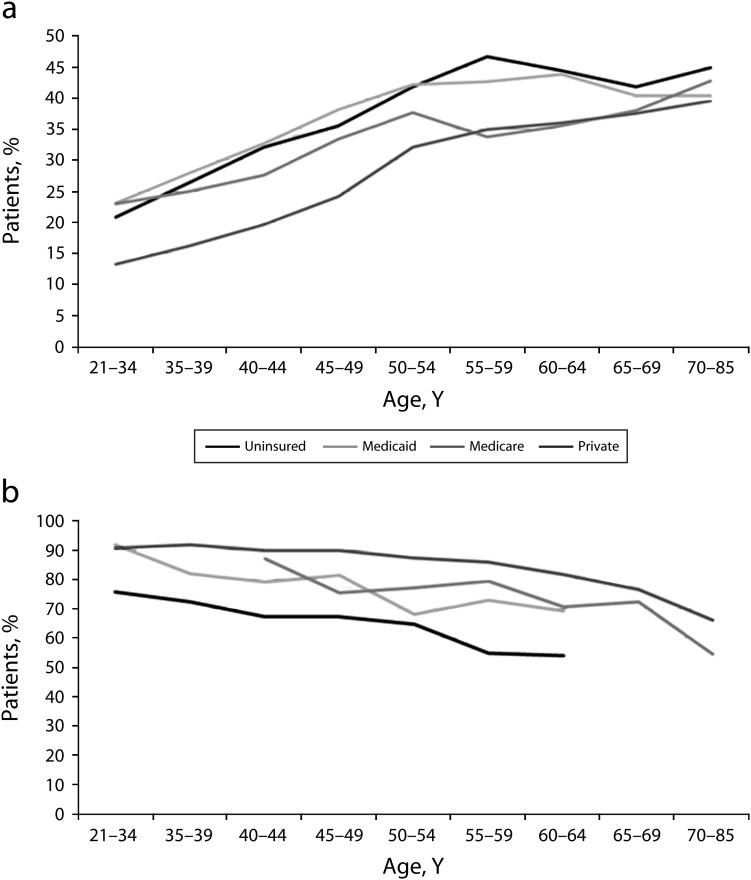
When we examined the combined effect of age and insurance type, we found that privately insured patients aged 18 to 50 years had much lower rates of advanced-stage disease than did patients with other insurance types (Figure 1a). However, the rates of late-stage disease among privately insured patients approached convergence with other patients with increasing age. Privately insured patients aged 18 to 34 years had the lowest rate of advanced-stage disease (13.31%); uninsured patients aged 55 to 59 years had the highest rate (46.72%). NHIS data also showed differences in cervical cancer screening by insurance type and age, with higher screening rates among privately insured and younger women (Figure 1b). Uninsured women aged 50 years and older had the lowest Pap test rates.
FIGURE 1—
Invasive cervical cancer diagnoses and screening, by age group and insurance category, for (a) late-stage diagnosis and (b) Papanicolaou test in past 3 years: United States.
Source. (a) National Cancer Database, 2000–200714; (b) National Health Interview Survey, 2000–2005.22
In multivariable models predicting moderate disease (stage II), age was the strongest predictor: patients aged 50 years and older were nearly twice as likely as patients aged 21 to 34 years to be diagnosed with stage II disease (Table 2). Uninsured and Medicaid-insured patients were respectively 1.43 and 1.32 times as likely as privately insured patients to be diagnosed with stage II disease. The likelihood of stage II disease was also higher among younger and older Medicare patients than among the privately insured. Patients diagnosed with adenocarcinoma or adenosquamous–glassy cell carcinomas were less likely than patients with squamous cell carcinoma to be diagnosed with stage II cancer. We observed only a tentative association between race/ethnicity and stage II disease.
TABLE 2—
Multivariable Models Predicting Stage II Versus Stage I and Stage III/IV Versus Stage I Among Cervical Cancer Patients: National Cancer Database, United States, 2000–2007
| Variable | Stage II vs Stage I, RR (95% CI) | Stage III/IV vs Stage I, RR (95% CI) |
| Age, y | ||
| 21–34 (Ref) | 1.00 | 1.00 |
| 35–39 | 1.22 (1.15, 1.29) | 1.25 (1.18, 1.32) |
| 40–44 | 1.44 (1.36, 1.52) | 1.50 (1.43, 1.58) |
| 45–49 | 1.72 (1.63, 1.82) | 1.83 (1.74, 1.92) |
| 50–54 | 1.92 (1.82, 2.03) | 2.20 (2.09, 2.31) |
| 55–59 | 1.98 (1.87, 2.10) | 2.32 (2.21, 2.43) |
| 60–64 | 1.98 (1.86, 2.11) | 2.31 (2.20, 2.43) |
| 65–69 | 1.98 (1.85, 2.13) | 2.24 (2.11, 2.38) |
| 70–85 | 2.19 (2.05, 2.35) | 2.49 (2.35, 2.63) |
| Insurance status | ||
| Private (Ref) | 1.00 | 1.00 |
| Uninsured | 1.43 (1.38, 1.49) | 1.44 (1.40, 1.49) |
| Medicaid | 1.32 (1.28, 1.37) | 1.37 (1.34, 1.41) |
| Younger Medicarea | 1.13 (1.05, 1.21) | 1.12 (1.06, 1.19) |
| Older Medicareb | 1.19 (1.12, 1.26) | 1.20 (1.15, 1.26) |
| Missing | 1.17 (1.10, 1.25) | 1.27 (1.21, 1.33) |
| Race/ethnicity | ||
| Non-Hispanic White (Ref) | 1.00 | 1.00 |
| Hispanic | 1.05 (1.01, 1.09) | 0.86 (0.83, 0.89) |
| African American | 1.05 (1.02, 1.09) | 1.05 (1.02, 1.08) |
| Other/Asian | 1.06 (1.01, 1.12) | 0.88 (0.84, 0.92) |
| Missing | 1.01 (0.97, 1.06) | 0.96 (0.93, 1.00) |
| Histology | ||
| SCC (Ref) | 1.00 | 1.00 |
| Adenocarcinoma | 0.71 (0.68, 0.73) | 0.69 (0.67, 0.71) |
| Adenosquamous/GC | 0.88 (0.83, 0.94) | 0.90 (0.86, 0.96) |
| Other specified carcinoma | 1.00 (0.90, 1.12) | 1.29 (1.24, 1.35) |
| Unspecified carcinoma | 0.91 (0.84, 0.99) | 1.23 (1.18, 1.28) |
| Diagnosis year | ||
| 2000–2001 (Ref) | 1.00 | 1.00 |
| 2002–2003 | 1.04 (1.01, 1.08) | 1.07 (1.04, 1.09) |
| 2004–2005 | 1.06 (1.03, 1.10) | 1.10 (1.07, 1.13) |
| 2006–2007 | 1.06 (1.02, 1.09) | 1.13 (1.10, 1.16) |
Note. CI = confidence interval; GC = glassy cell carcinoma; RR = risk ratio; SCC = squamous cell carcinoma. Model also adjusted for area-level education, geographic region, and urban or rural residence.
Aged 18–64 years.
Aged ≥ 65 years.
In multivariable models predicting advanced-stage disease (stages III and IV), age was the most important predictor, followed by insurance type (Table 2). The risk of advanced-stage disease among women aged 50 years and older was 2.2 to 2.5 times that of patients aged 21 to 34 years. Uninsured and Medicaid patients were respectively 1.44 and 1.37 times as likely to be diagnosed with stage III or IV as with stage I disease. Younger and older Medicare patients were also more likely than others to be diagnosed with stage III or IV disease; however, their risk of advanced disease was not as pronounced as that of uninsured and Medicaid patients. Women diagnosed with adenocarcinomas were less likely to be diagnosed with late stage compared to squamous cell carcinomas. African Americans had a slightly higher risk of advanced-stage disease, and Hispanics and other race/ethnicity groups had a lower risk than did Whites.
We also examined associations with age and other covariates in multivariable models stratified by insurance type (Table 3). Although privately insured patients had lower percentages of advanced-stage disease across all age groups, they also had the steepest gradient of increasing relative risk by age of all insurance groups. Among privately insured women, we found an approximately tripled relative risk for women aged 55 years and older; among women in other insurance categories, maximum relative risks were around 2. Among uninsured, Medicaid-insured, and Medicare-insured patients, Hispanics and women of other races were less likely to be diagnosed with late-stage disease than were White women. Among privately insured and older Medicare-insured women, African Americans were more likely than Whites to be diagnosed with late-stage disease. Patients with adenocarcinomas were less likely than women with squamous cell carcinoma to be diagnosed with late-stage disease, regardless of insurance type.
TABLE 3—
Multivariable Models Predicting Stage III/IV Versus Stage I by Insurance Type Among Cervical Cancer Patients: National Cancer Database, 2000–2007
| Uninsured |
Medicaid |
Younger Medicarea |
Older Medicareb |
Private |
||||||
| Variable | Late Stage, % | RR (95% CI) | Late Stage, % | RR (95% CI) | Late Stage, % | RR (95% CI) | Late Stage, % | RR (95% CI) | Late Stage, % | RR (95% CI) |
| Age | ||||||||||
| 21–34 (Ref) | 20.82 | 1.00 | 23.07 | 1.00 | 23.02 | 1.00 | … | … | 13.31 | 1.00 |
| 35–39 | 26.48 | 1.19 (1.03, 1.37) | 27.96 | 1.26 (1.14, 1.39) | 25.00 | 1.09 (0.74, 1.59) | … | … | 16.19 | 1.28 (1.17, 1.39) |
| 40–44 | 32.17 | 1.36 (1.19, 1.56) | 32.75 | 1.52 (1.39, 1.67) | 27.62 | 1.24 (0.88, 1.76) | … | … | 19.67 | 1.58 (1.46, 1.71) |
| 45–49 | 35.53 | 1.52 (1.34, 1.74) | 38.19 | 1.78 (1.63, 1.95) | 33.45 | 1.62 (1.17, 2.23) | … | … | 24.23 | 2.02 (1.87, 2.18) |
| 50–54 | 41.84 | 1.71 (1.50, 1.94) | 42.22 | 1.97 (1.80, 2.15) | 37.67 | 1.69 (1.22, 2.32) | … | … | 32.15 | 2.67 (2.48, 2.87) |
| 55–59 | 46.72 | 1.82 (1.60, 2.07) | 42.72 | 2.01 (1.83, 2.20) | 33.78 | 1.55 (1.13, 2.11) | … | … | 34.96 | 2.89 (2.68, 3.11) |
| 60–64 | 44.43 | 1.82 (1.58, 2.08) | 43.88 | 2.00 (1.82, 2.19) | 35.60 | 1.67 (1.23, 2.28) | … | … | 35.99 | 2.92 (2.71, 3.16) |
| 65–69 | 41.85 | 1.77 (1.45, 2.16) | 40.45 | 1.90 (1.66, 2.18) | … | … | 38.06 | 1.00 | 37.55 | 2.96 (2.67, 3.28) |
| 70–85 | 44.93 | 2.04 (1.70, 2.44) | 40.42 | 2.00 (1.76, 2.27) | … | … | 42.75 | 1.12 (1.07, 1.17) | 39.63 | 3.23 (2.95, 3.54) |
| Race/ethnicity | ||||||||||
| Non-Hispanic White (Ref) | 38.08 | 1.00 | 35.2 | 1.00 | 32.61 | 1.00 | 41.52 | 1.00 | 23.58 | 1.00 |
| Hispanic | 27.95 | 0.81 (0.75, 0.88) | 29.85 | 0.83 (0.77, 0.88) | 28.29 | 0.91 (0.73, 1.13) | 34.69 | 0.83 (0.75, 0.92) | 21.75 | 0.96 (0.90, 1.03) |
| African American | 40.31 | 0.98 (0.91, 1.06) | 39.06 | 1.04 (0.99, 1.10) | 35.27 | 1.02 (0.89, 1.17) | 45.32 | 1.06 (1.01, 1.12) | 28.82 | 1.11 (1.06, 1.16) |
| Other/Asian | 26.34 | 0.76 (0.65, 0.89) | 31.63 | 0.83 (0.75, 0.91) | 27.03 | 0.86 (0.61, 1.22) | 34.99 | 0.88 (0.78, 0.99) | 25.18 | 1.04 (0.97, 1.12) |
| Histology | ||||||||||
| SCC (Ref) | 35.87 | 1.00 | 35.33 | 1.00 | 32.53 | 1.00 | 40.73 | 1.00 | 26.22 | 1.00 |
| Adenocarcinoma | 27.02 | 0.78 (0.70, 0.86) | 25.23 | 0.70 (0.64, 0.76) | 25.08 | 0.78 (0.65, 0.94) | 38.84 | 0.89 (0.83, 0.94) | 15.36 | 0.58 (0.55, 0.61) |
| Adenosquamous/GC | 31.32 | 0.86 (0.73, 1.02) | 30.84 | 0.88 (0.77, 1.00) | 30.86 | 0.97 (0.72, 1.31) | 39.76 | 0.98 (0.86, 1.12) | 23.33 | 0.90 (0.82, 0.97) |
| Other specified carcinoma | 57.53 | 1.37 (1.10, 1.71) | 46.38 | 1.22 (1.06, 1.41) | 61.29 | 1.71 (1.35, 2.16) | 45.66 | 0.99 (0.86, 1.14) | 47.75 | 1.54 (1.44, 1.63) |
Note. CI = confidence interval; GC = glassy cell carcinoma; RR = risk ratio; SCC = squamous cell carcinoma. Ellipses indicate not applicable. Model also adjusted for area-level education, geographic region, and urban or rural residence.
Aged 18–64 years.
Aged ≥ 65 years.
The NCDB cervical cancer coverage rate was 88% of the North American Association of Central Cancer Registries' gold and silver registries20 in 2006 and 83% in 2004. The NCDB and SEER cases had similar age distributions, although SEER had a higher percentage (23%) of Hispanic patients and a lower percentage of unknown race/ethnicity (1%). The proportion of stage I patients in SEER (47.78%) was essentially the same as in the NCDB (46.84%); however, the proportion of stage II patients was higher in the NCDB (23.35%) than in SEER (16.38%). As a result, the NCDB had fewer stage III (19.61% vs 22.16%) and stage IV (10.20% vs 13.68%) cases than did SEER. These stage distributions point to slight differences between our study cohort and population-based registries. When we compared the 5097 (6.63%) NCDB cervical cancer patients who were missing staging data to the 69 739 (93.37%) who were not, we found no association with missing stage for race, geographic region, or area-level education in our multivariable analysis. Patients aged 70 to 85 years (RR = 1.19; 95% CI = 1.03, 1.37) and younger Medicare patients (RR = 1.25; 95% CI = 1.08, 1.45) were more likely to have missing data for stage.
DISCUSSION
In a large national sample of women diagnosed with cervical cancer in 2000 to 2007, the strongest predictor of late stage at diagnosis was age, followed by insurance. Late stage at diagnosis is likely attributable to underscreening as opposed to interval cancers (tumors arising between regular screenings), according to previous studies examining screening histories of women diagnosed with invasive cervical cancer.23–26 Although the risk of advanced-stage disease was highest among the oldest age group (70–85 years), the greatest change in advanced-stage disease occurred in women aged 40 to 44 years and 50 to 59 years, which coincide with, respectively, the periods in a woman’s life when reproduction typically ends and menopause begins.27,28
These changes in advanced-stage diagnoses with age corresponded with reported declines in cervical cancer screening between women aged 25 to 44 years (85.7% reported having a Pap test in the past 3 years) and women aged 45 to 54 years (81.0% had been screened). This decline was also reported in women aged 55 to 64 years (76.0%) and 65 to 74 years (61.6%).29 Our results suggest that interventions are needed to mitigate decreased screening compliance in middle-aged women (40–69 years), a period that saw the greatest shifts in advanced-stage diagnoses. Strengthening screening compliance in middle-aged women may offer an additional benefit in preventing advanced disease in older women (70–79 years), because it takes several years for tumors to progress.30 Guidelines for Pap tests vary by age; the most frequent screening (annual for conventional Pap tests and every 2 years for liquid-based Pap tests) is recommended for women aged 30 years and younger. Women aged 70 years and older who have had 3 or more normal Pap tests and no abnormal Pap tests in the past 10 years may choose to stop cervical cancer screening.
Reasons for screening noncompliance may also vary by age. In a study among women with deficient screening, postmenopausal women were more likely to report that screening was not recommended by a physician, and premenopasual women were more likely to identify procrastination as a reason for not being screened.31 Further study is needed to determine whether late stage at diagnosis among older women results from decreased screening attributable to patient-related factors or is related to providers’ (appropriate or inappropriate) application of the guidelines to low-risk women at older ages. In addition to variations in screening practices, clinical sampling errors, which may be caused in older women by a lack of cells in the area of the cervix (the cervical transformation zone) where cancer cells are typically found, may also play a role in increased rates of advanced-stage disease.32,33
The risk of advanced-stage cervical cancer was higher among patients without private insurance, particularly among uninsured women and Medicaid recipients. This finding is consistent with our NHIS data on Pap tests. Previous analyses of national survey data have also documented lower cervical cancer screening compliance among women lacking insurance and women without a usual care provider.11,12,34–38 Although general patterns between late stage at diagnosis and screening are similar, the specific shifts in stage in the NCDB do not precisely match NHIS screening data because the study populations may differ and self-reported data may overestimate cancer screening.39
In addition to lack of screening, uninsured and underinsured patients have higher rates of abnormal screening results40 and lower follow-up rates after an abnormal result. Such factors may also contribute to advanced-stage disease among these groups.41–43 Increasing the proportion of uninsured and Medicaid-insured cervical cancer patients diagnosed at an earlier stage through improved screening and follow-up after an abnormal result is not only important to lower morbidity and mortality, but may also offer cost savings, because advanced-stage cervical cancers are more expensive to treat than early-stage cancers.44 Even among privately insured patients, women aged 35 years and older were more likely to be diagnosed with advanced disease. Although we adjusted for education by zip code area, individual socioeconomic factors, such as lack of transportation and logistical challenges (time off of work and child care), may vary by age and negatively influence screening among women with access to care.45 Other studies have reported older age as the most prominent predictor of failure to screen among women within comprehensive health insurance plans,26 highlighting the need for increased Pap testing adherence even among middle-aged women with access to care for whom it is recommended.46
Similar to previous studies, we observed higher risks of advanced-stage disease in African Americans. However, when we stratified by insurance type, only privately insured and older Medicare-insured African Americans had statistically significant higher risks of advanced-stage disease than did older Medicare-insured and privately insured Whites. Previous studies of cervical cancer screening compliance by race/ethnicity among privately insured women reported mixed results: one study found lower screening rates among privately insured African Americans than Whites,47 but another group noted similar rates.26 Among uninsured and Medicaid-insured patients, Hispanics had lower risks of advanced-stage disease than did Whites. The negative association between stage and Hispanic ethnicity among uninsured and Medicaid-insured patients accords with a study reporting lower odds of cervical cancer screening among uninsured Whites than uninsured Hispanics and African Americans.48 Our results, along with those of others, provide some evidence that minority women are more savvy than White women about accessing subsidized services or safety net public services.48,49 In addition, national screening programs may be more effective at reaching minority women.
Although previous investigations suggested that Pap tests are more effective in detecting squamous cell carcinomas than adenocarcinomas,2,50,51 we observed lower risks of advanced stage among patients with adenocarcinomas. We initially hypothesized that insurance was confounding the relationship between stage and histologic type, because the proportion of adenocarcinomas among privately insured patients was much higher than among patients with other insurance types. Yet when we stratified our multivariable results by insurance type, we observed a decreased risk of advanced disease among patients with adenocarcinomas across all insurance types. The trend of lower rates of advanced-stage disease in adenocarcinomas has been observed in SEER data.50
Evidence suggests that adenocarcinomas are more likely to arise as interval cancers (developing between regular screenings).23 Interval cancers are more likely to be diagnosed at earlier stages than are cancers eventually detected in an underscreened population.23–25 In addition, some data indicate that the etiology of adenocarcinoma and squamous cell carcinoma differ: nulliparity and obesity may play a larger role in adenocarcinoma.51,52 How this difference affects the morphogenesis and stage progression of adenocarcinoma is unclear.52 More detailed studies to elucidate the paradoxical relationship between stage and histology among cervical cancers are needed.
Limitations
A limitation of our data source is that it only collects data on patients diagnosed or treated at facilities accredited by the Commission on Cancer, which are more likely to be located in urban areas and tend to be larger than nonaccredited facilities.14,19 However, when we compared cervical cancer case counts with North American Association of Central Cancer Registries data, the NCDB coverage rate was 85%, which is high relative to the estimated 70% NCDB coverage rate for all cancers combined.20 When we compared patient characteristics between the NCDB and SEER, the NCDB had fewer stage III and IV cancers, which should be considered when attempting to generalize our results to other populations. In addition, the impact of the National Breast and Cervical Cancer Early Detection Program on uninsured and Medicaid-insured patients in our study and the coverage rates are unclear. This program offers women with incomes below 250% of the federal poverty level the cervical cancer screening and treatment mandated by the federal Breast and Cervical Cancer Prevention and Treatment Act of 2000.53 Although the program screened more than 1.6 million women in 2004 to 2009, it reached fewer than 10% of eligible women.53,54
A sensitivity analysis examining factors related to missing staging data revealed higher rates of missing data among patients in the oldest age category and among younger Medicare patients. We believe the potential selection bias resulting from missing staging data elements was relatively minor, because only 7% of all selected cervical cancer patients were missing stage information. An additional limitation was that no formal validation study of the insurance status data in the NCDB has been conducted. Although this variable does not reflect changes in insurance type or specific covered benefits over time, it does reflect insurance coverage during initial treatment, with the exception of Medicaid recipients.55
Conclusions
Ours was the first study to examine the relationship between cervical cancer stage and insurance in a large contemporary cohort of women. We found age to be the strongest predictor of advanced-stage disease, regardless of insurance type, and uninsured and Medicaid-insured patients to be significantly more likely to present with advanced-stage cervical cancer than were women with private insurance. Advanced-stage disease leads not only to poorer quality of life and greater morbidity, but often to higher treatment costs as well.44 Screening should be made accessible and affordable for all women for whom it is recommended,46,56,57 especially for those at higher risk of advanced-stage disease, such as middle-aged women, Medicaid recipients, and uninsured women.
Acknowledgments
This study was funded by the American Cancer Society Intramural Research Department.
This research was presented at the Fourth Annual American Association for Cancer Research Conference on the Science of Cancer Health Disparities, Washington, DC, September 18–21, 2011.
Human Participant Protection
This study was granted exemption status from the Morehouse University institutional review board because it used only de-identified hospital registry data.
References
- 1.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61(4):212–236 [DOI] [PubMed] [Google Scholar]
- 2.Watson M, Saraiya M, Benard Vet al. Burden of cervical cancer in the United States, 1998–2003. Cancer. 2008;113(10 suppl):2855–2864 [DOI] [PubMed] [Google Scholar]
- 3.Altekruse SFKC, Krapcho M, Neyman Net al. SEER Cancer Statistics Review, 1975–2007. Bethesda, MD: National Cancer Institute; 2010 [Google Scholar]
- 4.Ferrante JM, Gonzalez EC, Roetzheim RG, Pal N, Woodard L. Clinical and demographic predictors of late-stage cervical cancer. Arch Fam Med. 2000;9(5):439–445 [DOI] [PubMed] [Google Scholar]
- 5.Free K, Roberts S, Bourne Ret al. Cancer of the cervix—old and young, now and then. Gynecol Oncol. 1991;43(2):129–136 [DOI] [PubMed] [Google Scholar]
- 6.Singh GK, Miller BA, Hankey BF, Edwards BK. Persistent area socioeconomic disparities in U.S. incidence of cervical cancer, mortality, stage, and survival, 1975–2000. Cancer. 2004;101(5):1051–1057 [DOI] [PubMed] [Google Scholar]
- 7.Virnig BA, Baxter NN, Habermann EB, Feldman RD, Bradley CJ. A matter of race: early- versus late-stage cancer diagnosis. Health Aff (Millwood). 2009;28(1):160–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitchell JB, McCormack LA. Time trends in late-stage diagnosis of cervical cancer. Differences by race/ethnicity and income. Med Care. 1997;35(12):1220–1224 [DOI] [PubMed] [Google Scholar]
- 9.Mandelblatt J, Andrews H, Kerner J, Zauber A, Burnett W. Determinants of late stage diagnosis of breast and cervical cancer: the impact of age, race, social class, and hospital type. Am J Public Health. 1991;81(5):646–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liff JM, Chow WH, Greenberg RS. Rural-urban differences in stage at diagnosis. Possible relationship to cancer screening. Cancer. 1991;67(5):1454–1459 [DOI] [PubMed] [Google Scholar]
- 11.Breen N, Wagener DK, Brown ML, Davis WW, Ballard-Barbash R. Progress in cancer screening over a decade: results of cancer screening from the 1987, 1992, and 1998 National Health Interview Surveys. J Natl Cancer Inst. 2001;93(22):1704–1713 [DOI] [PubMed] [Google Scholar]
- 12.Swan J, Breen N, Graubard BIet al. Data and trends in cancer screening in the United States: results from the 2005 National Health Interview Survey. Cancer;116(20):4872–4881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riley GF, Potosky AL, Lubitz JD, Brown ML. Stage of cancer at diagnosis for Medicare HMO and fee-for-service enrollees. Am J Public Health. 1994;84(10):1598–1604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Commission on Cancer National Cancer Database (NCDB);2010. Available at: http://www.facs.org/cancer/ncdb/index.html. Accessed October 20, 2009 [Google Scholar]
- 15.American Joint Commission on Cancer AJCCC Cancer Staging Manual. 6th ed. Chicago, IL: Springer; 2002 [Google Scholar]
- 16.US Census Bureau Census regions and divisions of the United States. 2009. Available at: http://www.census.gov/geo/www/us_regdiv.pdf. Accessed July 15, 2010 [Google Scholar]
- 17.US Census Bureau. Census. 2000 Available at: http://www.census.gov/main/www/cen2000.html. Accessed Novemeber 1, 2010. [Google Scholar]
- 18.Deddens JA, Petersen MR. Approaches for estimating prevalence ratios. Occup Environ Med. 2008;65(7):481, 501–506 [DOI] [PubMed] [Google Scholar]
- 19.Bilimoria KY, Bentrem DJ, Stewart AK, Winchester DP, Ko CY. Comparison of Commission on Cancer-approved and -nonapproved hospitals in the United States: implications for studies that use the National Cancer Data Base. J Clin Oncol. 2009;27(25):4177–4181 [DOI] [PubMed] [Google Scholar]
- 20.North American Association of Central Cancer Registries CINA+ cancer incidence rates in North America. 2010. Available at: http://www.cancer-rates.info/naaccr. Accessed March 25, 2011 [Google Scholar]
- 21. Surveillance Epidemiology and End Results (SEER) Program. SEER*Stat Database: Incidence—SEER 17 Regs Research, Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2009 Sub (1973–2007 varying)—Linked To County Attributes—Total U.S., 1969–2007 Counties, released April 2010, based on the November 2009 submission. National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch; 2010.
- 22. National Center for Health Statistics. National Health Interview Survey (NHIS), Public Use Data Release, NHIS Survey Description. Hyattsville, MD: Centers for Disease Control and Prevention; 2000, 2005.
- 23.Kirschner B, Poll S, Rygaard C, Wahlin A, Junge J. Screening history in women with cervical cancer in a Danish population-based screening program. Gynecol Oncol. 2011;120(1):68–72 [DOI] [PubMed] [Google Scholar]
- 24.Gök M, Rozendaal L, Berkhof J, Visser O, Meijer CJL, van Kemenade FJ. Cytology history preceding cervical cancer diagnosis: a regional analysis of 286 cases. Br J Cancer. 2011;104(4):685–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Subramaniam A, Fauci JM, Schneider KEet al. Invasive cervical cancer and screening: what are the rates of unscreened and underscreened women in the modern era? J Low Genit Tract Dis. 2011;15(2):110–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leyden WA, Manos MM, Geiger AMet al. Cervical cancer in women with comprehensive health care access: attributable factors in the screening process. J Natl Cancer Inst. 2005;97(9):675–683 [DOI] [PubMed] [Google Scholar]
- 27.Ventura SAJ, Mosher W. Estimated Pregnancy Rates by Outcome for the United States, 1990–2004. Hyattsville, MD: Center for Disease Control and Prevention; 2008 [PubMed] [Google Scholar]
- 28.Gold EB, Bromberger J, Crawford Set al. Factors associated with age at natural menopause in a multiethnic sample of midlife women. Am J Epidemiol. 2001;153(9):865–874 [DOI] [PubMed] [Google Scholar]
- 29. Health, United States, 2009: With Special Feature on Medical Technology. Hyattsville, MD: National Center for Health Statistics; 2010. [PubMed]
- 30.Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet. 2007;370(9590):890–907 [DOI] [PubMed] [Google Scholar]
- 31.Harlan LC, Bernstein AB, Kessler LG. Cervical cancer screening: who is not screened and why? Am J Public Health. 1991;81(7):885–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Janerich DT, Hadjimichael O, Schwartz PEet al. The screening histories of women with invasive cervical cancer, Connecticut. Am J Public Health. 1995;85(6):791–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nanda K, McCrory DC, Myers ERet al. Accuracy of the Papanicolaou test in screening for and follow-up of cervical cytologic abnormalities: a systematic review. Ann Intern Med. 2000;132(10):810–819 [DOI] [PubMed] [Google Scholar]
- 34.Hsia J, Kemper E, Kiefe Cet al. The importance of health insurance as a determinant of cancer screening: evidence from the Women’s Health Initiative. Prev Med. 2000;31(3):261–270 [DOI] [PubMed] [Google Scholar]
- 35.Potosky AL, Breen N, Graubard BI, Parsons PE. The association between health care coverage and the use of cancer screening tests. Results from the 1992 National Health Interview Survey. Med Care. 1998;36(3):257–270 [DOI] [PubMed] [Google Scholar]
- 36.Berk ML, Schur CL. Access to care: how much difference does Medicaid make? Health Aff (Millwood). 1998;17(3):169–180 [DOI] [PubMed] [Google Scholar]
- 37.Ward E, Jemal A, Cokkinides Vet al. Cancer disparities by Race/Ethnicity and Socioeconomic Status. CA Cancer J Clin. 2004;54(2):78–93 [DOI] [PubMed] [Google Scholar]
- 38.Nash D, Chan C, Horowitz D, Vlahov D. Barriers and missed opportunities in breast and cervical cancer screening among women aged 50 and over, New York City, 2002. J Womens Health (Larchmt). 2007;16(1):46–56. [DOI] [PubMed]
- 39.Gordon NP, Hiatt RA, Lampert DI. Concordance of self-reported data and medical record audit for six cancer screening procedures. J Natl Cancer Inst. 1993;85(7):566–570 [DOI] [PubMed] [Google Scholar]
- 40.Yabroff KR, Freedman A, Brown ML, Ballard-Barbash R, McNeel T, Taplin S. Trends in abnormal cancer screening results in the United States of America. J Med Screen. 2007;14(2):67–72 [DOI] [PubMed] [Google Scholar]
- 41.Yabroff KR, Washington KS, Leader A, Neilson E, Mandelblatt J. Is the promise of cancer-screening programs being compromised? Quality of follow-up care after abnormal screening results. Med Care Res Rev. 2003;60:294–331 [DOI] [PubMed] [Google Scholar]
- 42.Melnikow J, Chan BK, Stewart GK. Do follow-up recommendations for abnormal Papanicolaou smears influence patient adherence? Arch Fam Med. 1999;8(6):510–514 [DOI] [PubMed] [Google Scholar]
- 43.Zapka J, Taplin SH, Price RA, Cranos C, Yabroff R. Factors in quality care—the case of follow-up to abnormal cancer screening tests—problems in the steps and interfaces of care. J Natl Cancer Inst Monogr. 2010;2010(40):58–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Subramanian S, Trogdon J, Ekwueme DU, Gardner JG, Whitmire JT, Rao C. Cost of cervical cancer treatment: implications for providing coverage to low-income women under the Medicaid expansion for cancer care. Womens Health Issues. 2010;20(6):400–405 [DOI] [PubMed] [Google Scholar]
- 45.Daley E, Alio A, Anstey EH, Chandler R, Dyer K, Helmy H. Examining barriers to cervical cancer screening and treatment in Florida through a socio-ecological lens. J Community Health. 2011;36(1):121–131 [DOI] [PubMed] [Google Scholar]
- 46.Saslow D, Runowicz CD, Solomon Det al. American Cancer Society guideline for the early detection of cervical neoplasia and cancer. CA Cancer J Clin. 2002;52(6):342–362 [DOI] [PubMed] [Google Scholar]
- 47.Sung HY, Kearney KA, Miller M, Kinney W, Sawaya GF, Hiatt RA. Papanicolaou smear history and diagnosis of invasive cervical carcinoma among members of a large prepaid health plan. Cancer. 2000;88(10):2283–2289 [PubMed] [Google Scholar]
- 48.Adams EK, Breen N, Joski PJ. Impact of the National Breast and Cervical Cancer Early Detection Program on mammography and Pap test utilization among White, Hispanic, and African American women: 1996–2000. Cancer. 2007;109(2 suppl):348–358 [DOI] [PubMed] [Google Scholar]
- 49.Kaiser Family Foundation Racial and Ethnic Disparities in Women’s Health Care Coverage and Access to Care: Findings From the 2001 Kaiser Women’s Health Survey. Washington, DC: Kaiser Family Foundation; 2004 [Google Scholar]
- 50.Bansal S, Lewin SN, Burke WMet al. Sarcoma of the cervix: natural history and outcomes. Gynecol Oncol. 2010;118(2):134–138 [DOI] [PubMed] [Google Scholar]
- 51.Wang SS, Sherman ME, Hildesheim A, Lacey JV, Jr, Devesa S. Cervical adenocarcinoma and squamous cell carcinoma incidence trends among White women and Black women in the United States for 1976–2000. Cancer. 2004;100(5):1035–1044 [DOI] [PubMed] [Google Scholar]
- 52.Brinton LA, Tashima KT, Lehman HFet al. Epidemiology of cervical cancer by cell type. Cancer Res. 1987;47(6):1706–1711 [PubMed] [Google Scholar]
- 53.Tangka FK, O’Hara B, Gardner JGet al. Meeting the cervical cancer screening needs of underserved women: the National Breast and Cervical Cancer Early Detection Program, 2004–2006. Cancer Causes Control. 2010;21(7):1081–1090 [DOI] [PubMed] [Google Scholar]
- 54.Centers for Disease Conrtol and Prevention National Breast and Cervical Cancer Early Detection Program (NBCCEDP). 2010. Available at: http://www.cdc.gov/cancer/nbccedp/data/summaries/national_aggregate.htm. Accessed March 24, 2011 [Google Scholar]
- 55.Bradley CJ, Given CW, Roberts C. Late stage cancers in a Medicaid-insured population. Med Care. 2003;41(6):722–728 [DOI] [PubMed] [Google Scholar]
- 56.Wright TC, Jr, Cox JT, Massad LS, Twiggs LB, Wilkinson EJ. 2001 Consensus Guidelines for the management of women with cervical cytological abnormalities. JAMA. 2002;287(16):2120–2129 [DOI] [PubMed] [Google Scholar]
- 57.US Preventative Task Force Screening for Cervical Cancer US Preventative Task Force; 2003. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspscerv.htm. Accessed April 3, 2011 [Google Scholar]