Abstract
Dialysis patients have a tendency to bleed, and clinicians sometimes encounter cases with a significant amount of spontaneous hemorrhage. We herein report two cases of spontaneous renal hemorrhage in hemodialysis patients.
Case 1
A 70-year-old male who had received hemodialysis for 8 years presented with right abdominal pain. He had a history of renal failure due to diabetes mellitus. CT showed a right perirenal hemorrhage. Angiography revealed a right renal artery hemorrhage, and catheter embolization was performed.
Case 2
A 76-year-old male who had undergone 7 years of continuous ambulatory peritoneal dialysis and 1 year of hemodialysis presented with right abdominal pain. He had a history of renal failure due to IgA nephropathy. CT showed a right perirenal hemorrhage. He received a blood transfusion and was put on absolute bed rest. At 2 days after admission, his anemia was found to have improved.
Key Words: Renal hemorrhage, Hemodialysis, Spontaneous renal hematoma
Introduction
Spontaneous subcapsular or perirenal hematomas are relatively uncommon but often diagnostically challenging conditions. The appropriate treatment of such patients is based first on the diagnosis that a subcapsular or perirenal hemorrhage has occurred, and second on the determination of its cause. An accurate diagnosis of the cause requires a combination of clinical information and radiologic imaging [1]. In this report, we describe two cases of spontaneous renal hemorrhage in hemodialysis patients and discuss the diagnosis and management of renal hemorrhage.
Case Report
Case 1
Patient: 70 years old, male.
Chief complaint: Right abdominal pain.
Past history: Hypertension since age 55, diabetes mellitus since age 58, and chronic renal failure since age 68.
Current Medical History
Ten hours after regular hemodialysis, the patient presented with right abdominal pain and came to our hospital. His laboratory data showed slight anemia with a hemoglobin (Hb) level of 9.2 mg/dl, which was almost his normal level. Although his abdominal pain was improved, he was not able to leave his bed due to orthostatic hypotension with systolic blood pressure from 100 to 50 mm Hg, so he was admitted to our department.
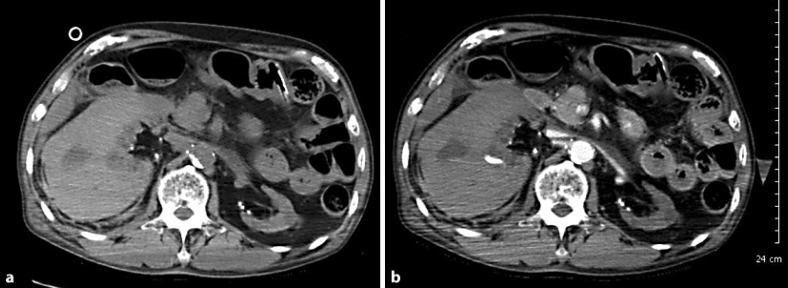
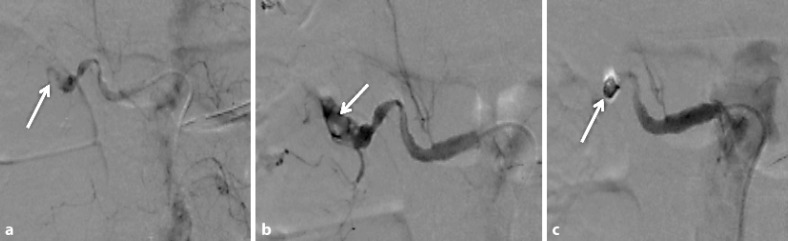
The next day (26 h from admission), he had cardiac arrest. After cardiopulmonary resuscitation was performed, an abdominal computed tomography (CT) showed right perirenal hemorrhage and the laboratory data showed a decreased Hb level of 4.2 mg/dl (fig. 1). Based on a diagnosis of hemorrhagic shock, an emergency angiography was performed. The right branch of his renal artery was leaking, so coiled catheter embolization was performed (fig. 2). Although the blood leakage was stopped, the patient's hemodynamics were unstable, and he died 47 h after admission. Pathological autopsy confirmed the diagnosis of right renal artery rupture.
Fig. 1.
Non-contrast (a) and contrast (b) axial CT images. A right perirenal hemorrhage was observed.
Fig. 2.
a Leakage from the renal artery. b, c Gel film and coiling for hemostasis and arrest of bleeding.
Case 2
Patient: 76 years old, male.
Chief complaint: Right abdominal pain.
Past history: Hypertension since age 61, IgA nephropathy since age 59, and chronic renal failure since age 67.
Current Medical History
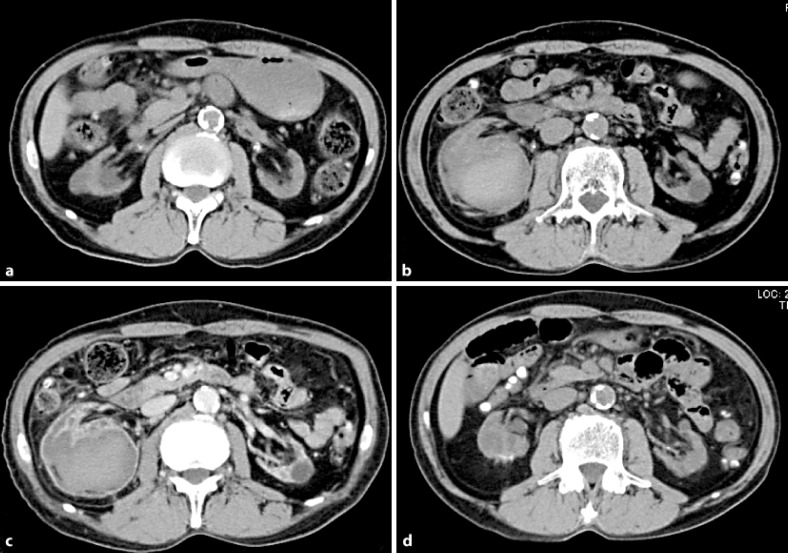
At the end of regular hemodialysis, the patient experienced right abdominal pain and was referred to our hospital for further examination. His laboratory data showed anemia, with a Hb level of 6.2 mg/dl and tachycardia of 96 beats per minute. CT examination showed a right perirenal hemorrhage (fig. 3b). Because his hemodynamics were stable after 4 units of blood had been transfused, emergency angiography was not performed, and the patient was put on absolute bed rest. Two days after admission, a CT scan revealed no widespread hemorrhage in the retroperipheral area. A contrast CT scan was performed for further examination, revealing acquired cystic disease of the kidney (ACDK), but no apparent renal tumor was detected (fig. 3b and c). Because the patient was suspected to have a renal tumor rupture and renal failure, we recommended a nephrectomy, but the patient refused the operation. Therefore, follow-up every 3 to 6 months by CT scanning was decided upon. At 18 months after the hemorrhage, the follow-up CT showed complete resorption of the perirenal hemorrhage (fig. 3d).
Fig. 3.
a Before hemorrhage. b Non-contrast, and c contrast axial CT images. d CT image 18 months after the hemorrhage.
Discussion
Platelet aggregometry appears to be of little help for clinically assessing the severity of uremic bleeding diathesis in hemodialysis patients [2, 3]. Uremic patients have a bleeding tendency associated with platelet dysfunction [4], because platelets from uremic patients have a reduced aggregating response to adenosine diphosphate, epinephrine, and collagen [5]. Uremic platelets also have a defective interaction with vessel subendothelium, and radioligand studies have indicated an impaired binding of fibrinogen to adenosine diphosphate-stimulated uremic platelets [6, 7]. Anemia, increases in nitric monoxide, and irregularities in von Willebrand factor are also related to a bleeding tendency, regardless of the platelet status.
Prevention and treatment options for bleeding include one or a combination of the following: erythropoietin, cryoprecipitate, desmopressin, and conjugated estrogens [8]. Iseki et al. [9] reported that the relative risk in chronic dialysis patients compared to normal subjects is 10.7 for cerebral hemorrhage and 4.0 for subarachnoid hemorrhage. In previous reports, the reason for spontaneous rupture of the kidney involved a malignant tumor in 33.3% of cases, a non-malignant tumor (such as an angiomyolipoma) in 24.4%, an arteriovenous malformation in 17.9%, an infection in 10.3%, nephritis in 5.1%, hematologic disease in 5.1%, and other causes in 3.0% of patients [10].
A total of 74% of patients who had received hemodialysis for more than 4 years had ACDK, and 80% of renal cell carcinomas in hemodialysis patients were related to ACDK [11]. A possible mechanism underlying the spontaneous rupture of renal cell carcinoma was thought to be renal vein congestion due to tumor thrombosis, vessel rupture due to exponential tumor growth, and direct invasion of the tumor into the renal vessels, but these are apparently not the major causes of ruptures [12].
Belville [13] suggested that CT scanning is the most valuable examination for patients with spontaneous renal hemorrhage. On the other hand, the evaluation of renal tumors which caused spontaneous hemorrhage was very difficult, and resulted in only about 60% detectability [12]. Moreover, the tumor size and rupture frequency were not correlated, and spontaneous renal rupture even when tumor size was only 1 cm has been reported [14]. Therefore, the potential risk of renal tumors should always be considered when choosing a conservative therapy for spontaneous renal rupture. Neither magnetic resonance imaging nor angiography was any more effective than CT for detecting renal tumors [10, 15].
The relative risk of renal cell carcinoma in hemodialysis patients was found to be 13.3- to 29-fold higher than in normal subjects, and the hemodialysis patients have a risk of 2.3 to 3.3% of developing renal cell carcinoma. Moreover, patients who have received hemodialysis for more than 10 years have a 3.8-fold higher relative risk of renal cell carcinoma than patients who have been on hemodialysis for a shorter period of time.
Kendall et al. [16] strongly propose radical nephrectomy as the appropriate approach for treating these tumors; however, Bosniak [1] reported that because these patients can be followed up carefully and relatively noninvasively with repeat CT studies, aggressive surgical intervention is not justified. The need to follow-up these patients until the hematoma is completely resorbed or a diagnosis is made has also been stressed because of the reported association between perinephric hemorrhage and small renal cell carcinoma [13]. Thus, radical nephrectomy or careful follow-up observations are required for spontaneous renal hemorrhage, even when there are no CT findings of a tumor.
References
- 1.Bosniak MA. Spontaneous subcapsular and perirenal hematomas. Radiology. 1989;172:601–602. doi: 10.1148/radiology.172.3.2772165. [DOI] [PubMed] [Google Scholar]
- 2.Steiner RW, Coggins C, Carvalho AC. Bleeding time in uremia: a useful test to assess clinical bleeding. Am J Hematol. 1979;7:107–117. doi: 10.1002/ajh.2830070203. [DOI] [PubMed] [Google Scholar]
- 3.Weigert AL, Schafer AI. Uremic bleeding: pathogenesis and therapy. Am J Med Sci. 1998;316:94–104. doi: 10.1097/00000441-199808000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Di Minno G, Martinez J, McKean ML, De La Rosa J, Burke JF, Murphy S. Platelet dysfunction in uremia. Multifaceted defect partially corrected by dialysis. Am J Med. 1985;79:552–559. doi: 10.1016/0002-9343(85)90051-8. [DOI] [PubMed] [Google Scholar]
- 5.Evans EP, Branch RA, Bloom AL. A clinical and experimental study of platelet function in chronic renal failure. J Clin Pathol. 1972;25:745–753. doi: 10.1136/jcp.25.9.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Minno G, Cerbone A, Usberti M, et al. Platelet dysfunction in uremia. II. Correction by arachidonic acid of the impaired exposure of fibrinogen receptors by adenosine diphosphate or collagen. J Lab Clin Med. 1986;108:246–252. [PubMed] [Google Scholar]
- 7.Castillo R, Lozano T, Escolar G, Revert L, Lopez J, Ordinas A. Defective platelet adhesion on vessel subendothelium in uremic patients. Blood. 1986;68:337–342. [PubMed] [Google Scholar]
- 8.Hedges SJ, Dehoney SB, Hooper JS, Amanzadeh J, Busti AJ. Evidence-based treatment recommendations for uremic bleeding. Nat Clin Pract Nephrol. 2007;3:138–153. doi: 10.1038/ncpneph0421. [DOI] [PubMed] [Google Scholar]
- 9.Iseki K, Kinjo K, Kimura Y, Osawa A, Fukiyama K. Evidence for high risk of cerebral hemorrhage in chronic dialysis patients. Kidney Int. 1993;44:1086–1090. doi: 10.1038/ki.1993.352. [DOI] [PubMed] [Google Scholar]
- 10.McDougal WS, Kursh ED, Persky L. Spontaneous rupture of the kidney with perirenal hematoma. J Urol. 1975;114:181–184. doi: 10.1016/s0022-5347(17)66981-7. [DOI] [PubMed] [Google Scholar]
- 11.Truong LD, Krishnan B, Cao JT, Barrios R, Suki WN. Renal neoplasm in acquired cystic kidney disease. Am J Kidney Dis. 1995;26:1–12. doi: 10.1016/0272-6386(95)90146-9. [DOI] [PubMed] [Google Scholar]
- 12.Ishikawa I. Development of adenocarcinoma and acquired cystic disease of the kidney in hemodialysis patients. Princess Takamatsu Symp. 1987;18:77–86. [PubMed] [Google Scholar]
- 13.Belville JS, Morgentaler A, Loughlin KR, Tumeh SS. Spontaneous perinephric and subcapsular renal hemorrhage: evaluation with CT, US, and angiography. Radiology. 1989;172:733–738. doi: 10.1148/radiology.172.3.2672096. [DOI] [PubMed] [Google Scholar]
- 14.Skinner DG, Colvin RB, Vermillion CD, Pfister RC, Leadbetter WF. Diagnosis and management of renal cell carcinoma. A clinical and pathologic study of 309 cases. Cancer. 1971;28:1165–1177. doi: 10.1002/1097-0142(1971)28:5<1165::aid-cncr2820280513>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 15.Levine E. Renal cell carcinoma in uremic acquired renal cystic disease: incidence, detection, and management. Urol Radiol. 1992;13:203–210. doi: 10.1007/BF02924624. [DOI] [PubMed] [Google Scholar]
- 16.Kendall AR, Senay BA, Coll ME. Spontaneous subcapsular renal hematoma: diagnosis and management. J Urol. 1988;139:246–250. doi: 10.1016/s0022-5347(17)42376-7. [DOI] [PubMed] [Google Scholar]