Abstract
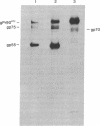
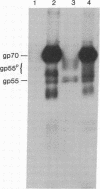
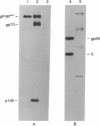
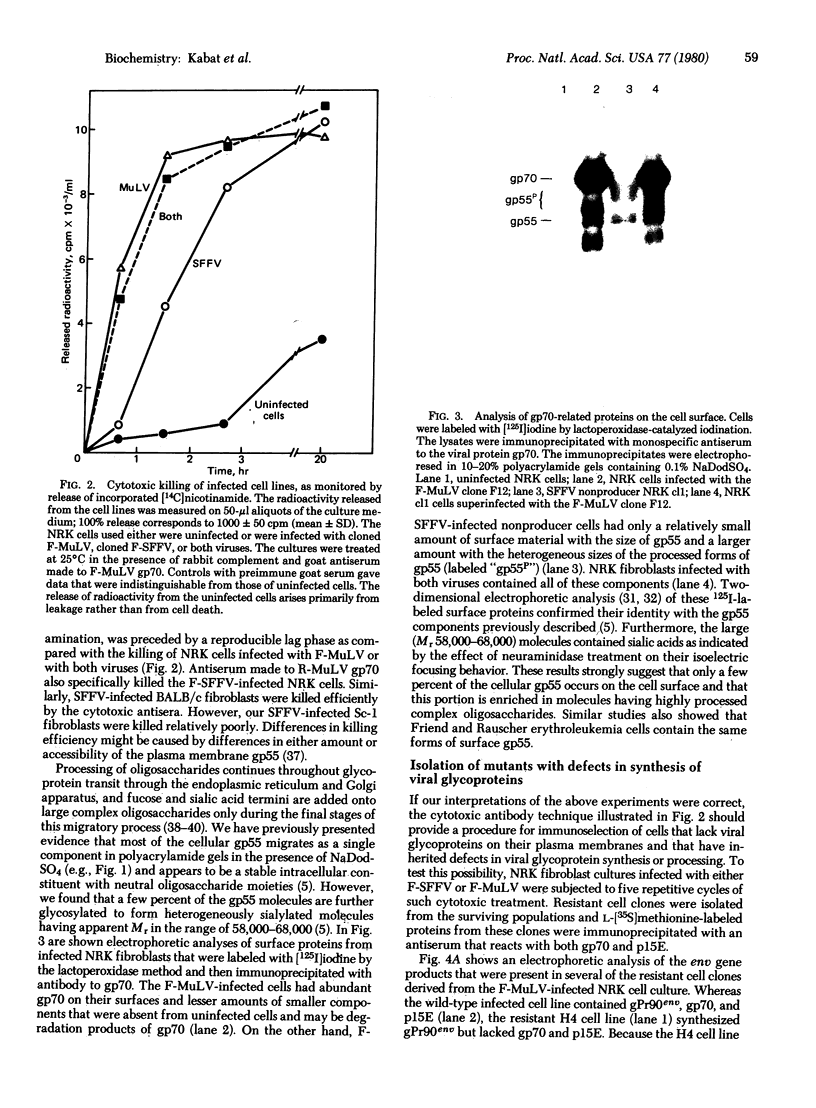
We have described a heterogeneously processed glycoprotein with an apparent molecular weight of 55,000 (gp55) that is encoded by the Friend spleen focus-forming virus, an acute erythroleukemia virus [Dresler, S., Ruta, M., Murray, M.J. & Kabat, D. (1976) J. Virol. 30, 564-573]. Several lines of evidence suggest that a small proportion of the gp55 in infected cells is located on the surface membranes. First, different nonproducer cell lines infected with cloned Friend spleen focus-forming virus were efficiently killed in the presence of complement by cytotoxic antisera that react with gp55. Furthermore, a clone of cells selected for resistance to the cytotoxic antibody synthesized an altered intracellular form of gp55. This immunoselection procedure appears to also be useful for isolating glycoprotein mutants of other RNA tumor viruses. Analysis of cell surface proteins labeled with [125I]iodine supported the idea that gp55 occurs on the plasma membranes. However, the cell surface gp55 had more highly processed oligosaccharides than the majority of the gp55, which occurs within the infected cells. In addition, we have found that leukemia cells from mice infected with the erythroleukemic Rauscher virus complex contain a membrane glycoprotein that appears similar to the gp55 encoded by Friend spleen focus-forming virus. The encoding of similar glycoproteins by independently isolated acute erythroleukemia viruses suggests that these glycoproteins may be important in leukemogenesis.
Full text
PDF

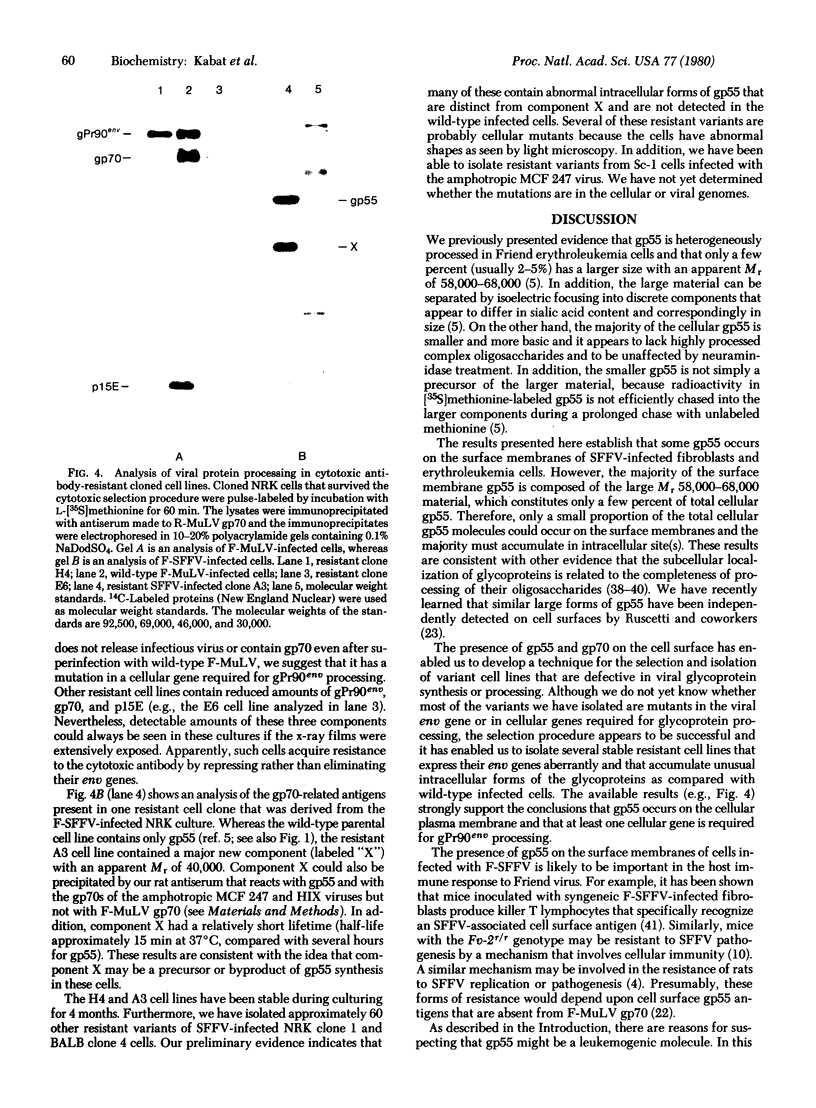

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AXELRAD A. A., STEEVES R. A. ASSAY FOR FRIEND LEUKEMIA VIRUS: RAPID QUANTITATIVE METHOD BASED ON ENUMERATION OF MACROSCOPIC SPLEEN FOCI IN MICE. Virology. 1964 Nov;24:513–518. doi: 10.1016/0042-6822(64)90199-0. [DOI] [PubMed] [Google Scholar]
- Bernstein A., Mak T. W., Stephenson J. R. The Friend virus genome: evidence for the stable association of MuLV sequences and sequences involved in erythroleukemic transformation. Cell. 1977 Sep;12(1):287–294. doi: 10.1016/0092-8674(77)90206-9. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Clarke B. J., Axelrad A. A., Shreeve M. M., McLeod D. L. Erythroid colony induction without erythropoietin by Friend leukemia virus in vitro. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3556–3560. doi: 10.1073/pnas.72.9.3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson P. J., Rose W. M., Fieldsteel A. H. Lymphatic leukaemia in rats and mice inoculated with Friend virus. Br J Cancer. 1966 Mar;20(1):114–121. doi: 10.1038/bjc.1966.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devare S. G., Rapp U. R., Todaro G. J., Stephenson J. R. Acquisition of oncogenicity by endogenous mouse type C viruses: effects of variations in env and gag genes. J Virol. 1978 Nov;28(2):457–465. doi: 10.1128/jvi.28.2.457-465.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dexter T. M., Scott D., Teich N. M. Infection of bone marrow cells in vitro with FLV: effects on stem cell proliferation, differentiation and leukemogenic capacity. Cell. 1977 Oct;12(2):355–364. doi: 10.1016/0092-8674(77)90111-8. [DOI] [PubMed] [Google Scholar]
- Dresler S., Ruta M., Murray M. J., Kabat D. Glycoprotein encoded by the Friend spleen focus-forming virus. J Virol. 1979 May;30(2):564–575. doi: 10.1128/jvi.30.2.564-575.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckner R. J., Hettrick K. L. Persistence and pathogenicity of defective Friend spleen focus-forming virus. Decreased transplantability of hemopoietic cells as a marker for preleukemic change. J Exp Med. 1979 Feb 1;149(2):340–357. doi: 10.1084/jem.149.2.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder J. H., Pickett R. A., 2nd, Hampton J., Lerner R. A. Radioiodination of proteins in single polyacrylamide gel slices. Tryptic peptide analysis of all the major members of complex multicomponent systems using microgram quantities of total protein. J Biol Chem. 1977 Sep 25;252(18):6510–6515. [PubMed] [Google Scholar]
- Evans L. H., Dresler S., Kabat D. Synthesis and glycosylation of polyprotein precursors to the internal core proteins of Friend murine leukemia virus. J Virol. 1977 Dec;24(3):865–874. doi: 10.1128/jvi.24.3.865-874.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRIEND C. Cell-free transmission in adult Swiss mice of a disease having the character of a leukemia. J Exp Med. 1957 Apr 1;105(4):307–318. doi: 10.1084/jem.105.4.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famulari N. G., Buchhagen D. L., Klenk H. D., Fleissner E. Presence of murine leukemia virus envelope proteins gp70 and p15(E) in a common polyprotein of infected cells. J Virol. 1976 Nov;20(2):501–508. doi: 10.1128/jvi.20.2.501-508.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faust C. H., Jr, Heim I., Moore J. Murine myeloma immunoglobulin heavy-chain mRNA. Isolation, partial purification, and characterization of gamma1, gamma2a, gamma2b, gamma3, micron and alpha heavy-chain mRNA'S. Biochemistry. 1979 Mar 20;18(6):1106–1119. doi: 10.1021/bi00573a027. [DOI] [PubMed] [Google Scholar]
- Fieldsteel A. H., Dawson P. J., Becker F. A., Kurahara C., Mitoma C. Rauscher virus-induced reticulum-cell sarcomas: Their growth in vitro and erythropoietic differentiation. Br J Cancer. 1977 Feb;35(2):199–207. doi: 10.1038/bjc.1977.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischinger P. J., Dunlop N. M., Blevins C. S. Identification of virus found in mouse lymphomas induced by HIX murine oncornavirus. J Virol. 1978 May;26(2):532–535. doi: 10.1128/jvi.26.2.532-535.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischinger P. J., Frankel A. E., Elder J. H., Lerner R. A., Ihle J. N., Bolognesi D. P. Biological, immunological, and biochemical evidence that HIX virus is a recombinant between Moloney leukemia virus and a murine xenotropic C type virus. Virology. 1978 Oct 15;90(2):241–254. doi: 10.1016/0042-6822(78)90308-2. [DOI] [PubMed] [Google Scholar]
- Fischinger P. J., Nomura S., Bolognesi D. P. A novel murine oncornavirus with dual eco- and xenotropic properties. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5150–5155. doi: 10.1073/pnas.72.12.5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forchhammer J., Turnock G. Glycoproteins from murine C-type virus are more acidic in virus derived from transformed cells than from nontransformed cells. Virology. 1978 Jul 1;88(1):177–182. doi: 10.1016/0042-6822(78)90121-6. [DOI] [PubMed] [Google Scholar]
- Gillis S., Gillis A. E., Smith K. A. The detection of a spleen focus-forming virus neoantigen by lymphocyte-mediated cytolysis. J Exp Med. 1978 Jul 1;148(1):18–31. doi: 10.1084/jem.148.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley J. W., Wolford N. K., Old L. J., Rowe W. P. A new class of murine leukemia virus associated with development of spontaneous lymphomas. Proc Natl Acad Sci U S A. 1977 Feb;74(2):789–792. doi: 10.1073/pnas.74.2.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioachim H. L., Sabbath M. Redistribution and modulation of Gross murine leukemia virus antigens induced by specific antibodies. J Natl Cancer Inst. 1979 Jan;62(1):169–180. [PubMed] [Google Scholar]
- Kawashima K., Ikeda H., Hartley J. W., Stockert E., Rowe W. P., Old L. J. Changes in expression of murine leukemia virus antigens and production of xenotropic virus in the late preleukemic period in AKR mice. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4680–4684. doi: 10.1073/pnas.73.12.4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Cell membrane antigen isolation with the staphylococcal protein A-antibody adsorbent. J Immunol. 1976 Nov;117(5 Pt 1):1482–1490. [PubMed] [Google Scholar]
- Kurth R., Medley G. A membrane permeability test for the detection of cell surface antigens. Immunology. 1975 Nov;29(5):803–811. [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Murray M. J., Kabat D. Genetic and sialylation sources of heterogeneity of the murine leukemia virus membrane envelope glycoproteins gp69/71. J Biol Chem. 1979 Feb 25;254(4):1340–1348. [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Robbins P. W., Hubbard S. C., Turco S. J., Wirth D. F. Proposal for a common oligosaccharide intermediate in the synthesis of membrane glycoproteins. Cell. 1977 Dec;12(4):893–900. doi: 10.1016/0092-8674(77)90153-2. [DOI] [PubMed] [Google Scholar]
- Ruscetti S. K., Linemeyer D., Feild J., Troxler D., Scolnick E. M. Characterization of a protein found in cells infected with the spleen focus-forming virus that shares immunological cross-reactivity with the gp70 found in mink cell focus-inducing virus particles. J Virol. 1979 Jun;30(3):787–798. doi: 10.1128/jvi.30.3.787-798.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscetti S., Linemeyer D., Field J., Troxler D., Scolnick E. Type-specific radioimmunoassays for the gp70s of mink cell focus-inducing murine leukemia viruses: expression of a cross-reacting antigen in cells infected with the Friend strain of the spleen focus-forming virus. J Exp Med. 1978 Sep 1;148(3):654–663. doi: 10.1084/jem.148.3.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherton C. C., Dresler S. L., Polonoff E., Webb M. C., Kabat D. Synthesis of murine leukemia virus proteins differentiating Friend erythroleukemia cells. Cancer Res. 1978 May;38(5):1426–1433. [PubMed] [Google Scholar]
- Steeves R. A. Editorial: Spleen focus-forming virus in Friend and Rauscher leukemia virus preparations. J Natl Cancer Inst. 1975 Feb;54(2):289–297. doi: 10.1093/jnci/54.2.289. [DOI] [PubMed] [Google Scholar]
- Tabas I., Schlesinger S., Kornfeld S. Processing of high mannose oligosaccharides to form complex type oligosaccharides on the newly synthesized polypeptides of the vesicular stomatitis virus G protein and the IgG heavy chain. J Biol Chem. 1978 Feb 10;253(3):716–722. [PubMed] [Google Scholar]
- Troxler D. H., Boyars J. K., Parks W. P., Scolnick E. M. Friend strain of spleen focus-forming virus: a recombinant between mouse type C ecotropic viral sequences and sequences related to xenotropic virus. J Virol. 1977 May;22(2):361–372. doi: 10.1128/jvi.22.2.361-372.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troxler D. H., Lowy D., Howk R., Young H., Scolnick E. M. Friend strain of spleen focus-forming virus is a recombinant between ecotropic murine type C virus and the env gene region of xenotropic type C virus. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4671–4675. doi: 10.1073/pnas.74.10.4671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troxler D. H., Parks W. P., Vass W. C., Scolnick E. M. Isolation of a fibroblast nonproducer cell line containing the Friend strain of the spleen focus-forming virus. Virology. 1977 Feb;76(2):602–615. doi: 10.1016/0042-6822(77)90242-2. [DOI] [PubMed] [Google Scholar]
- Troxler D. H., Scolnick E. M. Rapid leukemia induced by cloned friend strain of replicating murine type-C virus. Association with induction of xenotropic-related RNA sequences contained in spleen focus-forming virus. Virology. 1978 Mar;85(1):17–27. doi: 10.1016/0042-6822(78)90408-7. [DOI] [PubMed] [Google Scholar]
- Troxler D. H., Yuan E., Linemeyer D., Ruscetti S., Scolnick E. M. Helper-independent mink cell focus-inducing strains of Friend murine type-C virus: potential relationship to the origin of replication-defective spleen focus-forming virus. J Exp Med. 1978 Sep 1;148(3):639–653. doi: 10.1084/jem.148.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitetta E. S., Baur S., Uhr J. W. Cell surface immunoglobulin. II. Isolation and characterization of immunoglobulin from mouse splenic lymphocytes. J Exp Med. 1971 Jul 1;134(1):242–264. doi: 10.1084/jem.134.1.242. [DOI] [PMC free article] [PubMed] [Google Scholar]