Abstract
A 54-year-old man was diagnosed with Streptococcus mutans endocarditis of the mitral valve. Serological tests disclosed the presence of multiple autoantibodies including c-ANCA, anti-PR3 and anti-MPO. While the fever subsided with antibiotics, mental status and renal function deteriorated rapidly. Kidney biopsy revealed pauci-immune glomerulonephritis and acute eosinophilic interstitial nephritis. The abnormal clinical features improved rapidly after addition of corticosteroids and cyclophosphamide to the antibiotics. Immunosuppressive agents may be required in a fraction of the patients with infective endocarditis who develop ANCA and ANCA-mediated renal disease. Histological identification of the type of renal disease is imperative for the choice of the treatment.
Key Words: Infective endocarditis, Pauci-immune glomerulonephritis, Interstitial nephritis, Vasculitis, Renal failure, Anti-neutrophil cytoplasmic antibodies, ANCA
Introduction
Subacute bacterial endocarditis stimulates multiple immunological abnormalities [1]. These abnormalities can cause disease on their own. Anti-neutrophil cytoplasmic antibodies (ANCA) is one of the immunological abnormalities complicating the course of endocarditis [2, 3]. The development of ANCA-mediated disease during the course of endocarditis raises the question of specific treatment of the immunological disease. Treatment with immunosuppressive medications may increase the risk of septic death [4]. Also, treatment of the endocarditis with appropriate antibiotics usually leads to abolition of the immunological abnormalities and their clinical manifestations [5].
The question that has not been answered adequately is whether there is any indication for addition of ANCA-specific treatment to the regime of some patients with infectious endocarditis and ANCA positivity. To clarify this issue, we present a patient who received immunosuppressive treatment for life-threatening ANCA-mediated disease complicating subacute endocarditis.
Report of a Case
A 53-year-old man with mitral valve prolapse, dental caries and gingivitis, but no previous history of rheumatologic, renal or neurological disease was admitted with a 3-month history of anorexia, weight loss exceeding 27 kg, nocturnal chills and low-grade fevers, pronounced weakness, and changes in cognition forcing him to discontinue working. Complete blood count and serum creatinine were normal, while serum lipase and bilirubin were elevated (table 1) and urinalysis showed microscopic hematuria, few white blood cells (WBC), one WBC cast and 30 mg/dl of protein. Abdominal computed tomography and magnetic resonance imaging showed normal pancreas, splenomegaly, a simple left renal cyst and a cyst in the liver.
Table 1.
Hematological, biochemical and nutrition indices
| Index | Initial | Peak | Recovery |
|---|---|---|---|
| Blood hematocrit, Vol% | 45.5 | 25.9a | 40.5 |
| Blood hemoglobin, g/dl | 15.6 | 8.7a | 14.1 |
| Blood white cell count, k/mm3 | 9.9 | 12.6 | 4.2 |
| Blood platelet count, k/mm3 | 201 | 326 | 92 |
| Serum creatinine, mg/dl | 1.1 | 6.6 | 1.3 |
| Serum bilirubinb, mg/dl | 1.6 | 1.9 | 1.1 |
| Serum lipasec, U/l | 340 | 2,193 | 249 |
| Serum albumin, g/dl | 3.6 | 2.3 | 3.6 |
| Serum pre-albumind, mg/dl | 8 | <5 | Not measured |
| Body mass index | 30.0e | 21.6 | 29.7 |
With transfusions of packed red cells.
Alanine aminotrasferase, and lactate dehydrogenase levels slightly elevated at the peak value and normalized with treatment.
Normal range 23–300 U/l.
Normal range 18–50 mg/dl.
Initial value was obtained one year prior to the first admission.
Temporary improvement of the cognitive changes followed administration of an oral antidepressant. However, he was readmitted one month later with deterioration in his mental status plus great difficulty in swallowing. He had developed in the interim progressive weight loss, further decrease in cognitive function, increased oxygen requirements, profound weakness and difficulty in swallowing both liquids and solids and continuous low-grade fever. A grade II/VI apical systolic murmur with radiation to the left axilla, unchanged from previous examinations, and splenomegaly were noted. The rest of the physical examination, including the skin, was unremarkable. Echocardiogram showed a vegetation in the posterior mitral leaflet. Chest X-ray and computed tomography (CT) of the skull showed no abnormalities. Multiple blood cultures grew Streptococcus mutans.
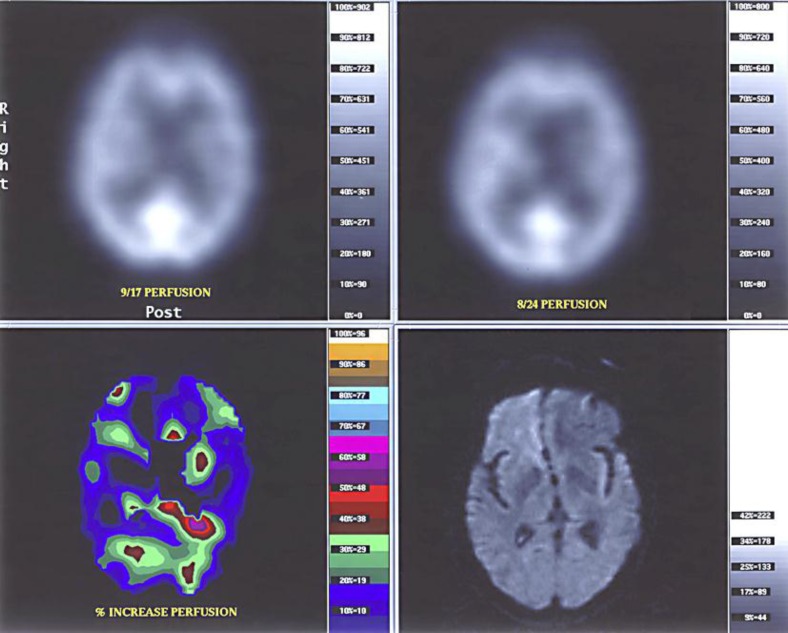
Treatment with piperacillin and tazobactam was initiated, followed by ampicillin, which was changed to vancomycin after a gallium scan showed diffuse uptake of the isotope by the kidneys consistent with interstitial nephritis. The fever subsided soon after initiation of antibiotics. However, his mental status did not improve and progressive renal insufficiency developed. Neurological examination showed profound confusion, swallowing difficulty and no other abnormalities. Lumbar puncture revealed 57 white cells, 34 lymphocytes, protein 74 mg/dl (normal 12–60 mg/dl) and glucose 44 mg/dl with corresponding serum glucose of 79 mg/dl. Electroencephalogram showed slow wave abnormality in the left temporal lobe. Computer tomography (CT) and magnetic resonance imaging (MRI) of the brain showed no abnormalities. However, a perfusion scintigraphy using Tc-99 HPCAC (SPECT) fused with an MRI showed symmetrically decreased brain perfusion more pronounced in the frontal lobes (fig. 1).
Fig. 1.
Tc-99m HMPAO (Ceretec) identical perfusion examinations performed with tarred doses of 30.0 mCi and acquisition beginning 15 min after injection with 20 min duration on a three-head gamma camera. The output pixels were co-registered with diffusion weighted MRI of the brain. Computer processing of the quantitative data shows a generalized increase in perfusion between the study prior to the initiation of immunosuppression and the study three weeks after initiation of immunosuppression.
Urinalysis revealed modest proteinuria, approximately 1 g per 24 h, hematuria with dysmorphic red cells, pyuria without bacteriuria, red cell casts and mixed red and white cell casts. A renal cortical perfusion scan fused with an abdominal CT showed no perfusion defects. Table 1 shows abnormal hematological and biochemical tests. Table 2 shows serological tests. Unless stated otherwise in the footnote of the tables, initial values were obtained at the first admission and recovery values were obtained one year later.
Table 2.
Serological tests
| Test | Normal range | Baseline | Peak | Recovery |
|---|---|---|---|---|
| ESR, mm/h | <20 | 47 | 63 | 1 |
| CRP, mg/dl | 0.01–1.00 | 7.65 | 10.30 | 0.10 |
| Ferritin, ng/ml | 100–270 | – | 991 | 386 |
| C3 complement, mg/dl | 88–201 | – | 58 | 108 |
| C4 complement, mg/dl | 16–47 | – | 20 | 34 |
| IgG, g/dl | 0.5–1.3 | – | 2.5a | 1.4 |
| ANA titer | ≤1:10 | – | 1:80b | ≤1:10 |
| RF, IU/ml | ≤43 | 163 | 316 | <6 |
| APL, IgG, U/ml | <10 | – | 91 | <10 |
| APL, IgM, U/ml | <10 | – | 21 | <10 |
| c-ANCA | negative | – | positive | negative |
| Anti-PR-3, IRR | <0.90 | – | 2.96 | <0.90 |
| p-ANCA | negative | – | negative | negative |
| Anti-MPO, IRR | <0.90 | – | 1.19 | <0.90 |
| Anti-smooth muscle antibody | negative | – | 1:320 | negative |
| Anti-F-actin antibody, U | <20 | – | 33 | <20 |
ESR = Erythrocyte sedimentation rate; CRP = C-reactive protein; IgG = gamma-globulin; APL = antiphospholipid antibody; PR-3 = proteinase-3; MPO = myeloperoxidase.
Polyclonal gammopathy; serum levels of IgA and IgM within normal range.
Cytoplasmic immunofluorescence pattern; SSA negative, SSB negative, Scl-70 negative.
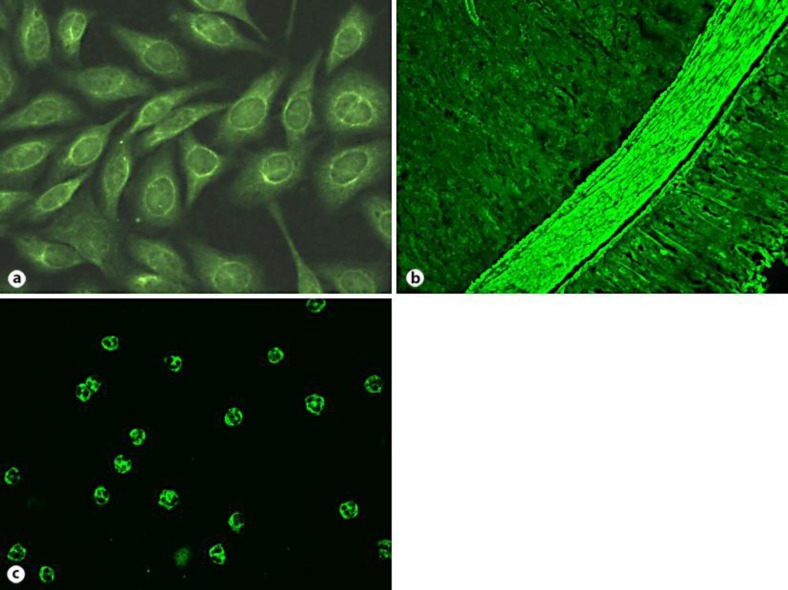
Erythrocyte sedimentation rate was elevated; serologies revealed elevated CRP, low C3, positive rheumatoid factor, polyclonal hypergammaglobulinemia, and the presence of multiple autoantibodies including low-titer anti-nuclear/anti-cytoplasmic antibodies on Hep2 cells (fig. 2a), anti-phospholipid antibodies, anti-smooth muscle antibody (fig. 2b), antibodies to F-actin, c-ANCA (fig. 2c), PR3-ANCA, and, at a weak titer, MPO-ANCA. Normal or negative serologies included antistreptolysine titer, HIV, hepatitis A, B and C, cryoglobulins, HLAB27, antiglomerular basement membrane antibody, IgA APL.
Fig. 2.
Indirect immunofluorescence showing cytoplasmic staining with perinuclear accentuation on HEp-2 cells (a), anti-smooth muscle staining on mouse kidney/stomach (b), and c-ANCA on ethanol-fixed neutrophils (c).
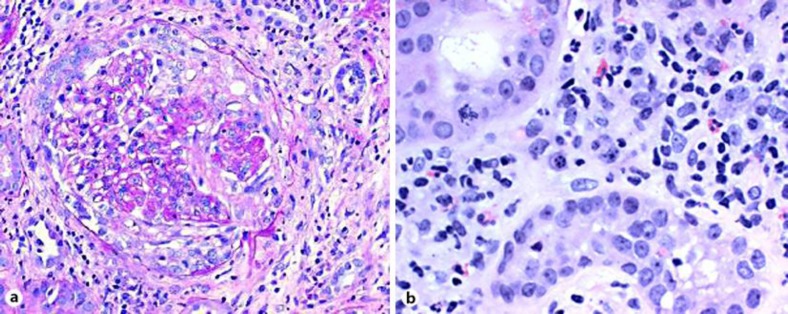
Percutaneous kidney biopsy showed crescentic glomerulonephritis and interstitial nephritis (fig. 3) with negative immunofluorescence and absence of glomerular deposits on electron microscopy. This histological picture is consistent with ANCA-associated pauci-immune glomerulonephritis. His neurological clinical condition and serum creatinine continued to deteriorate after two weeks of vancomycin to the point that a discussion about pursuing aggressive treatments, such as dialysis, or not took place with his family. A decision to treat ANCA-mediated disease (glomerulonephritis and probable cerebral vasculitis) was taken. In addition to antibiotics, he received intravenous, and later oral, cyclophosphamide and oral corticosteroids for one year. On this regime, his confusion and renal failure reversed rapidly, strength and nutrition recovered and only difficulty in swallowing persisted. Repeated fusion SPECT-MRI study of the brain one month after the first cyclophosphamide injection was normal (fig. 1).
Fig. 3.
Renal biopsy. a Glomerulus with a cellular crescent. Immunofluorence was negative and electron microscopy showed no deposits. b Interstitial nephritis. Eosinophils are noted among the cellular components of the interstitial infiltrate.
One year later, off any medications, he was asymptomatic and able to exercise regularly; repeated echocardiogram was consistent with healed vegetation in the posterior leaflet of his mitral valve. Serologies were normalized within one or two months of initiation of immunosuppressive medications. Six years after the end of treatment, he had no clinical manifestations of ANCA-mediated disease, serologies were normal, serum creatinine was 1.0 mg/dl, and urinalysis had no blood, but contained 20 mg/dl of protein.
Discussion
This case presents a cure of ANCA-mediated disease triggered by infective endocarditis by addition of corticosteroids and immunosuppressives to antibiotics in a patient who had rapid clinical deterioration during appropriate antibiotic treatment. The literature contains two other cases in which addition of immunosuppressive regimes led to rapid improvement in patients with infectious endocarditis, ANCA positivity and ANCA-mediated disease [6, 7]. In addition to the deterioration of the clinical course, these patients had histological evidence of ANCA-mediated disease, including necrotizing vasculitis found in a sural nerve biopsy [6] and pauci-immune crescentic glomerulonephritis [7].
Examination of histology of involved organs is imperative in patients with infective endocarditis that triggers formation of multiple autoantibodies (table 2) and appearance of clinical manifestations that could develop either in chronic infection or vasculitis. The kidneys are a frequent organ involved in infective endocarditis. Multiple histological pictures are found in patients with endocarditis and renal disease, including renal infarcts, glomerulonephritis of either postinfectious or vasculitic variety, acute tubular necrosis, acute interstitial nephritis and other conditions [8]. In addition, ANA and antiphospholipid antibodies may also cause renal disease.
The histological picture affects the treatment regime. Postinfectious glomerulonephritis usually responds to treatment of endocarditis with antibiotics and, in some cases, addition of corticosteroids. Pauci-immune glomerulonephritis or vasculitis may require addition of corticosteroids and immunosuppressives to the antibiotics [9]. Our patient illustrates this approach. Interstitial nephritis may be triggered by antibiotics, usually β-lactam, but is also found in patients with ANCA-associated disease [10, 11] and may require, in addition to changing the antibiotics, corticosteroids and immunosuppressives. Renal disease secondary to antiphospholipid antibodies is treated with warfarin.
The presence of smooth-muscle antibodies of anti-F-actin specificity, seen in autoimmune hepatitis and in low titers in acute and chronic infections, cancer, and systemic autoimmune diseases, may result in atypical c-ANCA pattern. In such cases, only the demonstration of PR3 or MPO specificity by ELISA will indicate that the neutrophil fluorescence is clinically significant. Animal studies have shown that ANCA are pathogenic [12]. Infections cause ANCA formation through various pathways [13, 14].
In addition to patients with infective endocarditis triggering ANCA-mediated manifestations that do not respond to antibiotics alone, immunosuppressive management is required for patients with sterile endocarditis caused by the vasculitic process and echocardiographic features similar to those of infective endocarditis [15]. Distinguishing between the two types of endocarditis is of paramount importance.
Histology is required for the differentiation of renal disease during the course of infective endocarditis. Identifying among patients with endocarditis and ANCA positivity those who have vasculitis and not infection as the cause of endocarditis and those with infective endocarditis who will require immunosuppressives in addition to the antibiotics are major clinical tasks.
References
- 1.Williams RC., Jr Kunkel H. Rheumatoid factor, complement, and conglutinin aberrations in patients with subacute bacterial endocarditis. J Clin Invest. 1962;41:666–675. doi: 10.1172/JCI104523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wagner AS, Andrassy K, Ritz E. Is vasculitis in subacute bacterial endocarditis associated with ANCA? Lancet. 1991;337:799–800. doi: 10.1016/0140-6736(91)91427-v. [DOI] [PubMed] [Google Scholar]
- 3.Choi HK, Labrecht P, Niles KL, Gross WL, Merkel PA. Subacute bacterial endocarditis with positive antineutrophil cytoplasmic amtibodies and anti-proteinase 3 antibodies. Arthr Rheum. 2000;43:226–231. doi: 10.1002/1529-0131(200001)43:1<226::AID-ANR27>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 4.Charlier C, Henegar C, Launay O, Pagnoux C, Berezné A, Bienvenu B, Cohen P, Mouthon L, Guilevin L. Risk factors for major infections in Wegener granulomatosis: analysis of 113 patients. Ann Rheum Dis. 2009;68:658–663. doi: 10.1136/ard.2008.088302. [DOI] [PubMed] [Google Scholar]
- 5.Robert SC, Forbes SH, Soleimanian S, Hadley JS. Complements do not lie. BMJ Case Reports. 2011 doi: 10.1136/bcr.08.2011.4705. DOI: 10.1136/bcr.08.2011.4705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Helmich B, Ehren M, Lindstaed M, Meyer M, Pfohl M, Schatz H. Anti-MPO-ANCA-positive microscopic polyangiitis following subacute bacterial endocarditis. Clin Rheumatol. 2001;20:441–443. doi: 10.1007/pl00011214. [DOI] [PubMed] [Google Scholar]
- 7.Couzi L, Morel D, Deminiere C, Merville P. An unusual endocarditis induced crescentic glomerulonephritis treated by plasmapheresis. Clin Nephrol. 2004;62:461–465. doi: 10.5414/cnp62461. [DOI] [PubMed] [Google Scholar]
- 8.Majumbar A, Chowdary S, Fereira MAS. Hammond LA. Howie AJ. Lipkin GW. Littler WA. Renal pathological findings in infective endocarditis. Nephrol Dial Transplant. 2000;15:1782–1787. doi: 10.1093/ndt/15.11.1782. [DOI] [PubMed] [Google Scholar]
- 9.Haseyama T, Imai H, Komasuda A, Hamai K, Ohtani H, Kibira S, Miura AB. Proteinase-3 positive crescentic glomerulonephritis in a patient with Down's syndrome and infectious endocarditis. Nephrol Dial Transplant. 1998;13:2142–2146. doi: 10.1093/ndt/13.8.2142. [DOI] [PubMed] [Google Scholar]
- 10.Yamamoto T, Yoshihara S, Suzuki H, Nagase M, Oka M, Hishida A. MPO ANCA-positive crescentic necrotizing glomerulonephritis with renal eosinophilic infiltration and peripheral blood eosinophils. Am J Kidney Dis. 1998;31:1032–1037. doi: 10.1053/ajkd.1998.v31.pm9631850. [DOI] [PubMed] [Google Scholar]
- 11.Son D, Kanda H, Yamaguchi A, Kawabata K, Kawakami T, Kubo K, Higashihara M, Shimizu J, Uozaki H, Kuramochi S, Misaki Y, Takeuchi F, Yamamoto K. Myeloperoxidase antineutrophil cytoplasmic antibody-associated vasculitis with diffuse tubulointerstitial nephritis. J Nephrol. 2009;22:417–420. [PubMed] [Google Scholar]
- 12.Jennette JC, Xiao H, Falk R, Gasim AM. Experimental models of vasculitis and glomerulonephritis induced by antineutrophil cytoplasmic antibodies. Contrib Nephrol. 2011;169:211–220. doi: 10.1159/000314776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schultz H. From infection to autoimmunity: a new model for induction of ANCA against the bactericidal/permeability increasing protein (BPI). Autoimmun Rev. 2007;6:223–227. doi: 10.1016/j.autrev.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 14.Kain R, Exner M, Brandes R, Ziebermayr R, Cunningham D, Aldesrson CA, Davidovits A, Raab J, Jahn R, Asour O, Spitzauer S, Sunder-Plassmann G, Fukuda M, Klemm P, Rees AJ, Kerjashki D. Molecular mimicry in pauci-immune focal necrotizing glomerulonephritis. Nat Med. 2008;14:1088–1096. doi: 10.1038/nm.1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mishell JM. Cases from the Osler Medical Service at Johns Hopkins University: cardiac valvular lesions in Wegener's granulomatosis. Am J Med. 2002;113:607–609. doi: 10.1016/s0002-9343(02)01349-9. [DOI] [PubMed] [Google Scholar]