Abstract
A 59-year-old man was diagnosed with IgG4-related tubulointerstitial nephritis. His symptoms as well as laboratory and imaging findings were improved after initiation of steroid therapy. Serologically, he showed hypocomplementemia (C3 23 mg/dl, C4 <2 mg/dl, CH50 <7 U/ml) with high levels of IgG (IgG4 1,970 mg/dl) and immune complexes (C1q assay 8.1 μg/ml) and a low level of C1q (<2.0 mg/dl). Histologically, he also showed linear depositions of IgG, IgM, C3, C4d, C1q, membrane attack complex and all IgG subclasses (IgG1, IgG2, IgG3 and IgG4) along the tubular basement membrane, as well as granular depositions of these components in the renal interstitium. However, mannose-binding lectin and L-ficolin were not detected in these tissues. Homogeneous electron-dense deposits were observed by electron microscopy in the tubular basement membrane. It appears that the immune complexes might activate the classical pathway of the complement in both blood and local tissues in a patient with IgG4-related tubulointerstitial nephritis.
Key Words: IgG4, Tubulointerstitial nephritis, Complement, Classical pathway, Immune complex
Introduction
Recently, a new concept of IgG4-related disease has been proposed. IgG4-related disease is a systemic disease characterized by high serum IgG4 levels and multi-organ fibrosis with infiltration of T lymphocytes and IgG4-positive plasma cells [1, 2]. The target organs in patients with IgG4-related disease are the pancreas, gallbladder, salivary glands, retroperitoneum, lungs, prostate and kidneys, and the representative disease is autoimmune pancreatitis. Clinically, IgG4-related disease predominantly affects middle-aged to elderly men. Lymphadenopathy is a common complication (40–50%) and hypocomplementemia is observed in 30–40% of cases [3, 4, 5, 6, 7, 8]. In IgG4-related nephropathy, the lesions are mainly located at the tubulointerstitium rather than at glomeruli [9, 10, 11, 12]. Massive infiltration of IgG4-positive plasma cells and serious fibrosis in the interstitium are distinctive findings rather than typical interstitial nephritis. There are some reports showing diffuse deposition of IgG and IgG subclasses in the interstitium [12].
Since steroid therapy is very effective and the prognosis is relatively good, severe tubulointerstitial nephritis (TIN) requiring maintenance hemodialysis sometimes occurs [12]. Furthermore, hypocomplementemia is observed in 60–70% of IgG4-related nephropathy cases [8]. Although the hypocomplementemia is a distinctive finding that may be associated with histological damage [7], the pathological associations of complement activation in sera and local tissues have not been fully elucidated [5, 13].
We present herein a patient diagnosed with IgG4-related TIN and with confirmed complement activation via the classical pathway in serological and histological examinations.
Case Report
A 59-year-old Japanese man with symptoms of dysgeusia, dysosmia and anorexia was admitted to our hospital on January 6, 2010. He had a history of pneumonia, but no other medical problems and no history of smoking, habitual drinking or family history of renal disease. On admission, his body height was 164.3 cm, body weight 71.3 kg, body temperature 36.3°C and blood pressure 170/110 mm Hg. Physical examination revealed lymphadenopathy in the left supraclavicular and bilateral inguinal regions. Laboratory findings showed hyperuricemia (uric acid 8.4 mg/dl), hyperproteinemia (total protein 11.1 g/dl, Alb 3.3 g/dl), IgG4-dominant-hypergammaglobulinemia (IgG 3,814 mg/dl, IgG4 1,970 mg/dl), hepatic dysfunction (AST 65 IU/l, ALT 54 IU/l), renal dysfunction (serum urea nitrogen 22 mg/dl, serum creatinine 2.69 mg/dl), hypocomplementemia (C3 23 mg/dl, C4 <2 mg/dl, CH50 <7 U/ml, C1q <2.0 mg/dl; normal range 8.8–15.3) and rouleaux formation of erythrocytes. Antinuclear antibody was positive with a titer of 20. Rheumatoid factor, cytoplasmic antineutrophil cytoplasmic antibodies and perinuclear antineutrophil cytoplasmic antibodies, SS-A and SS-B were all negative, cryoglobulin was not examined. Monoclonal protein was not detected in his serum and urine by immunoelectrophoresis. High levels of immune complexes (ICs) [C1q assay 8.1 μg/ml; normal range 0–3, monoclonal rheumatoid factor (mRF) assay 11.6 μg/ml; normal range 0–4.1, respectively] were observed (table 1). Urinalysis showed no proteinuria, hematuria or glucosuria, except for increases of tubular damage markers (β2-microglobulin 5,960 μg/l) and granular casts in the sediments. Bone marrow biopsy and lymph node biopsy (left inguinal) did not show plasma cell dyscrasia or other malignancy. Computed tomography (CT) showed bilateral mucosal thickening of the paranasal sinuses and bilateral renal swelling, and gallium (Ga) citrate scintigraphy showed abnormal isotope accumulation in the bilateral kidneys.
Table 1.
Laboratory findings
| Before Tx | After Tx (3 weeks later) | Normal range | |
|---|---|---|---|
| Hemoglobin, g/dl | 13.3 | 12.7 | 13.4–17.1 |
| WBC,/μl | 8,000 | 10,400 | 3,900–9,700 |
| Eosinophils,/μl | 984 | 20 | 70–440 |
| SUN/Cre, mg/dl | 22/2.69 | 38/1.51 | 9–21/0.6–1.0 |
| AST/ALT, U/l | 65/54 | 18/30 | 5–37/6–43 |
| Amylase, U/l | 64 | 108 | 43–124 |
| Uric acid, mg/dl | 8.4 | 5.5 | 3.5–6.9 |
| Urinary protein, mg/dl | – | – | 0–20 |
| eGFR, ml/min/1.73 m2 | 20.4 | 38.4 | ≥60 |
| Total protein, g/dl | 11.1 | 7 | 6.5–8.5 |
| Albumin, g/dl | 3.3 | 3.7 | 4.0–5.2 |
| IgG, mg/dl | 3,814 | 1,637 | 870–1,700 |
| IgG4, mg/dl | 1,970 | 969 | 4.8–105 |
| C3, mg/dl | 23 | 71 | 69–128 |
| C4, mg/dl | <2 | 12 | 14–36 |
| CH50, U/ml | <7 | 37.4 | 25–54 |
| C1q, μg/ml | <2 | NE | 8.8–15.3 |
| Immune complex, μg/ml | |||
| C1q assay/mRF assay | 8.1/11.6 | <1.5/NE | 0–3/0–4.1 |
| ANA, titer | 20 | 20 | 0–19 |
| Rheumatoid factor, U/l | 3 | NE | 0–20 |
| CRP, mg/dl | 0.4 | 0 | <0.2 |
Tx = Treatment (steroid therapy); WBC = white blood cells; SUN = serum urea nitrogen; Cre = creatinine; ANA = antineutrophil antibodies; NE = not examined.
Because of suspected TIN, ultrasound-guided percutaneous renal biopsy was performed on February 4, 2010. The renal biopsy specimens showed no glomerular abnormalities, but severe and widespread interstitial fibrosis and tubular atrophy associated with inflammatory infiltrates consisting mainly of lymphocytes and IgG4-positive plasma cells were observed (fig. 1). Routine immunofluorescence (IF) showed granular deposition of IgG (+) in the glomeruli, linear depositions of IgG (++), IgM (+), C3c (+) and C1q (++) along the tubular basement membrane (TBM) and granular depositions of IgG (++), IgM (+), C3c (+) and C1q (++) in the interstitium (fig. 2). Additional IF were performed with fluorescein isothiocyanate (FITC)-labeled monoclonal mouse anti-human IgG1, IgG2, IgG3 and IgG4 antibodies (SouthernBiotech, USA), monoclonal anti-human mannose-binding lectin (MBL) antibody (kindly provided from Prof. Matsushita, Tokai University, Kanagawa, Japan), polyclonal goat anti-human L-ficolin antibody (Santa Cruz Biotechnology, USA), polyclonal rabbit anti-human C4d antibody (DAKO, Denmark) and polyclonal rabbit anti-human membrane attack complex (MAC) antibody (Abcam, UK). The studies showed linear depositions of IgG1 (++), IgG2 (+), IgG3 (+), IgG4 (++), C4d (+) and MAC (+) along the TBM (fig. 2). In the renal interstitium, granular depositions of IgG1 (++), IgG2 (+), IgG3 (+), IgG4 (++), C4d (+) and MAC (+) were also detected. No staining of MBL and L-ficolin was observed in the renal specimens (not shown). To examine whether the patient's serum contained anti-TBM antibodies, indirect IF was performed. Renal tissue sections obtained from a patient with minimal change nephrotic syndrome were prepared and our patient's sera were diluted 1:1 with phosphate-buffered saline (PBS, pH 7.2). The renal sections were then incubated with the diluted sera, and stained with FITC-labeled rabbit anti-human IgM and IgG antisera. Anti-TBM antibodies were not detected. Electron microscopy (EM) showed many homogeneous electron-dense deposits in the TBM, but no glomerular deposition (fig. 3). Therefore, he was diagnosed with IgG4-related TIN and was treated with prednisolone 40 mg p.o. daily. His symptoms and systemic lymphadenopathy immediately disappeared and renal function was gradually improved (table 1). Elevated levels of serum IgG and hypocomplementemia returned to normal. CT scanning after a month on steroid therapy showed that the size of the kidneys was improved and Ga citrate scintigraphy showed disappearance of abnormal isotope accumulation in the bilateral kidneys. The dose of prednisolone was gradually tapered and he was discharged from our hospital.
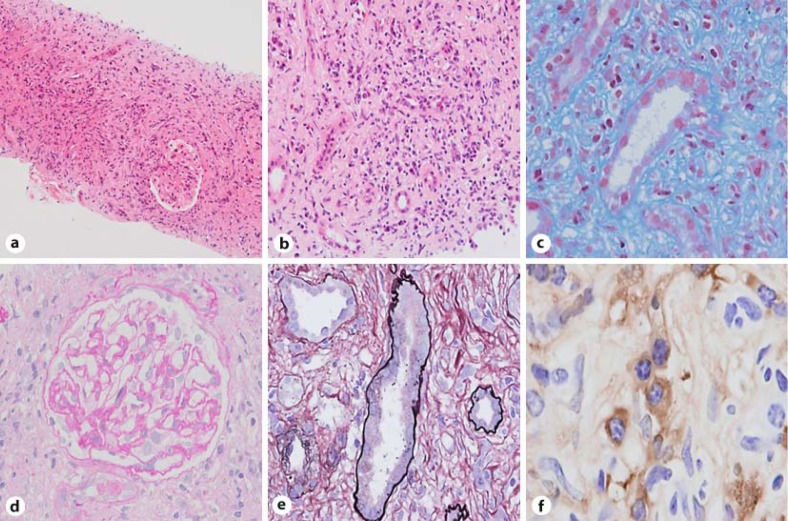
Fig. 1.
Light microscopic and immunohistochemical studies. Light microscopy shows diffuse inflammatory infiltrates consisting mainly of lymphocytes and plasma cells (a; HE stain, original magnification ×100, b; HE stain, original magnification ×200) with fibrosis (c; Masson trichrome stain, original magnification ×400) in the tubulointerstitium and atrophy of tubules (e; PAS stain, original magnification ×400). The glomeruli show no abnormality (d; HE stain, original magnification ×400). Immunohistochemistry shows renal interstitial inflammation composed of IgG4-positve plasma cells (f; original magnification ×400).
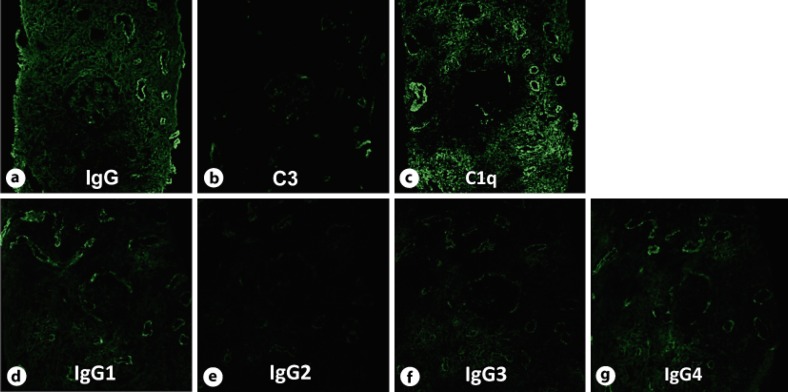
Fig. 2.
IF study. Routine IF shows strong positive staining for IgG (a) and C1q (c) and weak staining for C3 (b) in the tubulointerstitium (original magnification ×200). Additional IF shows strong positive staining for IgG1 (d) and IgG4 (g) and weak staining for IgG2 (e) and IgG3 (f) along the TBM and in the interstitium (original magnification ×200).
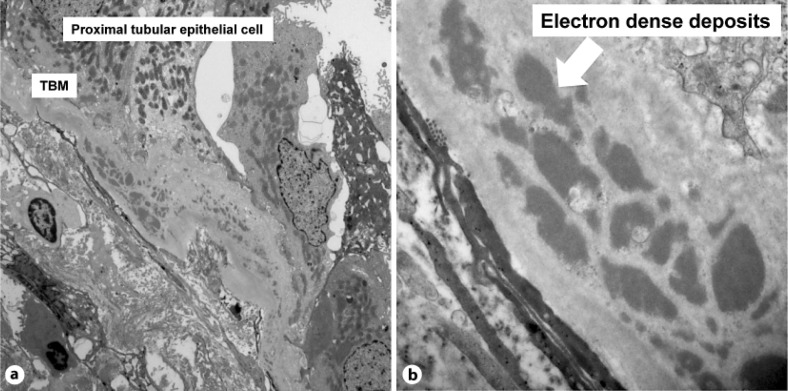
Fig. 3.
EM study. EM shows homogeneous electron-dense deposits in the TBM (arrow) (original magnification in a ×3,000, and in b ×12,000).
Discussion
IgG4-related disease is a new entity and shows various symptoms depending on affected organs. In our case, the patient showed symptoms of anorexia, dysgeusia and dysosmia. Anorexia is one of the common symptoms reflecting systemic inflammatory conditions and the other symptoms were thought to have resulted from sinusitis which was observed by CT scanning. Recently, chronic rhinosinusitis is suggested to be involved in IgG4-related disease and a large amount of IgG4-bearing plasma cell infiltrations in the nasal mucosa were found in that report [14]. Serologically, 30–40% of patients present with hypocomplementemia, which may affect complement activation. However, the triggering mechanism of the complement pathway has not been elucidated [5, 13]. Although IgG4 does not possess the ability to bind C1q complement [15], the classical pathway component, this disease shows low serum levels of C4 and low titers of total serum hemolytic activity (CH50). Therefore, we explored the mechanisms of complement activation in both sera and local tissues.
In our case, the levels of serum C3, C4, CH50 and C1q were decreased, and the level of ICs (C1q assay) was increased. The level of serum anti-C1q antibody as in lupus nephritis was not examined, and we could not define the constituents of ICs. However, these laboratory findings imply that the classical pathway is activated in the fluid phase.
Muraki et al. [5] suggested that the elevated levels of ICs were associated with increased levels of IgG1 rather than those of IgG4 and decreased levels of C3 and C4 in cases of autoimmune pancreatitis, since IgG1 is a molecule capable of activation of the classical pathway. Therefore, we assume that activation of complement via the classical pathway in the fluid phase might be caused by ICs including the IgG subclasses except for IgG4.
Next, we examined the histology in detail, and the depositions of various IgG subclasses, IgM, C1q, C4d and MAC, were observed along the TBM and in the interstitium, but no deposition of MBL and L-ficolin that are capable of activation of the lectin complement pathway was found. Yamamoto et al. [6] reported a case of Mikulicz’ disease, an IgG4-related disease, with deposits of C1q, C3, IgG and IgM observed along the TBM. Furthermore, we also identified electron-dense deposits in the TBM by EM. To exclude the possibility of autoantibodies that recognize TBM, the patient's serum was applied to renal tissues. However, we could not detect any anti-TBM antibodies in the sera.
In local tissues, ICs might contain homologous components such as IgG1, IgG2, IgG3, IgG4, IgM, C1q, C3 and C4. Therefore, we thought that complement activation via the classical pathway by ICs also might have occurred in the local tissues and be one of the causes of the pathogenesis of local tissue damage. Histopathological differences between IgG4-related TIN and other types of TIN are based on whether IC formation occurs in local tissues in IgG4-related TIN and not in other TIN. On the other hand, the characteristic histopathological features of IgG4-related nephropathy appear mainly in the tubulointerstitium, although some cases show glomerulonephritis and glomerular IgG deposition [8]. We also observed a positive signal of IgG in the glomeruli, but we could detect neither apparent signals of IgG subclasses by IF nor glomerular deposition by EM. The types of circulating IC may determine whether ICs are capable of accumulating in the glomerular basement membrane. In our case, interstitial deposits of ICs might have formed in the interstitium and/or leaked from peritubular capillaries. Further studies are necessary to elucidate the pathogenesis of IgG4-related disease and the mechanism of complement activation by not only IgG, including the IgG4 and IgG1 subclasses, but also other factors.
In conclusion, it appears that IC-mediated complement activation via the classical pathway might occur in the fluid phase and the renal interstitium in IgG4-related TIN.
References
- 1.Kamisawa T, Okamoto A. IgG4-related sclerosing disease. World J Gastroenterol. 2008;14:3948–3955. doi: 10.3748/wjg.14.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hamano H, Kawa S, Horiuchi A, Unno H, Furuya N, Akamatsu T, Fukushima M, Nikaido T, Nakayama K, Usuda N, Kiyosawa K. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Eng J Med. 2001;344:732–738. doi: 10.1056/NEJM200103083441005. [DOI] [PubMed] [Google Scholar]
- 3.Saeki T, Saito A, Yamazaki H, Emura I, Imai N, Ueno M, Nishi S, Ito T, Miyamura S, Gejyo F. Tubulointerstitial nephritis associated with IgG4-related systemic disease. Clin Exp Nephrol. 2007;11:168–173. doi: 10.1007/s10157-007-0464-9. [DOI] [PubMed] [Google Scholar]
- 4.Nishimori I, Suda K, Oi I, Ogawa M. Research on the clinical state of autoimmune pancreatitis. J Japan Pancreas Soc. 2002;17:619–627. [Google Scholar]
- 5.Muraki T, Hamano H, Ochi Y, Komatsu K, Komiyama Y, Arakura N, Yoshizawa K, Ota M, Kawa S, Kiyosawa K. Autoimmune pancreatitis and complement activation system. Pancreas. 2006;32:16–21. doi: 10.1097/01.mpa.0000188308.75043.e4. [DOI] [PubMed] [Google Scholar]
- 6.Yamamoto M, Takahashi H, Naishiro Y, Isshiki H, Ohara M, Suzuki C, Yamamoto H, Kokai Y, Himi T, Imai K, Shinomura Y. Mikulicz's disease and systemic IgG4-related plasmacytic syndrome (SIPS). Jpn J Clin Immunol. 2008;31:1–8. doi: 10.2177/jsci.31.1. [DOI] [PubMed] [Google Scholar]
- 7.Saeki T, Ito T, Yamazaki H, Imai N, Nishi S. Hypocomplementemia of unknown etiology: an opportunity to find cases of IgG4-positive multi-organ lymphoproliferative syndrome. Rheumatol Int. 2009;30:99–103. doi: 10.1007/s00296-009-0925-4. [DOI] [PubMed] [Google Scholar]
- 8.Saeki T, Nishi S, Imai N, Ito T, Yamazaki H, Kawano M, Yamamoto M, Takahashi H, Matsui S, Nakada S, Origuchi T, Hirabayashi A, Homma N, Tsubata Y, Takata T, Wada Y, Saito A, Fukase S, Ishioka K, Miyazaki K, Masaki Y, Umehara H, Sugai S, Narita I. Clinicopathological characteristics of patients with IgG4-related tubulointerstitial nephritis. Kidney Int. 2010;78:1016–1023. doi: 10.1038/ki.2010.271. [DOI] [PubMed] [Google Scholar]
- 9.Takeda S, Haratake J, Kasai T, Takaeda C, Takazakura E. IgG4-associated idiopathic tubulointerstitial nephritis complicating autoimmune pancreatitis. Nephrol Dial Transplant. 2004;19:474–476. doi: 10.1093/ndt/gfg477. [DOI] [PubMed] [Google Scholar]
- 10.Saeki T, Nishi S, Ito T, Yamazaki H, Miyamura S, Emura I, Imai N, Ueno M, Saito A, Gejyo F. Renal lesions in IgG4-related systemic disease. Intern Med. 2007;46:1365–1371. doi: 10.2169/internalmedicine.46.0183. [DOI] [PubMed] [Google Scholar]
- 11.Katano K, Hayatsu Y, Matsuda T, Miyazaki R, Yamada K, Kawano M, Takahashi N, Kimura H, Yoshida H. Endocapillary proliferative glomerulonephritis with crescent formation and concurrent tubulointerstitial nephritis complicating retroperitoneal fibrosis with a high serum level of IgG4. Clin Nephrol. 2007;68:308–314. doi: 10.5414/cnp68308. [DOI] [PubMed] [Google Scholar]
- 12.Saida Y, Homma N, Hama H, Ueno M, Imai N, Nishi S, Gejyo F. A case of IgG4-related tubulointerstitial nephritis showing the progression of renal dysfunction after a cure for autoimmune pancreatitis. Jpn J Nephrol. 2010;52:73–79. [PubMed] [Google Scholar]
- 13.Dhobale S, Bedetti C, Killian P, Ilyas M, Liput J, Jasnosz K, Giclas P. IgG4 related sclerosing disease with multiple organ involvements and response to corticosteroid treatment. J Clin Rheumatol. 2009;15:354–457. doi: 10.1097/RHU.0b013e3181b5d631. [DOI] [PubMed] [Google Scholar]
- 14.Moteki H, Yasuo M, Hamano H, Uehara T, Usami S. IgG4-related chronic rhinosinusitis: A new clinical entity of nasal disease. Acta Oto-Laryngologica. 2011;131:518–526. doi: 10.3109/00016489.2010.533699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van der Zee JS. van Swieten P. Aalberse RC. Inhibition of complement activation by IgG4 antibodies. Clin Exp Immunol. 1986;64:415–422. [PMC free article] [PubMed] [Google Scholar]