Abstract
Higher plants synthesize 24-methyl sterols and 24-ethyl sterols in defined proportions. As a first step in investigating the physiological function of this balance, an Arabidopsis cDNA encoding an S-adenosyl-l-methionine 24-methylene lophenol-C241-methyltransferase, the typical plant enzyme responsible for the production of 24-ethyl sterols, was expressed in tobacco (Nicotiana tabacum L.) under the control of a constitutive promoter. Transgenic plants displayed a novel 24-alkyl-Δ5-sterol profile: the ratio of 24-methyl cholesterol to sitosterol, which is close to 1 in the wild type, decreased dramatically to values ranging from 0.01 to 0.31. In succeeding generations of transgenic tobacco, a high S-adenosyl-l-methionine 24-methylene lophenol-C241-methyltransferase enzyme activity and, consequently, a low ratio of 24-methyl cholesterol to sitosterol, was associated with reduced growth compared with the wild type. However, this new morphological phenotype appeared only below the threshold ratio of 24-methyl cholesterol to sitosterol of approximately 0.1. Because the size of cells was unchanged in small, transgenic plants, we hypothesize that a radical decrease of 24-methyl cholesterol and/or a concomitant increase of sitosterol would be responsible for a change in cell division through as-yet unknown mechanisms.
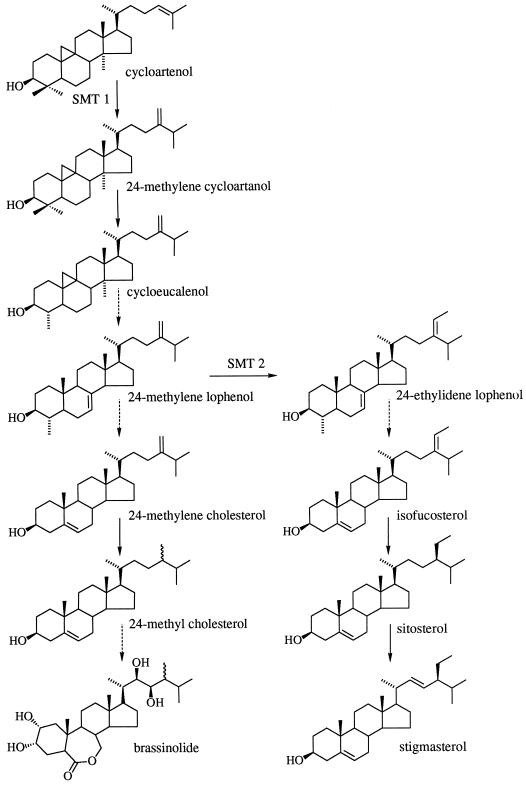
Sterol metabolism in the plant cell has features not seen in other kingdoms: unique enzymatic steps involved in the transformation of squalene into pathway end products (for review, see Benveniste, 1986; Ourisson, 1994). The occurrence in photosynthetic organisms of sterols bearing an additional alkyl group at position C24, 24-methyl sterols and 24-ethyl sterols, has been widely documented (for review, see Nes and McKean, 1977). Higher plants contain mixtures of 24-alkyl-Δ5-sterols in which 24-methyl cholesterol is the major 24-methyl sterol, and sitosterol and stigmasterol are the predominant 24-ethyl sterols (Fig. 1); the R is almost invariable with respect to species and is therefore controlled.
Figure 1.
Simplified biosynthetic pathway of sterols in higher plants. 24-Methyl cholesterol is present in higher plants as a mixture of its (24-R)- and (24-S)-epimers campesterol and dihydrobrassicasterol (Rubinstein et al., 1976; Rendell et al., 1986) and is a precursor for the synthesis of brassinosteroids. The dashed arrows indicate more than one biosynthetic step not shown here.
The presence of high proportions of 24-ethyl sterols in plant membranes correlates with their efficiency in developing specific interactions with plant phospholipids to reinforce and stabilize the bilayer architecture (Demel and De Kruyff, 1976; Bloch, 1983; Schuler et al., 1991). The extra carbon atoms are added on sterol intermediates via SMTs, enzymes that have always been looked at with considerable interest in terms of the chemistry and biochemistry of their catalyzed reactions (Rahier et al., 1984; Bladocha and Benveniste, 1985; Rendell et al., 1986; Janssen and Nes, 1992).
Two distinct SMTs are responsible for the two methyl transfers involved in the conversion of cycloartenol into sitosterol. As shown in Figure 1, cycloartenol is the natural substrate of the first methylation reaction (SMT1), which produces 24-methylene cycloartenol (Malhotra and Nes, 1971; Wojciechowski et al., 1973); 24-methylene lophenol is the natural substrate of the second methylation reaction (SMT2), which produces 24-ethylidene lophenol (Fonteneau et al., 1977). The occurrence of two distinct SMTs in plants was recently reinforced after cDNAs were cloned from Arabidopsis (Husselstein et al., 1996), soybean (Shi et al., 1996), maize (Grebenok et al., 1997), and tobacco (Bouvier-Navé et al., 1997); indeed, alignment of the deduced amino acid sequences indicated that they are distributed into two families (Bouvier-Navé et al., 1997). Moreover, expression of plant SMTs in the yeast null mutant erg6 (Gaber et al., 1989), in which the gene encoding the S-adenosyl-l-Met-zymosterol C24-methyltransferase has been knocked out, restored the synthesis of ergosterol in the case of SMTs from maize (Grebenok et al., 1997) and tobacco (Bouvier-Navé et al., 1998) and of ergosterol and 24-ethyl sterols in the case of SMT from Arabidopsis (Husselstein et al., 1996).
Further studies in the latter case showed that in a delipidated microsomal preparation of the complemented yeast, the catalytic efficiency of the expressed SMT was 17 times higher using 24-methylene lophenol as a substrate than when cycloartenol was used (Bouvier-Navé et al., 1997). This clearly indicates that in the yeast membrane environment this Arabidopsis SMT operates as a 24-methylene lophenol-C241-methyltransferase (Fig. 1, SMT2), catalyzing the second methyl transfer, which is responsible for the plant cell's capability to produce 24-ethyl sterols.
As a first step in investigating in more detail the physiological function of 24-methyl- and 24-ethyl sterols and the regulation of their respective concentrations in the plant cell, transgenic tobacco expressing an Arabidopsis SMT2 under control of a strong constitutive promoter was generated. We report that a dramatic decrease in the amount of 24-methyl cholesterol and a concomitant increase in sitosterol are associated with reduced growth in transgenic plants.
MATERIALS AND METHODS
Plasmid Constructs
A 1.45-kb XhoI-SmaI fragment containing an Arabidopsis cDNA encoding a sterol-C241-methyltransferase was excised from the plasmid pSK411 (Husselstein et al., 1996) and cloned into the XhoI-SmaI opened binary vector pFB8 (Atanassova et al., 1995). Correct insertion of the 1.45-kb fragment was determined by restriction of plasmid DNA extracted from Escherichia coli DH5α cultures transformed with the ligation mixture. Recombinant pFB8 derivatives, referred to as p35S-SMT2, and the corresponding void plasmid, referred to as p35S, were introduced into Agrobacterium tumefaciens LBA 4404 by triparental mating using pRK2013 in E. coli HB101 as a helper plasmid (Bevan, 1984).
Plant Transformation and Genetic Analysis
Tobacco (Nicotiana tabacum L. var Xanthi) was transformed with p35S-SMT2 and p35S via A. tumefaciens according to a modification of the method reported by Horsch et al. (1985). Leaf pieces were co-cultivated with an A. tumefaciens culture on Murashige and Skoog medium supplemented with Glc (30 g/L), ANA (0.1 mg/L), and 6-benzylaminopurine (1 mg/L) for 2 d and were then transferred onto the same medium supplemented with kanamycin (100 mg/L) as the plant-selective agent and cefotaxime (500 mg/L) to prevent further bacterial growth. Regenerated shoots were rooted on Murashige and Skoog medium with a half-reduced concentration of NH4NO3 and supplemented with Suc (30 g/L), kanamycin (200 mg/L), and cefotaxime (200 mg/L). In vitro cultures were grown under a 16-h light period at 24°C and an 8-h dark period at 20°C. Tobacco plants were transferred to soil and grown under standard greenhouse conditions.
Succeeding generations of transgenic plants were scored for kanamycin resistance according to two procedures: some of the seeds were germinated on Murashige and Skoog medium supplemented with 300 mg/L kanamycin; others were grown on Murashige and Skoog medium and then used to produce cotyledon-derived calli using callus-inducing medium (Bourgin et al., 1979) supplemented with 100 mg/L kanamycin. Integration of the transgene into the plant genome was determined by PCR on chromosomal DNA prepared according to the method of Krysan et al. (1996). Two different reactions were carried out with 50 ng of template DNA and 2 units of Dynazyme (Finnzymes Oy, Espoo, Finland) in the conditions recommended by the manufacturer. The first reaction used primers G1 (sense, 5′-GCCGGGATCCATCGCAGCGTAATGC-3′) and G2 (antisense, 5′-GCCTCCCTGCTGCGGTTT TTCACCG-3′) for the amplification of a 1298-bp internal fragment of the uidA gene carried on the pFB8 vector (Atanassova et al., 1995); the second reaction used primers A1 (sense, 5′-GGCTCAATCTCCGCCGAGAAAGTCC-3′) and A2 (antisense, 5′-CTCTCCTCCGGTGACTCCGG-3′) for the amplification of a 965-bp internal fragment of the SMT2 cDNA.
Transgene Expression Analysis
Total RNAs from leaf material of 2-month-old greenhouse-grown plants were extracted according to the method of Goodall et al. (1990). Northern analysis was done by separating 10-μg RNA samples on formaldehyde gels as described by Sambrook et al. (1989) and blotting them onto nylon filters (Hybond-N+, Amersham). Randomly primed (Stratagene) 32P-labeled probes polymerized from the SMT2 cDNA 411 (Husselstein et al., 1996) were hybridized to the filters as recommended by the manufacturer. Filters were then washed in 0.2× SSC containing 0.1% SDS at 65°C before autoradiography. RT-PCR reactions were started with 5 μg of total RNA, primer A2, and 200 units of RT (Moloney murine leukemia virus from BRL) with reagents and conditions as listed by the manufacturer. RT products were amplified in standard PCR conditions with primers A1 and A2. PCR products were further analyzed by Southern hybridization (Sambrook et al., 1989) with the SMT2 probe described above.
SMT Enzymatic Assay
Isolation of membranes from tobacco leaves was carried out as follows: 12 g of leaf tissue was ground in a 0.2 m KH2PO4 buffer (pH 7.5) containing 0.35 m sorbitol, 10 mm Na2EDTA·2H2O, 5 mm MgCl2·6H2O, 40 g/L PVP, and 10 mm dithioerythritol. The supernatant fraction resulting from a 15-min centrifugation at 10,000g was centrifuged for 1 h at 100,000g. The membrane fraction was then resuspended in a 0.1 m Tris-HCl buffer (pH 7.5) containing 1 mm β-mercaptoethanol and 20% (v/v) glycerol. Microsomal proteins were quantified by the Bio-Rad protein assay using BSA as a standard.
A radiochemical assay was set up based on that reported by Fonteneau et al. (1977). A standard assay for tobacco SMT2 consisted of 0.1 m Tris-HCl at pH 7.5, 1 mm β-mercaptoethanol, 20% glycerol (v/v), 0.1% Tween 80 (w/v), 30 μm sterol substrate, 100 μm [methyl-3H]-adenosyl-Met (475,000 cpm,) and 0.8 mg/mL microsomal proteins. The mixture was incubated at 30°C for 45 min, and then the reaction was stopped by adding 100 μL of 12% (w/v) ethanolic KOH. Sterol carriers were added before neutral lipids were extracted with n-hexane. Sterols were purified by TLC (two runs of dichloromethane), and the band corresponding to 4α-methyl sterols was scraped off (RF = 0.3) and collected into scintillation vials containing 10 mL of liquid-scintillation cocktail (Ready Organic, Beckman). Radioactivity was determined in a liquid-scintillation counter (Packard Instruments, Downers Grove, IL).
Extraction and Dosage of Sterols
Lipids from about 5 to 10 mg (small-scale qualitative analysis) or 100 to 200 mg (quantitative analysis) of ground, dry material were extracted at 70°C in dichloromethane:methanol (2:1, v/v). The dried residue was saponified with 6% (w/v) KOH in methanol at 90°C for 1 h to release the sterol moiety of steryl esters. Sterols were then extracted with 3 volumes of n-hexane, and an acetylation reaction was performed on the dried residue for 1 h at 60°C in toluene with a mixture of pyridine:acetic anhydride (1:1, v/v). Steryl-acetates were resolved by TLC using precoated silica plates (60F254, Merck, Darmstadt, Germany), with one run of dichloromethane as a single band at RF = 0.5. Purified steryl acetates were separated and identified using a gas chromatograph (model 8300, Varian, Les Ulis, France) with a flame-ionization detector and a glass capillary column (wall coated, open, and tubular; 30 m long; 0.25 mm i.d.; coated with DB1; J & W Scientific, Folsom, CA) using H2 as a carrier gas (2 mL/min). The temperature program included a fast increase from 60°C to 230°C (30°C/min) and a slow increase from 230°C to 280°C (2°C/min). Data from the detector were monitored with a computer program (Star, Varian). Sterol structures were confirmed by GC-MS (model MD800, Fisons Instruments, Beverly, MA) equipped with a glass capillary column (WCOT coated with DB5; J & W Scientific) as described previously (Rahier and Benveniste, 1989). Determination of the sterol content from microsomes was as indicated above except for the extraction procedure, which started with the saponification step.
Measurement of Cell Length
Stem and midrib epidermis from the middle of the fifth internode and from the middle of the fifth leaf were peeled off from 3-month-old greenhouse-grown plants. Five cells per epidermis sample were randomly chosen and their lengths were measured under a microscope with a micrometer (Zeiss). Cell measurements were done for five plants per line.
RESULTS
Generation of Tobacco Plants Expressing the Arabidopsis SMT2 cDNA
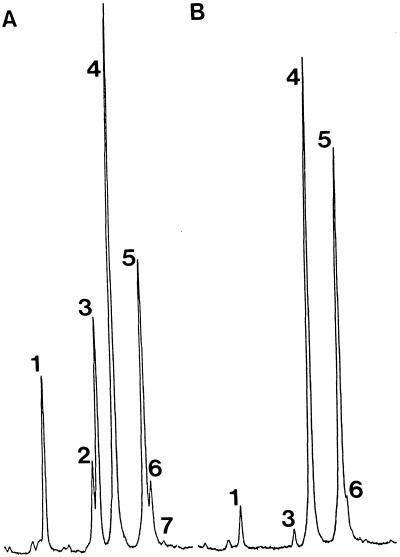
A set of 21 plants randomly chosen from the population of leaf-disc-derived kanamycin-resistant regenerants were subjected to a small-scale, rapid sterol analysis to point out any putative new trait(s). Analysis of leaf material from all 21 regenerants revealed a novel 24-alkyl-Δ5-sterol composition: a dramatic decrease in the proportion of 24-methyl sterols and a concomitant increase in the proportion of 24-ethyl sterols. Gas chromatograms shown in Figure 2 illustrate this first sterol screen; R (peaks 3–5) was calculated for each T1 transformant. Wild-type plants and transgenic controls (p35S) had R values of approximately 1, whereas p35S-SMT2 tobacco had R values ranging from 0.03 to 0.62 (data not shown).
Figure 2.
Gas chromatograms of steryl acetates from leaf material of primary tobacco transformants 1 month after their transfer to the greenhouse. A, Wild type; B, p35S-SMT2. Representative samples are shown. Peak 1, Cholesteryl-acetate; peak 2, 24-methylene cholesteryl-acetate; peak 3, 24-methyl cholesteryl-acetate; peak 4, stigmasteryl-acetate; peak 5, sitosteryl-acetate; peak 6, isofucosteryl-acetate; peak 7, cycloartenyl-acetate.
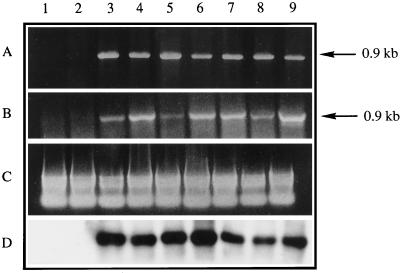
At this point in the study seven of these new lines, as well as one wild-type line and one p35S control line originating from the same leaf-disc culture, were chosen for further characterization. The integration of T-DNA(s) into the genome of kanamycin-resistant tobacco was demonstrated using internal primers recognizing specifically the uid A gene (data not shown) or the Arabidopsis SMT2 cDNA cloned into the T-DNA cassette (Fig. 3A). Expression of the p35S-driven SMT2 cDNA in T1 plants was checked by RT-PCR. As shown in Figure 3, RT-PCR assays gave conclusive evidence for the presence of an Arabidopsis-specific SMT2 mRNA in transgenic tobacco.
Figure 3.
Transgene integration and expression in tobacco. Lane 1, Wild type; lane 2, transgenic p35S control; lanes 3 to 9, transgenic p35S-SMT2 lines A, B, C, D, E, F, and G, respectively. A, Internal 0.9-kb fragment of the Arabidopsis cDNA encoding SMT2 cloned into the T-DNA of the plant-transformation vector was amplified from chromosomal DNA of primary transformants. B, Total leaf RNA subjected to a RT-PCR reaction using primers specific to the 0.9-kb internal fragment of the Arabidopsis cDNA encoding SMT2. C, Ethidium bromide-stained gel showing equal amounts of total RNA used in B. D, Southern hybridization of the PCR products obtained as described in B with a 32P-radiolabeled SMT2 probe. The arrows denote the size of the expected and obtained PCR products.
Sterol Composition of p35S-SMT2 Tobacco
Sterol composition of control and transgenic tobacco plants was accurately determined on leaf samples from the upper third of the plants. Results reported in Table I demonstrate clearly the striking difference between control and SMT2 lines: the latter show a considerable decrease in the amount of 24-methyl cholesterol with a concomitant increase of 24-ethyl sterols (sitosterol) compared with the controls. There were only slight increases in other 24-ethyl sterols (e.g. stigmasterol). The other minor variation of the sterol profile of SMT2 tobacco leaves was a faint decrease in cholesterol compared with control lines. We did not detect any significant variation in the amount of sterol in transgenic plants: all contained approximately 2 mg total sterols g−1 dry weight, the same as in the controls. From these overall observations, the shift occurring in the sterol profile of SMT2 plants may be clearly expressed by R. As mentioned above, R was approximately 1 for control plants, whereas it decreased radically for the seven T1 SMT2 plants analyzed, ranging from 0.01 to 0.31.
Table I.
Total sterol content of tobacco plants transformed with an Arabidopsis SMT2 cDNA
| Sterola | Control
|
p35S-smt2
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Wild type | p35S | A | B | C | D | E | F | G | |
| % of total | |||||||||
| Cycloartenol | 2 | 2 | 2 | 8 | 3 | 2.5 | 2 | 3 | 2 |
| Cholesterol | 7 | 7 | 2 | 2 | 5 | 2 | 3 | 6 | 4 |
| 24-Methylene cholesterol | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 |
| 24-Methyl cholesterol | 22 | 22 | 1.5 | 0.5 | 10 | 1.5 | 1 | 5 | 5 |
| Isofucosterol | 9 | 9 | 6.5 | 5 | 11 | 7 | 6 | 6 | 9 |
| Sitosterol | 20 | 18 | 40 | 40.5 | 32 | 47 | 43 | 35 | 32 |
| Stigmasterol | 39 | 41 | 48 | 44 | 38 | 40 | 45 | 44 | 48 |
| mg g−1 dry wt | |||||||||
| Total amount | 2.2 | 2.1 | 2.1 | 1.9 | 1.8 | 2.3 | 2.8 | 1.9 | 2.1 |
| R | 1.10 | 1.22 | 0.04 | 0.01 | 0.31 | 0.03 | 0.02 | 0.14 | 0.15 |
Analysis was performed on leaf material from T1 plants grown for 6 weeks in the greenhouse.
β-Amyrin, cycloeucalenol, and Δ7-sitostenol were found in trace amounts in all samples.
Growth of p35S-SMT2 Tobacco
Growth rates and morphological phenotypes were observed throughout succeeding generations of transgenic tobacco. During the early stages of growth (i.e. during germination of seeds in vitro on a nutrient medium or in soil in the greenhouse), the SMT2 lines exhibited the same morphological phenotype as controls; however, a reduction in growth of SMT2 seedlings from some transgenic lines became apparent after 3 weeks of culture (data not shown). When the plants were transferred to standard-size pots in the same greenhouse conditions, we observed a significant size reduction in a large number of SMT2 plants compared with control plants and with another set of wild-type-like SMT2 plants. The smallest SMT2 plants were one-half the size of control plants (Fig. 4). The low growth rate of some of the SMT2 plants was always associated with up to a 2-month delay in flowering. Delayed flowering tobacco plants displayed a reduced number of flowers per inflorescence compared with control lines; however, the morphology and fecundity of flowers were similar to those of controls (data not shown).
Figure 4.
Wild-type and p35S-SMT2 tobacco plants. Three-month-old plants grown in standard greenhouse conditions are shown: wild-type plants are on the left and T3 plants from line D are on the right.
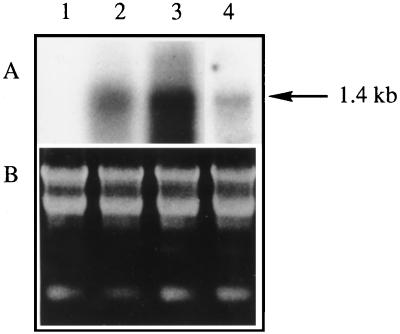
To describe more precisely the growth-reduction phenotype of the SMT2 overexpressors, plants from the T3 generation were compared with the wild type with respect to plant height, number of internodes, and size of cells. The data in Table II show that the growth-reduction trait was stably transmitted through generations. These data also show that, whatever the stem height of a plant, the average number of expanded leaves per plant remained stable at approximately 21; therefore, growth reduction could be calculated, on average, as a 36% internode length reduction compared with wild-type plants. It is crucial to point out that the length of stem epidermis cells and midrib epidermis cells was not significantly different in SMT2 plants compared with wild-type plants; therefore, the growth reduction reported here is not due to a reduction of the cell length but rather to a reduced number of cells per unit length. Finally, overexpression of SMT2 in T3 plants was verified by northern analysis (Fig. 5), and the proportion of 24-methyl- to 24-ethyl sterols was determined (Table II).
Table II.
Size measurements on 3-month-old greenhouse-grown p35S-SMT2 plants (vegetative stage) from T3 generations and corresponding mean sterol composition
| Line (n) | Plant Height | Expanded Leaves | Length of Epidermal Cells
|
R | |
|---|---|---|---|---|---|
| Stem | Midrib | ||||
| cm | no. plant−1 | μm | |||
| Wild type (11) | 80.8 ± 8.8a | 20.7 ± 1.9 | 179.0 ± 41.0 | 363.7 ± 112.8 | 1.08 |
| A (10) | 51.6 ± 5.3 | 21.1 ± 1.6 | 165.3 ± 36.5 | 427.5 ± 82.1 | 0.06 |
| D (33) | 52.5 ± 9.3 | 21.5 ± 2.6 | 176.7 ± 47.8 | 404.7 ± 103.7 | 0.07 |
| E (21) | 60.4 ± 5.4 | 22.0 ± 1.5 | 172.1 ± 33.1 | 351.1 ± 94.6 | 0.05 |
Mean ± sd.
Figure 5.
Northern analysis. Lane 1, Wild-type tobacco line; lanes 2 to 4, transgenic p35S-SMT2 plant lines A, D, and E, respectively. Ten micrograms of total leaf RNA from T3 plants was hybridized with a probe derived from the Arabidopsis SMT2. The arrow denotes the SMT2 1.4-kb transcript. The ethidium bromide-stained gel demonstrates equivalent RNA quantities loaded in each lane.
Modification of the Proportions of 24-Methyl- and 24-Ethyl Cholesterol and Growth Effect
To establish that a dramatic decrease in 24-methyl cholesterol and/or an increase in sitosterol may be associated with reduced plant growth and size, individuals within five independent T2 families were checked on the basis of the following three parameters: the height of the stem, the R, and the 24-methylene lophenol-C241-methyltransferase activity monitored in microsomes from leaf tissue. The population of transgenic plants was grown in the greenhouse after the wild-type individuals from a given T2 family were counterselected in vitro on a germination medium containing 200 mg/L kanamycin. Data collected from the analysis of 2-month-old plants are shown in Table III. The overall results assigned to wild-type or transgenic control plants define a pool of controls having an SMT2 activity of approximately 0.8 nmol mg−1 protein h−1, an R value of approximately 1 in microsomes from leaf tissue and from the total sterol fraction, and a stem height of approximately 60 cm.
Table III.
SMT2 enzyme assay, sterol profile, and plant size in T2 generations of p35S-SMT2 lines
| Line/No. of Transgenic Locia | Individual/Genetic Structureb | SMT2 Enzyme Activity | R
|
Plant Height | |
|---|---|---|---|---|---|
| Microsomes | Total sterols | ||||
| nmol mg−1 protein h−1 | cm | ||||
| Wild typec | – | 0.88 ± 0.33 | 0.99 ± 0.07 | 1.00 ± 0.03 (1.35 ± 0.20) | 59 ± 2 (142 ± 11) |
| p35Sc | – | 0.80 ± 0.30 | 0.90 ± 0.06 | 0.75 ± 0.06 (0.95 ± 0.34) | 60 ± 1 (146 ± 15) |
| A/1 | 1/hom | 5.9d | 0.05 | 0.02 (0.04) | 39 (64) |
| 2/hom | 3.2 | 0.09 | 0.13 (0.10) | 56 (135) | |
| 3/hom | 7.5 | 0.03 | 0.02 (0.04) | 54 (120) | |
| 4/het | 4.1 | 0.17 | 0.14 (0.17) | 63 (145) | |
| B/2 | 1/het | 3.9 | 0.14 | 0.15 (0.18) | 56 (130) |
| 2/het | 5.4 | 0.04 | 0.04 (0.02) | 42 (85) | |
| 3/hom | 2.8 | 0.07 | 0.10 (0.08) | 53 (130) | |
| 4/het | 8.0 | 0.03 | 0.02 (0.04) | 45 (110) | |
| C/1 | 1/het | 1.8 | 0.16 | 0.18 (0.18) | 53 (135) |
| 2/hom | 5.4 | 0.09 | 0.09 (0.14) | 48 (130) | |
| 3/het | 3.7 | 0.17 | 0.18 (0.19) | 50 (135) | |
| 4/− | 3.0 | 0.17 | 0.18 (0.20) | 58 (130) | |
| D/2 | 1/het | 5.7 | 0.03 | 0.04 (0.07) | 48 (120) |
| 2/het | 4.6 | 0.03 | 0.02 (0.08) | 51 (110) | |
| 3/het | 6.3 | 0.03 | 0.05 (0.04) | 39 (90) | |
| 4/het | 10.5 | 0.03 | 0.03 (0.03) | 28 (55) | |
| E/1 | 1/het | 3.7 | 0.15 | 0.15 (0.20) | 59 (130) |
| 2/het | 3.6 | 0.15 | 0.16 (0.17) | 56 (125) | |
| 3/hom | 12.6 | 0.03 | 0.05 (0.04) | 38 (90) | |
| 4/hom | 11.0 | 0.03 | 0.02 (0.04) | 36 (85) | |
Values in parentheses are data collected from the same plants after seed setting.
Primary transformants were selfed and the resulting T2 progenies were segregated for kanamycin resistance on a germination medium. Sample sizes ranged between 50 and 140 individuals. Hypotheses of the number of mendelian loci were challenged according to χ2 tests (data not shown).
T2 individuals were selfed and the resulting T3 progenies were scored for kanamycin resistance as described in footnote a. hom, Homozygote; het, heterozygote; –, not done.
Values presented for wild-type and transgenic controls are means ± sd of four individuals.
Mean value of two experiments; sd never exceeded 30%.
When control data were compared with the values obtained for the 20 T2 plants, four important points appeared. First, the SMT2 activity measured in leaf microsomes from each individual was increased up to 14-fold over the mean control value; this increase was greater than 5-fold for 60% of the analyzed population. Second, the R measured in total lipid extracts decreased to values ranging from 0.02 to 0.18. These values may be distributed into two groups: one comprising the lowest values ranging from 0.02 to 0.05 and corresponding to the highest SMT2 levels and the other ranging from 0.09 to 0.18, which includes plants displaying relatively low R values and corresponding to SMT2 levels found below the limit of 5-fold the control values. Third, when plant growth was monitored in terms of stem height, the first group of plants displaying the most dramatic change in sterol metabolism were those exhibiting a growth reduction matching values from 10% to 50% of the size of control plants; the second group of plants, although showing an important decrease in R but relatively moderate when compared with the extreme values (0.02–0.05 found in the first group), had a growth rate very close to that of the controls. These measurements were fully validated when sizes of plants having completed their growth and flowering were recorded, along with their R factor taken from a total leaf sterol extract: the lower the R value, the smaller the plant (Table III). Fourth, the R from a leaf sterol extract was without exception in the analyzed plant population almost identical to that measured in the corresponding microsomal fraction, which contains the cell membranes in which sterols are localized.
DISCUSSION
We have generated transgenic tobacco plants expressing an Arabidopsis cDNA encoding SMT2 under control of a strong constitutive promoter, and examined the biosynthetic and biological features of this new material over succeeding generations.
Tobacco plants expressing the SMT2 cDNA were characterized by a dramatic drop in the amount of 24-methyl sterols and a concomitant increase in the amount of 24-ethyl sterols. Analysis of the overall sterol profiles indicated that aside from minor variations, the major change occurring in the transgenic material was a strong shift from 24-methyl cholesterol to sitosterol; therefore, the R was calculated. Whereas R was approximately 1 in control lines (wild-type, transgenic controls), it ranged from 0.01 to 0.31 in p35S-SMT2 lines. The amount of other 24-ethyl sterols found in tobacco remained almost unchanged, particularly stigmasterol, which is thought to be the product of the desaturation at C22 of sitosterol (Fig. 1; for review, see Grünwald, 1975; Benveniste, 1986, and refs. therein). Although in plants a sterol-22-desaturase has not yet been characterized in terms of enzymatic activity or sequence information, it is tempting to speculate about its features. These would be either a rate-limiting enzymatic step in the pathway or a highly regulated point in the course of plant development for as yet unknown reasons. In summary, from the biosynthetic point of view, transgenic tobacco lines characterized in this work represent the first case, to our knowledge, of a stable modification of the sterol content whereby only two molecular species, 24-methyl cholesterol and sitosterol, are affected by a selective action on SMT2 enzyme activity. Although powerful inhibitors of plant SMTs do exist (Rahier et al., 1980; Schmitt et al., 1981), these molecules are not selective regarding SMT1 and SMT2.
SMT2 is located at a branching point in the sterol pathway and governs the final concentrations of 24-methyl cholesterol and sitosterol in the plant cell. In this study we have evaluated the biological effect of SMT2 overexpression, which results in a modified R in transgenic tobacco. Our results show that a dramatic decrease in R is associated with a reduction of plant growth. Precisely, plants exhibiting the highest SMT2 activity and therefore the lowest R value are those affected in growth: their height is reduced up to one-half the size of the wild type, their leaves are smaller, and they exhibit delayed flowering and produce fewer flowers compared with the wild type.
This biological effect was not linear with respect to R but was clearly below the threshold R value of approximately 0.1, suggesting that tobacco may tolerate a variable composition of 24-methyl- and 24-ethyl sterols in a range of R values between 0.1 and 1 without consequences on its development. Since growth is hampered below the threshold value of R, one could speculate that the isolation of primary transformants (on the basis of reporter gene expression selection) having an R value equal or close to 0 was counterselected. Growth reduction can be due to a reduction in cell length, a reduction in the number of cells, or a combination of both. Data collected from a population of T3 plants, for which the height reduction corresponded to a 36% reduction of the mean internode length, clearly showed that the cell length was not affected in SMT2 tobacco compared with the wild type. Therefore, these data indicate that the reduction in plant stature may have been due to a decrease in cell number. Although it is difficult to determine the effects on cell growth by looking at only the final size of cells, we hypothesize that it is the cell division frequency (the meristem activity) that is reduced in transgenic SMT2 tobacco. Based on these observations, we propose that a radical decrease in the concentration of 24-methyl cholesterol and/or an equivalent increase in that of sitosterol may be responsible for the reduced growth rate.
We should now investigate two hypotheses about the physiological relationship between such a novel sterol balance and its effect on growth. First, the new sterol content could modify the physical properties of the plasma membrane, which may affect cell growth. Some clues about this aspect may be found in our previous work, in which treatment of plants or plant cell cultures with post-squalene sterol biosynthesis inhibitors and selection of mutants resistant to some of these drugs indicated that replacement of most of the pathway end products (24-alkyl-Δ5-sterols) with sterol intermediates was highly toxic (Maillot-Vernier et al., 1990; Schaller et al., 1994). Moreover, tobacco lines overproducing sterols by means of an increased 3-hydroxy-3-methyl glutaryl CoA reductase activity were shown to regulate the concentration of free sterols present in membranes (Schaller et al., 1995). Likewise, a functional analysis of the physical properties of artificial plant membrane systems sets sitosterol at a given concentration as the optimal plant membrane reinforcer among various sterols (Schuler et al., 1991). This aspect in planta is very poorly documented; however, Crèvecoeur et al. (1992) reported physical modifications of the plasma membrane and modification of its sterol content in meristematic cells during the transition from vegetative to floral meristem.
The second hypothesis is that the new sterol content in plants overexpressing SMT2 could affect the biosynthetic interface between sterols and brassinosteroids. The role of the latter in plant growth and development has been recently demonstrated through molecular genetics (Clouse, 1996; Kauschmann et al., 1996; Li et al., 1996; Szekeres et al., 1996; Li and Chory, 1997; Choe et al., 1998), which were preceded by solid biochemical studies (for review, see Adam and Marquardt, 1986; Mandava, 1988; Suzuki et al., 1995; Schmidt et al., 1997). Altogether, these data show that 24-methyl cholesterol is the sterol precursor for the biosynthesis of brassinolide. It is of course tempting to suggest that a severe decrease in the amount of 24-methyl cholesterol would affect the production of brassinosteroids in concentrations required to fulfill normal development. However, it has been shown that all brassinosteroid-biosynthetic mutants characterized so far in Arabidopsis (which are rescued by exogenous brassinosteroids) display a dwarfed phenotype caused by the lack of cell elongation. This is true of det2, which is blocked in the reduction of 3-dehydro-Δ4(5)-campestenol to 3-dehydro campestanol (Fujioka et al., 1997); of dwf4, which is impaired in the C22α-hydroxylation of campestanol (Choe et al., 1998); and of cpd, which presents a defect in the C23α-hydroxylation of cathasterone (Szekeres et al., 1996). Likewise, Nomura et al. (1997) reported a blockage of brassinosteroid synthesis in the pea lkb mutant, in which considerable reduction of brassinolide and brassinosteroid intermediates were associated with a 50% reduction of the cell length. These overall data indicate that brassinosteroids are essential to controlling plant development and especially cell elongation (Clouse, 1996; Kauschmann et al., 1996).
We report here a reduced plant stature likely due to a reduction in cell number without alteration of cell size. This seems to contrast with what would be expected from a modification of the brassinosteroid pathway. In this respect, further investigations challenging the brassinosteroid profile of SMT2 tobacco will clarify this point. Finally, this novel plant material bearing a unique and stable modification of the post-squalene sterol pathway represents a starting point for investigation of the expression of SMT genes in higher plants.
ACKNOWLEDGMENTS
We thank Drs. R. Atanassova and M. Legrand (IBMP, Strasbourg) for the gift of the plant expression vector pFB8. We warmly acknowledge B. Bastian for typing the manuscript and A. Hoeft for GS-MS analysis.
Abbreviations:
- R
ratio of 24-methyl cholesterol to sitosterol
- RT
reverse transcriptase
- SMT
sterol-C-methyltransferase
- SMT1
S-adenosyl-l-Met cycloartenol C24-methyltransferase
- SMT2
S-adenosyl-l-Met 24-methylene lophenol-C241-methyltransferase
LITERATURE CITED
- Adam G, Marquardt V. Brassinosteroids. Phytochemistry. 1986;25:1787–1799. [Google Scholar]
- Atanassova R, Favet N, Martz F, Chabbert B, Tollier MT, Monties B, Fritig B, Legrand M. Altered lignin composition in transgenic tobacco expressing O-methyltransferase sequences in sense and antisense orientation. Plant J. 1995;8:465–477. [Google Scholar]
- Benveniste P. Sterol biosynthesis. Annu Rev Plant Physiol. 1986;37:275–307. [Google Scholar]
- Bevan M. Binary Agrobacterium vectors for plant transformation. Nucleic Acids Res. 1984;12:8711–8721. doi: 10.1093/nar/12.22.8711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bladocha M, Benveniste P. Stereochemical aspects of the biosynthesis of the side chain of 9β,19-cyclopropyl sterols in maize seedlings treated with Tridemorph. Plant Physiol. 1985;79:1098–1106. doi: 10.1104/pp.79.4.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch KE. Sterol structure and membrane function. Crit Rev Biochem. 1983;14:47–91. doi: 10.3109/10409238309102790. [DOI] [PubMed] [Google Scholar]
- Bourgin JP, Chupeau Y, Missonier C. Plant regeneration from mesophyll protoplasts of several Nicotiana species. Physiol Plant. 1979;45:288–292. [Google Scholar]
- Bouvier-Navé P, Husselstein T, Benveniste P. Two families of sterol methyltransferases are involved in the first and the second methylation steps of plant sterol biosynthesis. Eur J Biochem. 1998;256:88–96. doi: 10.1046/j.1432-1327.1998.2560088.x. [DOI] [PubMed] [Google Scholar]
- Bouvier-Navé P, Husselstein T, Desprez T, Benveniste P. Identification of cDNAs encoding sterol methyl-transferases involved in the second methylation step of plant sterol biosynthesis. Eur J Biochem. 1997;246:518–529. doi: 10.1111/j.1432-1033.1997.t01-1-00518.x. [DOI] [PubMed] [Google Scholar]
- Choe S, Dilkes BP, Fujioka S, Takatsuto S, Sakurai A, Feldmann KA. The DWF4 gene of Arabidopsis encodes a cytochrome P450 that mediates multiple 22α-hydroxylation steps in brassinosteroid biosynthesis. Plant Cell. 1998;10:231–243. doi: 10.1105/tpc.10.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clouse SD. Molecular genetic studies confirm the role of brassinosteroids in plant growth and development. Plant J. 1996;10:1–8. doi: 10.1046/j.1365-313x.1996.10010001.x. [DOI] [PubMed] [Google Scholar]
- Crèvecoeur M, Crespi P, Lefort F, Greppin H. Sterols and plasmalemma modifications in spinach apex during transition to flowering. J Plant Physiol. 1992;139:595–599. [Google Scholar]
- Demel RA, De Kruyff B. The function of sterols in membranes. Biochem Biophys Acta. 1976;457:109–132. doi: 10.1016/0304-4157(76)90008-3. [DOI] [PubMed] [Google Scholar]
- Fonteneau P, Hartmann MA, Benveniste P. A 24-methylene lophenol C-28 methyl transferase from suspension cultures of bramble cells. Plant Sci Lett. 1977;10:147–155. [Google Scholar]
- Fujioka S, Li J, Choi YH, Seto H, Takatsuto S, Noguchi T, Watanabe T, Kuriyama H, Yokota T, Chory J, Sakurai A. The Arabidopsis deetiolated2 mutant is blocked early in brassinosteroid biosynthesis. Plant Cell. 1997;9:1951–1962. doi: 10.1105/tpc.9.11.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaber RF, Copple DM, Kennedy BK, Vidal M, Bard M. The yeast gene ERG 6 is required for normal membrane functions but is not essential for biosynthesis of the cell-cycle-sparking sterol. Mol Cell Biol. 1989;9:3447–3456. doi: 10.1128/mcb.9.8.3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodall GJ, Wiebauer K, Filipowicz W. Analysis of pre-mRNA processing in transfected plant protoplasts. Methods Enzymol. 1990;88:148–161. doi: 10.1016/0076-6879(90)81117-d. [DOI] [PubMed] [Google Scholar]
- Grebenok RJ, Galbraight DW, Della Penna D. Characterization of Zea mays endosperm C-24 sterol methyltransferase: one of two types of sterol methyl-transferase in higher plants. Plant Mol Biol. 1997;34:891–896. doi: 10.1023/a:1005818210641. [DOI] [PubMed] [Google Scholar]
- Grünwald C. Plant sterols. Annu Rev Plant Physiol. 1975;26:209–236. [Google Scholar]
- Horsch RB, Fry JE, Hoffmann NL, Eichholtz D, Rogers SG, Fraley RT. A simple and general method for transferring genes into plants. Science. 1985;227:1229–1231. doi: 10.1126/science.227.4691.1229. [DOI] [PubMed] [Google Scholar]
- Husselstein T, Gachotte D, Desprez T, Bard M, Benveniste P. Transformation of Saccharomyces cerevisiae with a cDNA encoding a sterol C-methyltransferase from Arabidopsis thaliana results in the synthesis of 24-ethyl sterols. FEBS Lett. 1996;381:87–92. doi: 10.1016/0014-5793(96)00089-0. [DOI] [PubMed] [Google Scholar]
- Janssen GG, Nes WD. Structural requirements for transformation of substrates by the S-adenosyl-l-methionine: Δ24(25)-sterol methyltransferase. J Biol Chem. 1992;267:25856–25863. [PubMed] [Google Scholar]
- Kauschmann A, Jessop A, Koncz C, Szekeres M, Willmitzer L, Altmann T. Genetic evidence for an essential role of brassinosteroids in plant development. Plant J. 1996;9:701–713. [Google Scholar]
- Krysan PJ, Young JC, Tax F, Sussman MR. Identification of transferred DNA insertions within Arabidopsis genes involved in signal transduction and ion transport. Proc Natl Acad Sci USA. 1996;93:8145–8150. doi: 10.1073/pnas.93.15.8145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Chory J. A putative leucine-rich repeat receptor kinase involved in brassinosteroid signal transduction. Cell. 1997;90:929–938. doi: 10.1016/s0092-8674(00)80357-8. [DOI] [PubMed] [Google Scholar]
- Li J, Nagpal P, Vitart V, McMorris TC, Chory J. A role for brassinosteroids in light-dependent development of Arabidopsis. Science. 1996;272:398–401. doi: 10.1126/science.272.5260.398. [DOI] [PubMed] [Google Scholar]
- Maillot-Vernier P, Schaller H, Benveniste P, Belliard G. In vitro selection of calli resistant to a triazole cytochrome P-450-obtusifoliol-14-demethylase inhibitor from protoplasts of Nicotiana tabacum L. cv Xanthii. Plant Physiol. 1990;93:1190–1195. doi: 10.1104/pp.93.3.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra HC, Nes WR. The mechanism of introduction of alkyl groups at C-24 of sterols. IV. Inhibition by triparanol. J Biol Chem. 1971;246:4934–4937. [PubMed] [Google Scholar]
- Mandava NB. Plant growth-promoting brassinosteroids. Annu Rev Plant Physiol. 1988;39:23–52. [Google Scholar]
- Nes WR, McKean ML (1977) Occurrence, physiology and ecology of sterols. In Biochemistry of Steroids and Other Isopentenoids. University Park Press, Baltimore, MD, pp 411–533
- Nomura T, Nakayama M, Reid JB, Takeuchi Y, Yokota T. Blockage of brassinosteroid biosynthesis and sensitivity causes dwarfism in garden pea. Plant Physiol. 1997;113:31–37. doi: 10.1104/pp.113.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ourisson G. Peculiarities of sterol biosynthesis in plants. J Plant Physiol. 1994;143:434–439. [Google Scholar]
- Rahier A, Benveniste P. Mass spectral identification of phytosterols. In: Nes WD, Parish E, editors. Analysis of Sterols and Other Biologically Significant Steroids. San Diego, CA: Academic Press; 1989. pp. 223–250. [Google Scholar]
- Rahier A, Génot JC, Schuber F, Benveniste P, Narula AS. Inhibition of (S)-adenosyl-l-methionine sterol-C-24-methyltransferase by analogues of a carbonium ion high energy intermediate—structure activity relationship for C-25 heteroatoms (N,As,S) substituted triterpenoid derivatives. J Biol Chem. 1984;259:15215–15223. [PubMed] [Google Scholar]
- Rahier A, Narula AS, Benveniste P, Schmitt P. 25-Azacycloartanol, a potent inhibitor of SAM-sterol-C-24 and C-28 methyltransferase in higher plant cells. Biochem Biophys Res Commun. 1980;92:20–25. doi: 10.1016/0006-291x(80)91513-2. [DOI] [PubMed] [Google Scholar]
- Rendell N, Misso NLA, Goad LJ. Biosynthesis of 24-methylcholest-5-en-3β-ol and 24-ethylcholest-5-en-3β-ol in Zea mays. Lipids. 1986;21:63–68. doi: 10.1007/BF02534304. [DOI] [PubMed] [Google Scholar]
- Rubinstein I, Goad LJ, Clague ADH, Mulheim LJ. The 220 MHZ NMR spectra of phytosterols. Phytochemistry. 1976;15:195–200. [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual, Ed 2. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Schaller H, Gondet L, Maillot-Vernier P, Benveniste P. Sterol overproduction is the biochemical basis of resistance to a triazole in calli from a tobacco mutant. Planta. 1994;194:295–305. [Google Scholar]
- Schaller H, Grausem B, Benveniste P, Chye ML, Tan CT, Song YH, Chua NH. Expression of the Hevea brasiliensis (H.B.K.) Müll. Arg. 3-hydroxy-3-methylglutaryl-coenzyme A reductase 1 in tobacco results in sterol overproduction. Plant Physiol. 1995;109:761–770. doi: 10.1104/pp.109.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt J, Altmann T, Adam G. Brassinosteroids from seeds of Arabidopsis thaliana. Phytochemistry. 1997;45:1325–1327. doi: 10.1016/s0031-9422(97)00177-5. [DOI] [PubMed] [Google Scholar]
- Schmitt P, Narula AS, Benveniste P, Rahier A. Manipulation by 25-azacycloartanol of the relative percentage of C10, C9 and C8 side-chain sterols in suspension cultures of bramble cells. Phytochemistry. 1981;20:197–201. [Google Scholar]
- Schuler I, Milon A, Nakatani Y, Ourisson G, Albrecht AM, Benveniste P, Hartmann MA. Differential effects of plant sterols on water permeability and on acyl chain ordering of soybean phosphatidylcholine bilayers. Proc Natl Acad Sci USA. 1991;88:6926–6930. doi: 10.1073/pnas.88.16.6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Gonzales RA, Bhattacharyya MK. Identification and characterization of an S-adenosyl-l-methionine: Δ24-sterol-C-methyltransferase cDNA from soybean. J Biol Chem. 1996;271:9384–9389. doi: 10.1074/jbc.271.16.9384. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Fujioka S, Takatsuto S, Yokota T, Murofushi N, Sakurai A. Biosynthesis of Brassinosteroids in seedlings of Catharanthus roseus, Nicotiana tabacum, and Oryza sativa. Biosci Biotech Biochem. 1995;59:168–172. [Google Scholar]
- Szekeres M, Nemeth K, Koncz-Kalman Z, Mathur J, Kauschmann A, Altmann T, Redei GP, Nagy F, Schell J, Koncz C. Brassinosteroids rescue the deficiency of CYP90, a cytochrome P450, controlling cell elongation and de-etiolation in Arabidopsis. Cell. 1996;85:171–182. doi: 10.1016/s0092-8674(00)81094-6. [DOI] [PubMed] [Google Scholar]
- Wojciechowski ZA, Goad LJ, Goodwin TW. S-adenosyl-l-methionine-cycloartenol methyltransferase activity in cell-free systems from Trebouxia sp. and Scenedesmus obliquus. Biochem J. 1973;136:405–412. doi: 10.1042/bj1360405. [DOI] [PMC free article] [PubMed] [Google Scholar]