Summary
Secreted signaling molecules typically float in the outer leaflet of the plasma membrane, or freely diffuse away from the signaling cell, suggesting that a signal should be sensed equally by all neighboring cells. However, we demonstrate that Spitz (Spi) mediated Epidermal Growth Factor Receptor (EGFR) signaling is spatially biased to selectively determine the induction of a single bract cell on the proximal side of each mechanosensory organ on the Drosophila leg. Dynamic and oriented cellular protrusions emanating from the socket cell, the source of Spi, robustly favor the Spi/EGFR signaling response in a particular cell among equally competent neighbors. We propose that these protrusive structures enhance signaling by increasing contact between the signaling and responding cells. The planar polarized direction of the protrusions determines the direction of the signaling outcome. This asymmetric cell signaling serves as a developmental mechanism to generate spatially patterned cell fates.
Introduction
At least two mechanisms that tightly regulate spatial specificity of cell fate induction by intercellular signaling (Wolpert, 2007) are commonly described. First, “competence” allows only cells arising from a given development linage at a particular time and location to be capable of adopting the cell fate in response to the signal. Second, “combinatorial signaling” requires that cell fate decisions depend on the combination of two or more concurrent signal inputs from distinct sources. Spatial specificity of signaling might also be achieved by a third mechanism, involving directional presentation or delivery of signaling molecules by the signaling cell to a particular neighbor. The plausibility of this scenario is questionable if one assumes free diffusability of signaling molecules that would tend to produce a symmetrical response. However, in recent years, a large number of signaling molecules have been found to be either covalently modified by a lipid moiety (Amanai and Jiang, 2001; Chamoun et al., 2001; Chen et al., 2004; Micchelli et al., 2002; Miura et al., 2006; Steinhauer and Treisman, 2009; Takada et al., 2006; Willert et al., 2003) or shown to bind to extracellular lipid-protein particles (Panakova et al., 2005; Vyas et al., 2008). These modifications could lead to a significant restraint on their mobility potentially facilitating more spatially restricted signal presentation (Chuang and Kornberg, 2000; Miura et al., 2006; Tanaka et al., 2005; Vyas et al., 2008; Willert et al., 2003). Here, we show that such a signaling molecule, the Drosophila TGF-α homolog, Spitz, induces a cell fate in a single cell among equivalent neighbors, in a defined position, and we identify special adaptations of the signaling cell that facilitate such a directional signaling event.
Drosophila bract cells can be distinguished by a thick, pigmented trichome (a bract), and on the distal leg segments (femur, tibia, and tarsus) are found exclusively on the proximal side of mechanosensory bristles (Hannah-Alava, 1958) (Fig. 1A). The four cells of the mature mechanosensory organ (bristle, socket, sheath and neuron) arise from asymmetric divisions of the sensory organ precursor (SOP) cell (Jan and Jan, 2001). The bract cell is not lineally related, but is induced from the surrounding epithelium by cells within the SOP lineage (Garcia-Bellido, 1966; Tobler, 1966; Tokunaga, 1962). The specific cell within the sensory organ that produces the inductive signal was not easily discerned, as disrupting development of either the socket cell or shaft cell produced bractless organs (Tobler, 1969; Tobler et al., 1973). Subsequent investigations demonstrated that two signaling pathways are involved in bract cell fate induction: EGFR signaling promotes bract cell fate (del Alamo et al., 2002; Held, 2002), while Notch activation suppresses it (del Alamo et al., 2002). Though the role of Notch was suggested to be limited to excluding bract formation around chemosensory organs (Held, 2002; Layalle et al., 2004), this interpretation is subject to caveats, leaving open the possibility that EGF and Notch pathways act combinatorially to produce spatially biased bract cell induction.
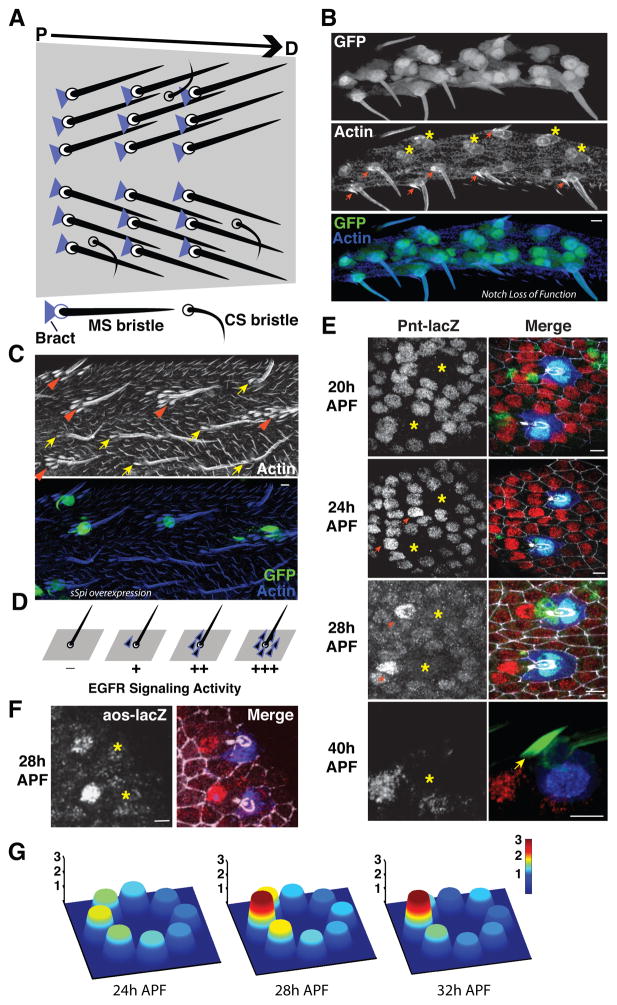
Figure 1. Proximal induction of bract cell fate depends on asymmetric EGFR signaling around sensory organs.
(A) Diagram of the Drosophila leg epithilium, modeled after a figure by Lewis Held (Held, 2002). Single bract cells (blue triangles) are specifically associated with the proximal side of each mechanosensory bristle (dark straight lines) but not chemosensory bristles (black curved lines). The proximal-distal axis of the tissue is from left to right in this and all subsequent panels. (B) GFP marks SOPs with Notch activity rescued in a Notch loss of function background. Note that high Notch activity causes some SOPs to produce double sockets/lack of shafts (asterisks). SOPs that develop normally have a single bract associated with the proximal side of the bristles (red arrows). (C) Over-expression of sSpi-GFP in sensory organs results in a circle of bract cells (arrowheads, upper panel) around the over-expressing organs. Neighboring wild type sensory organs have single bracts (arrows). (D) Summary diagram of bract cell fates around sensory organs with varying levels of EGF/Spi signaling. (E) EGFR signaling response assayed via Pnt-LacZ becomes asymmetric around sensory organs during the course of bract cell induction. Left panels, Pnt-lacZ. Asterisks label the position of sensory organs. Right panels, merge of cut (green, labeling all cells in the SOP linage), Pnt-LacZ (red), Su(H) (Blue, cell body of socket cells), and E-Cadherin (gray, adherens junctions). In the 40h APF merged image, the green channel displays F-actin, showing a characteristic bract structure (arrow) growing from the cell with high Pnt-lacZ activity. (F) EGFR signaling response assayed via Aos-LacZ reporter (left panel) is asymmetric around sensory organs during bract cell fate induction. Image captured at 28hAPF, comparable to the third panel in (E). Asterisks label positions of sensory organs. Right panel, merge of Aos-LacZ (red), Su(H) (Blue), and E-Cadherin (gray). (G) Quantifications of EGFR signaling asymmetry via Pnt-LacZ through the time course of bract cell fate induction. Both the height and peak color (see color bar) of each cell indicate the relative strength of signaling. Scale bars: 5 μm.
The planar cell polarity (PCP) pathway, while not directly involved in bract cell induction, is required to determine the direction of the bract cell induction event (Held et al., 1986a). In wild type, a single bract is induced strictly on the proximal side of the mechanosensory organ. In contrast, in PCP mutants, bracts are frequently induced in an incorrect position relative to the mechanosensory organ, while continuing to respect the one bract per sensory organ rule. This observation led del Alamo and colleagues to hypothesize that polarization of the SOP cell polarizes EGF/Spi signaling to determine the position of the bract (del Alamo et al., 2002). The PCP signaling pathway has been intensively studied in recent years in various model organisms, and has been found to organize a large variety of tissues along their planar axis (Vladar et al., 2009). However, it is unknown how PCP influences directionality of Spitz-mediated bract induction.
This study describes a PCP-controlled lamellipodia-like protrusive cellular structure that potentiates the directional delivery of an EGF signal mediating a cell fate induction event. We identify the socket cell as the source of the inductive EGF/Spi signal. EGF/Spi induces the bract cell fate selectively in a proximal neighbor among equivalent neighboring epithelial cells. Socket cells produce planar polarized and highly dynamic protrusions from their basolateral surfaces, and the direction of those processes determines the direction of enhanced EFG/Spi signaling. We propose that the protrusions potentiate delivery of the EGF/Spi signal by increasing contact between the signaling and responding cell, resulting in the selective enhancement of signaling efficiency in a specified direction.
Results
Spatial bias of bract cell induction is due to asymmetry of EGFR signaling levels
Loss- and gain-of-function experiments have shown that EGF signaling by Spitz (Spi) induces bract cell fate (del Alamo et al., 2002; Held, 2002). Previous studies in which widespread ectopic Ras activation was produced demonstrated that almost all leg epithelial cells have the potential to adopt the bract cell fate (del Alamo et al., 2002; Held, 2002). However, manipulations that modestly increased the magnitude of Spi/EGFR signaling from the sensory organs resulted in induction of extra bracts only on the proximal side (del Alamo et al., 2002; Held, 2002). To address whether the cells neighboring the sensory organ on all sides are competent to become bract cells, we further increased the strength of the Spi/EGF signal from the sensory organs using Neu-Gal4 to drive sSpi-GFP (secreted Spitz ligand) and observed induction of bract cell fate in all the surrounding cells, although in some cases at a greater distance on the proximal side (Fig. 1C,D). Therefore, all neighbors of the sensory organ are competent for bract fate induction by responding to a Spi/EGF signal from the SOP.
We then investigated the possibility that spatial bias of bract induction might result from the combination of symmetric activation and asymmetric inhibitory activity from an independent signaling pathway. Notch signaling has previously been shown to suppress bract cell fate (del Alamo et al., 2002). It was proposed that the only role for Notch was suppression of bract cell induction near chemosensory bristles where bracts do not normally form (Held, 1990; Layalle et al., 2004). However, a potential contribution of Notch signaling to biasing bract cell fate induction could not be assessed during the overlapping periods of competence for bract formation (12–30h APF; Held, 2002), and Notch-dependent asymmetric divisions of SOP cells (roughly from 17–21h APF; Gho et al., 1999). Failure of asymmetric SOP cell division itself could also cause an absence of the associated bract cell (Held, 1990). To circumvent this complication, we inactivated Notch from 12h APF onward while restoring Notch activity selectively in the sensory organs. We observed normal development of the majority of mechanosensory organs, and each of those correctly induced a single proximal bract (Fig. 1B). Furthermore, single bract cells were induced on the proximal side of chemosensory bristles (not shown). These results provide compelling evidence that Notch signaling does not contribute to the spatial bias of bract cell induction, though it does prevent chemosensory organs from inducing bracts (Held, 1990; Layalle et al., 2004).
To directly visualize whether EGFR signaling itself might be asymmetric, we took advantage of a LacZ reporter line that indicates the expression of Argos (Aos), a bona fide EGFR signaling target (Golembo et al., 1996). We observed high Aos-LacZ reporter activity selectively in the proximal neighbors of the sensory organs, presumably the prospective bract cells (Fig. 1F and data not shown). We further characterized the development of asymmetry using a Pnt-LacZ reporter line (Fig. 1E,G) that reflects the expression of Pnt2, a known EGFR/MAPK pathway target (Brunner et al., 1994; Gabay et al., 1996; Klaes et al., 1994; Scholz et al., 1993). The Pnt-LacZ reporter has the advantage that its activity is suppressed within the sensory organ lineage (Frankfort and Mardon, 2004) (Fig. 1E, asterisks). In the cells surrounding sensory organs, Pnt-LacZ is modestly elevated by 20hAPF, but there is no apparent difference in levels among those neighbors. Similar patterns of Aos and Pnt reporter expression have recently been noted by others (Mou et al., 2012). By 24h APF, Pnt-LacZ activity begins to show obvious spatial bias around many sensory organs, favoring the proximal neighbors. This asymmetry is gradually consolidated, such that by 28h APF, there are one or two cells on the proximal side of each sensory organ that show substantially elevated Pnt-LacZ expression. Consolidated Pnt-LacZ expression faithfully indicates the Bract fate, since it persists even after the bract cell is morphologically differentiated (Fig. 1E). The proximal most neighbor of the sensory organ accumulates, on average, approximately 3 times the reporter activity of the distal most neighbor (Fig. 1G). Our analysis therefore shows that during bract cell fate induction, there is a low-level signaling response in all surrounding cells, and a gradual but robust enhancement of EGF signaling activation specifically in the proximal neighbors. Since neither competency nor combinational signaling with Notch is responsible for the spatial bias of cell fate, we postulate that the asymmetry of EGFR signaling is directly responsible for the selective induction of a bract cell on the proximal side of the sensory organ.
The socket cell is the Spitz signal-producing cell responsible for inducing bract cell fate
To understand how the EGF signaling response might be biased toward the proximal side, we determined which cell in the SOP lineage is responsible for sending the Spi/EGF signal (Fig. 2A). The SOP cell itself cannot induce bract cell fate, because bract cells are not induced when asymmetric SOP cell divisions are disrupted (Held, 1990). This is consistent with our observation that asymmetry of the EGF signaling response, as reported by Pnt-LacZ and Aos-LacZ, is first detected around 24h APF (Fig. 1D). By then, the four granddaughter cells of the SOP have been born, suggesting that correct fate determination of one or more of them is necessary. We therefore focused on this 24h APF time point to identify the signaling cell. Rhomboid (Rho), an intra-membrane serine protease, is the key regulator mediating proteolytic activation of the Spi precursor, and its restricted expression usually identifies signal-producing cells (Freeman et al., 1992a). Using a Rho-LacZ enhancer trap line (Freeman et al., 1992a), Rho was found to be specifically expressed in leg SOPs as early as 8h APF (del Alamo et al., 2002). At 24h APF, when the asymmetry of signaling is first detected, we consistently observed Rho-LacZ in the socket cell and the shaft cell, the two outer cells arising from PIIa, (Fig. 2B). Conversely, the two inner cells (sheath and neuron) had no detectable Rho-LacZ activity, ruling them out as signal producing cells. This is confirmed by the observation that organs consisting of only a sheath and neuron, resulting from overexpression of Tribbles (Fichelson and Gho, 2004), fail to induce a bract cell (data not shown).
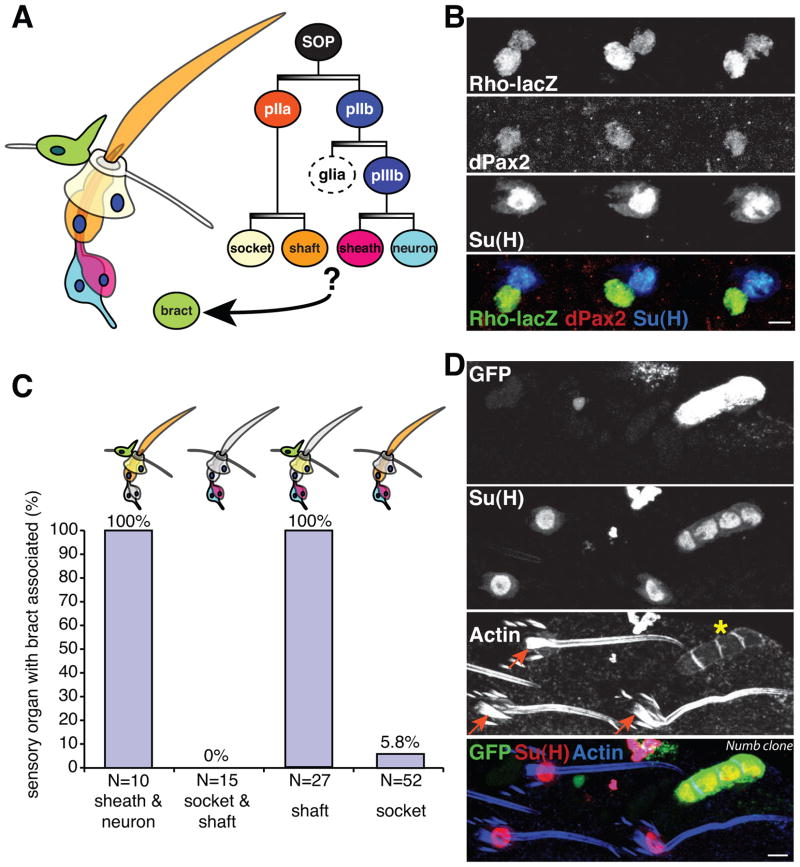
Figure 2. Within the SOP lineage, the socket cell is the key signaling cell for inducing bract cell fate.
(A) Schematic of sensory organ with associated bract cell. Asymmetric divisions (darker side of graded bars indicates higher Notch activity) generate four mature daughter cells with distinct fates. (B) Expression of Rhomboid (Rho-lacZ) is restricted to the two outer cells within each mature sensory organ: the shaft (expressing dPax2) and the socket (expressing Su(H)). Three adjacent sensory organs on a 24h APF leg are shown. (C) Percentage of mosaic spi mutant sensory organs with associated bract cells. The mutant cell type(s) is indicated. See Figure S1 for representative images. (D) On a 40h APF leg, a GFP positive numb15 mutant clone with 4 socket cells positive for Su(H) (asterisk), induces no bract; a single proximally-associated bract is associated with each wild type sensory organ (arrows), Scale bars: 5 μm.
Next, we directly investigated the signaling roles of socket and shaft cells by making labeled spi mutant clones within the SOP lineage using a SOP-Flpase strain (see Experimental Procedures), and correlating bract induction with the capacity for Spi signaling in each cell type (Fig. 2C and Fig. S1). In the case of two-cell mutant clones, bract induction is normal when the inner cells (sheath and neuron) lack spi, confirming that they and their immediate mother cell, pIIIb (also mutant for spi), is dispensable for inducing the bract fate. In contrast, bracts are not induced when two-cell clones of outer cells (shaft and socket) lack spi, consistent with the observed Rho expression in these cells. Dramatically different roles for socket and shaft cells were revealed when we analyzed single cell mutant clones: most socket cell clones failed to induce bract cells, while spi clones within the shaft cell had no effect on bract cell induction (Fig. 2C). Although we cannot rule out a possible signaling contribution from pIIa, the immediate precursor to both the socket and shaft cell, pIIa itself is not sufficient, as pIIa retains signaling capacity in single socket cell spi clones that do not induce bracts. We therefore conclude that the socket cell is the key signal-producing cell for inducing the bract cell fate.
By blocking development of either the shaft cell (via Hairless or shaven mutants; Tobler et al., 1973), or socket cell (via applying mitomycin; Tobler, 1969), Tobler found that bract cells are not induced without either of these two cell types. Our genetic mosaic analysis distinguished the different signaling roles of those two cell types while keeping stereotypical cellular composition and structural integrity of the sensory organs intact. Nonetheless, Tobler’s observations suggest a requirement for the shaft cell as well, which, taken in the context of our results, may be to facilitate the signaling function of the socket cell. A facilitating role for the shaft cell is further confirmed by the observation that sensory organs comprising only socket cells (produced in numb mutant clones; Guo et al., 1996) fail to induce bract cells (Fig. 2D). This failure is likely not due to inability of the numb mutant socket cells to generate active ligand, since Rho-LacZ reporter levels within these mutant sensory organs are equivalent to those in neighboring wild type socket cells (Fig. 3B). Apparently, socket cells within sensory organs lacking other key cell types (most likely shaft cells) are defective in some essential attribute required for effective signaling.
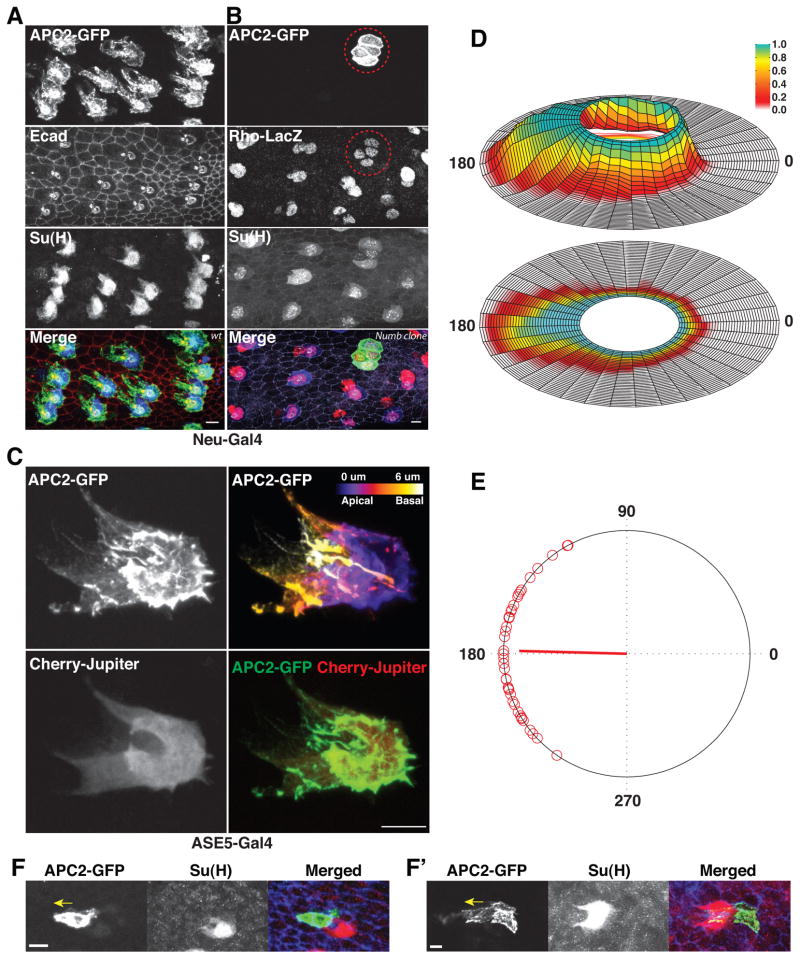
Figure 3. Socket cells produce proximally oriented planar polarized protrusions.
(A) Planar polarized protrusions on a 24h APF leg (portion of femur shown) are revealed by expression of APC2-GFP driven by Neu-Gal4. E-Cad and Su(H) label adherens junctions and socket cell bodies, respectively. All protrusions (as identified by GFP positive cables extending beyond socket cell body) project proximally. (B) A numb15 mutant sensory organ (dashed circles), labelled with APC2-GFP, does not exhibit protrusions, but expresses approximately wild-type levels Rhomboid-LacZ. (C) Planar polarized protrusions originate from socket cells. Protrusions are visualized by expression of APC2-GFP (top panels) or Cherry-Jupiter (bottom panels) driven by a socket cell specific ASE5-Gal4 driver. The depth of the protrusions seen by APC2-GFP within the epidermis is color coded in the top right panel (see color bar). Bottom right panel shows an overlay of the maximal projections from GFP and Cherry. Protrusions are not induced as a consequence of the EGF signaling response; see Fig S2. (D) Probability plot of protrusions from a single socket cell reaching a given distance from the cell body in any given sector. Data are derived from a three-hour movie, starting from 24hAPF, of a socket cell labelled with ASE5-Gal4 UAS-APC2-GFP. The distance is normalized against the size of socket cell body (unit circle in the center). Height (top panel) and color (top and bottom panels) indicate the probability that the protrusions reach each area in a single frame of the movie. (E) Distributions of protrusion directions for wild type sensory organs (n=40, from 24h APF fixed tarsal segments). Each red circle on the polar plot represents the mean angle of protrusions from an individual sensory organ. The mean vector is indicated by the red line from the center of the plot. (F, F′) Small clones of cells expressing APC2-GFP reveal proximal protrusions from most or all leg epithelial cells at 24h APF. Protrusions in epithelial cells on the proximal (F) or distal (F′) side of the sensory organ (socket cell labelled by Su(H)) point proximally (yellow arrows). Scale bars: 5 μm.
Socket cells extend planar polarized protrusions toward proximal neighbors
The nature of the defect became apparent when we compared wild type sensory organs with those comprising only socket cells by expressing APC2-GFP to reveal cytoskeletal structures (see Experimental Procedures for details). At 24h APF, we observed prominent cable-like protrusions projecting proximally from almost every wild type sensory organ (Fig. 3A). The protrusions typically reach one to two cells away from the sensory organ, contact two to three of the proximal neighbors at any given time, and are highly dynamic (Movies S1, S2). Labeling only a single cell using a socket cell specific Gal4 driver (Barolo et al., 2000), we demonstrated that the protrusions originate from the socket cell (Fig. 3C and Movie S2). The protrusions contain actin cables, labeled by APC2-GFP as well as other F-actin markers (not shown), and are also visible with Cherry-Jupiter, a known microtubule reporter (Cabernard and Doe, 2009). These lamellipodia-like protrusive structures project from the basolateral surface on the proximal side of the socket cells (Fig. 3C, Movie S1), are less than 1μm in thickness and appear to contact or envelop the basolateral surface of several neighboring epithelial cells (Movie S2). Importantly, when we used APC2-GFP to label sensory organs consisting of only socket cells, no similar protrusions were observed (Fig. 3B). While this difference indicates that the protrusions might depend on the presence of one or more of the other sensory organ cell types, it also suggests that the lack of proximal protrusions might explain why sensory organs comprising only four socket cells fail to induce bracts.
Indeed, the temporal and spatial characteristics of the protrusions suggest they may be responsible for breaking the symmetry of EGFR signaling. First, they are observed as early as 20h APF, before signaling asymmetry is readily apparent (Fig. 1E), and persist for the next approximately ten hours, during which time EGFR signaling asymmetry becomes robust (Movie S3). Second, although the protrusions are sufficiently dynamic that with our current frame rate of live imaging (1 frame every 2 minutes), while we typically observed the protrusions to explore different neighborhoods between adjacent frames, they rarely drastically change their overall direction of projection (Movie S3 and Fig. 4D). Since the presence of these protrusive structures could potentially increase the contact between the signaling and recipient cells, as a surrogate measure of such enhanced contact, we quantified the area of the protrusions as a function of direction. Using a movie recorded from 24–27h APF, we confirmed that the socket cell protrusions overwhelmingly prefer to explore the cell’s proximal neighborhood, such that roughly two thirds of the overall potential contact via these protrusions falls within a ±30 degree angle from its mean orientation (Fig. 3D). 50% of the time, protrusions extend in the proximal direction to a distance of 9.49 μm beyond the range of the cell body, but only 1.33 μm in the distal direction. The proximal bias of protrusions among different sensory organs is also very robust (Fig. 3A,E); the overall orientation of protrusions from almost all sensory organs examined at 24h APF falls within a neighborhood of roughly ±60 degrees from the proximal direction, with the mean orientation of the population indistinguishable from the proximal direction and a circular standard deviation of 29.3 degrees (Fig. 3E). Importantly, the protrusions are not induced as a consequence of the Spi/EGFR signaling response, because the protrusions are present in sensory organs in which the EGFR pathway has been suppressed by either overexpression of the inhibitor, Argos, or removal the ligand, Spitz (Fig. S2).
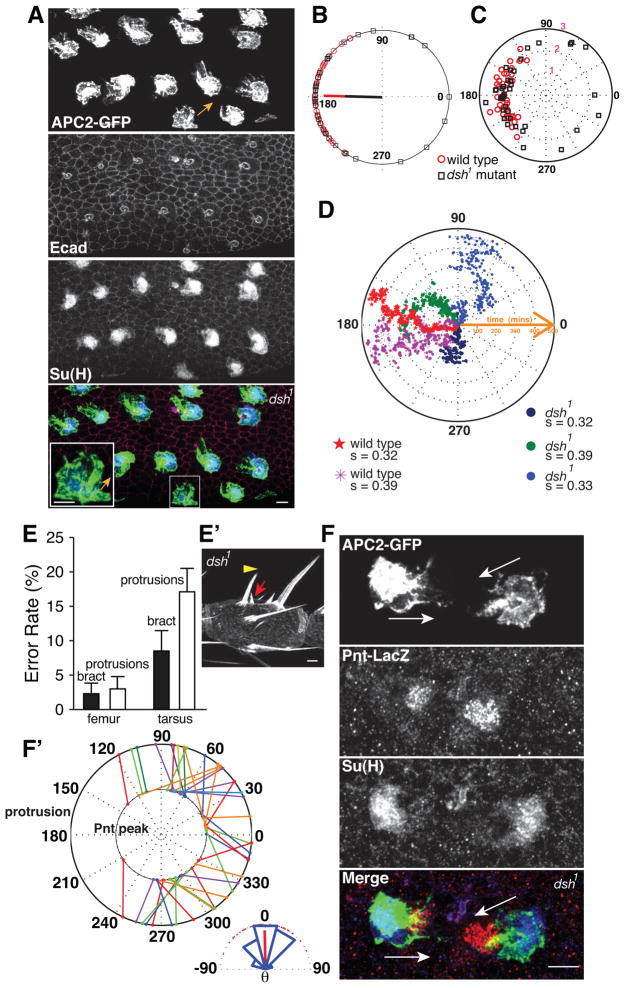
Figure 4. Misorientation of polarized protrusions leads to mistakes of bract cell orientation.
(A) Planar polarized protrusions on a 24h APF dsh1 mutant leg (portion of femur shown) revealed by Neu-Gal4 driven expression of APC2-GFP (top panels). E-Cad and Su(H) label adherens junctions and socket cell bodies, respectively. Most protrusions project proximally as in wild type tissue (Fig 3A), while misorientation of protrusions (arrow) is occasionally observed (magnified view shown in inset) See Fig S3. (B) Distributions of protrusion directions in dsh1 mutant sensory organs (n=37, from 24h APF fixed tarsal segments) and comparison with wild type sensory organs (n=40). Black squares (dsh1 mutant) and red circles (wild type) on the unit circle represent the mean angle of protrusions from individual sensory organs. Mean vectors for dsh1 and wild type cells are represented by black and red lines respectively. (C) Comparison of protrusion area and orientation among individual dsh1 (black squares) and wild type (red circles) sensory organs. The position of each sensory organ on the plot is determined by the overall orientation of protrusion (angle) and the ratio, area of cell body + protrusion/area of cell body (radius). (D) Persistence of protrusion orientation during the course of bract cell fate induction. Data points from different movies are represented by dots with unique color. The circular standard deviation and genotype of each cell are shown. (E) Correlation of protrusion error rate and bract orientation mistakes (example in E′) in femur and tarsal leg segments of dsh1 mutant flies. Error bars show standard deviation (n=14 femurs; n=17 tarsi). (E′) The orientation of mis-positioned bract (red arrow) can be independent of the direction of associated shaft (yellow arrowhead). Portion of a dsh1 mutant tarsus is shown. See Fig S3. (F) Two oppositely oriented sensory organs on a 28h APF dsh1 mutant leg, showing orientation of protrusions (APC2-GFP) correlates with the direction of peak EGFR signaling (Pnt-LacZ). Arrows indicate protrusion directions. (F′) Correlations between protrusion directions and the direction of EGF signaling asymmetry (n=40, from 28h APF misoriented tarsal SOPs of dsh1 mutant). Protrusion orientation of each SOP and direction of adjacent cell showing peak pnt-LacZ activity are represented by dots with the same color on the outer and inner circles respectively and linked by a solid line. The angular distribution of the difference between these two angles for each sensory organ is shown in the inset. Scale bars: 5 μm.
By expressing the APC2-GFP reporter in small clones, we found that in addition to the socket cells, epithelial cells also produce proximally directed basolateral protrusions, including those immediately proximal or distal to the sensory organs (Fig. 3F, F′).
Orientation of socket cell protrusions and EGFR signaling asymmetry are controlled by the planar cell polarity pathway
The observation of proximal basolateral protrusions on both developing leg epithelial cells and socket cells suggested that they might be a relatively general manifestation of epithelial PCP. We investigated how the orientation of protrusions is controlled, focusing on socket cell protrusions since these appear to be associated with bract fate induction (Fig. 2D, 3B). The planar cell polarity pathway was previously shown to affect the orientation of bract cells relative to the mechanosensory bristles (del Alamo et al., 2002; Held et al., 1986b). To address whether the direction of socket cell protrusions determines the direction of bract cell induction, we first assessed the correlation between protrusion direction and the direction of bract cells relative to the sensory organs in several PCP mutants.
In dsh1 mutant legs at 24hAPF, a majority of sensory organs project protrusions indistinguishable in morphology and orientation compared to their wild type counterparts (Fig. 4A). Improperly positioned bract cells and misdirected protrusions (defined as a deviation of ≥90 degrees from the proximal direction) are observed at similar, low, frequencies in the femur (2.3±1.4% and 3.0±1.6%, respectively), and at similar, but higher frequencies in the tarsi (8.5±2.9% and 17.1±3.3%, respectively) (Fig. 4A, E and Fig. S3). Nearly identical observations were made in pk-sple13 legs (not shown). It is important to note that we have never observed these errors in wild type legs, and that the maximum possible error rate is 50% if directed randomly. Thus, there is an approximate correlation between the frequency of incorrectly positioned bracts and misoriented protrusions in PCP mutants, suggesting that the direction of protrusions may determine the position at which the bract is induced.
To further characterize the protrusion orientation defects in the tarsal segments of dsh1 mutant legs compared to wild type, we plotted the angle of protrusions from individual sensory organs (Fig. 4B). As expected, the means are similarly proximal for both dsh1 and wild type. However, the distribution of their orientations is substantially more dispersed in dsh1 (circular standard deviation=54.4 degrees; circular kurtosis=0.36) compared to wild type (circular standard deviation=29.3 degrees, circular kurtosis=0.55), with a significant portion of outliers protruding toward the distal side. Importantly, disrupting the core planar cell polarity pathway does not significantly change other geometric and dynamic properties of the protrusions. For example, protrusions from mutant sensory organs have roughly equivalent areas compared to wild type (132 vs 141 μm2) (Fig. 4C). Similarly, individual mutant protrusions do not substantially change their overall orientations over time, irrespective of how far they deviate from the proximal direction (Fig. 4D, Movie S3).
Since the above data show that a reasonable estimate of the overall orientation of any protrusion over the period of bract fate induction can be obtained from a measurement made at any particular time point, we simultaneously assayed protrusion orientation and EGF signaling responses using Pnt-LacZ, in 28h APF dsh1 legs. If the direction of protrusions determines the ultimate position of bract induction, we would expect to observe a tight correlation between the orientation of protrusions and the consolidating EGFR signaling asymmetry at this time. Indeed, among samples in which the protrusions are misoriented by over 60 degrees, we found that peak LacZ reporter activity points in approximately the same direction (±45 degrees) as protrusion orientation in around 90% of cases, and none differed by more than 75 degrees (Fig. 4F, F′). Indeed, their distributions strongly correlate, with a circular correlation coefficient of 0.90 and a p-value of 1.6e-07. This correlation is surprisingly strong, given the dynamic nature of the protrusions (Fig. 4D, Movie S3). It is essential to note that the direction of bract cell induction does not necessarily reflect the orientation of the sensory organ as a whole. In some cases, the tilt of the shaft is altered to correspond to bract cell direction, but in many other cases, bract direction is abnormal (distal or lateral) while the tilt of the shaft is not correspondingly affected (Fig. 4E′, Fig. S3B′). Thus, the orientation of bract induction is tightly correlated to the direction of socket cell protrusions but not to the overall orientation of the bristle. We conclude that the planar cell polarity pathway regulates the orientation of the socket cell protrusions, and this, in turn determines the orientation of EGFR signaling asymmetry and thus bract cell positioning.
Protrusions potentiate EGFR signaling to facilitate achieving the signaling threshold for bract cell fate induction
While the correlation between the direction of protrusion extension and activation of signaling suggests that the protrusions are responsible for signaling asymmetry, we wished to directly suppress protrusions to confirm their role in signaling. We found that when the activity of the formin Diaphanous (Dia; Castrillon and Wasserman, 1994), was compromised during sensory organ development by inducing large mutant clones of the hypomorphic allele dia5 in leg epidermis, the four cells within the mutant SOP lineage developed in correct number and position, but the socket cell protrusions were significantly suppressed and disorganized (Fig. 5A). Compared to wild type, the APC2-GFP positive F-actin cables from mutant sensory organs usually extend only a short distance beyond the cell cortex. This phenotype is reminiscent of the shortened protrusions from dia5 mutant leading edge cells during dorsal closure in the fly embryo (Homem and Peifer, 2009) and is consistent with the well-established role of Dia in promoting actin filament assembly and bundling at the tips of filopodia (Block et al., 2008; Schirenbeck et al., 2005; Yang et al., 2007). The size of mutant protrusions is diminished compared to wild type, covering on average 55% less area at 24hAPF (mean area of 68 vs 141 μm2 for wild type) (Fig. 5B). Nevertheless, compromising Dia function does not abolish these protrusions, nor does it dramatically change the overall proximal orientation of the residual protrusive structures (Fig. 5B).
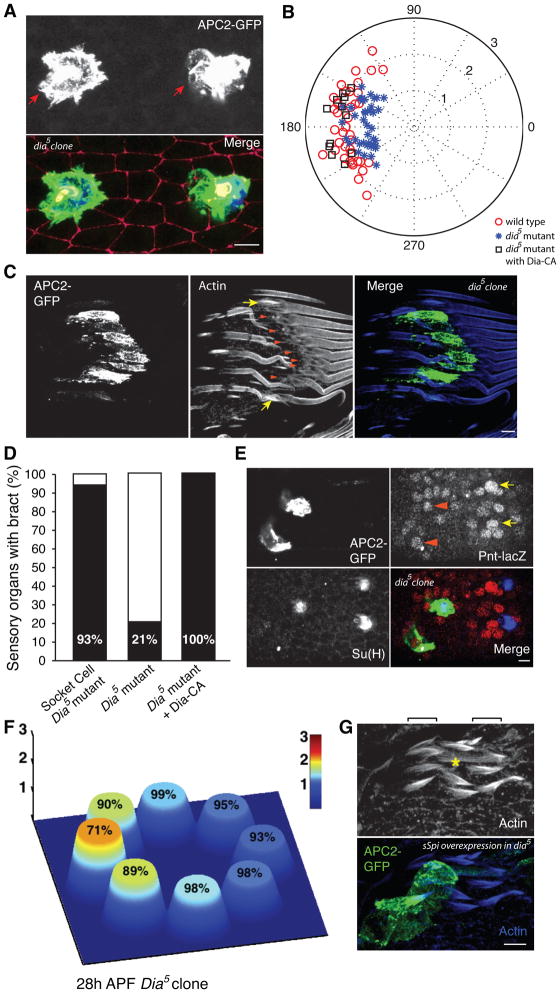
Figure 5. Suppression of planar polarized protrusions results in decreased EGFR signaling asymmetry and the failure of bract cell induction.
(A) Planar polarized protrusions (red arrows) from dia5 mutant sensory organs (labeled by APC2-GFP) are less extensive than wild type (compare with Figure 3A). Bottom panel shows an overlay of APC2-GFP (green); E-Cad (red; adherens junctions) and Su(H) (blue; socket cell bodies). Portion of a ~24h APF femur segment is shown. See Fig S4. (B) Comparison of protrusion area and orientation from individual sensory organs between dia5 mutant (blue asterisks), dia5 mutant rescued with Dia-CA (black squares) and wild type (red circles). Position of each sensory organ on the plot is determined by the overall orientation of protrusion (angle) and the ratio, area of cell body + protrusion/area of cell body (radius). (C) Bracts (revealed by F-actin) are not induced (arrowheads) adjacent to dia5 mutant sensory organs in a large dia5 clone (expressing APC2-GFP). Note adjacent wild type sensory organs have associated bracts (arrows). Portion of a ~40h APF leg is shown. (D) Percentage of dia5 mutant mechanosensory organs with associated bract cells (black portion of the bars) with three different manipulations: small socket cell clones generated with SOP-Flpase, large clones generated with hs-Flpase, and large clones within which Dia is rescued in SOP cells. See Figure S4 for representative images. (E) Comparison of EGFR signaling asymmetry (Pnt-lacZ) around dia5 mutant sensory organs (labeled with APC2-GFP) and adjacent wild type sensory organs (GFP negative, Su(H) positive). Note the peak reporter activities proximal to mutant sensory organs (arrowheads) are significantly lower than wild type (arrows). (F), Quantification of EGFR signaling asymmetry around dia5 mutant sensory organs at 28h APF (comparable with the middle panel in Fig. 1E). Percentages on top of each cell indicate the ratio of signal intensity compared to the corresponding cell around wild type sensory organs. (G) Rescue of missing bract phenotype in large dia5 clones by expressing sSpi-GFP within mutant sensory organs. Note ectopic bracts (seen by F-actin staining at 40 APF) are induced around the shaft of sensory organ expressing sSpi (asterisk). This is similar to the result of sSpi-GFP overexpression in wild type sensory organs, except that extra bracts at a greater distance on the proximal side are not observed (compare to Fig. 1C). Scale bar: 5 μm.
We next asked whether SOPs with compromised protrusions are impaired in their ability to induce bract cells. When we made small dia5 mutant clones encompassing socket cells, using SOP-Flpase, a modest fraction of sensory organs (6 of 83 clones examined) did not induce an associated bract cell (Fig. 5D, Fig S4). We hypothesized that the low penetrance might result from the perdurance of residual wild type protein that small dia5 clones inherit from their progenitor cells. To test this interpretation, we examined large dia5 clones generated by hs-FLP at larval stages, and found a highly penetrant absence of bract cell induction within the clones (53 of 67 mutant SOPs examined) (Fig. 5C,D). To rule out the possibility that the absence of bract cells within large dia clones might be due to absence of Dia in the prospective bract cell, we rescued dia selectively in the SOP linage using a constitutively active Dia construct, which lacks the C-terminal autoinhibitory DAD domain (Homem and Peifer, 2008, 2009; Wallar et al., 2006). Such rescue restored both protrusions (mean area of 158 vs 67 μm2 in unrescued clones and 141 μm2, in wild type; Fig. 5B, Fig. S5) and associated bract cells (17 of 17 SOP examined) (Fig. 5C, Fig. S5). Thus, epithelial cells do not need dia to become bract cells, and the loss of bract induction in large dia mutant clones can be directly attributed to the mutant SOPs.
Finally, we investigated whether failure of bract cell induction within the large dia clones is due to compromised EGF signaling when the protrusions are suppressed. We observed that Pnt-LacZ asymmetry began to develop around mutant sensory organs at roughly the same time (24h APF) as in wild type (not shown), but we detected a quantitative difference in EGF signaling around mutant and wild type sensory organs (Fig. 5E,F). At 28h APF, while Pnt-LacZ activity was substantially higher in one or two proximal neighbors around wild type sensory organs, the extent of asymmetry around mutant sensory organs was decreased significantly compared to wild type (~70% of the wild type gradient; compare Fig. 5F to Fig. 1F, middle panel). The difference was largely due to reduced levels on the proximal side of the sensory organ, while distal levels remained similar (Fig. 5F). We interpret this to mean that the mutant socket cells produced a Spi signal, but that in the absence of intact protrusions, it was not selectively and efficiently delivered to the proximal neighbors. Hence a minimal threshold for bract cell induction was frequently not reached. Consistent with this, when expressed with a socket cell specific driver, we observed the presence of sSpi-GFP within wild type socket cell protrusions (Movie S4), proving active ligand can be delivered to the protrusions. This interpretation was further confirmed by the observation that increasing the amount of secreted Spi within dia mutant sensory organs rescued bract cell induction and even induced ectopic bract cells uniformly around mutant SOPs (Fig. 5G). Thus, the protrusions appear to facilitate achieving a critical threshold of EGF signaling in a specific subset of SOP neighbors, and the sub-threshold signaling level when the protrusions are impaired can be overcome with overwhelming amounts of free ligand.
These results also strongly argue that it is asymmetry of the socket cell but not that of the prospective bract cell that contributes to the polarized response. Protrusions from the proximal neighbor point away from the socket cell, while those from the distal neighbor point toward the socket cell (Fig. 3F, F′). If polarity of the receiving cells controls signal directionality, one would therefore have to hypothesize that EGF signal receptivity is anti-correlated to the protrusion direction. Though conceivable, this would not explain the reduction of signaling with manipulations that specifically affect the signaling cell (by perturbation of dia selectively in the socket cell, or by making a socket only sensory organ). This difference is also seen in the altered range of bract induction when sensory organs expressing ectopic sSpi are made mutant for dia. Therefore, the only parsimonious conclusion is that the socket cell protrusion potentiates the EGF/Spi signal and its orientation determines the direction of signaling.
Discussion
This study has demonstrated that a signaling cell can use a secreted molecule to initiate cell fate induction in a selected cell among equivalent competent neighbors. We refer to this mechanism as ‘asymmetric cell signaling’. The robust asymmetry of EGF/Spi signaling we describe is the biological basis for the spatial bias of bract cell fate induction on the Drosophila leg. Cellular protrusions projected by the socket cell to its proximal neighbors generate the spatially biased signaling outcome. The PCP pathway provides directional information that aligns the direction of this inductive signal with the tissue axes.
Production and polarization of socket cell protrusions
Prior studies demonstrated that both the socket and the shaft cell are required for bract cell induction, but failed to identify which is responsible for sending the inductive signal (Tobler, 1969; Tobler et al., 1973). Identification of Spi as the inductive signal (del Alamo et al., 2002) and development of sensory organ specific genetic mosaic analysis (this study), have allowed us to demonstrate that Spi from the socket cell alone is necessary and sufficient for bract cell induction. As it expresses Rhomboid, the shaft cell is likely also generating activated Spi ligand, but mutant clones of Spi affecting only this cell do not reveal an obvious developmental process for which it is required.
However, consistent with the previously identified requirement for the shaft cell (Tobler et al., 1973), we found that sensory organs made of only socket cells do not induce a bract cell. The socket cells in these sensory organs fail to elaborate protrusions, suggesting that the shaft cell facilitates extension of socket cell protrusions. We envision that these two cells undergo interdependent morphogenesis during development (Le Borgne et al., 2002), and that this interaction might be related to production of the protrusions. An alternate interpretation is that the presence of multiple socket cells suppresses the protrusions.
How does the socket cell sense polarity information? We speculate that directional information is inherited from the PCP-dependent orientation of the SOP division through the stereotypically oriented cell divisions that give rise to the sensory organ (Gho and Schweisguth, 1998; Le Borgne et al., 2002). We note that the disruption of protrusion orientation, and the disruption of bract position, in core PCP mutants are incomplete. This may be due to the architecture of the PCP signaling pathway (Axelrod, 2009), or to additional mechanisms contributing to orientation of SOP progenitors, socket cell polarization and bract induction. Nevertheless, the misorientation of socket cell protrusions in PCP mutants has proven to be an invaluable tool in demonstrating that the protrusions are responsible for the asymmetry of EGF/Spi signaling.
Breaking symmetry: how asymmetric cell signaling is achieved
The finding that the responsive epithelial cells surrounding the sensory organ are polarized raises the possibility that competence to become a bract and orientation of the protrusions are each independently under PCP control. In such a scenario, one would predict that upon loss of the PCP signal each would be randomly misdirected; therefore one would also have to explain why, in cases when induction is incorrectly oriented, these independent processes are coordinately misdirected. The possibility that PCP mutants cause incorrect bract location by changing competence in the epithelial cells is more definitively ruled out by manipulations that affect just the SOP.
Two sets of data, considered together, make a strong argument that socket cell protrusions enhance the efficacy of EGF/Spi signaling in a specified direction to produce asymmetric cell signaling. First, in PCP mutants, we find a tight correlation between orientation of protrusions and the position of enhanced EGFR signaling and bract induction. Second, we find that diminishing protrusions by mutating dia interferes with bract induction. Given the competence of all surrounding epithelial cells, these two observations argue strongly that the protrusion is responsible for facilitating the induction event.
Although we cannot completely rule out possible contributions of bract induction by cryptic polarized features other than protrusions that might be affected in PCP mutants, the orientation of bracts can be uncoupled from the orientation of the SOP as a whole [(Held et al., 1986b); see for examples Fig. 4E′, Fig. S3B′]. By contrast, the observation that we cannot experimentally dissociate protrusion polarity, the socket-shaft vector, and the subsequent position of bract induction (Fig. 4F′, and data not shown), even in cases where other structural features of the sensory organ are not correspondingly aligned, argues in favor of a biologically relevant causal relationship. Furthermore, such potential cryptic, polarized features of sensory organs are highly unlikely to be similarly diminished with impaired Dia function. We cannot completely rule out the possibility that compromising Dia function could affect EGF signaling by unknown mechanisms other than perturbing protrusions. However, we view other possibilities as unlikely for the following reasons. First, we selected a hypomorphic allele, dia5 for these experiments, and sensory organs with compromised Dia are grossly normal in cell morphology and are still capable of making protrusions, albeit smaller ones. Second, the signaling defect in dia5 mutant sensory organs is overcome by overexpressing sSpi, indicating that Spi can still be secreted from dia5 mutant cells, but with little or no directional bias, as indicated by the presence of uniformly induced ectopic bracts. Finally, and most compellingly, compared to wild type sensory organs, in dia5 mutants it is the cells proximal to mutant sensory organs that show the most significant drop in EGFR signaling strength, while the reduction in distal neighbors is negligible. This difference argues strongly against any general, non-polarized process as the cause of signaling defects within dia5 clones. In the extreme case of absent protrusions, observed in the four-socket-cell sensory organs, symmetric weak activation of EGF signaling results in failure of bract induction. The correlation between protrusion direction and stronger signaling, and the reduction in elevated signaling specifically in the direction of protrusions when protrusions are diminished, therefore strongly suggest that the protrusions are responsible for generating EGFR signaling asymmetry by potentiating signaling in a specified direction.
Though we cannot readily visualize the dynamic distribution of activated ligand, based on prior work (Miura et al., 2006), we know that EGF/Spi is palmitoylated, and upon secretion, either remains tethered to the membrane, or if freed from the cell, is expected to have limited mobility. In either case, the observed asymmetric signaling response suggests the necessity of a regulated mode of ligand delivery. Since the protrusions have been demonstrated to potentiate the signal, we hypothesize that they do so either by creating direct contact between signaling and responding cells, or by locally increasing the concentration of a poorly diffusing ligand.
Signal integration
The levels of Pnt-LacZ or Aos-LacZ we used as assays of EGFR signaling activity reflect the cumulative history of EGFR signaling, in effect integrating signaling activity over time. We hypothesize that EGFR pathway activity fluctuates as a function of protrusion contact, with the cumulative duration of signal activation read out as increasing levels of Pnt-LacZ or Aos-LacZ. Direct visualization and measurement of this process will require the development of live, real time probes of pathway activity and gene expression. We propose that a cumulative threshold is reached at which a bistable switch fixes the cell fate decision.
We also postulate that signal integration might be involved in limiting supra-threshold EGF/Spi response to a single cell. Our time-lapse analysis (Fig. 3D) is consistent with the notion that integration over time produces an increasingly regular peak signal in the most proximal cell, with weaker signal on either side. In addition, or alternatively, Argos, a potent EGF inhibitor usually expressed from cells responding to high levels of EGF/Spi signaling (Fig. 1F), could provide a negative feedback that limits the response (Golembo et al., 1996). Mutation of Argos frequently produces one or two extra proximal bracts (del Alamo et al., 2002). Whether or how much these mechanisms might contribute to limiting the response to a single cell is unknown, and it is also possible that an as yet unrecognized lateral inhibition mechanism also contributes.
Experimental Procedures
Fly genetics
Fly culture and crosses were performed according to standard procedures and cultures raised at room temperature unless otherwise stated. To generate the SOP-Flpase strain, a derivative of the 1.0 kb BamHI-XhoI enhancer element from the E(spl)mα gene with five Su(H) binding sites mutated (Castro et al., 2005) was cloned upstream of the flipase coding region. Transgenic flies were generated by phiC31 integrase mediated transformation. To generate mitotic clones using hs-Flpase, flies were heat shocked for ~2h at 37°C during late 2nd/early 3rd instar. Newly eclosed pupae were heat shocked at 37°C for 5 minutes to induce single cell flip-on clones.
Immunohistochemistry
Pupae collected at the white prepupal stage and aged for precise times, as indicated, were dissected in PBS and fixed in PBS containing 4% formaldehyde for 30 min at room temperature. After fixation, pupal legs were dissected from the cuticle and stained following standard procedures. The primary antibodies used were: anti-beta-gal (Promega, 1:200), anti-ECAD2 (DSHB, 1:100), anti-dPax2 (gift from Marcus Noll, 1:50), anti-Su(H) (Santa Cruz Bio, 1:1000), anti-Cut (DSHB, 1:100).
Due to the difficulty of removing cuticles from legs younger than 20h APF, we only focused on developmental stages from this point on. To visualize bracts and bristles, legs staged between 36h and 48h APF were incubated with 1% Alexa Dye-conjugated phalloidin (Molecular Probes) for 1 hour before imaging.
Live imaging of pupal legs
After carefully removing the pupal case of ~24h APF pupae, ‘naked’ pupae were mounted on a translucent hydrophobic membrane dish (VIVA Science) with the ventral side of the animal abutting the membrane. The dish was moistened with drops of water and inverted for live imaging using an upright Leica SP5 confocal microscope with an oil-immersion objective lens directly above the membrane.
Visualization of protrusions
Cable-like proximal protrusions from sensory organs in the pupal legs are obvious when UAS-APC2-GFP (Drosophila Adenomatous polyposis coli homolog 2 (APC2; McCartney et al., 1999; Webb et al., 2009) is expressed within all SOP cells under control of the Neu-Gal4 driver or a socket cell specific driver ASE5-Gal4 (Barolo et al., 2000). For fixed samples, ~10um Z-axis stacked projections were generated from the surface of the epidermis, in order to image the full extent of protrusions. A second reporter, Cherry-Jupiter (Karpova et al., 2006), was also used to visualize the protrusions.. The GFP signal in protrusions imaged using ASE5-Gal4 was weaker than with Neu-Gal4, perhaps reflecting weaker activity of the ASE5-Gal4 driver, and it labels an increasing portion of socket cells through 36h APF. For this reason, the Neur-Gal4 driver was preferred for MARCM based clonal analysis due to its strong labelling from every sensory organ from all stages. For live imaging using ASE5-Gal4; UAS-APC2-GFP reporter, a Histone2Av-mRFP transgene was used occasionally in the background to indicate the position of the socket cell nucleus as well as those of neighboring cells. To visualize protrusions from other epithelial cells, a flip-on Act-Gal4 driver line is used to express UAS-APC2-GFP reporter in randomly generated single cell Flp activated clones.
Temperature shift schemes
Flies with Notchts1 in the background were kept at 19°C. For Notch inactivation during bract development, pupae were shifted to 29°C from the equivalent of 12h APF until sacrificed around 40h APF.
For mosaic analysis using dia5 mutants, flies were raised at room temperature with the exception of a heat shock pulse to induce clones. Because of the temperature sensitive nature of the dia5 allele (Homem and Peifer, 2008), mosaic animals were moved to 29°C from the wandering 3rd instar stage until the time of sacrifice. The age at which pupae were sacrificed was calculated by accounting for the faster rate of development at 29°C.
Supplementary Material
Highlights.
Asymmetric EGFR signaling around sensory organs biases proximal bract induction.
The signaling cell emanates protrusions specifically towards proximal neighbors.
Signaling direction is perturbed concurrently with a shift of protrusion direction.
Protrusions potentiate signaling, thus breaking signaling symmetry.
Acknowledgments
We are grateful to Drs. Matthew Freeman, Jessica Treisman, Hugo Bellen, James Posakony, Bingwei Lu, Michel Gho, Markus Noll, Developmental Studies Hybridoma Bank and Bloomington Stock Center for fly stocks, plasmid and antibodies. We thank Dr. Yi Guo for artistic input as well as assistance in fly husbandry; Dr. Zhigang Chen for the angular distribution module for image analysis using the CellProfiler framework; Dr. Tom Kornberg for valuable input; Drs. Matthew Scott, Joe Lipsick, Mike Simon, Lewis Held and Axelrod lab members for critical readings of the manuscript. Y.P. and C.H. were generously supported by Jane Coffin Childs postdoctoral fellowships. Supported by NIH grants GM059823 and GM075311 to J.D.A.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amanai K, Jiang J. Distinct roles of Central missing and Dispatched in sending the Hedgehog signal. Development. 2001;128:5119–5127. doi: 10.1242/dev.128.24.5119. [DOI] [PubMed] [Google Scholar]
- Axelrod JD. Progress and challenges in understanding planar cell polarity signaling. Semin Cell Dev Biol. 2009;20:964–971. doi: 10.1016/j.semcdb.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Barolo S, Walker RG, Polyanovsky AD, Freschi G, Keil T, Posakony JW. A notch-independent activity of suppressor of hairless is required for normal mechanoreceptor physiology. Cell. 2000;103:957–969. doi: 10.1016/s0092-8674(00)00198-7. [DOI] [PubMed] [Google Scholar]
- Block J, Stradal TE, Hanisch J, Geffers R, Kostler SA, Urban E, Small JV, Rottner K, Faix J. Filopodia formation induced by active mDia2/Drf3. J Microsc. 2008;231:506–517. doi: 10.1111/j.1365-2818.2008.02063.x. [DOI] [PubMed] [Google Scholar]
- Brunner D, Ducker K, Oellers N, Hafen E, Scholz H, Klambt C. The ETS domain protein pointed-P2 is a target of MAP kinase in the sevenless signal transduction pathway. Nature. 1994;370:386–389. doi: 10.1038/370386a0. [DOI] [PubMed] [Google Scholar]
- Cabernard C, Doe CQ. Apical/basal spindle orientation is required for neuroblast homeostasis and neuronal differentiation in Drosophila. Dev Cell. 2009;17:134–141. doi: 10.1016/j.devcel.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Castrillon DH, Wasserman SA. Diaphanous is required for cytokinesis in Drosophila and shares domains of similarity with the products of the limb deformity gene. Development. 1994;120:3367–3377. doi: 10.1242/dev.120.12.3367. [DOI] [PubMed] [Google Scholar]
- Castro B, Barolo S, Bailey AM, Posakony JW. Lateral inhibition in proneural clusters: cis-regulatory logic and default repression by Suppressor of Hairless. Development. 2005;132:3333–3344. doi: 10.1242/dev.01920. [DOI] [PubMed] [Google Scholar]
- Chamoun Z, Mann RK, Nellen D, von Kessler DP, Bellotto M, Beachy PA, Basler K. Skinny hedgehog, an acyltransferase required for palmitoylation and activity of the hedgehog signal. Science. 2001;293:2080–2084. doi: 10.1126/science.1064437. [DOI] [PubMed] [Google Scholar]
- Chen MH, Li YJ, Kawakami T, Xu SM, Chuang PT. Palmitoylation is required for the production of a soluble multimeric Hedgehog protein complex and long-range signaling in vertebrates. Genes Dev. 2004;18:641–659. doi: 10.1101/gad.1185804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang PT, Kornberg TB. On the range of hedgehog signaling. Curr Opin Genet Dev. 2000;10:515–522. doi: 10.1016/s0959-437x(00)00121-0. [DOI] [PubMed] [Google Scholar]
- del Alamo D, Terriente J, Diaz-Benjumea FJ. Spitz/EGFr signalling via the Ras/MAPK pathway mediates the induction of bract cells in Drosophila legs. Development. 2002;129:1975–1982. doi: 10.1242/dev.129.8.1975. [DOI] [PubMed] [Google Scholar]
- Fichelson P, Gho M. Mother-daughter precursor cell fate transformation after Cdc2 down-regulation in the Drosophila bristle lineage. Dev Biol. 2004;276:367–377. doi: 10.1016/j.ydbio.2004.08.043. [DOI] [PubMed] [Google Scholar]
- Frankfort BJ, Mardon G. Senseless represses nuclear transduction of Egfr pathway activation. Development. 2004;131:563–570. doi: 10.1242/dev.00941. [DOI] [PubMed] [Google Scholar]
- Freeman M, Kimmel BE, Rubin GM. Identifying targets of the rough homeobox gene of Drosophila: evidence that rhomboid functions in eye development. Development. 1992a;116:335–346. doi: 10.1242/dev.116.2.335. [DOI] [PubMed] [Google Scholar]
- Gabay L, Scholz H, Golembo M, Klaes A, Shilo BZ, Klambt C. EGF receptor signaling induces pointed P1 transcription and inactivates Yan protein in the Drosophila embryonic ventral ectoderm. Development. 1996;122:3355–3362. doi: 10.1242/dev.122.11.3355. [DOI] [PubMed] [Google Scholar]
- Garcia-Bellido A. Pattern reconstruction by dissociated imaginal disk cells of Drosophila melanogaster. Dev Biol. 1966;14:278–306. doi: 10.1016/0012-1606(66)90017-0. [DOI] [PubMed] [Google Scholar]
- Gho M, Bellaiche Y, Schweisguth F. Revisiting the Drosophila microchaete lineage: a novel intrinsically asymmetric cell division generates a glial cell. Development. 1999;126:3573–3584. doi: 10.1242/dev.126.16.3573. [DOI] [PubMed] [Google Scholar]
- Gho M, Schweisguth F. Frizzled signalling controls orientation of asymmetric sense organ precursor cell divisions in Drosophila. Nature. 1998;393:178–181. doi: 10.1038/30265. [DOI] [PubMed] [Google Scholar]
- Golembo M, Schweitzer R, Freeman M, Shilo BZ. Argos transcription is induced by the Drosophila EGF receptor pathway to form an inhibitory feedback loop. Development. 1996;122:223–230. doi: 10.1242/dev.122.1.223. [DOI] [PubMed] [Google Scholar]
- Guo M, Jan LY, Jan YN. Control of daughter cell fates during asymmetric division: interaction of Numb and Notch. Neuron. 1996;17:27–41. doi: 10.1016/s0896-6273(00)80278-0. [DOI] [PubMed] [Google Scholar]
- Held LI., Jr Sensitive periods for abnormal patterning on a leg segment in Drosophila melanogaster. Roux’s Arch Dev Biol. 1990;199:31–47. doi: 10.1007/BF01681531. [DOI] [PubMed] [Google Scholar]
- Held LI., Jr Bristles induce bracts via the EGFR pathway on Drosophila legs. Mech Dev. 2002;117:225–234. doi: 10.1016/s0925-4773(02)00212-5. [DOI] [PubMed] [Google Scholar]
- Held LI, Jr, Duarte CM, Derakhshanian K. Extra joints and misoriented bristles on Drosophila legs. Prog Clin Biol Res. 1986a;217A:293–296. [PubMed] [Google Scholar]
- Held LI, Jr, Duarte CM, Derakhshanian K. Extra tarsal joints and abnormal cuticular polarities in various mutants of Drosophila melanogaster. Roux’s Arch Dev Biol. 1986b;195:145–157. doi: 10.1007/BF02439432. [DOI] [PubMed] [Google Scholar]
- Homem CC, Peifer M. Diaphanous regulates myosin and adherens junctions to control cell contractility and protrusive behavior during morphogenesis. Development. 2008;135:1005–1018. doi: 10.1242/dev.016337. [DOI] [PubMed] [Google Scholar]
- Homem CC, Peifer M. Exploring the roles of diaphanous and enabled activity in shaping the balance between filopodia and lamellipodia. Mol Biol Cell. 2009;20:5138–5155. doi: 10.1091/mbc.E09-02-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan YN, Jan LY. Asymmetric cell division in the Drosophila nervous system. Nat Rev Neurosci. 2001;2:772–779. doi: 10.1038/35097516. [DOI] [PubMed] [Google Scholar]
- Karpova N, Bobinnec Y, Fouix S, Huitorel P, Debec A. Jupiter, a new Drosophila protein associated with microtubules. Cell Motil Cytoskeleton. 2006;63:301–312. doi: 10.1002/cm.20124. [DOI] [PubMed] [Google Scholar]
- Klaes A, Menne T, Stollewerk A, Scholz H, Klambt C. The Ets transcription factors encoded by the Drosophila gene pointed direct glial cell differentiation in the embryonic CNS. Cell. 1994;78:149–160. doi: 10.1016/0092-8674(94)90581-9. [DOI] [PubMed] [Google Scholar]
- Layalle S, Ragone G, Giangrande A, Ghysen A, Dambly-Chaudiere C. Control of bract formation in Drosophila: poxn, kek1, and the EGF-R pathway. Genesis. 2004;39:246–255. doi: 10.1002/gene.20052. [DOI] [PubMed] [Google Scholar]
- Le Borgne R, Bellaiche Y, Schweisguth F. Drosophila E-cadherin regulates the orientation of asymmetric cell division in the sensory organ lineage. Curr Biol. 2002;12:95–104. doi: 10.1016/s0960-9822(01)00648-0. [DOI] [PubMed] [Google Scholar]
- McCartney BM, Dierick HA, Kirkpatrick C, Moline MM, Baas A, Peifer M, Bejsovec A. Drosophila APC2 is a cytoskeletally-associated protein that regulates wingless signaling in the embryonic epidermis. J Cell Biol. 1999;146:1303–1318. doi: 10.1083/jcb.146.6.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micchelli CA, The I, Selva E, Mogila V, Perrimon N. Rasp, a putative transmembrane acyltransferase, is required for Hedgehog signaling. Development. 2002;129:843–851. doi: 10.1242/dev.129.4.843. [DOI] [PubMed] [Google Scholar]
- Miura GI, Buglino J, Alvarado D, Lemmon MA, Resh MD, Treisman JE. Palmitoylation of the EGFR ligand Spitz by Rasp increases Spitz activity by restricting its diffusion. Dev Cell. 2006;10:167–176. doi: 10.1016/j.devcel.2005.11.017. [DOI] [PubMed] [Google Scholar]
- Mou X, Duncan DM, Baehrecke EH, Duncan I. Control of target gene specificity during metamorphosis by the steroid response gene E93. Proc Natl Acad Sci U S A. 2012;109:2949–2954. doi: 10.1073/pnas.1117559109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panakova D, Sprong H, Marois E, Thiele C, Eaton S. Lipoprotein particles are required for Hedgehog and Wingless signalling. Nature. 2005;435:58–65. doi: 10.1038/nature03504. [DOI] [PubMed] [Google Scholar]
- Schirenbeck A, Bretschneider T, Arasada R, Schleicher M, Faix J. The Diaphanous-related formin dDia2 is required for the formation and maintenance of filopodia. Nat Cell Biol. 2005;7:619–625. doi: 10.1038/ncb1266. [DOI] [PubMed] [Google Scholar]
- Takada R, Satomi Y, Kurata T, Ueno N, Norioka S, Kondoh H, Takao T, Takada S. Monounsaturated fatty acid modification of Wnt protein: its role in Wnt secretion. Dev Cell. 2006;11:791–801. doi: 10.1016/j.devcel.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Okada Y, Hirokawa N. FGF-induced vesicular release of Sonic hedgehog and retinoic acid in leftward nodal flow is critical for left-right determination. Nature. 2005;435:172–177. doi: 10.1038/nature03494. [DOI] [PubMed] [Google Scholar]
- Tobler H. Cell specific determination and the relationship between proliferation and transdetermination in leg and wing primordia in Drosophila melanogaster. J Embryol Exp Morphol. 1966;16:609–633. [PubMed] [Google Scholar]
- Tobler H. Influencing of bristle differentiation and pattern formation by mitomycin in Drosophila melanogaster. Experientia. 1969;25:213–214. doi: 10.1007/BF01899131. [DOI] [PubMed] [Google Scholar]
- Tobler H, Rothenbuhler V, Nothiger R. A study of the differentiation of bracts in Drosophila melanogaster using two mutations, H2 and svde. Experientia. 1973;29:370–371. doi: 10.1007/BF01926537. [DOI] [PubMed] [Google Scholar]
- Tokunaga C. Cell lineage and differentiation on the male foreleg of Drosophila melanogaster. Dev Biol. 1962;4:489–516. doi: 10.1016/0012-1606(62)90054-4. [DOI] [PubMed] [Google Scholar]
- Tree DR, Ma D, Axelrod JD. A three-tiered mechanism for regulation of planar cell polarity. Semin Cell Dev Biol. 2002;13:217–224. doi: 10.1016/s1084-9521(02)00042-3. [DOI] [PubMed] [Google Scholar]
- Vladar EK, Antic D, Axelrod JD. Planar cell polarity signaling: the developing cell’s compass. Cold Spring Harb Perspect Biol. 2009;1:a002964. doi: 10.1101/cshperspect.a002964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas N, Goswami D, Manonmani A, Sharma P, Ranganath HA, VijayRaghavan K, Shashidhara LS, Sowdhamini R, Mayor S. Nanoscale organization of hedgehog is essential for long-range signaling. Cell. 2008;133:1214–1227. doi: 10.1016/j.cell.2008.05.026. [DOI] [PubMed] [Google Scholar]
- Wallar BJ, Stropich BN, Schoenherr JA, Holman HA, Kitchen SM, Alberts AS. The basic region of the diaphanous-autoregulatory domain (DAD) is required for autoregulatory interactions with the diaphanous-related formin inhibitory domain. J Biol Chem. 2006;281:4300–4307. doi: 10.1074/jbc.M510277200. [DOI] [PubMed] [Google Scholar]
- Wallingford JB. Planar cell polarity signaling, cilia and polarized ciliary beating. Curr Opin Cell Biol. 2010;22:597–604. doi: 10.1016/j.ceb.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb RL, Zhou MN, McCartney BM. A novel role for an APC2-Diaphanous complex in regulating actin organization in Drosophila. Development. 2009;136:1283–1293. doi: 10.1242/dev.026963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willert K, Brown JD, Danenberg E, Duncan AW, Weissman IL, Reya T, Yates JR, 3rd, Nusse R. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448–452. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- Wolpert L. Principles of development. 3. Oxford ; New York: Oxford University Press; 2007. [Google Scholar]
- Yang C, Czech L, Gerboth S, Kojima S, Scita G, Svitkina T. Novel roles of formin mDia2 in lamellipodia and filopodia formation in motile cells. PLoS Biol. 2007;5:e317. doi: 10.1371/journal.pbio.0050317. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.