Abstract
Ribonucleotide reductase inhibitors enhance the anti-HIV-1 activities of a variety of nucleoside analogs, including those that act as chain terminators and those that increase the HIV-1 mutation rate. However the use of these ribonucleotide reductase inhibitors is limited by their associated toxicities. The hydroxylated phytostilbene resveratrol has activity in a host of systems including inhibition of ribonucleotide reductase and has minimal toxicity. Here we synthesized derivatives of resveratrol and examined them for anti-HIV-1 activity and their ability to enhance the antiviral activity of decitabine, a nucleoside analog that decreases viral replication by increasing the HIV-1 mutation rate. The data demonstrates six of the derivatives have anti-HIV-1 activity greater than resveratrol. However, only resveratrol acted in synergy with decitabine to inhibit HIV-1 infectivity. These results reveal novel resveratrol derivatives with anti-HIV-1 activity that may have mechanisms of action that differ from the drugs currently used to treat HIV-1.
Keywords: HIV-1, Resveratrol, Antiviral, Mutagenesis, Decitabine
Human immunodeficiency virus type 1 (HIV-1), the causative agent of AIDS, currently infects more than 30 million individuals worldwide.1 While drug therapy effectively suppresses viral replication, resistance to these drugs is a significant problem and necessitates the development of novel drug therapies.2 The development of drug resistance is facilitated by the high mutation rate of HIV-1 as well as the large number of virus particles produced by infected cells. Although the mutation rate facilitates emergence of drug resistance, it also renders HIV-1 particularly susceptible to modest increases in mutation rate.3 An antiviral strategy termed lethal mutagenesis aims to take advantage of this susceptibility by intentionally increasing the mutation rate of RNA viruses such that these viruses are unable to replicate their genome with enough fidelity to remain infectious.4 A number of RNA viruses have been shown to be susceptible to lethal mutagenesis including hepatitic C virus, poliovirus, vesicular stomatitis virus and HIV-1. 5–10 In fact, hepatitis C virus is treated with ribavirin, a drug that has been shown to inhibit viral replication through lethal mutagenesis in cell culture.11, 12 Whether ribavirin has the same mechanism in vivo is not yet clear.13, 14
Although none of the clinically approved anti-HIV-1 therapies act through lethal mutagenesis, we have reported the ability of the cytosine analog decitabine (5-aza-2'-deoxycytidine) and its riboside analog, 5-azacytidine to lethally mutagenize HIV-1 in a cell culture system.10, 15 Additionally, we have demonstrated that select ribonucleotide reductase inhibitors (RNRIs) such as hydroxyurea and gemcitabine dramatically enhance the antiviral activity of decitabine.15 RNRIs have also been shown to increase the antiviral activity of nucleoside analogs that act as chain terminators such as zidovudine and didanosine.10, 16, 17 RNRIs may increase the anti-HIV-1 activity of nucleoside analogs by decreasing endogenous nucleotide concentrations thereby increasing incorporation of the antiviral nucleoside analog. Although gemcitabine and hydroxyurea are clinically approved, their use is limited by the associated toxicities.18–20
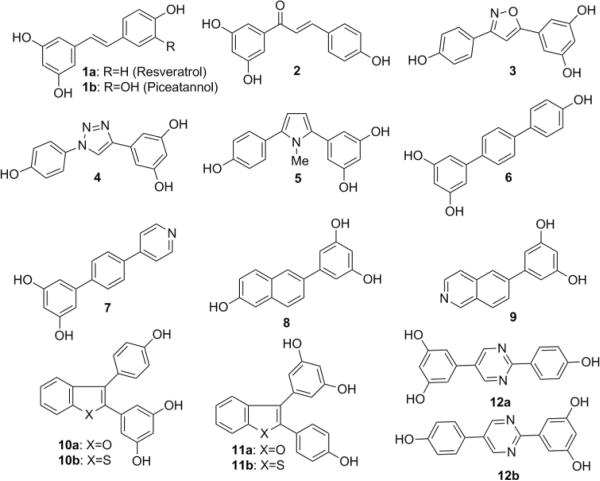
Resveratrol (Figure 1, 1a) is a natural product that is well tolerated and has a number of biological activities including the ability to inhibit HIV-1 replication.21–23 Resveratrol's anti-HIV-1 activity has been related to its ability to increase activity of SIRT1, a protein that may decrease transcription of the proviral genome.23, 24 Additionally, resveratrol synergistically increased the antiviral activity of nucleoside derivatives and this anti-HIV-1 activity may be attributed to its ability to inhibit RNRI.25 Besides being a RNRI with anti-HIV-1 activity, resveratrol has limited toxicity and is relatively inexpensive, making it a strong lead compound for the development of new anti-HIV-1 compounds that could be used alone or in combination with nucleoside analogs such as decitabine.
Figure 1.
Resveratrol and analogues
Using resveratrol as a lead compound, 15 derivatives were screened against HIV-1 alone and in combination with decitabine using an assay that quantifies their ability to inhibit HIV-1 infectivity within a single round of replication. We report here the anti-HIV-1 activity of six of the resveratrol derivatives and demonstrate that resveratrol potentiates the antiviral activity of decitabine.
Compounds 1a and 1b were purchased from Sigma-Aldrich and used without further purification. Compound 2 was purchased from Tokyo Chemical Industry. Compounds 62, and 82 were prepared following literature procedures. The synthesis of 5-membered heterocyclic compounds 3, 4 and 5 are as follows (Scheme 1). Aldehyde 13 was converted to the corresponding imidoyl chloride before cyclization with 1-Ethynyl-3,5-dimethoxybenzene. Deprotection with boron trifluoride afforded the isoxazole 3. The 1, 4-substituted triazole 4, was accessed through the corresponding azide precursor by copper catalyzed Huisgen [3+2] cycloaddition with 1-Ethynyl-3,5-dimethoxybenzene, followed by deprotection. The pyrrole analogue 5 was synthesized by condensation of 1,3-dicarbonyl intermediate with methyl amine followed by treatment with boron tribromide.
Scheme 1.
Reagents and conditions: (i) (a) NH2OH·HCl, NaHCO3; (b) NCS; (ii) (a) 1-Ethynyl-3,5-dimethoxybenzene, NEt3; (b) 1M BBr3, DCM, rt, 20 h; (iii) NaN3, cat. CuSO4; (iv) (a) 1-Ethynyl-3,5-dimethoxybenzene, sodium ascorbate; (b) 1 M BBr3, DCM, rt, 20 h; (v) 3,5-dimethoxy benzaldehyde, NEt3, triazolium salt; (vi) (a) NH2Me.HCl, AcOH, p-TsOH; (b) 1 M BBr3, DCM, rt, 20 h.
Derivatives 7, 9, 10 and 12 were prepared (Scheme 2) by employing the palladium catalyzed Suzuki-Miyauru cross coupling reaction. Thus, 3,5-dimethoxyphenylboronic acid 16 was reacted with 4-bromoiodobenzene followed by a second coupling reaction with 4-pyridylboronic acid to afford polyaromatic 17. Treatment of the boronic acid 16 with 6-bromoiosoquinoline generated intermediate 6-phenylisoquinoline 18. Benzofuran 19a and benzothiophene 19b were synthesized in a similar manner from 2,3-dibromoheteroaromatics respectively. Mono-coupling with 3,5-dimethoxyphenylboronic acid was highly selective for the 2-position, followed by coupling with second boronic acid to give oxygen and sulfur derivatives, respectively. 2,5-aryl substituted pyrimidine 20, was synthesized from 2,5-bromochloro pyrimidine, first by coupling to the bromide, then chloride with dimethoxy- and methoxy- boronic acids, respectively. Finally, the demethylation of the methoxy derivatives 17–20 was achieved with boron tribromide to afford compounds 7, 9, 10a,b and 12a. In a similar fashion (Scheme 3), the regioisomers of compounds 10a,b and 12a were synthesized by inverting the order of the boronic acid coupling partner followed by deprotection to give compounds 11a,b and 12b.
Scheme 2.
Reagents and conditions: (i) Pd(PPh3)4, 2 M Na2CO3, toluene/EtOH 3:1, relux, 6 h; (ii) 1 M BBr3, DCM, rt, 20 h.
Scheme 3.
Reagents and conditions: (i) Pd(PPh3)4, 2 M Na2CO3, toluene/EtOH 3:1, relux, 6 h; (ii) 1 M BBr3, DCM, rt, 20 h.
The antiviral activity of resveratrol and its derivatives was examined using the single-round replication assay10, 15 where marker genes present in the HIV-1 vector were used to assess infectivity and mutant frequency in the presence or absence of compounds. Six compounds demonstrated concentration-dependent inhibition of HIV-1 infectivity with EC50s below 75 μM (Table 1). Consistent with a previous report,23 resveratrol showed anti-HIV-1 activity which was concentration-dependent (Fig 2). However the EC50 of resveratrol was relatively high (>75 μM). Resveratrol and the six compounds showing improved potency were examined for cytotoxicity as described the supplementary information. The data show that 1b and 6 had an approximate 5-fold or greater selectivity index relative to the other derivatives.
Table 1.
Anti-HIV-1 activity (EC50) and toxicity (TC50) of resveratrol and derivatives.
| Compound | EC50 (μM)a | TC50 (μM)b | SIc |
|---|---|---|---|
| 1a | >75 | >300 | ND |
| 1b | 21.4 (17.8–25.1) | >400 | >18.7 |
| 2 | >75 | ND | ND |
| 3 | >75 | NDd | ND |
| 4 | >75 | ND | ND |
| 5 | >75 | ND | ND |
| 6 | 8.8 (7.2–10.9) | 179 (107–298) | 20.3 |
| 7 | >100 | ND | ND |
| 8 | >75 | ND | ND |
| 9 | >75 | ND | ND |
| 10a | 35.0 (25.9–47.3) | 84.8 (75.1–95.7) | 2.4 |
| 10b | 34.4 (28.3–41.9) | 131 (109–158) | 3.8 |
| 11a | 65.1 (56.4–75.2) | 108 (95.2–123) | 1.5 |
| 11b | 45.1 (31.1–65.5) | 118 (93.7–148) | 2.6 |
| 12a | >75 | ND | ND |
| 12b | >75 | ND | ND |
The concentration of compound that inhibits 50% of HIV-1 infection. The 95% Confidence Intervals are shown in parentheses after the EC50 value.
Concentration of compound that induces toxicity in 50% of the host cells.
Selectivity index: TC50/EC50
Not determined.
Figure 2.
Resveratrol inhibits HIV-1 in a concentration-dependent manner. The data shows the average ± standard deviation of 3 independent experiments.
We next evaluated the ability of resveratrol and three derivatives (1b, 11b, and 6) to inhibit HIV-1 replication when used in combination with decitabine, a mutagenic nucleoside with anti-HIV-1 activity. Decitabine and each derivative was used at a concentration that would have minimal anti-HIV-1 activity when used individually (Table 2) and examined for the ability of the combination to 1) enhance the antiviral activity of decitabine, and 2) increase the mutant frequency of HIV-1 using the single cycle assay we described previously.10, 15
Table 2.
Effect of Compounds on Infectivity and HIV-1 Mutant Frequency
| Compound (concentration) | % Infected Cellsa | Mutant Frequencyb |
|---|---|---|
| ND | 100 | 1.00 |
| Dec (70 nM) | 93±18 | 1.26 ±0.23 |
| 1a (20 μM) | 91±15 | 1.08±0.22 |
| 1a (20 μM) + Dec (70 nM) | 36±18c | 1.96±0.78 |
| 1b (6 μM) | 88±12 | 1.16±0.22 |
| 1b (6 μM) + Dec (70 nM) | 71±15 | 1.54±0.53 |
| 6 (6 μM) | 96±14 | 1.10±0.09 |
| 6(6 μM) + Dec (70 nM) | 74±16 | 1.72±0.72 |
| 11b (30 μM) | 99±14 | 1.08±0.12 |
| 11b (30 μM) + Dec (70 nM) | 80±34 | 1.94±0.69 |
ND (No Drug) was set to 100% and all others converted as described in the supplementary information. The data show the mean ± Standard Deviation.
ND (No Drug) was set to 1.00 and all others converted as described in supplementary information. The data show the mean ± Standard Deviation.
Statistically significant difference from individual drug treatment by One-Way ANOVA followed by Tukey's post-test with a p<0.05 considered significant.
Mutant frequencies of these compounds were calculated as described in the supplementary material. The data, (Table 2), indicate that resveratrol was the only compound that potentiated the antiviral activity of decitabine while increasing HIV-1 mutant frequency. While the average mutant frequency increased when either resveratrol or 11b was used in combination with decitabine compared to the no drug control, this was not statistically different than the mutant frequency seen with decitabine alone.
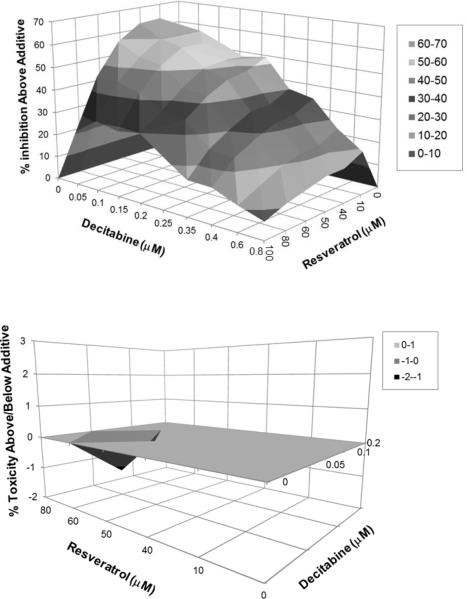
To further examine the ability of resveratrol to act in synergy with decitabine to inhibit HIV-1 activity, we used the MacSynergy II program.26 MacSynergy II determines if the interaction between two compounds can be described as additive, antagonistic, or synergistic. A 10 by 7 matrix was used to assess the anti-HIV-1 activity of decitabine (0–800 nM) and resveratrol (0–100 μM) alone and in combination. The MacSynergy II program calculates theoretical additive interactions from the individual dose responses. This calculated additivity which represents predicted anti-HIV-1 activity of the drug combinations was subtracted from the experimental data to reveal regions of synergy. Interactions that were additive are expected to lie on a zero plane of additivity (since predicted additive subtracted from experimental data would equal zero in cases of additivity), while any peaks above the plane of additivity indicate areas of synergy. Similarly, and peaks below the plane of additivity represent areas of antagonism. The data was assessed statistically by using the 95% confidence intervals around the experimental dose-responses. Synergy was considered to be significant if the lower 95% confidence limit of the experimental data was still greater than the calculated additive surface. Figure 3 shows that all combinations of resveratrol and decitabine lie above the plane of additivity with the highest peaks found at combinations containing between 10 and 60 μM of resveratrol and between 50 and 200 nM of decitabine.
Figure 3.
Resveratrol and decitabine act synergistically to inhibit HIV-1 infectivity in the absence of cellular toxicity.
These results indicate that the combination of resveratrol and decitabine are highly synergistic. The ability of resveratrol and decitabine to synergistically decrease HIV-1 infectivity could correlate with their ability to potentiate cellular toxicity. Therefore, the MacSynergy II program was used to assess the possibility that decitabine and resveratrol act synergistically to induce cell toxicity. As shown in Figure 3, the combinations of resveratrol and decitabine that showed the highest antiviral synergy, did not act synergistically to induce cellular toxicity.
In summary, we have synthesized novel resveratrol derivatives and discovered six with anti-HIV-1 activity superior to that of resveratrol. More importantly we have demonstrated that the combination of resveratrol and decitabine act synergistically to inhibit HIV-1 infectivity without a corresponding synergistic increase in cellular toxicity. Future studies will address why resveratrol, but not its derivatives potentiate the antiviral activity of decitabine
Supplementary Material
Acknowledgments
This research was supported by a University of Minnesota Academic Health Center Translational Research Grant as well as by NIH Grant GM56615. C.L.C. was supported in part from NIH Grant T32 CA09138 (Cancer Biology Training Grant); M.A.B. was supported in part from NIH Grant T32 GM008700 (Chemistry-Biology Interface Training Grant).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.(UNAIDS), J. U. N. P. o. H. A. WHO. 2010;Vol. 2010 [Google Scholar]
- 2.Hammer SM, Eron JJJ, Reiss P, Schooley RT, Thompson MA, Walmsley S, Cahn P, Fischl MA, Gatell JM, Hirsch MS, Jacobsen DM, Montaner JS, Richman DD, Yeni PG, Volberding PA. JAMA. 2008;300:555. doi: 10.1001/jama.300.5.555. [DOI] [PubMed] [Google Scholar]
- 3.Coffin JM. Science. 1995;267:483. doi: 10.1126/science.7824947. [DOI] [PubMed] [Google Scholar]
- 4.Drake JW, Holland JJ. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:13910. doi: 10.1073/pnas.96.24.13910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crotty S, Andino R. Microbes and Infection. 2002;4:1301. doi: 10.1016/s1286-4579(02)00008-4. [DOI] [PubMed] [Google Scholar]
- 6.Graci JD, Harki DA, Korneeva VS, Edathil JP, Too K, Franco D, Smidansky ED, Paul AV, Peterson BR, Brown DM, Loakes D, Cameron CE. Journal of Virology. 2007;81:11256. doi: 10.1128/JVI.01028-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loeb LA, Essigmann JM, Kazazi F, Zhang J, Rose KD, Mullins JI. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:1492. doi: 10.1073/pnas.96.4.1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruiz-Jarabo CM, Ly C, Domingo E, de la Torre JC. Virology. 2003;308:37. doi: 10.1016/s0042-6822(02)00046-6. [DOI] [PubMed] [Google Scholar]
- 9.Smith RA, Loeb LA, Preston BD. Virus Research. 2005;107:215. doi: 10.1016/j.virusres.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 10.Dapp MJ, Clouser CL, Patterson S, Mansky LM. Journal of Virology. 2009;83:11950. doi: 10.1128/JVI.01406-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Severson WE, Schmaljohn CS, Javadian A, Jonsson CB. Journal of Virology. 2003;77:481. doi: 10.1128/JVI.77.1.481-488.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Contreras AM, Hiasa Y, He W, Terella A, Schmidt EV, Chung RT. Journal of Virology. 2002;76:8505. doi: 10.1128/JVI.76.17.8505-8517.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cuevas JM, Gonzalez-Candelas F, Moya A, Sanjuan R. Journal of Virology. 2009;83:5760. doi: 10.1128/JVI.00201-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lutchman G, Danehower S, Song BC, Liang TJ, Hoofnagle JH, Thomson M, Ghany MG. Gastroenterology. 2007;132:1757. doi: 10.1053/j.gastro.2007.03.035. [DOI] [PubMed] [Google Scholar]
- 15.Clouser CL, Patterson SE, Mansky LM. Journal of Virology. 2010;84:9301. doi: 10.1128/JVI.01006-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sumpter LR, Inayat MS, Yost EE, Duvall W, Hagan E, Mayhew CN, Elford HL, Gallicchio VS. Antiviral Research. 2004;62:111. doi: 10.1016/j.antiviral.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 17.Lori F, Malykh A, Cara A, Sun D, Weinstein JN, Lisziewicz J, Gallo RC. Science. 1994;266:801. doi: 10.1126/science.7973634. [DOI] [PubMed] [Google Scholar]
- 18.Davidoff S, Shah RD, Arunabh T. Respiratory Medicine. 2006;100:760. doi: 10.1016/j.rmed.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 19.Gupta N, Ahmed I, Steinberg H, Patel D, Nissel-Horowitz S, Mehrotra B. American Journal of Clinical Oncology. 2002;25:96. doi: 10.1097/00000421-200202000-00021. [DOI] [PubMed] [Google Scholar]
- 20.Lisziewicz J, Foli A, Wainberg M, Lori F. Drug Safety. 2003;26:605. doi: 10.2165/00002018-200326090-00002. [DOI] [PubMed] [Google Scholar]
- 21.Heredia A, Davis C, Redfield R. Journal of Acquired Immune Deficiency Syndromes. 2000;25:246. doi: 10.1097/00126334-200011010-00006. [DOI] [PubMed] [Google Scholar]
- 22.Wang LX, Heredia A, Song H, Zhang Z, Yu B, Davis C, Redfield R. Journal of Pharmaceutical Sciences. 2004;93:2448. doi: 10.1002/jps.20156. [DOI] [PubMed] [Google Scholar]
- 23.Zhang HS, Zhou Y, Wu MR, Zhou HS, Xu F. Life Sciences. 2009;85:484. doi: 10.1016/j.lfs.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 24.Zhang HS, Wu MR. Virus Research. 2009;146:51. doi: 10.1016/j.virusres.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 25.Fontecave M, Lepoivre M, Elleingand E, Gerez C, Guittet O. FEBS Letters. 1998;421:277. doi: 10.1016/s0014-5793(97)01572-x. [DOI] [PubMed] [Google Scholar]
- 26.Prichard MN, Shipman C., Jr. Antiviral Research. 1990;14:181. doi: 10.1016/0166-3542(90)90001-n. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.