Abstract
Oxidative stress in the brain is implicated in increased sympathetic drive, inflammatory status and vascular dysfunctions, associated with development and establishment of hypertension. However, little is known about the mechanism of this impaired brain-vascular communication. Here, we tested the hypothesis that increased oxidative stress in the brain cardioregulatory areas, such as the paraventricular nucleus (PVN) of the hypothalamus, is driven by mitochondrial reactive oxygen species (ROS) and leads to increased inflammatory cells (ICs) and decreased/dysfunctional endothelial progenitor cells (EPCs), thereby compromising vasculature repair and accelerating hypertension. Chronic angiotensin II (Ang II) infusion resulted in elevated blood pressure and sympathetic vasomotor drive, decreased spontaneous baroreflex gain, and increased microglia activation in the PVN. This was associated with 46% decrease in BM EPCs and 250% increase in BM ICs, resulting in 5 fold decrease of EPCs/ICs ratio in the BM. Treatment with mitoTEMPO, a scavenger of mitochondrial O2−• intracerebroventricularly but not subcutaneously, attenuated Ang II-induced hypertension, decreased activation of microglia in the PVN, and normalized EPCs/ICs. This functional communication between the brain and BM was confirmed by retrograde neuronal labeling from the BM with GFP-tagged pseudorabies virus (PRV). Administration of GFP-PRV into the BM resulted in predominant labeling of PVN neurons within 3 days, with some fluorescence in the NTS, RVLM and SFO. Taken together, these data demonstrate that inhibition of mitochondrial ROS attenuates Ang II-induced hypertension and corrects the imbalance in EPCs/ICs in the BM. They suggest that an imbalance in vascular reparative and ICs may perpetuate vascular pathophysiology in this model of hypertension.
Keywords: mitochondrial ROS, endothelial progenitor cells, MAP, neurogenic hypertension
Introduction
The autonomic nervous system (ANS) and the immune system (IS) play major roles in the pathophysiology of hypertension and other cardiovascular diseases. Increased sympathetic drive has been shown to precede hypertension1, and, together with the decreased parasympathetic drive and the baroreceptor reflex, is a consequence of the elevated renin-angiotensin system (RAS) activity in the central cardioregulatory regions such as the paraventricular nucleus (PVN) and subfornical organ (SFO) amongst others2. Angiotensin II (Ang II) – dependent hypertension is also associated with an overactive IS, as the elevated levels of inflammatory cells (ICs) and cytokines contribute to both the pressor effects of Ang II3 as well as the cardiovascular pathophysiology4, 5. A functional link between the ANS and bone marrow cells has long been appreciated, and a cellular association between bone marrow stromal cells and hematopioetic stem cells and nerve terminals has been described as the ‘neuro-reticular complex’ 6,7.
Recent studies support that the ANS can modulate e the effects of the IS and influence the pathophysiology of cardiovascular diseases8–10. A direct sympathetic innervation of the immune organs, such as the spleen11 and increased splenic sympathetic nerve activity by central Ang II-infusion are directly related to the enhanced splenic immune response10. Moreover, stimulation of the vagus nerve exerts anti-inflammatory effects by decreasing levels of the inflammatory cytokines and suppressing the activation of ICs9. Therefore, the possibility exists that Ang II-dependent dysfunction of ANS precedes the activation of the IS, and that the ANS-IS synergetic action initiates the development of hypertension, as inferred by the recent observations of Abboud et al and Harrison et al12–13.
Endothelial dysfunction is a well-accepted hallmark of early hypertension. Since mature endothelial cells have limited reparative capacity, the bone marrow-derived endothelial progenitor cells (BM EPCs) contribute to the repair and maintenance of the damaged endothelium. However, EPC numbers and function are inversely correlated in patients with hypertension and other cardiovascular diseases14–17. Similar to the ICs, BM EPCs also appear to be neuro-regulated18. Evidence exists that the BM is densely innervated by the sympathetic and parasympathetic fibers19–22, and dysregulation of the neuronal input to the BM impairs EPC function in diabetes18. Moreover, both the EPC function and the numbers are improved by some RAS blockers23, 24, suggesting that one of the consequences of the overactive RAS in hypertension may also be the impairment of the endothelial reparative processes, which could lead to accelerated vascular dysfunction and hypertension-associated pathophysiology.
In line with this, we recently proposed a hypothesis of a dysfunctional autonomic-immune-vascular mechanism in neurogenic hypertension25. This involves Ang II-mediated neuroinflammation as exhibited by activation of the brain microglia, primarily in the PVN, resulting in excessive production of reactive oxygen species (ROS), elevated sympathetic activity and modulation of BM inflammatory and vascular reparative cells in the periphery, all playing important roles in the development and establishment of neurogenic hypertension25–27. In the brain, mitochondria are one of the major sources of intracellular ROS production, resulting in excessive oxidative stress, and altering neuronal redox state, which disrupts the sympathetic drive26. Collectively, we propose that increase in mitochondrial ROS is central in the dysfunctional neural-vascular communication associated with neurogenic hypertension. Furthermore, we postulate that impaired central-BM regulation of ICs and EPCs will impact the levels of activated ICs as well as the vasoreparative potential of EPCs, and that this shift in the cardiovascular damage/repair ratio will contribute to cardiovascular pathophysiology in hypertension. Hence, we investigated the effect of central administration of mitochondrial-targeted antioxidant (mitoTEMPO) on the brain inflammatory status and the behavior of BM-derived EPCs and ICs following a chronic Ang II-infusion in the rat model of neurogenic hypertension.
Methods
All experimental protocols are presented in the Methods section and are available in the Online Supplementary Data. All animal procedures were approved by the University of Florida Institute Animal Care and Use Committee.
Results
Chronic Ang II causes hypertension, perturbs the autonomic nervous system, and impairs BM cell activity
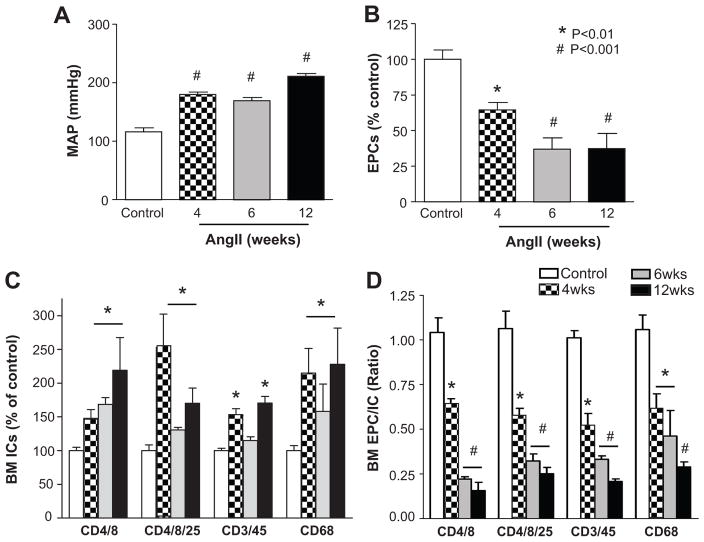
Ang II-infusion resulted in an increase in MAP to 177±6 mmHg (n=8) at 4 weeks, and to 202±4 mmHg (n=4) at 12 weeks of infusion, compared to the MAP of 98±2 mmHg in the age-matched controls (n=8), (Fig. 1A). Spectral analysis of telemetry blood pressure signal was performed, and the variables LF (SBP) and sBRG (PI) indicated 6- fold increase in the sympathetic vasomotor drive, and 3- fold decrease in the cardiac spontaneous baroreflex gain respectively, after 4 weeks of Ang II-infusion (Fig. S1A & S1B). The cardiac parasympathetic drive measured by HF (PI) did not show changes among the groups (Fig. S1C), yet the ratio of LF to HF, which is an indicator of vasovagal balance was 2- fold elevated by Ang II (Fig. S1D). This was associated with a 46–70% decrease in EPCs in the BM (Fig. 1B). In addition, there were significant increases in CD4+/8+ (200%), CD4+/8+/25+ (230%), CD3+/45+ (50%), and CD68+ (220%) cells in the BM of Ang II-infused rats (Fig. 1C). As a result, a 2–5-fold decrease in the EPCs/ICs ratio was observed in the BM of the Ang II-infused rats (Fig. 1D). Similar trends were observed in blood EPCs and IC (Fig. S2). To determine if Ang II hypertension was associated with dysfunctional BM mononuclear cells (MNCs), tube formation assays were performed. We found that both the tube length and the number of branches in the Ang II- infused rats were 20–30% lower than those grown from the control rats’ MNCs (Fig. S3).
Figure 1. Effect of chronic Ang II infusion on BM EPCs and ICs.
A, MAP measured by tail cuff after 4–12 weeks of Ang II infusion. B, Decreased BM-derived EPCs by chronic Ang II infusion. C, Increase in BM inflammatory cells (IC; CD4+/8+: T lymphocytes, CD4+/8+/25+: T regulatory cells, CD45+/3+: T lymphocytes, CD68+: macrophages) by chronic Ang II infusion. D, The ratio of EPCs to ICs. *P<0.01, #P<0.001 vs control, N=6
Six week s.c. infusion of phenylephrine, a vasoconstrictor which increases MAP without crossing the blood brain barrier27, increased MAP by ~22mmHg (Fig. S4A and S4B). This chronic increase in MAP was associated with a trend of decreases in EPC/IC ratios in the BM, which were not significant for the most part (Fig. S4C and S4D). Only EPC/CD4/8+ ratio showed a significant (20%) decrease in the BM of the phenylephrine-infused animals. These data suggest that Ang II-dependent elevation in sympathetic drive may be critical in inducing dysfunctional BM cell activity and that increase in BP alone is not sufficient for inducing this imbalance.
Intracerebroventricular but not subcutaneous mitoTEMPO infusion prevents hypertension, balances the autonomic nervous system, and restores BM cell activity
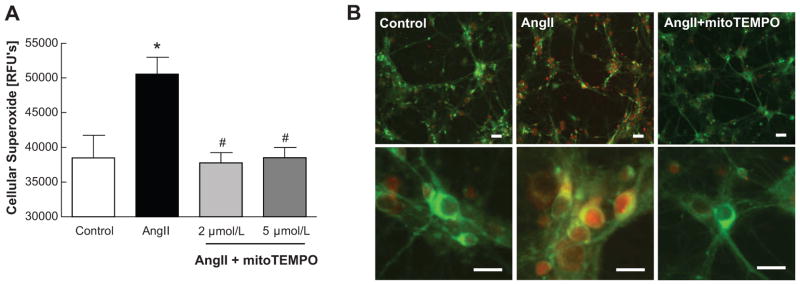
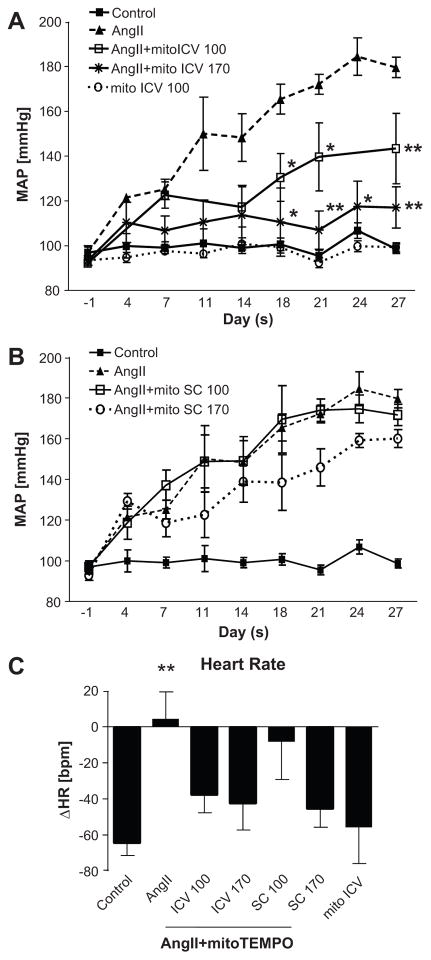
Ang II treatment of the rat neuronal cultures resulted in a 30% increase in cellular O2−•, which was completely inhibited by co-treatment with 2–5 μmol/L of mitoTEMPO, a selective scavenger of mitochondrial O2−• (Fig. 2A). This inhibition occurred primarily within the mitochondria, as evidenced by the co-staining with mitoTracker Green (a mitochondrial specific fluorescence dye) and mitoSOX Red (a superoxide marker) (Fig. 2B). Furthermore, chronic ICV infusion of mitoTEMPO (4 weeks) attenuated hypertension induced by Ang II (Fig. 3A). This attenuation was significant by 18 days of mitoTEMPO ICV infusion and was dose-dependent (100ng/kg/min; 146±12 mmHg; n=6, 170ng/kg/min; 112±13 mmHg; n=8). In contrast, subcutaneous infusion of 100ng/kg/min of mitoTEMPO did not influence Ang II-induced increases in MAP. However, 170 ng/kg/min dose of mitoTEMPO administrated subcutaneously showed a trend toward a decrease in MAP, but this effect was not significant (Fig.3B). In addition, there were no significant differences in heart rates between control and mitoTEMPO treatment groups (Fig. 3C). mitoTEMPO ICV alone did not affect MAP or HR (Fig. 3A & 3C). Inhibition of MAP by ICV mitoTEMPO (170ng/kg/min) was associated with the attenuation of heart weight/body weight ratio, cardiac myocyte diameter as an indicator of hypertrophy and cardiac fibrosis (Fig. S5). Spectral analysis of telemetry recording was performed to investigate if mitochondrial ROS influenced autonomic nerve activity. ICV mitoTEMPO (170ng/kg/min) was able to normalize the Ang II-perturbed LF (SBP) and sBRG (PI) (Fig. S1A & B) as well as the elevated LF/HF ratio (Fig. S1D). Together, these observations demonstrate that scavenging of brain mitochondrial O2−• inhibits sympathetic vasomotor drive, normalizes vasovagal balance, and attenuates high BP and cardiac hypertrophy induced by chronic Ang II infusion.
Figure 2. Cellular and mitochondrial superoxide scavenging by mitoTEMPO in Ang II-treated cultured neuron.
A, Cellular superoxide stained by dehydroethidium. Relative fluorescence were detected by microplate reader. *P<0.05 vs control, #P<0.05 vs Ang II. B, Representative images of neurons. mitoSOX (red) staining is used to detect mitochondrial superoxide and mitoTracker (green) is used for mitochondrial localization. Scale bars=50μm.
Figure 3. Effects of mitoTEMPO on MAP and heart rate.
A, ICV mitoTEMPO significantly attenuated MAP in Ang II-induced hypertension in a dose dependent manner (100 and 170ng/kg/min). B, Neither does of SC mitoTEMPO did not attenuate MAP. C, Heart rate did not show differences between mitoTEMPO treatment groups but Ang II infusion group. Bar graph is mean±SEM. *P<0.05, **P<0.01 vs control. N=7–8
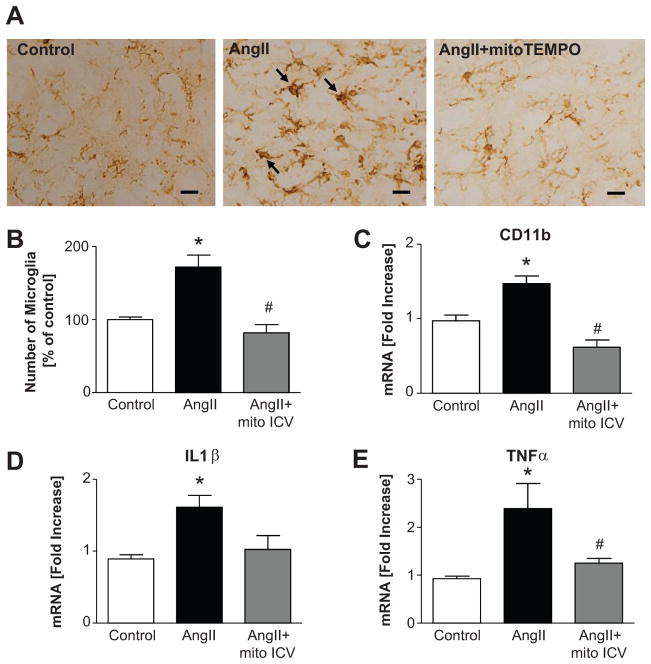
Our next objective was to determine effects of ICV mitoTEMPO on PVN microglial activation by determining the increase in microglial numbers and changes in their size and shape, which are the main indicators of microglial activation28. Ang II caused a 180% increase in the number of microglia, and a 1.5 fold increase in CD11b mRNA (Fig. 4B & 4C). Furthermore, the microglia appeared enlarged and their shape changed following Ang II infusion (Fig. 4A). Additionally, 1.6 fold and 2.3 fold increase in IL1β and TNFα mRNA levels were observed (Fig. 4D & 4E). ICV mitoTEMPO treatment that attenuates hypertension normalized these parameters.
Figure 4. Effects of ICV mitoTEMPO on activated microglia in the PVN.
A, Ang II-induced Iba-1 positive activated microglia within PVN were reduced to control level by ICV mitoTEMPO treatment. B, Quantification of the number of Iba-1 positive microglia in the PVN. C, mRNA of CD11b, a marker of activated microglia. D, mRNA of IL1β. E, mRNA of TNFα. *P<0.05 vs control, #P<0.05 vs Ang II. Scale bars=10μm
Finally, the effects of ICV mitoTEMPO on BM EPCs and ICs were studied to confirm a brain-BM communication in this neurogenic model of hypertension. Ang II-induced decrease in the number of BM EPCs was completely restored by ICV mitoTEMPO treatment (Fig. 5A). In addition, BM ICs were normalized by ICV mitoTEMPO (Fig. 5B). As a result, there was a 5 fold decrease in the EPCs/ICs ratio in the BM of Ang II-treated animals, and ICV mitoTEMPO normalized this imbalance (Fig. 5C). In contrast, SC infusion of mitoTEMPO failed to normalize the decrease in BM EPCs/ICs ratio.
Figure 5. Effects of ICV mitoTEMPO on BM EPCs and ICs and BM-neural communication.
A, ICV mitoTEMPO restored decreased number of EPCs (CD4−/5−/8−/90+) by Ang II infusion in BM. B, ICV mitoTEMPO normalized increases in BM derived inflammatory cells (CD4+/8+: T lymphocytes, CD4+/8+/25+: T regulatory cells, CD45+/3+: T lymphocytes, CD68+: macrophages). C, The ratio of EPCs to ICs. *P<0.05 vs control, #P<0.05 vs Ang II by 1-way ANOVA followed by Bonferoni. D, Retrograde tracing with GFP-PRV confirmed BM-neural communication in cardiovascular relevant brain regions. Scale bars=100μm
BM-PVN connection traced by PRV-GFP
Retrograde tracing with a replication-competent strain of pseudo rabies virus (PRV) containing GFP was carried out to confirm brain-BM connections. Microinjection of PRV-GFP in the femur BM resulted in GFP positive neurons in the PVN, predominantly. However, modest GFP fluorescence was also observed in the nucleus tractus solitarius (NTS), the rostral ventrolateral medulla (RVLM), and subfornical organ (SFO) (Fig. 5D). This retrograde labeling was specific to BM since a similar microinjection of PRV-GFP in the adjacent skeletal muscle did not show any GFP in the PVN. Furthermore severing sciatic and femoral nerves completely attenuated BM-PVN retrograde transport of this virus.
Discussion
The present study for the first time indicates the functional interactions between the cardiovascular relevant brain regions, particularly the PVN, and the BM. We have shown that Ang II-induced hypertension, which exhibits a neurogenic component, is associated with a decrease in the EPCs/ICs ratio. This may compromise the ability of EPCs to repair vasculature, and perpetuate hypertension-linked vascular pathophysiology. Retrograde labeling of the neurons in the cardioregulatory brain regions in general and the PVN in particular following PRV-GFP administration into the BM further supports a neural-BM connection, albeit an anatomical one. The functional interaction between the brain and BM is however implied by specific inhibition of the brain mitochondrial ROS, which apparently restores the imbalance of EPCs/ICs ratio within the BM to control levels, as well as attenuates the Ang II-induced hypertension. To confirm that this effect is specific, we used another vasoconstrictor, phenylephrine, to increase blood pressure without the CNS involvement, as phenylephrine is not known to cross the blood brain barrier27. For the most part, we found no significant effect of chronic phenylephrine infusion on the BM EPCs and ICs or their ratio. Therefore, we conclude that the effect we see on the EPC/IC ratio in the BM is not the consequence of the hypertension per se, but it may directly be linked to the elevated sympathetic drive caused by brain Ang II-dependent mitochondrial ROS. We would predict that this brain-mediated dysfunction of the BM activity would be inhibited by blocking the sympathetic drive to the BM. However, this would be difficult to demonstrate at present as it would require either a specific nerve ablation, or a complete sympathectomy, both of which may be too severe for the time-line in the present study. We have, however, recently been able to isolate and record activity from a specific nerve entering the rat femur via the nutrient foramen, which has characteristics of a sympathetic nerve (unpublished preliminary data). Our future studies will attempt to isolate and characterize the sympathetic activity of this nerve in hypertensive rats in order to relate it to the BM EPC/IC activity.
We have demonstrated that ICV but not SC infusion of mitoTEMPO attenuates hypertension by modulating the sympathetic vasomotor drive and cardiac baroreflex gain. Furthermore, mitoTEMPO treatment prevented the development of Ang II-induced cardiac hypertrophy, presumably through an indirect effect on attenuation of the blood pressure increase. It is also possible that the mitoTEMPO-induced prevention of cardiac hypertrophy may have been a result of a direct effect of lowering of the cardiac sympathetic drive; however, since we did not measure the sympathetic drive to the heart, we are unable to make this conclusion at the present time. However, Dikalova et al. observed an attenuation of hypertension even by SC administration of mitoTEMPO in mice29, which is in contrast to our present results. This discrepancy may likely be due to an increased accessibility of mitoTEMPO in the brains of mice, as a result of the previously reported altered permeability of the blood brain barrier (BBB) in mice subjected to twice the concentration of Ang II compared to the one used in the rats in the present study30. This is particularly relevant in view of evidence that autonomic regions of the brain are highly vascularized31. However, we cannot at this point rule out the possibility of functional differences between the ICV and SC mitoTEMPO delivery, as the higher levels of SC mitoTEMPO showed some albeit insignificant response, which could have resulted from the mitoTEMPO effect on the peripheral tissue mitochondria (e.g. smooth muscle of the blood vessels). Additional differences in other humoral responses and metabolic processing between the two species cannot be ruled out at the present time.
Dysfunctional/decreased EPCs and increased ICs in hypertension and cardiovascular disease are well established based on both clinical and animal studies32,33,34. Harrison’s group was among the first to demonstrate the role of T cells and adaptive immune system in the pathogenesis of hypertension34. Our present study appears to support the role of the CD4+ T cell inflammatory lymphocytes in Ang II- induced hypertension. As it has been reported, the activation of a subset of the proinflammatory CD4+ cells by Ang II is directly dependent on the activation of the third ventricle regions of the brain, as targeted lesions of this brain area prevented the Ang II-induced CD4+ activation34. Therefore, the ANS is implicated in the activation of the immune response in hypertension. This is in line with our present observations, as ICV infusion of mitoTEMPO inhibits Ang II – induced hypertension/sympathetic drive, as well as normalizes the CD4+ levels. On the other hand, the CD4+CD25+ regulatory T cells (T Regs) are able to suppress innate and adaptive immune responses by suppressing proinflammatory actions of other T lymphocyte subtypes as well as those of macrophages35. Thus, the increase in the CD4+CD25+ T Regs we observed in the present study may be compensatory to the elevation in other ICs, as the system seeks to restore its balance. Furthermore, we observed elevation in the CD8+ cells, which have been implicated in the process of killing of adjacent cells as well as in the release of proinflammatory cytokines and the activation of macrophages, thereby contributing to the Ang II-dependent hypertensive pathophysiology35,36. Similarly, the Ang II-dependent increase in the CD3+CD45+ inflammatory leukocytes has previously been shown in hypertension, as these cells infiltrate the vasculature and damage the endothelium34. Accumulation of inflammatory leukocytes in the NTS of the spontaneously hypertensive rat apparently contributes to the dysfunctional baroreflex processing37. Similarly, activation of macrophages (CD68+ cells) contributes to Ang II-dependent vascular dysfunction, hypertension and oxidative stress38,39. Peripheral circulating macrophages may also infiltrate specific brain regions40 and contribute to the central oxidative stress by releasing ROS and proinflammatory cytokines41,42, which could lead to modulation of the sympathetic drive. It is possible that the activation of ICs and the subsequent ROS and the proinflammatory cytokine release may directly influence the BM EPCs in Ang II- dependent hypertension. However, future studies are needed to adequately address this issue.
The novelty of the current study is that it provides evidence of the existence of a brain-BM axis whose dysfunction seems to be apparent in the pathogenesis of neurogenic hypertension in our model. The question whether the deleterious effects result from ICs restricted to the peripheral system or the ability of these cells to infiltrate into the CNS, and participate in neuroinflammation remains to be answered. However, based on recent evidence43,44 it is tempting to suggest that both extravasation of ICs and their progenitors into the PVN and their differentiation into microglia together with resident microglial activation are critical in neural dysregulation in hypertension. Thus, the feed-forward loop between the brain and BM, in addition to local activation of microglia and generation of mitochondrial ROS, may contribute to this pathophysiology. We postulate that Ang II-infusion activates resident microglia in the PVN and induces production of mitochondrial ROS initiating a cascade of signaling events involving cytokines/chemokines leading to impaired BM activity. Extravasation of BM-derived IC progenitors into the PVN perpetuates these deleterious effects. However, alternate sources of ROS and targets of Ang II cannot be ruled out at the present time in view of observations that Ang II also stimulates neuronal ROS. Further studies on Ang II-mediated interactions between microglia and neurons are needed to establish the precise mechanism.
Perspectives
In this study we present evidence for the existence of a brain-BM axis, the dysfunction of which may perpetuate the vascular pathophysiology in hypertension. We present the hypothesis that the central activation of angiotensinergic pathways influences the PVN microglia, which directly or indirectly raises cytokines, chemokines and ROS, thereby stimulating neuronal activity and leading to the increased sympathetic drive. The resulting alteration in the sympathetic drive to the BM promotes impairment in the EPCs/ICs ratio resulting in compromised vascular repair, and the increased ICs and impaired BBB perpetuates this pathophysiology. This hypothesis is supported by the following: (i) Ang II hypertension increases activated microglia and proinflammatory cytokines in the PVN28, (ii) interruption of microglia activation by either minocycline28 or by mitoTEMPO attenuates hypertension, hypertension-induced imbalance in BM activity is prevented by central mitoTEMPO treatment, and (iii) existence of neural connections between autonomic brain regions and the BM. Thus, targeting the microglia and the sympathetic drive to the BM presents a novel strategy for consideration in neurogenic hypertension.
Supplementary Material
Novelty and Significance.
What is New?
The hypothesis of a dysfunctional brain-bone marrow communication in hypertension
Mitochondrial oxidative stress in the brain autonomic areas is responsible for angiotensin II hypertension
An imbalance between the vascular reparative cells (EPCs) and the damaging inflammatory cells (ICs) is associated with increased sympathetic drive and hypertension
What is Relevant?
First evidence that a dysfunctional brain-bone marrow communication may be responsible for the vascular pathophysiology of hypertension.
Summary.
Hypertension is associated with a decrease in EPC/IC ratio
Inhibition of mitochondrial ROS in the brain attenuates the sympathetic drive and corrects the imbalance in EPC/IC ratio
Acknowledgments
We thank Fan Lin for her excellent technical support.
Source of Funding
This work was supported by NIH grants of HL33610.
Non-standard Abbreviations and Acronyms
- ROS
reactive oxygen species
- EPCs
endothelial progenitor cells
- ICs
Inflammatory cells
- BM
bone marrow
- HR
heart rate
- MAP
mean arterial pressure
- PVN
paraventricular nucleus
- SFO
subfornical organ
- OVLT
organum vasculosum of lamina terminals
- RVLM
rostral ventrolateral medulla
- NTS
nucleus of the solitary tract
Footnotes
Conflict(s) of Interest/Disclosure(s)
None
References
- 1.Cabassi A, Vinci S, Calzolari M, Bruschi G, Borghetti A. Regional sympathetic activity in pre-hypertensive phase of spontaneously hypertensive rats. Life Sci. 1998;62:1111–1118. doi: 10.1016/s0024-3205(98)00034-4. [DOI] [PubMed] [Google Scholar]
- 2.Osborn JW, Fink GD, Sved AF, Toney GM, Raizada MK. Circulating angiotensin II and dietary salt: converging signals for neurogenic hypertension. Curr Hypertens Rep. 2007;9:228–235. doi: 10.1007/s11906-007-0041-3. [DOI] [PubMed] [Google Scholar]
- 3.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 2007;204:2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brands MW, Banes-Berceli AK, Inscho EW, Al-Azawi H, Allen AJ, Labazi H. Interleukin 6 knockout prevents angiotensin II hypertension: role of renal vasoconstriction and janus kinase 2/signal transducer and activator of transcription 3 activation. Hypertension. 2010;56:879–884. doi: 10.1161/HYPERTENSIONAHA.110.158071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Melendez GC, McLarty JL, Levick SP, Du Y, Janicki JS, Brower GL. Interleukin 6 mediates myocardial fibrosis, concentric hypertrophy, and diastolic dysfunction in rats. Hypertension. 2010;56:225–231. doi: 10.1161/HYPERTENSIONAHA.109.148635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamazaki K, Allen TD. Ultrastructural morphometric study of efferent nerve terminals on murine bone marrow stromal cells, and the recognition of a novel anatomical unit: the “neuro-reticular complex”. Am J Anat. 1990;187:261–276. doi: 10.1002/aja.1001870306. [DOI] [PubMed] [Google Scholar]
- 7.Miyan JA, Broome CS, Whetton AD. Neural regulation of bone marrow. Blood. 1998;92:2971–2973. [PubMed] [Google Scholar]
- 8.Rosas-Ballina M, Olofsson PS, Ochani M, Valdes-Ferrer SI, Levine YA, Reardon C, Tusche MW, Pavlov VA, Andersson U, Chavan S, Mak TW, Tracey KJ. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science. 2011;334:98–101. doi: 10.1126/science.1209985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Yang H, Ulloa L, Al-Abed Y, Czura CJ, Tracey KJ. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 10.Ganta CK, Lu N, Helwig BG, Blecha F, Ganta RR, Zheng L, Ross CR, Musch TI, Fels RJ, Kenney MJ. Central angiotensin II-enhanced splenic cytokine gene expression is mediated by the sympathetic nervous system. Am J Physiol Heart Circ Physiol. 2005;289:H1683–1691. doi: 10.1152/ajpheart.00125.2005. [DOI] [PubMed] [Google Scholar]
- 11.Nance DM, Sanders VM. Autonomic innervation and regulation of the immune system (1987–2007) Brain Behav Immun. 2007;21:736–745. doi: 10.1016/j.bbi.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abboud FM, Harwani SC, Chapleau MW. Autonomic neural regulation of the immune system: implications for hypertension and cardiovascular disease. Hypertension. 2012;59:755–762. doi: 10.1161/HYPERTENSIONAHA.111.186833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harrison DG, Marvar PJ, Titze JM. Vascular inflammatory cells in hypertension. Front Physiol. 2012;3:128. doi: 10.3389/fphys.2012.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MacEneaney OJ, DeSouza CA, Weil BR, Kushner EJ, Van Guilder GP, Mestek ML, Greiner JJ, Stauffer BL. Prehypertension and endothelial progenitor cell function. J Hum Hypertens. 2009;25:57–62. doi: 10.1038/jhh.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giannotti G, Doerries C, Mocharla PS, Mueller MF, Bahlmann FH, Horvath T, Jiang H, Sorrentino SA, Steenken N, Manes C, Marzilli M, Rudolph KL, Luscher TF, Drexler H, Landmesser U. Impaired endothelial repair capacity of early endothelial progenitor cells in prehypertension: relation to endothelial dysfunction. Hypertension. 2010;55:1389–1397. doi: 10.1161/HYPERTENSIONAHA.109.141614. [DOI] [PubMed] [Google Scholar]
- 16.Yao EH, Yu Y, Fukuda N. Oxidative stress on progenitor and stem cells in cardiovascular diseases. Curr Pharm Biotechnol. 2006;7:101–108. doi: 10.2174/138920106776597685. [DOI] [PubMed] [Google Scholar]
- 17.Imanishi T, Hano T, Nishio I. Estrogen reduces endothelial progenitor cell senescence through augmentation of telomerase activity. J Hypertens. 2005;23:1699–1706. doi: 10.1097/01.hjh.0000176788.12376.20. [DOI] [PubMed] [Google Scholar]
- 18.Busik JV, Tikhonenko M, Bhatwadekar A, Opreanu M, Yakubova N, Caballero S, Player D, Nakagawa T, Afzal A, Kielczewski J, Sochacki A, Hasty S, Li Calzi S, Kim S, Duclas SK, Segal MS, Guberski DL, Esselman WJ, Boulton ME, Grant MB. Diabetic retinopathy is associated with bone marrow neuropathy and a depressed peripheral clock. J Exp Med. 2009;206:2897–2906. doi: 10.1084/jem.20090889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Artico M, Bosco S, Cavallotti C, Agostinelli E, Giuliani-Piccari G, Sciorio S, Cocco L, Vitale M. Noradrenergic and cholinergic innervation of the bone marrow. Int J Mol Med. 2002;10:77–80. [PubMed] [Google Scholar]
- 20.Katayama Y, Battista M, Kao WM, Hidalgo A, Peired AJ, Thomas SA, Frenette PS. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006;124:407–421. doi: 10.1016/j.cell.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 21.Mignini F, Streccioni V, Amenta F. Autonomic innervation of immune organs and neuroimmune modulation. Auton Autacoid Pharmacol. 2003;23:1–25. doi: 10.1046/j.1474-8673.2003.00280.x. [DOI] [PubMed] [Google Scholar]
- 22.Serre CM, Farlay D, Delmas PD, Chenu C. Evidence for a dense and intimate innervation of the bone tissue, including glutamate-containing fibers. Bone. 1999;25:623–629. doi: 10.1016/s8756-3282(99)00215-x. [DOI] [PubMed] [Google Scholar]
- 23.Honda A, Matsuura K, Fukushima N, Tsurumi Y, Kasanuki H, Hagiwara N. Telmisartan induces proliferation of human endothelial progenitor cells via PPARgamma-dependent PI3K/Akt pathway. Atherosclerosis. 2009;205:376–384. doi: 10.1016/j.atherosclerosis.2008.12.036. [DOI] [PubMed] [Google Scholar]
- 24.Muller P, Kazakov A, Jagoda P, Semenov A, Bohm M, Laufs U. ACE inhibition promotes upregulation of endothelial progenitor cells and neoangiogenesis in cardiac pressure overload. Cardiovasc Res. 2009;83:106–114. doi: 10.1093/cvr/cvp123. [DOI] [PubMed] [Google Scholar]
- 25.Zubcevic J, Waki H, Raizada MK, Paton JF. Autonomic-immune-vascular interaction: an emerging concept for neurogenic hypertension. Hypertension. 2011;57:1026–1033. doi: 10.1161/HYPERTENSIONAHA.111.169748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirooka Y. Oxidative stress in the cardiovascular center has a pivotal role in the sympathetic activation in hypertension. Hypertens Res. 2011;34:407–412. doi: 10.1038/hr.2011.14. [DOI] [PubMed] [Google Scholar]
- 27.Thurston CL, Helton ES. Effects of intravenous phenylephrine on blood pressure, nociception, and neural activity in the rostral ventral medulla in rats. Brain Res. 1996;717:81–90. doi: 10.1016/0006-8993(96)00007-8. [DOI] [PubMed] [Google Scholar]
- 28.Shi P, Diez-Freire C, Jun JY, Qi Y, Katovich MJ, Li Q, Sriramula S, Francis J, Sumners C, Raizada MK. Brain microglial cytokines in neurogenic hypertension. Hypertension. 2010;56:297–303. doi: 10.1161/HYPERTENSIONAHA.110.150409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dikalova AE, Bikineyeva AT, Budzyn K, Nazarewicz RR, McCann L, Lewis W, Harrison DG, Dikalov SI. Therapeutic targeting of mitochondrial superoxide in hypertension. Circ Res. 2010;107:106–116. doi: 10.1161/CIRCRESAHA.109.214601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cassis LA, Huang J, Gong MC, Daugherty A. Role of metabolism and receptor responsiveness in the attenuated responses to Angiotensin II in mice compared to rats. Regul Pept. 2004;117:107–116. doi: 10.1016/j.regpep.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 31.Zhang M, Mao Y, Ramirez SH, Tuma RF, Chabrashvili T. Angiotensin II induced cerebral microvascular inflammation and increased blood-brain barrier permeability via oxidative stress. Neuroscience. 2010;171:852–858. doi: 10.1016/j.neuroscience.2010.09.029. [DOI] [PubMed] [Google Scholar]
- 32.Lee CW, Huang PH, Huang SS, Leu HB, Huang CC, Wu TC, Chen JW, Lin SJ. Decreased circulating endothelial progenitor cell levels and function in essential hypertensive patients with electrocardiographic left ventricular hypertrophy. Hypertens Res. 2011;34:999–1003. doi: 10.1038/hr.2011.68. [DOI] [PubMed] [Google Scholar]
- 33.Endtmann C, Ebrahimian T, Czech T, Arfa O, Laufs U, Fritz M, Wassmann K, Werner N, Petoumenos V, Nickenig G, Wassmann S. Angiotensin II Impairs Endothelial Progenitor Cell Number and Function In Vitro and In Vivo: Implications for Vascular Regeneration. Hypertension. 2011;58:394–403. doi: 10.1161/HYPERTENSIONAHA.110.169193. [DOI] [PubMed] [Google Scholar]
- 34.Marvar PJ, Thabet SR, Guzik TJ, Lob HE, McCann LA, Weyand C, Gordon FJ, Harrison DG. Central and peripheral mechanisms of T-lymphocyte activation and vascular inflammation produced by angiotensin II-induced hypertension. Circ Res. 2010;107:263–270. doi: 10.1161/CIRCRESAHA.110.217299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Akdis CA, Akdis M. Mechanisms and treatment of allergic disease in the big picture of regulatory T cells. J Allergy Clin Immunol. 2009;123:735–746. doi: 10.1016/j.jaci.2009.02.030. quiz 747–738. [DOI] [PubMed] [Google Scholar]
- 36.Harrison DG, Guzik TJ, Lob HE, Madhur MS, Marvar PJ, Thabet SR, Vinh A, Weyand CM. Inflammation, immunity, and hypertension. Hypertension. 2010;57:132–140. doi: 10.1161/HYPERTENSIONAHA.110.163576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Waki H, Gouraud SS, Maeda M, Paton JF. Evidence of specific inflammatory condition in nucleus tractus solitarii of spontaneously hypertensive rats. Exp Physiol. 2009;95:595–600. doi: 10.1113/expphysiol.2009.047324. [DOI] [PubMed] [Google Scholar]
- 38.De Ciuceis C, Amiri F, Brassard P, Endemann DH, Touyz RM, Schiffrin EL. Reduced vascular remodeling, endothelial dysfunction, and oxidative stress in resistance arteries of angiotensin II-infused macrophage colony-stimulating factor-deficient mice: evidence for a role in inflammation in angiotensin-induced vascular injury. Arterioscler Thromb Vasc Biol. 2005;25:2106–2113. doi: 10.1161/01.ATV.0000181743.28028.57. [DOI] [PubMed] [Google Scholar]
- 39.Wenzel P, Knorr M, Kossmann S, Stratmann J, Hausding M, Schuhmacher S, Karbach SH, Schwenk M, Yogev N, Schulz E, Oelze M, Grabbe S, Jonuleit H, Becker C, Daiber A, Waisman A, Munzel T. Lysozyme M-positive monocytes mediate angiotensin II-induced arterial hypertension and vascular dysfunction. Circulation. 2011;124:1370–1381. doi: 10.1161/CIRCULATIONAHA.111.034470. [DOI] [PubMed] [Google Scholar]
- 40.Fiala M, Liu QN, Sayre J, Pop V, Brahmandam V, Graves MC, Vinters HV. Cyclooxygenase-2-positive macrophages infiltrate the Alzheimer’s disease brain and damage the blood-brain barrier. Eur J Clin Invest. 2002;32:360–371. doi: 10.1046/j.1365-2362.2002.00994.x. [DOI] [PubMed] [Google Scholar]
- 41.Smith JA, Das A, Ray SK, Banik NL. Role of pro-inflammatory cytokines released from microglia in neurodegenerative diseases. Brain Res Bull. 2012;87:10–20. doi: 10.1016/j.brainresbull.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu Y, Zhang ZH, Wei SG, Serrats J, Weiss RM, Felder RB. Brain perivascular macrophages and the sympathetic response to inflammation in rats after myocardial infarction. Hypertension. 55:652–659. doi: 10.1161/HYPERTENSIONAHA.109.142836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen SK, Tvrdik P, Peden E, Cho S, Wu S, Spangrude G, Capecchi MR. Hematopoietic origin of pathological grooming in Hoxb8 mutant mice. Cell. 2010;141:775–785. doi: 10.1016/j.cell.2010.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Longo B, Romariz S, Blanco MM, Vasconcelos JF, Bahia L, Soares MB, Mello LE, Ribeiro-dos-Santos R. Distribution and proliferation of bone marrow cells in the brain after pilocarpine-induced status epilepticus in mice. Epilepsia. 2010;51:1628–1632. doi: 10.1111/j.1528-1167.2010.02570.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.