Abstract
l-glutamate (Glu) is the main excitatory neurotransmitter in the central nervous system (CNS) and is associated with motor behavior and sensory perception. While microdialysis methods have been used to record tonic levels of Glu, little is known about the more rapid changes in Glu signals that may be observed in awake rats. We have reported acute recording methods using enzyme-based microelectrode arrays (MEA) with fast response time and low detection levels of Glu in anesthetized animals with minimal interference. The current paper concerns modification of the MEA design to allow for reliable measures in the brain of conscious rats. In this study, we characterized the effects of chronic implantation of the MEA into the brains of rats. We were capable of measuring Glu levels for 7 days without loss of sensitivity. We performed studies of tail-pinch induced stress, which caused a robust biphasic increase in Glu. Histological data show chronic implantation of the MEAs caused minimal injury to the CNS. Taken together, our data show that chronic recordings of tonic and phasic Glu can be carried out in awake rats for up to 17 days in vivo allowing longer term studies of Glu regulation in behaving rats.
Keywords: amperometry, chronic recordings, prefrontal cortex, striatum
Glu is the main excitatory neurotransmitter in the mammalian CNS, playing a role in development, plasticity, learning and memory, cognition, sensory systems and motor systems. Historically, Glu has been studied in brain tissue using microdialysis and electrophysiological methods such as patch-clamp recordings (Bagley and Moghaddam 1997; Tucci et al. 1997; Fitzpatrick et al. 2001; Kennedy et al. 2002; Shou et al. 2004). Currently, the most common technique employed to study Glu in the intact mammalian brain is in vivo microdialysis. Many microdialysis studies of Glu have been performed in anesthetized and awake animal models (Bagley and Moghaddam 1997; Tucci et al. 1997; Kennedy et al. 2002; Shou et al. 2004). Microdialysis allows for multiple analytes to be measured simultaneously with low detection limits. However, the fast dynamics of Glu regulation may be muted when using microdialysis due to the limits in response time of this method, even with the great strides that have been made in increasing the response time by coupling microdialysis with capillary electrophoresis (Tucci et al. 1997; Kennedy et al. 2002; Rossell et al. 2003). In addition, several microdialysis studies have reported that the Glu overflow measured with this technique is not tetrodotoxin-dependent, supporting that the Glu signals measured using microdialysis were not neuronally derived (Timmerman and Westerink 1997; Baker et al. 2002; Melendez et al. 2005). Furthermore, it has been shown that there was extensive damage to the brain tissue up to 1.4 mm away from the microdialysis probe implant site (Clapp-Lilly et al. 1999; Borland et al. 2005). Even with the recent improvements, it is doubtful that microdialysis will be able to achieve the response time required to see the second-by-second dynamics of Glu, mainly because of the physical limitations due to the flow rates and diffusion process required to cross the dialysis membrane. While it may be possible to collect and analyze microdialysis samples that correspond to one second sampling intervals (Rossell et al. 2003), Kennedy’s group (Shou et al. 2004) has shown when using microdialysis the fastest response time achievable was 12–18 s. As neurochemical levels and behaviors are capable of changing rapidly (subsecond), it is important to develop a technique that can routinely measure these fast changes with an adequate response time.
Our laboratory has recently developed an enzyme-based microelectrode array (MEA) that is capable of selectively detecting low levels of Glu with subsecond (500–800 ms) response time that is virtually free from CNS interferents, such as 3,4-dihydroxyphenylacetic acid and ascorbate (Burmeister et al. 2000, 2002). The present study concerns the adaptation and validation of MEA recordings in the awake, freely moving animal, which will allow for second-by-second detection of Glu neurotransmission in the behaving animal. Firstly, we have adapted our methodology for chronic recordings of tonic (resting) and phasic (rapid) changes in Glu in awake rats. Secondly, we investigated the reproducibility of such methods for measures in the rat prefrontal cortex and striatum over several days. Thirdly, the effects of a tail-pinch stress on extracellular levels of Glu were investigated in the striatum of awake behaving rats. Finally, histopathological studies were carried out to determine the damage produced by the MEA on surrounding brain tissue.
Materials and methods
Animals
Male Long Evans or Fischer 344 (Harlan) rats 3–6 months of age at the time of surgery were used for all experiments. Fischer rats weighed 375–450 g, while Long Evans rats weighed 400–600 g at the time of surgery. The animals were individually housed in a 12 h light/dark cycle with free access to food and water in the Association for Assessment and Accreditation of Laboratory Animal Care International approved animal resource center at the University of Kentucky. Animals were allowed at least 1 week to acclimate to the environment prior to any experiments. All appropriate animal care (food, water, bedding, cage cleaning etc.) was performed by the Animal Resource Center staff. There were no procedures involving undue discomfort to the animals. Following surgery, rats were individually housed under the same conditions. Animal care was approved by the University of Kentucky Institutional Animal Care and Use Committee and was in accordance with the Guide for the Care and Use of Laboratory Animals.
Enzyme-based microelectrode design
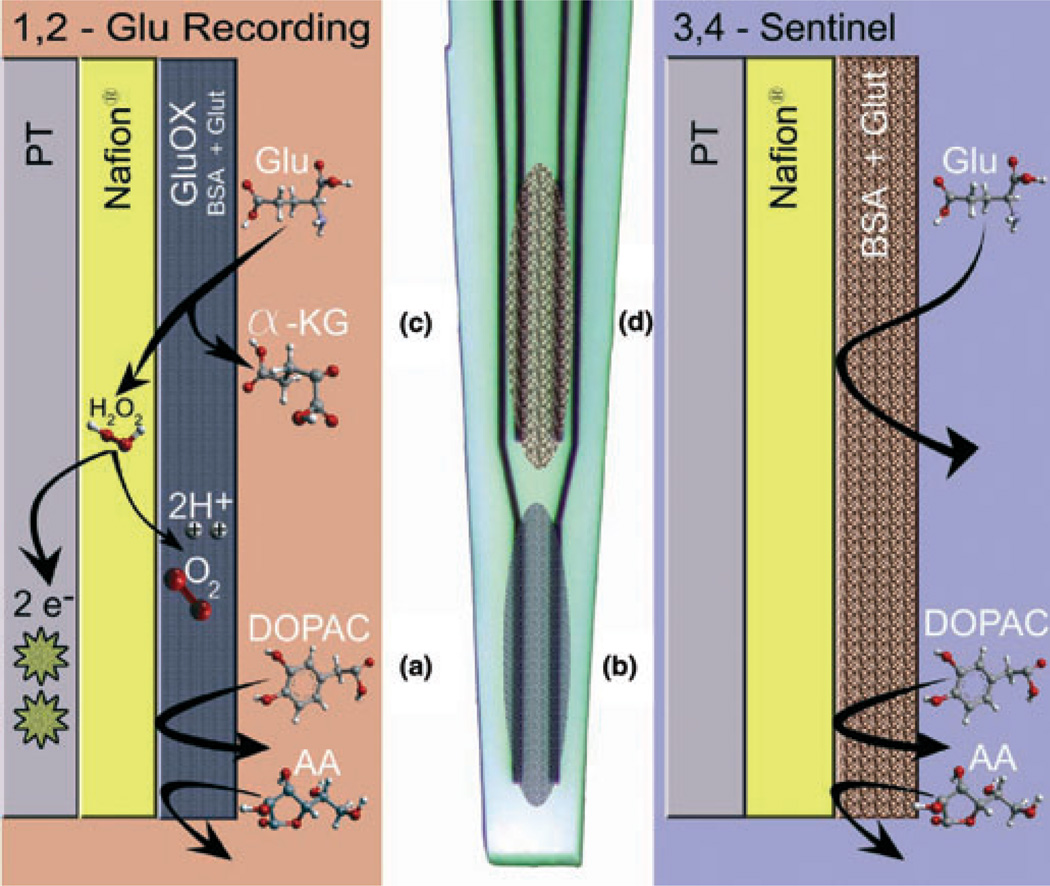
Ceramic-based microelectrodes were assembled and selected for in vivo recordings as previously described (Gerhardt et al. 1984; Burmeister et al. 2000, 2002). Preparation of the MEA for recording has been extensively described in Burmeister et al. 2002; Briefly, the platinum (Pt) recording sites were dip coated with Nafion® (Sigma-Aldrich Corp., St Louis, MO, USA) to repel anions as previously described (Burmeister and Gerhardt 2001). Microelectrode recording sites 1 and 2 (Fig. 1) were coated with a Glu oxidase (GluOx) (Seikagaku America, Inc., East Falmouth, MA, USA) coating solution as previously described (Nickell et al. 2005). In order to induce cross-linking of GluOx and increase the adhesion to the microelectrode, glutaraldehyde and bovine serum albumin (Sigma-Aldrich Corp.) were added to the GluOx solution. The GluOx layer was required by our approach to measure Glu, as it caused the enzymatic break-down of Glu to α-ketoglutarate and H2O2. When a potential of +0.7 V versus a Ag/AgCl reference electrode was applied to the MEA, the reporter molecule, H2O2, oxidized and gave up two electrons. The resulting current was then amplified and recorded by a FAST-16 recording system (Quanteon, LLC, Nicholasville, KY, USA). Microelectrode recording sites 3 and 4 (self-referencing or sentinel sites) were coated similar to sites 1 and 2, with the exception that the coating solution did not contain GluOx (Fig. 1). This means that sites 3 and 4 recorded everything, including dopamine, not repelled by Nafion® (any possible interferents, etc.) except Glu. When site 3 and 4 were subtracted from site 1 and 2, the resulting signal represented Glu measures (Burmeister and Gerhardt 2001; Burmeister et al. 2002).
Fig. 1.
Microelectrode arrays depicting self-referencing capabilities and coating techniques for enzyme-based measures of Glu and schematic diagram of coating layers used for the self-referencing enzyme-based recordings of Glu using our system. (a,b) Glu is broken down into H2O2 and α-ketoglutarate by the outermost layer containing GluOx. (c,d) The self-referencing sites do not contain GluOx in this layer. The layer closest to the Pt recording site is the Nafion® exclusion layer. Nafion® repels anions such as 3,4-dihydroxyphenylacetic acid and ascorbic acid. H2O2 can freely diffuse across the Nafion® layer, coming into contact with the Pt recording sire, where it is oxidized when a +0.7 V versus Ag/AgCl is applied. The resulting two electrons per H2O2 molecule are measured by our recording system.
Reference electrodes
Miniature Ag/AgCl reference electrodes were prepared by first stripping the Teflon off the silver wire (0.08 in bare, 0.110 in coated; A-M Systems Inc., Carlsborg, WA, USA) ¼ inch on each end. One of the stripped ends was soldered to a gold-plated socket (Ginder Scientific, Ottawa, ON, USA) and the other end was coated with AgCl by placing the tip of the stripped sliver wire (cathode) into a 1 mol/L HCl plating bath saturated with NaCl containing a Pt wire (anode) and applying 9 V DC current using a power supply to the cathode versus the anode.
Ceramic electrode preparation and rat hat pedestal system
MEA paddle modification
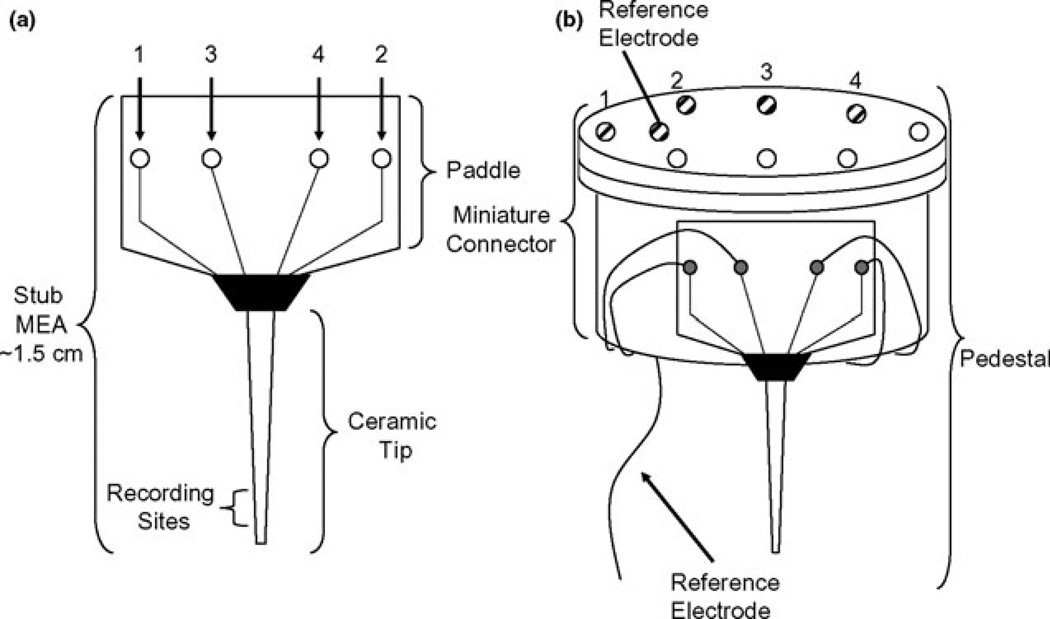
The pedestal was the portion of the system that was chronically implanted on the rat’s head and contained the microelectrode (Fig. 2). During recording sessions, the pedestal connected to the miniaturized rat hat, which contained the miniature 4-channel amplifier that was connected to a low torque commutator (Fig. 3). The paddle was altered during MEA production to change the electrode paddle from the design used in slice and anesthetized animal recordings to a smaller design needed for use in freely moving animals (Fig. 2). The resulting shorter paddle was termed the ‘stub’ MEA. The ceramic-based Glu selective MEAs were prepared for freely moving recordings first by cleaning the MEA. This was performed by dipping the bottom portion of the ceramic electrode tip in filtered, stirred isopropyl alcohol for 5 min. This was followed by a 5-min wash with stirred ddH2O to aid in removing any residues from the recording sites. The Pt recording surfaces were carefully wiped twice with a lab wipe to further remove any residual particles that were on the recording sites and would hinder the adhesion of the enzyme matrix. MEAs were dried for 15 min at 105–115°C to make sure there was no residual water on the sites. Next, the MEAs were coated with Nafion®, cured at 170°C for 5 min followed by the coating solutions (either containing or not containing GluOx) as described above.
Fig. 2.
Schematic of the assembly of the freely moving microelectrode arrays (MEA) pedestal. (a) Stub MEA designed for small size and light weight. The length of the stub MEA including the paddle and ceramic tip is approximately 1.5 cm. (b) Schematic of the fully assembled pedestal MEA.
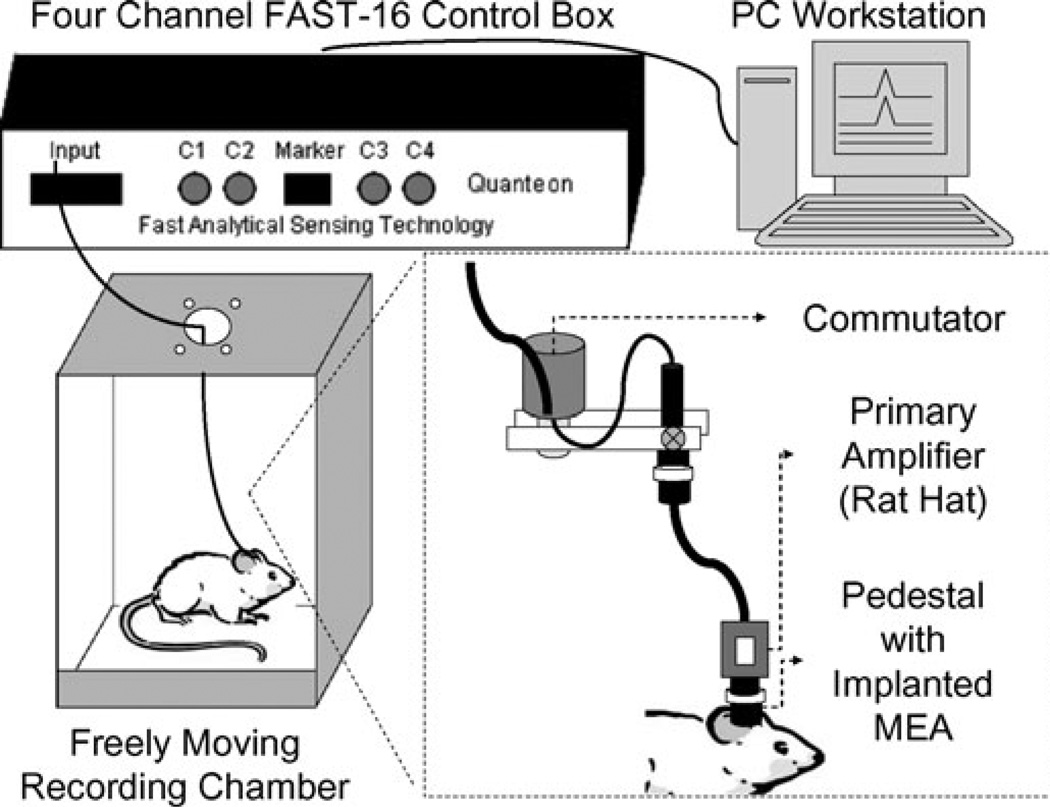
Fig. 3.
Schematic diagram of the in vivo freely moving recording system. The pedestal microelectrode arrays (MEA) pedestal is implanted into the rat brain. When the MEA is connected to the FAST-16 recording system, the signal travels from the implanted MEA to the pre-amplifier rat hat. The signal then travels through the commutator, which is located at the top of the recording chamber, and finally into the FAST-16 control box and PC workstation. The commutator allows for the connecting wires to freely rotate as the rat moves throughout the recording chamber without hindrance. Gold sockets from the miniamplifier/rat hat connect to the connecting pins embedded in the miniature connector of the pedestal to allow for continuous recordings. Dental acrylic surrounds the implanted pedestal apparatus. Rat is able to move to all areas of the recording chamber, performing everyday activities while Glu is simultaneously recorded from the central nervous system of the rat.
Miniature connector and MEA attachment
In order to attach the miniature connector to the modified MEA for freely moving recordings (together termed the pedestal), connecting wires were prepared by first stripping both ends of 30 American wire gauge varnished copper wire (Radioshack, Fort Worth, TX, USA). One end of the copper connecting wire was soldered to a gold-plated socket (Ginder Scientific). The other end of the wire was soldered to the microelectrode for each of the four corresponding recording sites, using eutectic solder, a low heat soldering iron (200°C) and heat sinks to prevent heat transfer damage to the Pt recording sites. 5 min epoxy was used to secure the MEA assembly and protect the wire board interface from moisture. The epoxy was allowed to dry for ~1 h. The gold-plated socket ends of the reference and microelectrode wires were inserted into a nine pin miniature connector (Ginder Scientific), so that the sockets were completely encompassed by the connector. The copper wires were tucked around the miniature connector and the MEA tip was carefully positioned parallel to the connector. Correct positioning of the MEA tip was essential for accurate microelectrode placement in the brain. Water proof 5 min epoxy was used to secure the paddle and wires to the round connector, making sure that the exposed part of the sockets on the end of the connector closest to the microelectrode tip and wires were covered with epoxy to ensure that moisture did not penetrate the pedestal. The completed assembly was allowed to air dry for at least 1 h.
Microelectrode array/cannula assembly
In order to locally apply small quantities of Glu and other chemicals at the MEA tip, a 26-gauge stainless steel guide cannula (Plastics One, Roanoke, VA, USA) was attached to the MEA assembly using sticky wax (Kerr Corp., Orange, CA, USA). A stainless steel dummy cannula, 1-mm longer than the guide, was inserted into the guide and screwed into place, such that the dummy cannula tip was aimed between the four recording sites at a distance of approximately 100 microns away from MEA tip. Ejections of Glu or drugs were performed through a 33-gauge internal cannula, or ejection cannula, which protruded 1-mm past the guide cannula. A stainless steel dummy cannula remained in the guide whenever the animal was not in the recording chamber.
Recording apparatus
The recording apparatus consisted of a large wooden box with a Plexiglas® (Cyro Industries, Rockaway, NJ, USA) insert. The recording headstage consisted of a round miniature connector with five connector pins (one connecting each of the four channels and one connecting the reference electrode). The connector pins lead to the 4-channel low-noise potentiostat (Rat Hat 4; Quanteon), which once connected was located as close as possible to the animal in order to minimize noise artifacts. Connecting wires, encompassed by an insulator, lead to the low torque commutator at the top of the box. The recording assembly hung from the top center of the box from a 12 lead commutator (Airflyte, Bayonne, NJ, USA) (Fig. 3). This allowed the animal to freely move to all areas of the box. The outputs of the commutator were connected to a Fast Analytical Sensing Technology (FAST-16) system (Quanteon) where the analog signals were amplified and then digitized in the microcomputer using a fast A/D-D/A board (National Instruments, Austin, TX, USA). In addition, control voltages for amperometric measurements were controlled by the data acquisition board. The digitized representation of the data was then visualized using the FAST-16 software (Quanteon).
Electrode calibration
Microelectrodes were calibrated to determine their sensitivity and selectivity against ascorbic acid as previously described (Nickell et al. 2005). Briefly, Constant potential amperometry was performed using a FAST-16 system designed for recording simultaneously from the four-channel microelectrodes with a final gain of 0.2 nA/V. The tip of the modified MEA was placed in a continuously stirred solution of 0.05 mol/L phosphate-buffered saline (PBS) (pH 7.4) (operated at a constant applied potential of +0.7 V vs. a Ag/AgCl). A recirculating water bath (Gaymar Co.) was used to maintain a constant buffer temperature of 37°C to allow the enzyme layer to function properly. Calibrations were performed by achieving final buffer concentrations of 250 µmol/L ascorbic acid and 20, 40, 60 and 80 µmol/L Glu through additions of aliquots of 20 mmol/L ascorbic acid and 20 mmol/L Glu stock solutions. Selectivity ratios for Glu over ascorbic acid were calculated in addition to the slope (sensitivity), limit of detection and linearity (R2) for Glu for all MEAs. The MEA’s had average slopes of −0.0076 ± 0.0011 nA/µmol/L (n = 15 MEA, 26 Pt recording sites) and limit of detections of 0.92 ± 0.21 µmol/L (n = 15 MEA, 26 Pt recording sites). The MEAs were also tested to determine the recording capability among the four Pt recording sites using dopamine (2 µmol/L final concentration) and H2O2 (8.8 µmol/L final concentration) as test substances. MEAs were only used if all four Pt recording sites had in vitro responses to dopamine and H2O2 that were within 10% of each other.
Electrode implantation
Rats were anesthetized with sodium pentobarbital solution (50 mg/mL), administered in two intraperitoneal (i.p.) doses or 2% isoflurane and placed in a stereotaxic apparatus (Kopf Instruments, Tujunga, CA, USA). Animal body temperature was maintained at 37°C with a heating pad (Braintree Scientific, Braintree, MA, USA). The animals’ eyes were coated with artificial tears (The Buttler Company, Columbus, OH, USA) to help maintain moisture and prevent infection. All surgeries were performed in a Vertical Laminar Flow Workstation (Microzone Corp., Ottawa, ON, USA), which filtered lab air through a high efficiency particulate air filter. Prior to incision, the skin directly on top of the animals head (between the ears and from just behind the eyes to the neck) was wiped with Betadine solution to keep the incision area clean and to prevent infection. The skin on top of the rat’s head was reflected, making as small an incision as possible. Three small holes were drilled in the skull in the opposite quadrants of where the MEA was implanted for placement of stainless steel scull screws. A fourth hole was drilled contralateral from the recording site for insertion of the miniature Ag/AgCl reference electrode. Following a successful calibration of the electrode, the animals underwent a 2 × 2 mm craniotomy and were implanted with a Glu selective MEA pedestal assembly into the right striatum (anterior-posterior: +1.0 mm; medical-lateral: −2.5 mm; dorsal-ventral: −4.3 mm vs. bregma) or the right prefrontal cortex (AP: +3.2 mm; ML: −0.8 mm, DV: −4.5 mm vs. bregma), based on the coordinates from Paxinos and Watson (1998) with the incisor bar set so that the scull was level (−2.3 mm). Three small stainless steel screws (Small Parts Inc., Miami Lakes, FL, USA) were threaded into the scull to serve as anchors and care was taken so that the tip of the screw did not touch the brain. The assembly was secured with approximately four layers of dental acrylic (Lang Dental MFG, Wheeling, IL, USA), taking care to cover as much of the MEA assembly as possible. The dental acrylic had a smooth texture and care was taken to remove excess from the skin surface so as not to promote the rat to scratch its head.
Following surgery, rats were placed on a heating pad to help maintain body temperature until the rat recovered from anesthesia. Three subcutaneous injections of 1 mL each of Ringer’s solution, Mammal (Fisher Scientific, Burr Ridge, IL, USA) were administered immediately following surgery. Rimadyl pellets were given for post-surgery inflammation and pain. Rats were allowed to recover for a minimum of 2 days prior to initial recordings.
Recording protocol
Typical recording sessions involved allowing the rat to freely roam around the recording chamber for 10 min to acclimate to the surroundings before connecting the pedestal to the potentiostat and started data acquisition. The rat underwent a minimum 30 min acclimation period, or until a stable baseline was established. Following this period, either an ejection cannula was inserted or animal manipulation was initiated. The ejection cannula was only inserted on days when chemicals needed to be ejected. After the ejection cannula was inserted (connected to a 10 µL Hamilton syringe, Reno, NV, USA), another acclimation period of ~30 min was allowed so that baseline was again established. Following this period, resting Glu, the effects of locally applied Glu, urethane injections (i.p.) or a 5-min tail-pinch were studied for up to three additional hours. The volumes of locally applied Glu were kept constant at 1 µL.
Following completion of the experiments (after multiple days of recordings), rats were anesthetized with isoflurane and transcardially perfused with 0.9% saline followed by 4% paraformaldehyde. The brain was removed and stored in 4% paraformaldehyde for 3 days followed by storage in 0.1 mol/L phosphate buffer (10% sucrose) for sectioning and staining to confirm electrode placement and examine possible tissue damage.
Histopathology
Male Long Evans rats between the age of 3 and 6 months at the time of surgery were chronically implanted with pedestal microelectrodes as previously described. Animals were then anesthetized with isoflurane and transcardially perfused after 2, 4 or 8 weeks. The brains were stored as previously described. After rinsing in sucrose, cryostat sections (14 µm) were collected from the area of implantation. The sections were post-fixed in acetone for 3 min prior to being processed for indirect immunohistochemistry. Antibodies used were raised against glial fibrillary acidic protein (GFAP anti-mouse, diluted 1 : 400; Chemicon, Temecula, CA, USA) as a marker for astrocytes and Iba1 (anti-rabbit, diluted 1 : 1000; Wako Chemicals, Germany), a pan-microglia marker. Incubations were performed for 48 h at 4°C. After washing, the sections were incubated in Alexa 488 and Alexa 594 secondary antibodies (diluted 1 : 500, Molecular Probes, Carlsbad, CA, USA) for 1 h at 22°C. Antibodies were diluted in PBS containing 0.3% Triton-X. After additional rinsing the sections were mounted in 90% glycerin in PBS. Double labeling of GFAP and Iba1 were performed in sequence such that each type of antibody was applied one at a time. Sections were evaluated using image analysis and Improvision software (Lexington, MA, USA). The densities of GFAP and Iba1 immunoreactivities were calculated using NIH image software on binary images and expressed as mean grey density. Mean values from four sections from each brain were analyzed using a one-way anova followed by Tukey’s post hoc analyses. All measurements were performed on blind-coded slices.
Data analysis
The FAST-16 recording system saved amperometric data, time and pressure ejection data marks for all four recording channels. Unless otherwise noted, calibration data, in conjunction with a modified spreadsheet program, was used to determine parameters for the signal differences recorded between data marks. Measures derived from the data file included: (i) Maximum amplitude, the peak concentration of the signal, (ii) Rise time, the time in seconds from ejection to peak Glu concentration, (iii) T80, the time in seconds from maximum rise to 80% decay, (iv) Clearance rate (Tc), (change in amplitude/change in time) between T20 and T60 (µmol/L/s). T20 and T60 are times in seconds from maximum rise to 20% and 60% decay, respectively. (v) k−1, exponential rate, curve fit based on first order fitting of the signal decay (s−1). (vi) Uptake rate, (k−1* amplitude) (µmol/L/s). An analysis of variance (anova) with a Tukey’s post hoc test was used to analyze the significance of signal amplitude over time, with time as the dependent variable. Significance was defined as p < 0.05.
Results
Validation of Glu signals in vivo
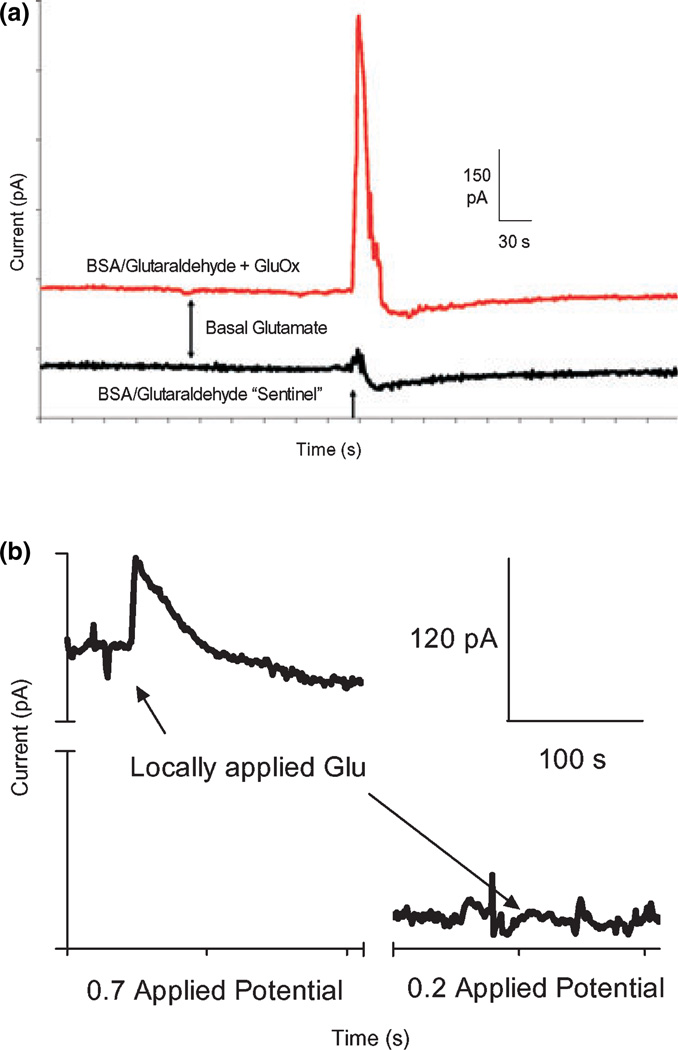
The FAST-16 recording system (Fig. 3) allowed for reliable second-by-second recordings of Glu in the striatum and prefrontal cortex of awake, freely behaving rats. We were able to consistently implant the MEA assemblies and record Glu for as long as 3 weeks in awake rats. Glu recordings were verified in vivo by locally applying a Glu solution (5 mmol/L isotonic, pH 7.4, 1 µL) with the use of the cannula directed between the four recording sites approximately 100 lm from the microelectrode surface. In Fig. 4a, the top tracing shows a GluOx (Glu recording) coated site, while the bottom tracing shows the self-referencing, or sentinel site, not coated with GluOx. GluOx was required for the enzymatic break-down of Glu to the reporter molecule H2O2, which was readily oxidized at the Pt recording sites operated at +0.7 V versus Ag/AgCl (Fig. 1). The self-referencing, or sentinel, site, can therefore detect possible interferents recorded on the GluOx coated site, with the exception of Glu. The difference between the two peak heights (maximum amplitude) show that the actual change in the electrochemical signal was due to locally applied Glu. This measure was repeatable within animals, between animals and over multiple days (Fig. 5). This self-referencing subtracting technique (Burmeister and Gerhardt 2001) was also used to measure resting Glu levels as described below.
Fig. 4.
(a) Representative tracing of resting Glu levels and locally applied Glu in the prefrontal cortex using a self-referencing microelectrode arrays (MEA) pedestal recording. Self-referencing microelectrodes were implanted in the right hemisphere of the prefrontal cortex. Glu (1 µL, 5 mmol/L) was locally applied, as indicated by the arrow. A rapid spike in Glu concentration was recorded on the GluOx coated site, similar to what is observed in anesthetized rats with very little change in signal on the self-referencing or sentinel site. Differences in maximum amplitudes suggests actual in vivo Glu signal, while differences in baseline measures suggests resting Glu levels. (b) Glu (5 mmol/L, 1 µL) was locally applied and detected using a self-referencing MEA with Nafion® as the exclusion layer. The selfreferencing sites were subtracted from the GluOx coated sites to yield the signal pictured above. (a) Locally applied Glu was recorded at +0.7 applied potential versus a Ag/AgCl reference electrode, optimal for Glu signals. (b) Locally applied Glu was recorded at +0.2 applied potential versus a Ag/AgCl reference electrode to asses for the presence of a dopamine signals.
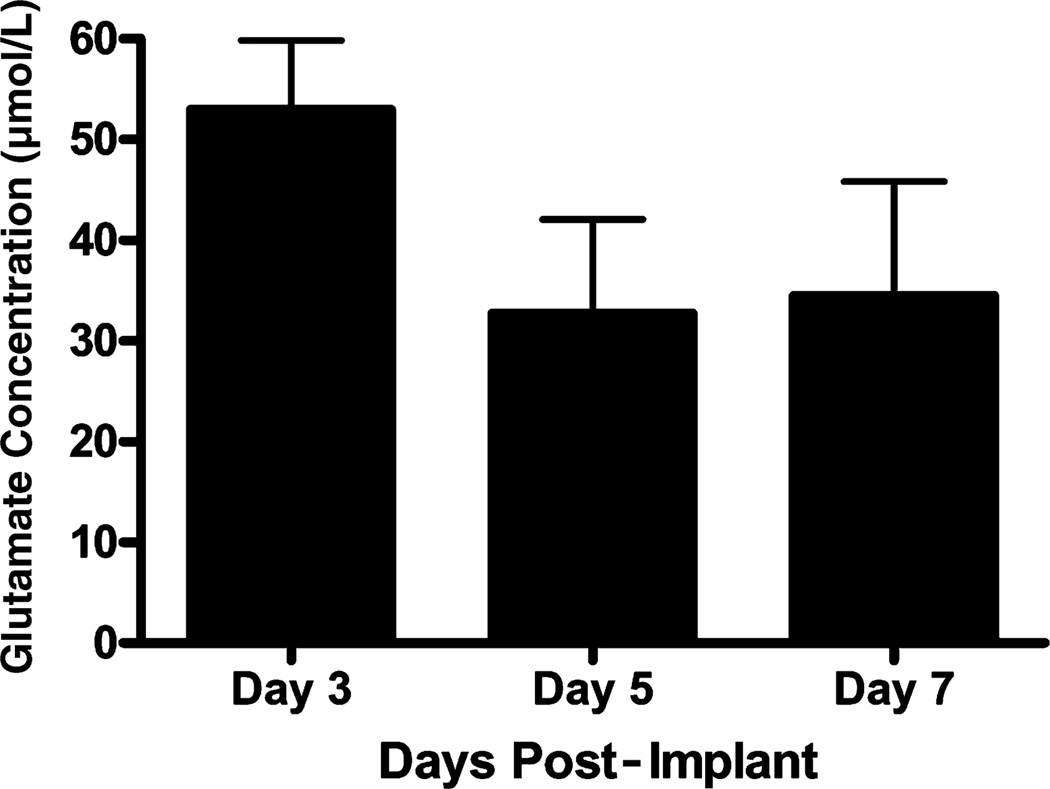
Fig. 5.
Locally applied Glu data through day 7 post-implant. Glu (5 mmol/L) was locally applied in the prefrontal cortex of Long Evans rats over days (n = 4) and recorded using a self-referencing microelectrode. Substantial and stable Glu recordings were observed on days 3, 5 and 7 post-implantation.
Another method for verifying Glu signals in vivo was performed by locally applying Glu (1 µL) while recording at applied potentials of either +0.7 Vor +0.2 Vversus a Ag/AgCl reference electrode. As can be seen in Fig. 4b, a sharp, robust peak was observed with locally applied Glu recorded at +0.7 V versus Ag/AgCl. However, when Glu was applied while the electrodes were polarized at +0.2 V versus Ag/AgCl, little to no change in signal was observed. Our prior studies have shown that H2O2 is oxidized primarily at +0.7 V versus Ag/AgCl at the Pt recording sites (Pomerleau et al. 2003). Thus, this approach demonstrated that the in vivo signal derived from Glu is converted to H2O2 by the enzyme layer.
Patency of chronically implanted MEAs in awake animals
Locally applied Glu recordings in the prefrontal cortex of Long Evans rats were reproducible in maximum amplitude from day 3 through day 7 post-implant (Fig. 5). Signals induced by locally applied Glu were also measured in the striatum of unanesthetized Long Evans rats. In the striatum, Glu signals were reproducible through day 7, as in the prefrontal cortex. However, the average maximum amplitudes of the Glu signals were smaller compared with the prefrontal cortex (data not shown), showing potential differential regulation of Glu in the rat prefrontal cortex versus rat striatum.
Tonic Glu measures (resting levels)
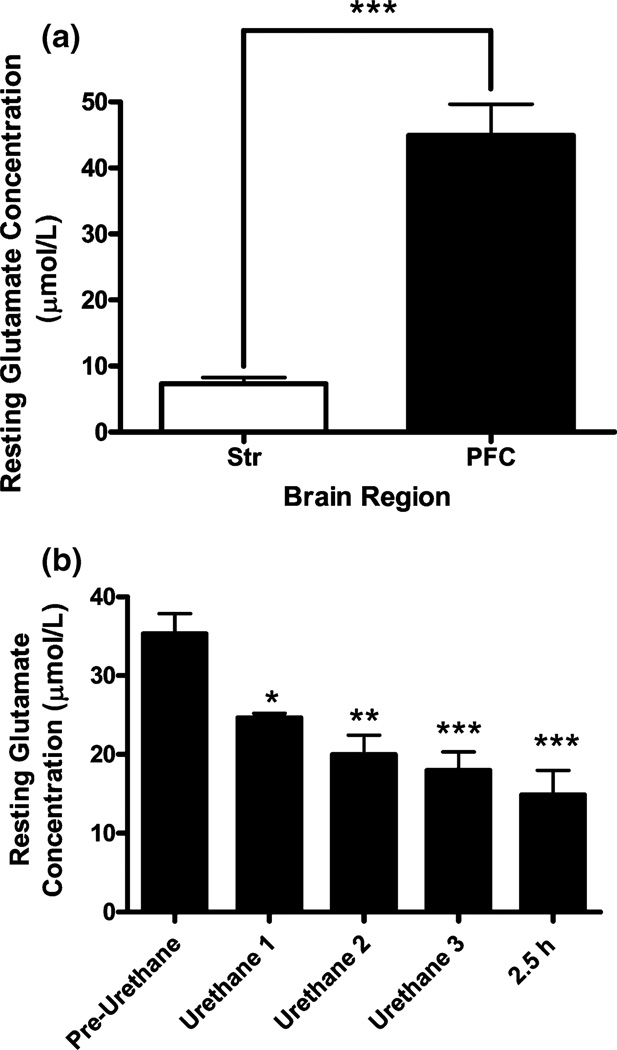
Resting Glu levels in awake rats were measured in the striatum and prefrontal cortex of Long Evans rats using the self-referencing technique described above. Briefly, the self-referencing site values were subtracted from the Glu recording site during unstimulated recordings. We observed a highly significant six-fold difference in resting levels of Glu in the prefrontal cortex (44.9 ± 4.7 µmol/L Glu) compared with resting measures in the striatum (7.3 ± 0.9 µmol/L Glu) (Fig. 6a) of the Long Evans rats.
Fig. 6.
(a) Resting Glu levels were assessed on day 3 post-implant in the striatum (Str) and prefrontal cortex (PFC) of Long Evans rats. Self-referencing microelectrode recordings were used to determine resting Glu levels. The self-referencing site signal was subtracted from the GluOx coated site signal to yield the resting Glu level. Average resting Glu levels were significantly different between the Str (7.3 ± 0.9 µmol/L; n = 3) and PFC (44.9 ± 4.7 µmol/L; n = 4) (mean ± SEM) (p < 0.001). (b) Effects of urethane on resting levels of Glu. Baseline was established and urethane (totaling 1.25 g/kg) was injected. Resting Glu levels were established prior to urethane injection (Pre-Urethane), 15 min following each of 3 urethane injections (Urethane 1, 2 and 3, respectively) and at 2.5 h following the initial (Urethane 1) injection (2.5 h). There was an approximate 20 µmol/L decrease in Glu signal (~58% decrease) over 2.5 h following urethane injections (n = 4).
Effects of urethane on resting Glu levels in awake rats
After verifying that our MEA recording system reliably measured Glu in chronically implanted and behaving rats over multiple days, we wanted to determine the effects of anesthesia (specifically urethane) on resting Glu levels in the brain. After verifying that the MEA was functioning properly by locally applying Glu on day 5 post-implantation, the rat was administered urethane (1.25 mg/kg, i.p.) as per our usual protocols for anesthetizing rats in our laboratory for acute in vivo amperometry measures (Nickell et al. 2005; Day et al. 2006). In this study, the resting Glu levels significantly decreased by 58% (resting measures of 35.3 ± 2.5 µmol/L dropped to 14.9 ± 3.1 µmol/L; p < 0.001) in the 2.5 h following the initial urethane injection (Fig. 6b).
Stress-induced Glu signals in awake rats
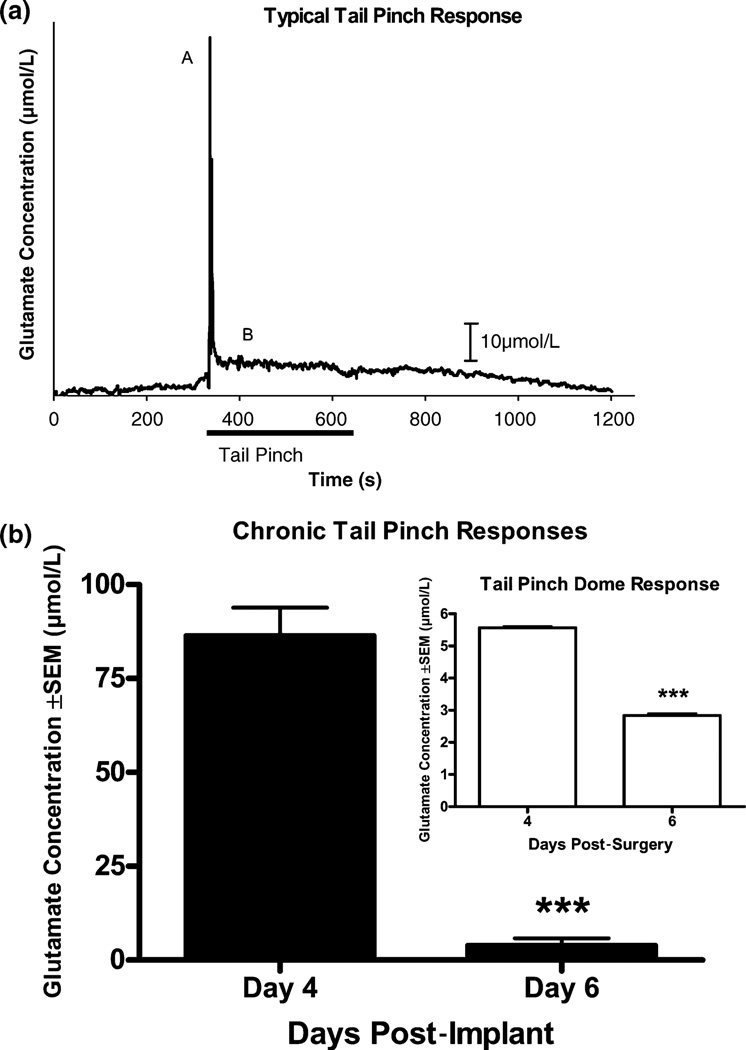
We wanted to determine if we could measure changes in Glu levels in a behaviorally relevant situation. Our laboratory and others are interested in stress and how it affects Glu levels in the striatum and prefrontal cortex. Glu tracings of a typical tail-pinch stress in the striatum of Fischer 344 rats can be seen in Fig. 7a. The Glu response due to a 5 min tail-pinch yielded a bimodal response. The initial phase was a rapid spike (Fig. 7a–A) lasting approximately 20 s. The second phase (Fig. 7a–B) of the response had a plateau-like appearance with a sustained increase in Glu levels compared with baseline. Susequent tail-pinches in the striatum of Fischer 344 rats resulted in a significantly decreased Glu response in both the spike (day 4 = 86.4 ± 7.5 µmol/L vs. day 6 = 3.8 ± 1.9 µmol/L) and plateau portions (day 4 = 5.6 ± 0.05 µmol/L vs. day 6 = 2.8 ± 0.007 µmol/L; p < 0.001) (Fig. 7b).
Fig. 7.
Effects of tail-pinch stress on extracellular levels of Glu in the striatum. Microelectrodes were implanted in the right hemisphere of the striatum. Stress was induced by a 5 min tail-pinch producing a bimodal Glu response with (a–A) an initial rapid large spike with totalduration approximately 20 s, followed by (a–B) a prolonged dome sustained the duration of the tail-pinch with a gradual return to basline. Glu challenge (locally applied Glu) was performed prior to stress to verify electrode response. (b) Graph depicting spike and plateau (inset) maximum Glu responses for the first and second tail-pinches, days 4 and 6, respectively. There was a significant difference in both the spike (day 4 = 86.4 ± 7.5 µmol/L vs. day 6 = 3.8 ± 1.9 µmol/L) and dome (day 4 = 5.6 ± 0.05 µmol/L vs. day 6 = 2.8 ± 0.007 µmol/L) (n = 3; p < 0.001) Glu responses between days 4 and 6.
Histology of the MEA implantation sites
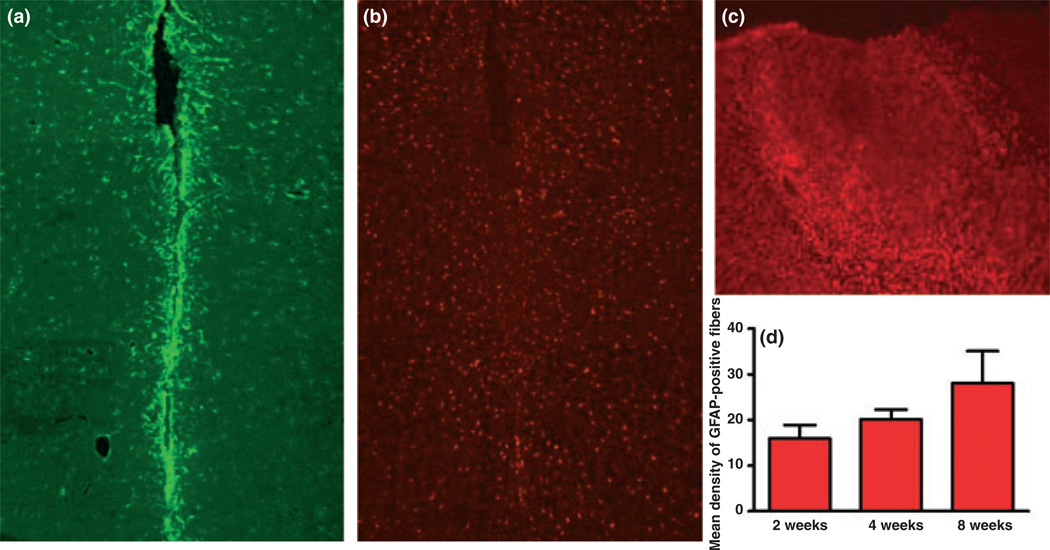
We investigated the histopathological effects from chronic MEA implantation. The three different implant durations (2, 4 and 8 weeks) yielded similar findings for GFAP (astrocytes) or Iba1 (microglia) staining. At close evaluation, both GFAP and Iba1 staining were seen to surround the MEA tracts, extending into tissue on average by 50–100 µm. No further staining was seen in adjacent areas to the MEA tracts (Figs 8a and b). There was a slight increase observed in number of astrocytes immediately surrounding the site of implant that increased as the implant duration increased. However, this increase was not significant and did not extend into the surrounding tissue (Fig. 8d). Furthermore, the number of microglia was not significantly different compared with control hemispheres. Fig. 8b shows microglia observed at the site of implantation. The degree of microglia activation that would be observed in an injury is shown in Fig. 8c. This was produced by a skull screw that was placed too deep into the tissue.
Fig. 8.
Histological evaluations of a chronic microelectrode arrays pedestal implantation in the right PFC of Long Evans rat. Rats were anesthetized with isoflurane and transcardially perfused after 2, 4 and 8 weeks with the implant. Brains were sectioned and stained with GFAP (red) and Iba1 (green). (a) GFAP staining at the microelectrode implant site (8 weeks). (b) Iba1 staining at microelectrode implant site (8 weeks). There was a slight increase in macroglia immediately surrounding implant site. However, very few of the microglia were rounded cells (activated). (c) Iba1 staining at showing screw site (penetrating the brain tissue; 8 weeks) depicting increased microglia levels at a true injury site. There was a drastic increase in microglia surrounding the showing screw penetration as compared with the rat hat microelectrode implant sites. (d) Graph depicting relative GFAP staining for weeks 2, 4 and 8 with implant. No difference in Iba1 staining was observed.
Discussion
This study demonstrates that chronic enzyme-based MEA recordings in the awake freely moving rat offer a robust method for monitoring Glu in vivo with minimal tissue damage at the site of implant. Current microdialysis methods are very limited in response time (routinely in minutes) and are only capable of recording over a single day per animal in the anesthetized or unanesthetized rat models (Shou et al. 2004). In our studies, we extended this recording capacity to 1 week routinely. Additionally, freely moving microdialysis studies yielded a high degree of damage to the brain at the site of implant with extended damage observed up to 1.4 mm from the site of implantation within 30 h (Clapp-Lilly et al. 1999; Borland et al. 2005). By contrast, our MEAs did not distrupt brain tissue more than 50–100 lm from the microelectrode tracts. The objective of this study was to adapt our current method for enzyme-based second-by-second Glu detection used in anesthetized rats or isolated preparations, to a system that can be used chronically in awake freely moving animals over a prolonged period of time. The small size and light weight of our design allowed for chronic recordings with limited affect on the rats’ behavior and ability to perform everyday activities.
The current ceramic-based MEA design used for acute recordings in anesthetized rats was successfully modified to produce a chronic implantation device that can reliably record Glu in the conscious, freely moving rat on a second-by-second basis. It is anticipated that the method will be useful for studying Glu levels associated with various behaviors. In this study, rats underwent successful chronic MEA implantations that yielded minimal damage to surrounding tissue and were reliable for measuring Glu up to 1-week post-implant and in some instances, up to 23 days while maintaining a high level of signal to noise (data not shown). This method has detection limits of 0.2 µmol/L and a response time of 500–800 ms, although data presented here were recorded at one second intervals. Additionally, pedestal MEA implants were cable of measuring H2O2 for up to 90 days in the awake, freely moving rat with no apparent adverse effects to the rat (data not shown). This suggests that as we improve upon our enzyme-based technology, through cross-linking and coating procedures, we will be able to increase the number of consecutive days we can record Glu in the unanesthetized animal. Furthermore, our H2O2 data show that the loss of ability to record Glu was due to an enzyme coating related function, not due to loss of MEA function. The ability to record H2O2 longer than it was possible to detect Glu suggests that the enzyme layer was either be degrading or the enzyme may be dysfunctional (Valdes and Moussy 2000) or detaching from the surface of the MEA. However, this must be further analyzed to determine the exact cause of the loss of enzyme function.
When using in vivo amperometry, identifying the molecule of interest is frequently questioned. First the ability of the enzyme-based MEA to differentially record for a specific molecule, in this case Glu, was due to the high selectivity of the enzyme (GluOx) for its substrate (Glu). Another way we addressed this issue is by varying the applied potential of the recordings. The reporter molecule, H2O2, resulting from the enzymatic breakdown of Glu by GluOx is oxidizable at predominately +0.7 V versus a Ag/AgCl reference electrode. However, as we have shown, when one drops the applied potential to +0.2 V versus a Ag/AgCl reference electrode, we lost the Glu signal as H2O2 was no longer oxidized at the surface of the MEA. To further show the ability of our enzyme-based MEA to detect Glu, we included an important and unique feature of our MEAs; the simultaneous self-referencing of the measured signal. By subtracting the signals obtained on the two adjacent sites (sentinel sites) that are not coated with GluOx from the signals obtained from the sites that measure Glu, we ascertained that the resulting signal was derived from Glu. All possible interferents detected by the Pt sites and/or noise are subtracted out of the signal. Thus self-referencing methods were employed throughout this study to ensure that resting levels and peak responses (maximum amplitude) were due primarily to changes in extracellular Glu levels.
The current self-referencing recording techniques with MEAs can be used to effectively and reliably measure resting (tonic) Glu levels. Our data show that there was a significant difference in resting Glu levels between the striatum (7.3 ± 0.9 µmol/L Glu) and prefrontal cortex (44.9 ± 4.7 µmol/L Glu). Previous studies using the same method for detecting Glu have reported that the resting Glu levels in the frontal cortex of the anesthetized rat were 1.6 ± 0.3 µmol/L and in the striatum they were 1.4 ± 0.2 µmol/L (Day et al. 2006), or approximately five- to 30-fold less compared with our freely moving recordings. The differences observed in resting Glu were not surprising. Studies using microdialysis reported resting Glu in the anesthetized rat striatum were approximately 1.3–2.7 µmol/L when sampling 20–24 s, which is comparable with our value of 1.4 µmol/L (Cellar et al. 2005). However, in an awake rat model using microdialysis measures in the striatum, resting Glu levels were reported to be 0.202–0.82 µmol/L when sampling every 15–20 min (Di Cara et al. 2001; Segovia et al. 2001). Additionally, 10–20 min microdialysis measures of resting Glu in the frontal cortex of the awake rat ranged between ~2 µmol/L and ~9 µmol/L (Boatell et al. 1995; Rocha et al. 1996). Resting Glu discrepancies using microdialysis may be explained by the sampling time and method, the extent of damage and the origin of the Glu pool being sampled. In addition, our faster sampling rates coupled with decreased tissue damage may contribute to the higher observed resting levels. Westerink’s group (Oldenziel et al. 2006a) has also reported on elevated resting glutamate levels (18.2 ± 9.3 µmol/L) in the striatum of anesthetized rats using an enzyme-based hydrogel-coated microsensor with an osmium-containing redox polymer. However, previous studies using redox hydrogel-coated enzyme-based Glu biosensors are limited by their response time of 8–10 s (Belay et al. 1998; Oldenziel et al. 2006a,b).
Current microdialysis studies report significant damage to brain tissue within 45 h within 1.4 mm of the area surrounding the probe implant site including altered mitochondria and endoplasmic reticulum. This supports an intracellular chemical disruption in the neuronal processes, in addition to an observed neuronal density loss up to 400 µm away from the microdialysis implant site (Clapp-Lilly et al. 1999). Additionally, Borland et al. (2005) observed disrupted dopamine release and uptake at least 220 µm remote from the microdialysis probe approximately 2 h following probe placement. However, histological studies performed to examine the effects of chronic implantation of our MEA showed minimal damage (50–100 µm) on surrounding brain tissue up to 8-weeks post-implant as measured by microglia and astrocyte markers.
We hypothesize that observed differences in resting levels of Glu between the awake and anesthetized animals using our enzyme-based MEAs were most likely due to an effect of the anesthetic (urethane) on resting Glu levels. We tested the effects of urethane on resting Glu by administering urethane to freely behaving rats using the same protocols used in our anesthetized studies. Our freely moving studies in the prefrontal cortex showed a 58% decrease in resting Glu levels following urethane dosing. The point at which there is a 58% decrease in Glu levels is the time point at which surgery would begin for typical anesthetized recordings. However, typical anesthetized recordings in our studies would occur following approximately 0.5–3 h additional time allowing for a deeper plane of surgical anesthesia. These data show that anesthetics affect resting Glu levels in the rat brain and contribute to poor estimates of resting Glu levels.
Our laboratory was interested in determining if we could measure behaviorally induced changes in extracellular Glu. To examine this, we performed a tail-pinch stress. Stress-induced Glu release that embodied rapid spike (~20 s) and prolonged plateau properties was recorded with chronically implanted MEAs in the striatum of Fischer 344 rats. These fast changes in Glu levels that occurred in the freely behaving rats due to stress would not be accurately detectable using other current in vivo techniques for measuring Glu. The prolonged plateau properties observed in the Glu signals due to stress is most likely the response observed in current freely moving microdialysis studies (Bagley and Moghaddam 1997). Our laboratory also observed a decrease in Glu spike and plateau responses due to tail-pinch stress with successive trials. The plateau portion of the response was similar to what has been observed in other laboratories previously using microdialysis (Bagley and Moghaddam 1997). Additionally, Moghaddam (2002) has reported that subsequent tail-pinch responses when performed on the same day show a decrease in response compared with the previous tail-pinch response, giving precedence to the decreases we observed in tail-pinch response over days. Taken together, these studies show that the improved response time of enzyme-based MEAs can be used for reliable second-by-second measures of Glu in the CNS of conscious freely moving rats for up to 17 days and recordings in unanesthetized animals are necessary to accurately determine the dynamics of glutamatergic function.
In Summary, the present studies using MEAs configured to record Glu on a second-by-second basis in the awake, freely behaving rat have helped us to begin to understand resting levels and behavior associated levels of Glu in the unanesthetized rat brain. We observed differences in resting Glu levels between the striatum and prefrontal cortex of awake rats. Furthermore, resting Glu levels in both brain areas were elevated in the awake rat compared with the anesthetized rat model and a 58% decrease in resting Glu was observed with urethane. We also found fast, robust changes in extracellular Glu due to a behavioral event, tail-pinch stress. Finally, histological studies showed that chronic implantation of our MEAs result in minimal damage to surrounding tissue up to 8-weeks post-implantation. These findings provide evidence that the high temporal and spatial resolution provided by our MEA technology are necessary for both resting and behavior-evoked Glu studies in awake rats.
Acknowledgements
Thanks to Kevin N. Hascup for his help with the photographs and schematics. This work was supported by DARPA N66001-02-C-8085, NSF DBI-0352848, USPHS grants and DA017186 and NIDA training grant T32DA16176.
Abbreviations used
- FAST-16
fast analytical sensing technology
- GFAP
glial fibrillary acidic protein
- Glu
l-glutamate
- MEA
microelectrode array
- PBS
phosphate buffered saline
- Pt
platinum
References
- Bagley J, Moghaddam B. Temporal dynamics of glutamate efflux in the prefrontal cortex and in the hippocampus following repeated stress: effects of pretreatment with saline or diazepam. Neuroscience. 1997;77:65–73. doi: 10.1016/s0306-4522(96)00435-6. [DOI] [PubMed] [Google Scholar]
- Baker DA, Xi ZX, Shen H, Swanson CJ, Kalivas PW. The orgin and neuronal function of in vivo nonsynaptic glutamate. J. Neurosci. 2002;22:9134–9141. doi: 10.1523/JNEUROSCI.22-20-09134.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belay A, Collins A, Ruzgas T, Kissinger PT, Gorton L, Csöregi E. Redox hydrogel based bienzyme electrode forl-glutamate monitoring. J. Pharmaceut. Biomed. Anal. 1998;19:93–105. doi: 10.1016/s0731-7085(98)00199-x. [DOI] [PubMed] [Google Scholar]
- Boatell ML, Bendahan G, Mahy N. Time-related cortical amino acid changes after basal forebrain lesion: a microdialysis study. J. Neurochem. 1995;64:285. doi: 10.1046/j.1471-4159.1995.64010285.x. [DOI] [PubMed] [Google Scholar]
- Borland LM, Guoyue S, Hua Y, Michael AC. Voltammetric study of extracellular dopamine near microdialysis probes acutely implanted in the striatum of anesthetized rat. J. Neurosci. Methods. 2005;146:149–158. doi: 10.1016/j.jneumeth.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Burmeister JJ, Gerhardt GA. Self-referencing ceramicbased multisite microelectrodes for the detection and elimination of interferences from the measurement of L-glutamate and other analytes. Anal.Chem. 2001;73:1037–1042. doi: 10.1021/ac0010429. [DOI] [PubMed] [Google Scholar]
- Burmeister JJ, Moxon K, Gerhardt GA. Ceramic-based multisite microelectrodes for electrochemical recordings. Anal. Chem. 2000;72:187–192. doi: 10.1021/ac9907991. [DOI] [PubMed] [Google Scholar]
- Burmeister JJ, Pomerleau F, Palmer M, Day BK, Huettl P, Gerhardt GA. Improved ceramic-based multisite microelectrode for rapid measurements of L-glutamate in the CNS. J. Neurosci. Methods. 2002;119:163–171. doi: 10.1016/s0165-0270(02)00172-3. [DOI] [PubMed] [Google Scholar]
- Cellar NA, Burns ST, Meiners JC, Chen H, Kennedy RT. Microfluidic chip for low-flow push-pull perfusion sampling in vivo with on-line analysis of amino acids. Anal. Chem. 2005;77:7067–7073. doi: 10.1021/ac0510033. [DOI] [PubMed] [Google Scholar]
- Clapp-Lilly KL, Roberts RC, Duffy LK, Irons KP, Hu Y, Drew KL. An ultrastructural analysis of tissue surrounding a microdialysis probe. J. Neurosci. Methods. 1999;90:129–142. doi: 10.1016/s0165-0270(99)00064-3. [DOI] [PubMed] [Google Scholar]
- Day BK, Pomerleau F, Burmeister JJ, Huettl PF, Gerhardt GA. Microelectrode array studies of basal and potassium evoked release of L-glutamate in the anesthetized rat brain. J. Neurochem. 2006;96:1626–1635. doi: 10.1111/j.1471-4159.2006.03673.x. [DOI] [PubMed] [Google Scholar]
- Di Cara B, Dusticier N, Forni C, Lievens JC, Daszuta A. Serotonin depletion produces long lasting increase in striatal glutamatergic transmission. J. Neurochem. 2001;78:240. doi: 10.1046/j.1471-4159.2001.00242.x. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick JS, Akopian G, Walsh JP. Short-term plasticity at inhibitory synapses in rat striatum and its effects on striatal output. J. Neurophysiol. 2001;85:2088–2099. doi: 10.1152/jn.2001.85.5.2088. [DOI] [PubMed] [Google Scholar]
- Gerhardt GA, Oke AF, Nagy G, Moghaddam B, Adams RN. Nafion-coated electrodes with high selectivity for CNS electrochemistry. Brain Res. 1984;290:390–395. doi: 10.1016/0006-8993(84)90963-6. [DOI] [PubMed] [Google Scholar]
- Kennedy RT, Watson CJ, Haskins WE, Powell DH, Strecker RE. In vivo neurochemical monitoring by microdialysis and capillary separations. Curr. Opin. Chem. Biol. 2002;6:659–665. doi: 10.1016/s1367-5931(02)00373-3. [DOI] [PubMed] [Google Scholar]
- Melendez RI, Vuthiganon J, Kalivas PW. Regulation of extracellular glutamate in the prefrontal cortex: focus on the cystine glutamate exchanger and group I metabotropic glutamate receptors. J. Pharm. Exp. Ther. 2005;314:139–147. doi: 10.1124/jpet.104.081521. [DOI] [PubMed] [Google Scholar]
- Moghaddam B. Stress activation of glutamate neurotransmission in the prefrontal cortex: implications for dopamine-associated psychiatric disorders. Soc. Biol. Psych. 2002;51:775–787. doi: 10.1016/s0006-3223(01)01362-2. [DOI] [PubMed] [Google Scholar]
- Nickell J, Pomerleau F, Allen J, Gerhardt GA. Agerelated changes in the dynamics of potassium-evoked L-glutamate release in the striatum of Fischer 344 rats. J.Neural Transm. 2005;112:87–96. doi: 10.1007/s00702-004-0151-x. [DOI] [PubMed] [Google Scholar]
- Oldenziel WH, Dijkstra G, Cremers TIFH, Westerink BHC. In vivo monitoring of extracellular glutamate in the brain with a microsensor. Brain Res. 2006a;1118:34–42. doi: 10.1016/j.brainres.2006.08.015. [DOI] [PubMed] [Google Scholar]
- Oldenziel WH, Dijkstra G, Cremers TIFH, Westerink BHC. Evaluation of hydrogel-coated glutamate microsensors. Anal. Chem. 2006b;78:3366–3378. doi: 10.1021/ac052146s. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain: Stereotaxic Coordinates. New York: Academic Press; 1998. [Google Scholar]
- Pomerleau F, Day BK, Huettl P, Burmeister JJ, Gerhardt GA. Real time in vivo measures of L-glutamate in the rat central nervous system using ceramic-based multisite microelectrode arrays. Ann. NY Acad. Sci. 2003;1003:454–457. doi: 10.1196/annals.1300.051. [DOI] [PubMed] [Google Scholar]
- Rocha L, Briones M, Ackermann RF, Anton B, Maidment NT, Evans CJ, Engel J., Jr Pentylenetetrazol-induced kindling: early involvement of excitatory and inhibitory systems. Epilepsy Res. 1996;26:105–113. doi: 10.1016/s0920-1211(96)00046-0. [DOI] [PubMed] [Google Scholar]
- Rossell S, Gonzalez LE, Hernandez L. One-second resolution brain microdialysis in fully awake rats Protocol for the collection, separation, and sorting of nanoliter dialsate volumes. J. Chromatogr. B. 2003;784:385–393. doi: 10.1016/s1570-0232(02)00826-7. [DOI] [PubMed] [Google Scholar]
- Segovia G, Del Arco A, Prieto L, Mora F. Glutamateglutamine cycle and aging in striatum of the awake rat: effects of a glutamate transporter blocker. Neurochem. Res. 2001;26:37–41. doi: 10.1023/a:1007624531077. [DOI] [PubMed] [Google Scholar]
- Shou M, Smith AD, Shackman JG, Peris J, Kennedy RT. In vivo monitoring of amino acids by microdialysis sampling with on-line derivatization by naphthalene-2,3-dicarboxyaldehyde and rapid micellar electrokinetic capillary chromatography. J. Neurosci. Methods. 2004;138:189–197. doi: 10.1016/j.jneumeth.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Timmerman W, Westerink BHC. Brain microdialysis of GABA and glutamate: what does it signify? Synapse. 1997;27:242–261. doi: 10.1002/(SICI)1098-2396(199711)27:3<242::AID-SYN9>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Tucci S, Rada P, Sepulveda MJ, Hernandez L. Glutamate measured by 6-s resolution brain microdialysis: capillary electrophoretic and laser-induced fluorescence detection application. J. Chromatogr. 1997;694:343–349. doi: 10.1016/s0378-4347(96)00488-4. [DOI] [PubMed] [Google Scholar]
- Valdes TI, Moussy F. In vitro and in vivo degradation of glucose oxidase enzyme used for implantable glucose biosensor. Diabetes Technol. Therapuet. 2000;2:367–376. doi: 10.1089/15209150050194233. [DOI] [PubMed] [Google Scholar]