Abstract
Recent functional connectivity magnetic resonance imaging and diffusion tensor imaging (DTI) studies have suggested atypical functional connectivity and reduced integrity of long-distance white matter fibers in autism spectrum disorder (ASD). However, evidence for short-distance white matter fibers is still limited, despite some speculation of potential sparing of local connectivity in ASD. Short-distance U-fibers are an important component of neural networks and are thought to play a crucial role in cognitive function. In the present study, we applied tract-based spatial statistics to derive short- and long-distance white matter fiber tracts in frontal, parietal, and temporal lobes in both hemispheres. DTI data were acquired from 26 children with ASD and 24 typically developing (TD) children. A mean fractional anisotropy (FA) image was created and thinned to represent centers of all common tracts. Evidence of compromised short-distance tracts for the ASD group was found in frontal lobe (reduced FA, increased mean diffusivity [MD] and radial diffusivity) as well as in temporal and parietal lobes (increased MD and radial diffusivity). Significant positive correlations between age and FA and negative correlations between age and MD and radial diffusivity were also found for short-distance tracts in each lobe in the TD, but not the ASD group. These results suggest white matter compromise in short-distance tracts in ASD. Absence of typical age-related correlations with DTI indices may reflect altered maturation of short-distance tracts in ASD. Our results are inconsistent with a notion of selective sparing of short-distance connectivity in ASD.
Keywords: Autism spectrum disorder, Diffusion tensor imaging, Brain connectivity, Local connectivity
1. Introduction
Autism spectrum disorder (ASD) is marked by deficits in social reciprocity and impaired communication (A.P.A., 2000). Despite extensive evidence of cognitive and behavioral abnormalities in ASD, the underlying brain impairments are not fully understood. However, there is growing consensus that ASD is not localized disorder, but one of distributed networks.
Diffusion tensor imaging (DTI) studies in ASD have shown microstructural abnormalities in corpus callosum (Alexander et al., 2007), arcuate fasciculus (Fletcher et al., 2010), inferior fronto-occipital and superior longitudinal fasciculi (Sahyoun et al., 2010), as well as limbic (Pugliese et al., 2009), hippocampo-fusiform, and amygdalo-fusiform pathways (Conturo et al., 2008), internal capsule (Keller et al., 2007), cerebellar peduncle (Brito et al., 2009), and corticospinal tract (Brito et al., 2009). One diffusion tensor tractography study of the frontal lobe showed reduced FA and increased mean diffusivity (MD) for short association fibers in both hemispheres, in addition to increased MD for long fibers, in children with ASD compared to typically developing (TD) children (Sundaram et al., 2008).
While most of the above studies have focused on long-distance tracts, evidence for short-distance white matter fibers in ASD thus remains limited. This is surprising in view of published speculation that aberrant long-distance connectivity in ASD may be accompanied by relatively intact local connectivity (Belmonte et al., 2004; Courchesne and Pierce, 2005; Casanova et al., 2002; Rubenstein and Merzenich, 2003). In the neurotypical brain, short-distance connections are crucial for the hierarchy from unimodal sensory to multimodal cognitive processing and for feedback connectivity back from multimodal to unimodal cortices (Mesulam, 1990). A recent functional connectivity MRI study further suggests distinct developmental patterns with intralobe short-distance connectivity being strongly developed in children, but long-distance tracts strengthening during adolescence and young adulthood (Dosenbach et al., 2010).
In the present study, we applied tract-based spatial statistics (Smith et al., 2006) to derive short- and long-distance white matter tracts in frontal, parietal and temporal lobes of both hemispheres. Based on evidence reviewed above, we hypothesized white matter compromise (reduced FA, increased MD and radial diffusivity) in long-distance tracts. While we expected such compromise also for short-distance tracts, we examined whether abnormalities for these might be less pronounced in ASD.
2. Methods
Twenty-six children with ASD (15 autism, 11 Asperger’s syndrome; 1 female) were compared with twenty-four TD (1 female) children. The two groups were matched for age (ASD: 12.6±0.6 [mean±sem], range 9–18 years; TD: 13.0±0.6, range 9–19 years), verbal IQ (ASD: 106.0±3.6, range 71–147; TD: 108.2±2.6, range 74–130) and nonverbal IQ (ASD: 109.1±3.3, range 69–140; TD: 110.3±2.5, range 85–129). Clinical diagnoses were confirmed by an expert clinical psychologist using the Autism Diagnostic Interview – Revised (ADI-R; Rutter et al., 2003) and the Autism Diagnostic Observation Schedule (ADOS; Lord et al., 2001). Children with associated medical conditions were excluded. TD children had no reported personal or family history of autism or any other neurological or psychiatric conditions. Independent-sample t-test confirmed that ASD and TD groups were matched on age, t(48)=0.3; p=.76; verbal IQ, t(48)=0.8; p=.38; and nonverbal IQ, t(48)=0.4; p=.71, as determined using the Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler, 1999). The research protocol was approved by the Institutional Review Boards of the San Diego State University and University of California, San Diego. Written informed assent and consent was obtained from all participants and their caregivers at the time of their visit.
MRI scans were performed on a 3T GE Signa Excite HD scanner (GE Healthcare, Milwaukee, WI), using a standard 8-channel head coil to acquire single-shot echoplanar diffusion weighted images (repetition time 10000ms, echo time 99.4ms, field-of-view 240mm, 128×128 matrix, in-plane resolution 1.875mm2, slice thickness 5mm [no gap], 27 axial slices). Two degrees of diffusion weighting (b=0 and 2000s/mm2) were used. Data were acquired in 15 non-linear directions with four repetitions. Head movement was minimized with foam pillows around participants’ heads.
DTI data were preprocessed using the diffusion toolbox in FSL (Smith et al., 2004). Distortions due to magnetic field inhomogeneities were corrected using field maps derived from the phase difference images obtained from two images with different echo times (Jezzard and Balaban, 1995). FA, MD and radial diffusivity images were created by fitting a tensor model to the raw diffusion data. FA images were aligned into a common space using the nonlinear registration tool FNIRT as part of tract-based spatial statistics (Smith et al., 2006) to create a mean FA skeleton, which represents the centers of all tracts common to both TD and ASD groups. Short-distance white matter tracts with a maximum length of 35mm adjacent to the cortical boundary were masked from the mean FA skeleton for frontal, temporal and parietal lobes in both hemispheres, using the Johns Hopkins University white matter tractography atlas (Wakana et al., 2004). These tracts include subcortical U-fibers (approximately 20mm in length) and fibers that conformed to the U-shape but were shorter (Supplementary Figure 1). Short tracts at a distance >4mm from the gray-white matter boundary in deep white matter were excluded from the analysis. Only very few short-distance tracts were identified in the occipital lobe, which were therefore excluded from the analysis. Tracts greater than 65mm in length that did not overlap with short-distance tracts were identified as long-distance tracts. Masks of short- and long-distance tracts were then superimposed on FA, MD and radial diffusivity maps of each participant to extract their mean values.
3. Results
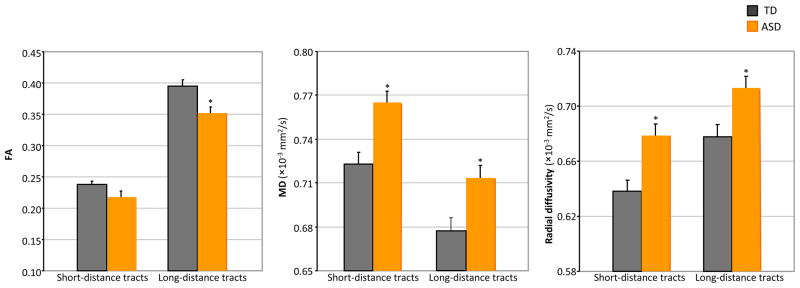
A mixed-model ANOVA with between subjects factor group (ASD, TD) and within subjects factor tract length (short, long) was conducted on FA, MD, and radial diffusivity for short- and long-distance tracts averaged across the whole brain (Figure 1). For all diffusion indices there were significant main effects of tract length and group (ps <.05). The interaction between tract length and group addressed the critical factor of short-distance sparing relative to abnormal long-distance connectivity. The interactions between tract length and group were not significant for MD, F(1, 48) = 1.3, p > .2, and radial diffusivity, F(1, 48) = .6, p > .4; however, there was a significant interaction between tract length and group for FA, F(1, 48) = 8.0, p < .01. Subsequent T-tests showed that while FA of long-distance tracts was significantly lower in the ASD group compared to the TD group, t(48) = 2.9, p < .01, the difference was only marginally significant for FA of short-distance tracts between ASD and TD groups, t(48) = 1.9, p = .06. No significant differences between ASD subgroups (autism versus Asperger’s Syndrome) were found for short- or long-distance tracts (p>.6).
Figure 1.
Whole brain FA, MD and radial diffusivity for short-distance and long-distance tracts by group (*, p<.05).
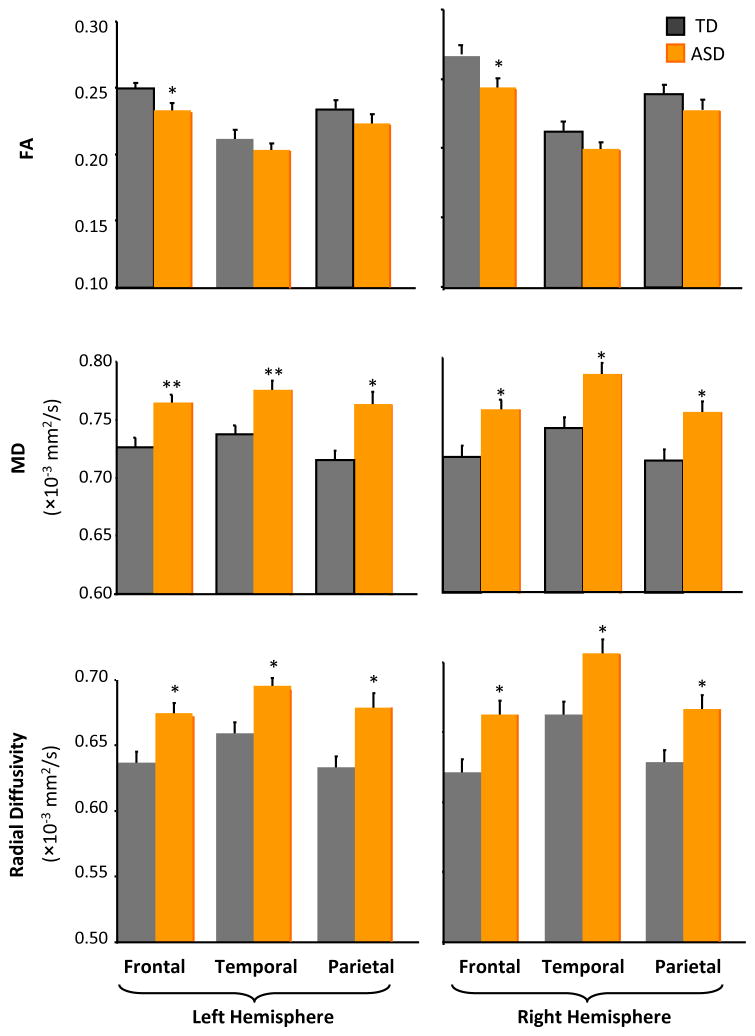
Next, to examine lobe-specific differences of white matter integrity for short-distance tracts, a mixed-model ANOVA with between subjects factor group and within subjects factors hemisphere (left, right) and lobe (frontal, temporal, parietal) was conducted on diffusion parameters for short-distance tracts (Figure 2). For FA, there were significant main effects of hemisphere and lobe and a significant hemisphere by lobe interaction. There was no significant main effect of group, nor were there any group interaction effects. T-tests for each lobe revealed reduced FA in left and right frontal lobes in the ASD group. For MD, there were significant main effects of group and lobe and a significant interaction between hemisphere and lobe. Group did not significantly interact with any other factor. Significantly increased MD was found in frontal, temporal, and parietal lobes for both left and right hemispheres in the ASD group. For radial diffusivity, there were significant main effects of group, hemisphere, and lobe, and a significant interaction between hemisphere and lobe; group did not significantly interact with any other factor. Significantly increased radial diffusivity was also found in frontal, parietal and temporal lobes for both left and right hemispheres in the ASD group. There was no significant difference between diagnostic subtypes (autism vs. Asperger’s Syndrome) within the ASD group for lobe-specific short-distance tracts (p>.5).
Figure 2.
FA, MD and radial diffusivity in short-distance white matter tracts by group, lobe (frontal, temporal, parietal lobes), and hemisphere (*, p<.05; **, p<.005).
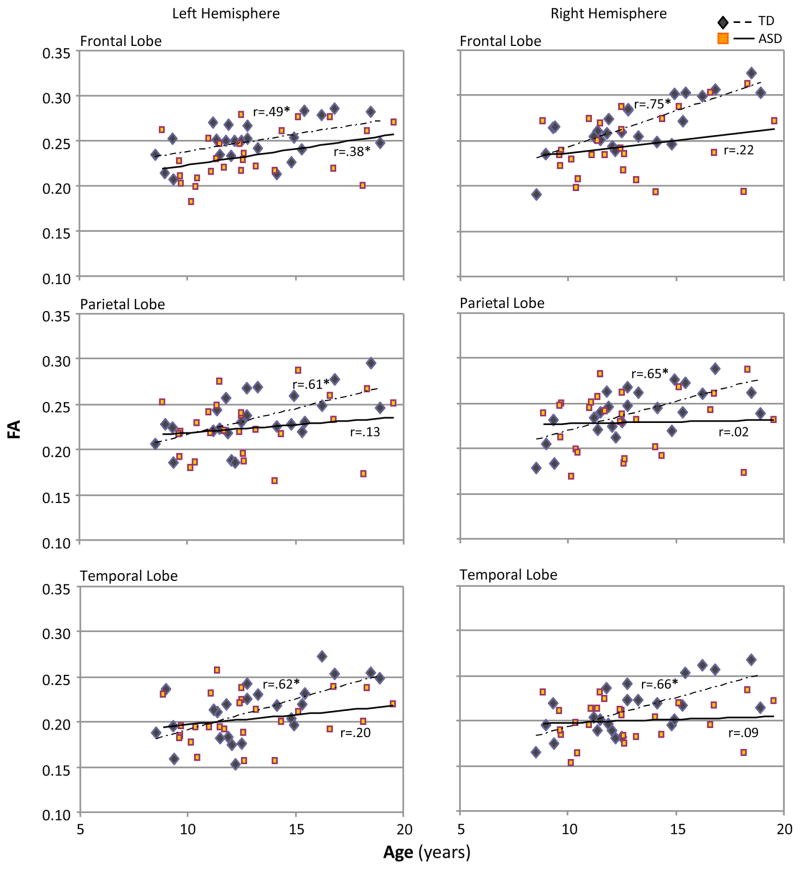
Pearson's correlation analyses showed significant positive correlation between age and FA and negative correlation between age and MD and radial diffusivity for short-distance tracts in each lobe in the TD group, whereas in the ASD group correlations between age and diffusion indices were moderate or absent and reached significance only in left frontal lobe (Figure 3). No correlations between DTI results and neuropsychological measures were found (p>.3).
Figure 3.
Pearson correlations between age and FA for short-distance white matter tracts in left and right frontal, temporal and parietal lobes (*, p<.05).
4. Discussion
The present study expands on previous DTI research in ASD, which has mostly focused on long-distance tracts, to examine potential white matter compromise in short-distance tracts. Such tracts, also called U-fibers, provide cascading connections from primary unimodal to sensory association and multimodal areas and are thus vital for linking sensorimotor and higher cognitive brain functions (Nieuwenhuys et al., 1988; Mesulam, 1990). We found evidence of white matter compromise for short-distance tracts in children and adolescents with ASD, including significantly increased MD and radial diffusivity in frontal, temporal, and parietal lobes of both hemispheres. FA was also reduced in bilateral frontal lobes, while FA differences for temporal and parietal lobes were not significant. This in combination with the finding of a significant group by tract length interaction for FA leaves open the possibility of a subtly different profile of compromise for short versus long-distance tracts. Correlations with age, positive for FA and negative for MD and radial diffusivity, were consistent for frontal, temporal, and parietal lobes in the TD group, whereas they were absent in the ASD group for temporal and parietal lobes.
Volumetric MRI studies in ASD have found white matter abnormalities in frontal (Herbert et al., 2004), parietal (Courchesne et al., 1993), and temporal (Lee et al., 2007) lobes. Our findings complement these earlier results and indicate microstructural abnormalities of short-distance tracts, which may be due to reduced myelination or axonal density, or aberrant fiber organization in children with ASD. Together with a previous report of reduced FA and increased diffusivity of short association fibers in the frontal lobe in ASD (Sundaram et al., 2008), our results provide evidence supporting our expectation of anomalous short-distance circuitry in ASD.
Postmortem studies have reported increased density of cortical minicolumns (Casanova et al., 2002) and poor differentiation of the gray-white matter boundary (Bailey et al., 1998). It has been suggested that the lack of local inhibitory interneurons may cause an imbalanced excitation/inhibition ratio and impaired local functional differentiation in ASD (Rubenstein and Merzenich, 2003). This has led to speculations about potentially spared or enhanced local connectivity in ASD (Courchesne and Pierce, 2005; Belmonte et al., 2004). However, note that some of the supporting evidence, in particular related to minicolumnar abnormalities, relates to a more microscopic level of organization compared to our investigation of short-distance tracts.
Our results may be compared to previous reports from other types of studies related to local connectivity in ASD. Using magnetoencephalography, Wilson et al. (2007) observed reduced gamma band power of auditory magnetic steady-state responses, which are considered important for coordinating local cortical activity, in children and adolescents with autism. Tommerdahl et al. (2008) reported absence of stimulus-driven synchronization effects on sensory perception in autism, suggesting local underconnectivity. Our findings could also be related to recent studies in children with ASD showing atypical regional homogeneity of functional MRI time series, which may be an indirect measure of local excitatory functional connectivity (Shukla et al., 2010; Paakki et al., 2010).
One potential limitation in our DTI data relates to spatial resolution, with voxel volumes of 17.6μl. While such resolution is comparable to published DTI studies of ASD, it is expected to affect FA values in regions containing crossing fibers and may have also resulted in subtle partial volume effects specifically in short-distance tracts that were in closer vicinity to the gray-white boundary than long-distance tracts. However, any potential partial volume effects would have been unlikely to affect ASD and TD groups differentially. Furthermore, acquisition of only 15 non-linear diffusion directions in the present study, although comparable to previous ROI studies, was not optimal for detailed information about fiber orientations and restricted diffusion. Future studies with higher angular resolution will be able to better delineate white matter tracts and will provide more insight into their anomalies in ASD.
The present investigation is one of the first ASD studies to examine short-distance connectivity. Our findings suggest that short-distance tracts in frontal, temporal, and parietal lobes are not selectively spared in ASD. Short-distance tracts lie tangentially at the junction of gray and white matter facilitating connections between neighboring cortical areas. Therefore, white matter compromise observed in short-distance tracts may relate to disturbances in functional segregation in ASD. Furthermore, reductions of age-related correlations with DTI indices in ASD may reflect altered maturation of short-distance tracts.
Supplementary Material
Short-distance (red) and long-distance tracts (blue) obtained from the mean FA skeleton (green), overlaid on the standard MNI template.
Research Highlights.
Evidence of white matter compromise in ASD is currently limited for short-distance tracts.
We provide quantitative estimation of DTI indices for short and long-distance tracts in ASD.
Anomalies for short-distance tracts in frontal, parietal, and temporal lobes in ASD were found.
Maturational changes in short-distance fibers seen in TD children were reduced or absent in ASD.
Findings do not support the idea of selective sparing of short-distance connectivity in ASD.
Acknowledgments
This study was supported by the National Institutes of Health, R01-DC006155 and R01-MH081023, with additional funding from NIDCD 1T32 DC007361-03 (author BK). Special thanks to the children and families who participated.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- APA. Diagnostic and Statistical Manual of Mental Disorders. 4. American Psychiatry Association; Washington, DC: 2000. (DSM-IV-TR) [Google Scholar]
- Alexander AL, Lee JE, Lazar M, Boudos R, DuBray MB, Oakes TR, et al. Diffusion tensor imaging of the corpus callosum in Autism. Neuroimage. 2007;34:61–73. doi: 10.1016/j.neuroimage.2006.08.032. [DOI] [PubMed] [Google Scholar]
- Bailey A, Luthert P, Dean A, Harding B, Janota I, Montgomery M, et al. A clinicopathological study of autism. Brain. 1998;121 ( Pt 5):889–905. doi: 10.1093/brain/121.5.889. [DOI] [PubMed] [Google Scholar]
- Belmonte MK, Allen G, Beckel-Mitchener A, Boulanger LM, Carper RA, Webb SJ. Autism and abnormal development of brain connectivity. J Neurosci. 2004;24:9228–9231. doi: 10.1523/JNEUROSCI.3340-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito AR, Vasconcelos MM, Domingues RC, Hygino da Cruz LC, Jr, Rodrigues LS, Gasparetto EL, et al. Diffusion tensor imaging findings in school-aged autistic children. J Neuroimaging. 2009;19:337–343. doi: 10.1111/j.1552-6569.2009.00366.x. [DOI] [PubMed] [Google Scholar]
- Casanova MF, Buxhoeveden DP, Switala AE, Roy E. Minicolumnar pathology in autism. Neurology. 2002;58:428–432. doi: 10.1212/wnl.58.3.428. [DOI] [PubMed] [Google Scholar]
- Conturo TE, Williams DL, Smith CD, Gultepe E, Akbudak E, Minshew NJ. Neuronal fiber pathway abnormalities in autism: an initial MRI diffusion tensor tracking study of hippocampo-fusiform and amygdalo-fusiform pathways. J Int Neuropsychol Soc. 2008;14:933–946. doi: 10.1017/S1355617708081381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E, Pierce K. Why the frontal cortex in autism might be talking only to itself: local over-connectivity but long-distance disconnection. Curr Opin Neurobiol. 2005;15:225–230. doi: 10.1016/j.conb.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Press GA, Yeung-Courchesne R. Parietal lobe abnormalities detected with MR in patients with infantile autism. AJR Am J Roentgenol. 1993;160:387–393. doi: 10.2214/ajr.160.2.8424359. [DOI] [PubMed] [Google Scholar]
- Dosenbach NU, Nardos B, Cohen AL, Fair DA, Power JD, Church JA, et al. Prediction of individual brain maturity using fMRI. Science. 2010;329:1358–1361. doi: 10.1126/science.1194144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher PT, Whitaker RT, Tao R, DuBray MB, Froehlich A, Ravichandran C, et al. Microstructural connectivity of the arcuate fasciculus in adolescents with high-functioning autism. Neuroimage. 2010;51:1117–1125. doi: 10.1016/j.neuroimage.2010.01.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert MR, Ziegler DA, Makris N, Filipek PA, Kemper TL, Normandin JJ, et al. Localization of white matter volume increase in autism and developmental language disorder. Ann Neurol. 2004;55:530–540. doi: 10.1002/ana.20032. [DOI] [PubMed] [Google Scholar]
- Jezzard P, Balaban RS. Correction for geometric distortion in echo planar images from B0 field variations. Magn Reson Med. 1995;34:65–73. doi: 10.1002/mrm.1910340111. [DOI] [PubMed] [Google Scholar]
- Keller TA, Kana RK, Just MA. A developmental study of the structural integrity of white matter in autism. Neuroreport. 2007;18:23–27. doi: 10.1097/01.wnr.0000239965.21685.99. [DOI] [PubMed] [Google Scholar]
- Lee JE, Bigler ED, Alexander AL, Lazar M, DuBray MB, Chung MK, et al. Diffusion tensor imaging of white matter in the superior temporal gyrus and temporal stem in autism. Neurosci Lett. 2007;424:127–132. doi: 10.1016/j.neulet.2007.07.042. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore P, Risi S. Autism Diagnostic Observation Schedule. Western Psychological Services; Los Angeles, CA: 2001. [Google Scholar]
- Mesulam MM. Large-scale neurocognitive networks and distributed processing for attention, language, and memory. Ann Neurol. 1990;28:597–613. doi: 10.1002/ana.410280502. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuys R, Voogd J, van Huijzen C. The Human Central Nervous System. Springer-Verlag; Berlin: 1988. [Google Scholar]
- Paakki JJ, Rahko J, Long X, Moilanen I, Tervonen O, Nikkinen J, et al. Alterations in regional homogeneity of resting-state brain activity in autism spectrum disorders. Brain Res. 2010;1321:169–179. doi: 10.1016/j.brainres.2009.12.081. [DOI] [PubMed] [Google Scholar]
- Pugliese L, Catani M, Ameis S, Dell'Acqua F, Thiebaut de SM, Murphy C, et al. The anatomy of extended limbic pathways in Asperger syndrome: a preliminary diffusion tensor imaging tractography study. Neuroimage. 2009;47:427–434. doi: 10.1016/j.neuroimage.2009.05.014. [DOI] [PubMed] [Google Scholar]
- Rubenstein JL, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003;2:255–267. doi: 10.1034/j.1601-183x.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M, Le Couteur A, Lord . Autism Diagnostic Interview - Revised. Western Psychological Services; Los Angeles, CA: 2003. [Google Scholar]
- Sahyoun CP, Belliveau JW, Mody M. White matter integrity and pictorial reasoning in high-functioning children with autism. Brain Cogn. 2010;73:180–188. doi: 10.1016/j.bandc.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla DK, Keehn B, Müller RA. Regional homogeneity of fMRI time series in autism spectrum disorders. Neurosci Lett. 2010;476:46–51. doi: 10.1016/j.neulet.2010.03.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Sundaram SK, Kumar A, Makki MI, Behen ME, Chugani HT, Chugani DC. Diffusion tensor imaging of frontal lobe in autism spectrum disorder. Cereb Cortex. 2008;18:2659–2665. doi: 10.1093/cercor/bhn031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tommerdahl M, Tannan V, Holden JK, Baranek GT. Absence of stimulus-driven synchronization effects on sensory perception in autism: Evidence for local underconnectivity? Behav Brain Funct. 2008;4:19. doi: 10.1186/1744-9081-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakana S, Jiang H, Nagae-Poetscher LM, van Zijl PC, Mori S. Fiber tract-based atlas of human white matter anatomy. Radiology. 2004;230:77–87. doi: 10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
- Wilson TW, Rojas DC, Reite ML, Teale PD, Rogers SJ. Children and adolescents with autism exhibit reduced MEG steady-state gamma responses. Biol Psychiatry. 2007;62:192–197. doi: 10.1016/j.biopsych.2006.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Short-distance (red) and long-distance tracts (blue) obtained from the mean FA skeleton (green), overlaid on the standard MNI template.