Abstract
All nervous systems are subject to neuromodulation. Neuromodulators can be delivered as local hormones, as cotransmitters in projection neurons, and through the general circulation. Because neuromodulators can transform the intrinsic firing properties of circuit neurons and alter effective synaptic strength, neuromodulatory substances reconfigure neuronal circuits, often massively altering their output. Thus, the anatomical connectome provides a minimal structure and the neuromodulatory environment constructs and specifies the functional circuits that give rise to behavior.
Introduction
Neuromodulation adds extraordinary richness to the dynamics that networks can display. It also adds confounds of many kinds that require that we relinquish our wish for simple and linear answers to how brain circuits work. In this review, my goal is to summarize many of the take-home lessons from old and new work on neuromodulation that can inform the trajectory of future work on circuits, large and small.
Historians say that we should study history to avoid repeating the mistakes of the past. Remarkable advances in anatomical methods, genetics, optogenetics and optical recordings are providing extraordinary opportunities for understanding circuit structure and function in brains, large and small. The present era of circuit exploration is tremendously exciting. At the same time, I see numerous examples of today’s researchers effectively “reinventing the wheel”, albeit elegantly enough for publication in our elite journals, partially because the new work is done with state-of-the art techniques, and partially because the pioneering work on modulation and dynamics of small circuits has been partially obscured by the mists of time. Those interested in how circuit dynamics arise from the properties of neurons and their connections should read Getting ’s prescient 1989 review (Getting, 1989).
Studies of some of the substances that we now term neuromodulators have a long and venerable history. The pharmacologists who worked 80 and 100 years ago already knew that there were multiple receptors for acetylcholine and norephinephrine (Dale, 1935), and that these were pharmacologically separable. By the early ‘70’s it was already clear that different classes of neurons released different neurotransmitters (Barker et al., 1972; Carraway and Leeman, 1973; Chang and Leeman, 1970; Kerkut and Cottrell, 1963; Kerkut and Walker, 1966; Otsuka et al., 1967; Walker et al., 1968), and that there were a large number of signaling molecules used in the brains of all animals including ACh, dopamine, norepinephrine, GABA, glycine, glutamate, serotonin, histamine, octopamine, and neuropeptides.
Although the diversity of signaling molecules was fascinating neurochemists of the day, many of the earliest workers interested in the neuronal circuits that gave rise to behavior saw no relevance of what they called “pharmacology” or “neurochemistry”. Instead, many of the early circuit electrophysiologists came from the traditions of engineering and electronics, and sought to develop a connectivity diagram (or connectome in today’s parlance) that would be the biological equivalent of an electronic circuit diagram, taking advantage of the identifiable neurons in invertebrate sensory and motor circuits (Burrows, 1975a, b; Calabrese and Peterson, 1983; Getting, 1981; Heitler and Burrows, 1977; Kristan and Calabrese, 1976; Kristan et al., 1974; Mulloney and Selverston, 1974a, b; Stent et al., 1978; Stent et al., 1979; Willows et al., 1973; Wilson, 1961; Wilson, 1966).
I was once told by one of the leaders in the field that the neurotransmitter that mediated a synaptic connection was irrelevant, and the only thing that mattered was the sign of the synapse, excitatory or inhibitory. Although today’s anatomists must know that neuromodulatory neurons can release their cotransmitters at a distance from their targets (Blitz et al., 2008; Brezina, 2010; Jan and Jan, 1982), the underlying assumption of today’s electron microscope connectome projects (Briggman et al., 2011; Chklovskii et al., 2010; Denk et al., 2012; Lichtman and Denk, 2011; Seung, 2011) is that the conventional close-apposition synapses provide most, if not all, of the information needed to characterize the circuit, the same assumption that was made 35 years ago the small-circuit physiologists.
The Early Era of Neuromodulation
In their preface, Kaczmarek and Levitan (1987) wrote that their book, Neuromodulation: The Biochemical Control of Neuronal Excitability, was intended to create a working understanding between electrophysiologists and biophysicists on one hand and neurochemists on the other hand, to understand the modulation of neuronal excitability and its consequences for neural processing. By 1987 it was clear that:
Neuronal intrinsic properties, action potential waveforms and membrane currents could be altered by manipulating the intracellular concentrations of second messengers such as cAMP (DeRiemer et al., 1985; Hockberger and Connor, 1984; Kaczmarek et al., 1986; Levitan, 1978; Siegelbaum et al., 1982).
Exogenous application of muscarinic agonists, amines and neuropeptides can increase or decrease the amplitude of a variety of voltage-dependent currents (Adams and Brown, 1980; Brown and Adams, 1980; Camardo et al., 1983; Dunlap and Fischbach, 1981).
Exogenous application of neuromodulators could alter the strength of synapses (Dudel, 1965; Glusman and Kravitz, 1982; Klein et al., 1982; Klein and Kandel, 1978), with implications for experience-dependent changes in behavior (Kandel and Schwartz, 1982).
Neuromodulation was part of a paradigm shift in the study of small circuits-the first “beyond the connectome realization”
By the end of the 1980’s there was an almost complete paradigm shift in the study of small circuits for six reasons:
It saw the end of the hope that similar motor patterns found in different species would be generated by similar circuits (Getting, 1989). By this time, enough was known about the specifics of rhythmic pattern generation in different animals to show that the details of each circuit was different, but there were certain canonical principles, or “building blocks” across preparations (Getting, 1989).
It brought the realization that it was going to be extremely difficult to obtain data sufficient to constrain detailed models of all but the simplest circuits (Selverston, 1980). This remains one of the most thorny problems in understanding biological circuits today. Because the output of all biological circuits results from the interaction of many non-linear elements, computational models are needed to understand them. How realistic do these models need to be, and what data are needed to constrain these models? How will modulation alter these processes?
It gave us the beginnings of the cellular mechanisms underlying neuromodulation of excitability (DeRiemer et al., 1985; Dunlap and Fischbach, 1981; Kaczmarek et al., 1986; Levitan et al., 1979).
It was the beginning of the understanding that neuronal dynamics and neuromodulatory mechanisms reconfigure circuits so that they could no longer be viewed as “hard-wired” (Eisen and Marder, 1984; Getting, 1989; Marder, 1984; Marder and Hooper, 1985), but capable of variable outputs under modulator control.
It brought the realization that circulating hormones and local neurohormones could alter behavior by acting at every level from sensory neuron (Pasztor and Bush, 1987), to central circuits (Harris-Warrick and Kravitz, 1984; Hooper and Marder, 1984; Marder and Hooper, 1985), to neuromuscular junctions and muscles (Lingle, 1981; Schwarz et al., 1980). This raised the possibility that the same modulator could act at different sites within a circuit to keep outputs coordinated, or that different modulators could compensate for changes at one site with changes elsewhere, or that modulators can effectively change the gain of one portion of a circuit or process without altering others (Brezina, 2010).
It demonstrated the prevalence of cotransmission in neurons of all kinds, including diffuse modulatory projection neurons that can liberate their transmitter at some distance from receptors (Adams and O’Shea, 1983; Bishop et al., 1987; Jan and Jan, 1982; Kupfermann, 1991; Nusbaum and Marder, 1989a; Siwicki et al., 1987).
Diffuse projections, hormones, and local hormones determine the modulatory tone of the brain
One of the most remarkable features of biological systems is that they are endlessly adaptable while usually maintaining their functional integrity. Moreover, many brain disorders, such as schizophrenia, depression, and epilepsy, are likely associated with some degree of dysfunction in modulatory control systems. Many of the other contributions in this issue will deal with the modulation of disparate regions of the vertebrate brain by the diffuse aminergic projections, local interneurons with peptide cotransmitters, and peptidergic systems that are important for pain regulation and other physiological processes. In their outstanding review in this issue, Taghert and Nitabach (2012) describe much of the wonderful recent work in flies and worms describing the roles of neuropeptides in specific behaviors. Consequently, in this review I will focus on “take-home messages” that have come from the study of neuromodulation primarily using crustacean and molluscan systems, and I draw heavily on specific examples from the crustacean stomatogastric nervous system.
Intrinsic versus Extrinsic Modulation
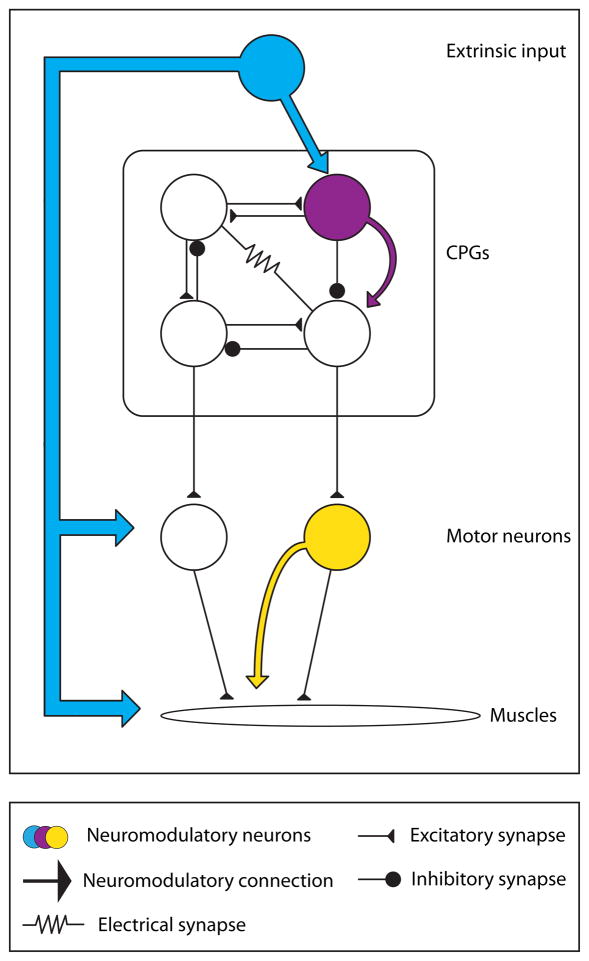
It can be useful to distinguish between neuromodulation that is intrinsic to the system or circuit being considered, and modulation that is delivered from an extrinsic source (Cropper et al., 1987; Katz, 1995; Katz and Frost, 1996; Morgan et al., 2000). In the former case, the modulatory substance is released by one of the circuit components, while in the latter case the modulatory substance is released from a source not directly part of the circuit at hand (Fig. 1). In the simplest case, a neuron that releases a cotransmitters that alters the excitability of its postysynaptic targets is intrinsic (Cropper et al., 1987; Katz and Frost, 1995a, b; Weiss et al., 1992; Weiss et al., 1978), while a neurohormone that is liberated by a neurosecretory structure and travels through the circulation is unambiguously extrinsic (Christie et al., 1995). While at some level this is an artificial distinction, it points out neurons can alter the configuration of the networks with which they are active in complex and rich ways (Katz and Frost, 1995a, b). Moreover, if the cotransmitters liberated from the same neuron are differentially released as a function of the dynamics of presynaptic activity (Brezina et al., 2000a; Karhunen et al., 2001; Peng and Horn, 1991; Peng and Zucker, 1993), this can alter the extent to which these substances influence postsynaptic function under different conditions.
Figure 1.
Intrinsic and Extrinsic Modulation. Extrinsic modulation comes from outside the circuit or modulated target. Intrinsic modulation refers to neurons that are part of a circuit and release modulators that can alter the properties of other circuit elements. Drawing loosely after Katz and Frost (Katz and Frost, 1996).
Circuits are multiply modulated
Neuromodulation of circuit function has been studied for more than 40 years in crustaceans and mollusks. The crustacean stomatogastric ganglion (STG) contains ~30 neurons and the crustacean cardiac ganglion contains only 9 neurons. Both are central pattern generating circuits that generate fictive motor patterns when removed from the animal, and both are modulated by a large number of different substances (Blitz and Nusbaum, 2011; Cruz-Bermudez and Marder, 2007; Johnson et al., 2011; Marder and Bucher, 2007; Stein, 2009; Wiwatpanit et al., 2012).
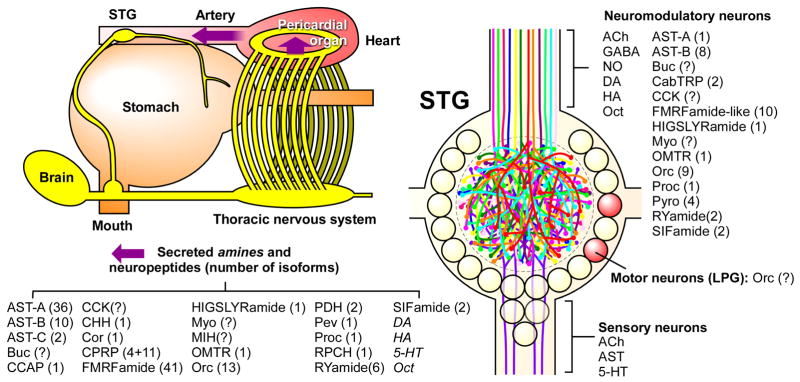
Figure 2 summarizes a partial list of what is known about the neuromodulatory control of the crab STG. These data were accumulated over the years by many laboratories using a combination of immunocytochemistry and biochemical techniques. Most recently, mass spectrometry has allowed the identification and characterization of many individual members of a number of different peptide families (Dickinson et al., 2009; Ma et al., 2009a; Ma et al., 2009b; Ma et al., 2009c; Stemmler et al., 2010). Many of the same substances are released both by descending modulatory neurons and by neurosecretory structures as hormone.
Figure 2.
Partial Summary of Neuromodulation of the crab stomatogastric ganglion (STG). The STG sits anterior to the heart within an artery that brings modulatory amines and peptides from neurosecretory structures such as the pericardial organs (bottom list). 25 pairs of descending modulatory neurons bring a host of substances into the neuropil of the STG (right). The number of family members of the neuropeptides are shown in parentheses. Figure was made by D. Bucher, summarizing work from the Li and Stemmler labs and numerous collaborators.
It is unlikely that the STG is unusual in the number of its modulatory inputs. A large number of neuromodulators are known to have important functions in the Aplysia feeding circuits (Brezina and Weiss, 1997; Furukawa et al., 2003; Koh and Weiss, 2007; Li et al., 2001; Proekt et al., 2005; Sweedler et al., 2002; Vilim et al., 2010; Wu et al., 2010), another system in which the search for modulators has been intense. And certainly, the number of important peptide modulators known in C. elegans and Drosophila is also large (Bargmann, 2012; Taghert and Nitabach, 2012). In contrast, there are relatively few vertebrate circuits, in which there have been determined attempts to find all of the modulatory inputs to the circuit. But, whether there are 5 or 12 or 25 modulators that can influence the output of a given circuit in the brain, no circuit is likely to be modulated by only one or two substances, no matter how tempting it is to think that a single substance is solely responsible for controlling a significant piece of the brain.
Neuromodulators and neuromodulatory neurons alter circuit dynamics
The exogenous application of neuromodulatory substances and the stimulation of modulatory projection neurons can significantly alter circuit output (Blitz et al., 2004; Blitz et al., 1999; Blitz et al., 1995; Blitz et al., 2008; Dando and Selverston, 1972; Dickinson et al., 2001; Dickinson and Marder, 1989; Dickinson et al., 1990; Dickinson and Nagy, 1983; Eisen and Marder, 1984; Flamm and Harris-Warrick, 1986a, b; Hooper and Marder, 1984; Hooper and Marder, 1987; Nagy and Dickinson, 1983; Nagy et al., 1988; Nusbaum and Marder, 1988; Nusbaum and Marder, 1989a; Nusbaum and Marder, 1989b; Saideman et al., 2006; Saideman et al., 2007).
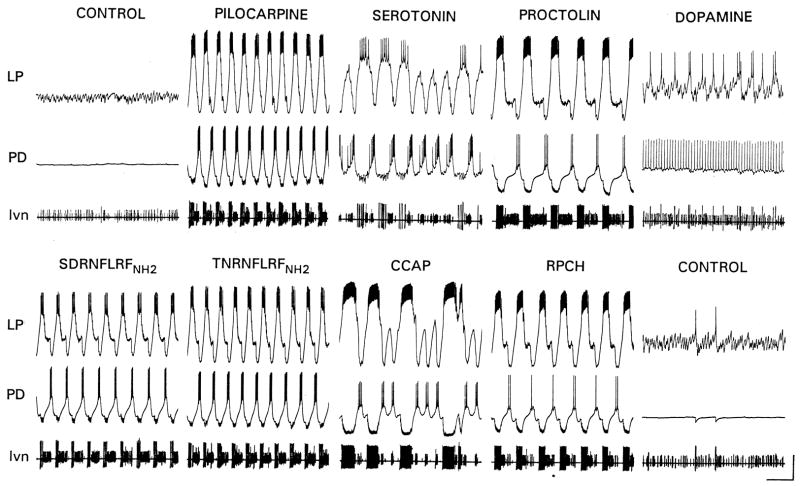
When the effects of descending modulatory projection neurons on the STG are removed by either cutting or blocking the input nerve to the STG, the fast pyloric rhythm either stops completely or slows down (Fig. 3, control). Under these conditions, exogenous application of a large number of different substances can elicit a triphasic motor pattern (Fig. 3), although each substance produces a different form of the rhythm. These data were initially interpreted as showing that the same neuronal circuitry can be reconfigured differently by each of a large number of neuromodulators. That interpretation still holds. But these data also make a second point: there are a large number of different neuromodulators that can activate the network. To some extent these constitute degenerate mechanisms that can, as a first approximation substitute for each other, if it is more important that a rhythm exist than its exact form. This is especially the case, if the neuromuscular junctions activated by these motor neurons act as a temporal filter (Brezina, 2010; Hooper and Weaver, 2000; Morris and Hooper, 1998). Modulators may also stabilize motor patterns (Zhao et al., 2011).
Figure 3.
Multiple neuromodulators can activate different forms of the pyloric rhythm. In each panel the top two traces are intracellular recordings from the Lateral Pyloric (LP) and Pyloric Dilator (PD) neurons. The bottom trace is an extracellular recording from the lateral ventricular nerve (lvn) that carries the axons of the LP, PD, and Pyloric (PY) neurons. (Marder and Weimann, 1992).
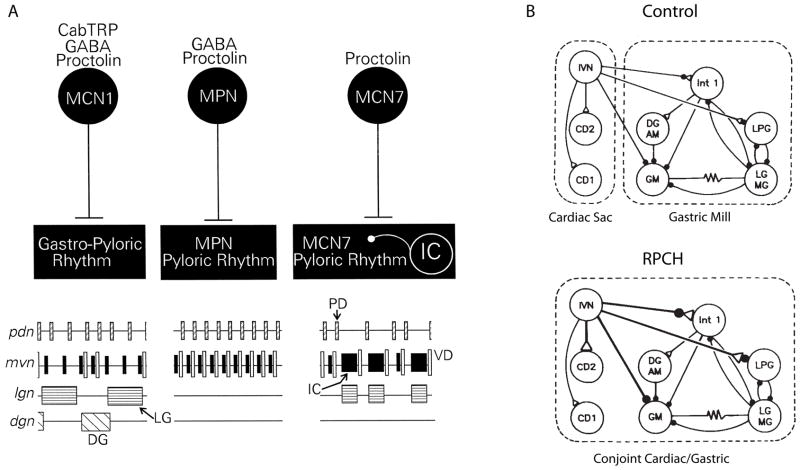
In addition to the fast pyloric rhythm, the STG also expresses two slower rhythms, the gastric mill rhythm and the cardiac sac rhythm. These rhythms require descending modulatory inputs for their expression. Figure 4A shows a cartoon comparing the effects of stimulating three different proctolin-containing modulatory projection neurons on the pyloric and gastric rhythms of the crab. While each of these neurons contains and releases proctolin, the cotransmitter complement of these three neurons is different (Blitz et al., 1999), and stimulation of these neurons elicits different motor patterns from the STG. A full gastric rhythm is elicited by MCN1, MPN increases the frequency of the fast pyloric rhythm, while MCN7 activates still a different rhythm.
Figure 4.
Modulatory reconfiguration of circuits. A) Three different proctolin-containing modulatory neurons each evoke different changes in STG motor patterns. (Blitz et al., 1999; Nusbaum et al., 2001). B) Bath application of RPCH constructs a conjoint rhythm from previously separate cardiac sac and gastric mill circuit elements. Modified from Dickinson et al (1990).
Not only can modulators alter the motor patterns produced by a single circuit, but they can also combine elements from two circuits into one. The schematic shown in Figure 4B shows that the neuropeptide, Red Pigment Concentrating Hormone (RPCH) strengthens synapses from the IVN neurons to STG network neurons and creates a single, conjoint rhythm from neurons that ordinarily are part of the cardiac sac and gastric rhythm (Dickinson et al., 1990). This is one of many examples of circuit switching in the STG, in which neurons switch from being part of the pyloric or gastric circuits (Weimann and Marder, 1994; Weimann et al., 1991).
While some aspects of the effects of a cotransmitter-containing projection neuron may be recapitulated with bath application of one of its substances, it is unlikely that exogenous bath applications will reproduce the concentration profiles that are produced by neural stimulation. In contrast, there are substances that only reach the neuropil of the STG as circulating hormones (Saideman et al., 2006; Weimann et al., 1997). In this case, bath-applications at realistic concentrations are far more likely to elicit responses similar to those evoked in vivo.
Determining the cellular mechanisms underlying circuit modulation
One of the goals of much of the work on the modulation of the STG has been to determine the mechanisms that account for the changes in circuit performance elicited by modulators on the basis of the modulator’s action on specific cellular and synaptic targets (Eisen and Marder, 1984; Flamm and Harris-Warrick, 1986a, b; Hooper and Marder, 1987; Marder and Eisen, 1984a). In these experiments pharmacological blockade of the glutamatergic inhibitory synapses was combined with photoinactivation of specific dye-filled neurons (Miller and Selverston, 1979) to isolate individual neurons for study.
These studies demonstrated: a) electrically coupled neurons could respond differently to the same modulatory substance (Marder and Eisen, 1984a), b) a given neuron could be a direct target for multiple modulatory substances (Flamm and Harris-Warrick, 1986b; Hooper and Marder, 1987; Marder and Eisen, 1984a; Swensen and Marder, 2000), c) multiple circuit neurons were simultaneous targets of the same neuromodulator (Flamm and Harris-Warrick, 1986b; Harris-Warrick and Johnson, 2010; Hooper and Marder, 1987), d) all circuit neurons are the subject of modulation (Harris-Warrick and Johnson, 2010; Swensen and Marder, 2001).
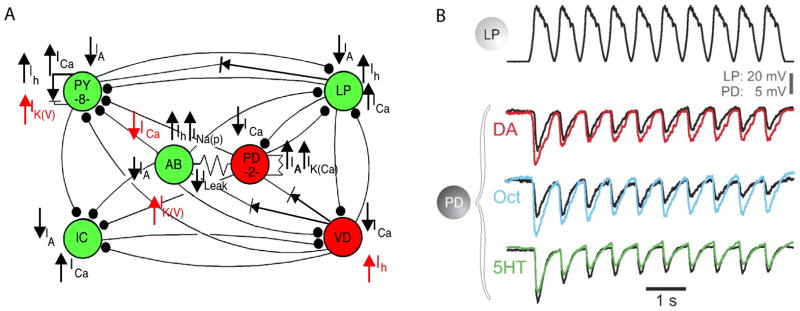
The effects of dopamine on membrane currents and receptors in STG neurons has been extensively studied (Clark and Baro, 2006, 2007; Clark et al., 2008; Harris-Warrick et al., 1995a; Harris-Warrick et al., 1995b; Harris-Warrick and Johnson, 2010; Peck et al., 2006; Zhang et al., 2010). An unexpected result from this work is that dopamine modulates several currents in the same neuron, and that the same current can be modulated differently in different target neurons (Fig. 5A).
Figure 5.
Aminergic modulation of pyloric circuit elements. A) The actions of dopamine on ionic currents in the indicated neurons are shown. From (Harris-Warrick, 2011). B) Graded IPSPs evoked in the postsynaptic PD neuron by depolarization of the LP neuron in control (black traces), dopamine (red), octopamine (blue) and serotonin (green). Modified from Johnston et al (2011).
Every synapse is subject to neuromodulation
The dynamics of circuit modulation in the STG also involves modulation of synaptic strength (Dickinson et al., 1990; Eisen and Marder, 1984; Harris-Warrick and Johnson, 2010; Johnson et al., 2011; Johnson and Harris-Warrick, 1990; Kloppenburg et al., 2000; Thirumalai et al., 2006; Zhao et al., 2011). Figure 5B shows that the same synapse is subject to modulation by dopamine, serotonin, and octopamine. Additionally, the extent of the modulation is altered as a function of synaptic depression (Johnson et al., 2011). This shows that there is an interaction between neuromodulation and other use-dependent processes that also influence synaptic strength during ongoing circuit activity.
The interaction between basal neuromodulatory tone and phasic activation of neuromodulatory inputs
Many of the same substances are delivered by specific modulatory projections into the STG and also are released into the hemolymph from neurosecretory structures such as the pericardial organs (Figure 2). This same dual function is a general feature of many nervous systems (Keller, 1992). The concentration of neuromodulators in the hemolymph are in the nanomolar range, while release from nerve terminals can produce substantially higher concentrations, at least for short periods of time in response to bursts of presynaptic activity (Rodgers et al., 2011a; Rodgers et al., 2011b). Neurons in the STG show DA receptors at non-synaptic regions (Oginsky et al., 2010), consistent with their role as signaling a tonic modulatory tone. Moreover, tonic low concentrations of DA seem to be important for maintaining circuit basal function, while phasic, higher concentrations produce shorter-term modulation (Rodgers et al., 2011a; Rodgers et al., 2011b).
How can highly modulated circuits be stable in the face of parameter changes brought about by modulation?
One of the most puzzling questions arising from extensive neuromodulation is how the integrity of the modulated circuits is maintained, although so may circuit parameters can be altered? If one tries to build a computational model of either a single neuron, or a circuit, it can be quite hard to find a set of parameters that are consistent with the desired output. Indeed, random assignment of parameters to a single neuron or a circuit will lead to significantly more failures than successful models (Prinz, 2010; Prinz et al., 2003a; Prinz et al., 2004; Taylor et al., 2009). Nonetheless, there are many different sets of parameters that can produce similar output patterns (Goldman et al., 2001; Prinz et al., 2004; Taylor et al., 2009). There are circumstances in which neuromodulators are used to qualitatively transform the behavior of a circuit, such as during transitions from sleep to wakefulness (McCormick, 1989, 1992; McCormick and Bal, 1997), or when a hormonal pathway is used to trigger eclosion (Kim et al., 2006) or molting (Webster et al., 2012). There are also neuromodulatory influences that reshape networks during ongoing behavior, and the sets of parameters that are produced by neuromodulator action must be consistent with stable and appropriate cellular and circuit function (Goldman et al., 2001).
Understanding how circuits can be stable in the face of ubiquitous neuromodulation is an important and deep problem. Why don’t the circuits important for behavior become “over-modulated” more often, and what mechanisms might protect against over-modulation? The answers to this question may be partially idiosyncratic to each circuit, but I suggest some general mechanisms that may play a role in maintaining functional circuit performance during modulation.
Stability Mechanism #1- Modulators that Coordinately Act on Opposing Processes
Harris-Warrick and Johnson (2010) suggest that the pattern of dopamine modulation of STG neurons at the cellular level (Fig. 5) is ideally suited to maintain stable function. Specifically, by acting on both inward and outward currents, dopamine actions can keep individual neurons, and therefore the network, within their operating range (Harris-Warrick and Johnson, 2010).
Stability Mechanism #2- Voltage-dependence of modulator actions
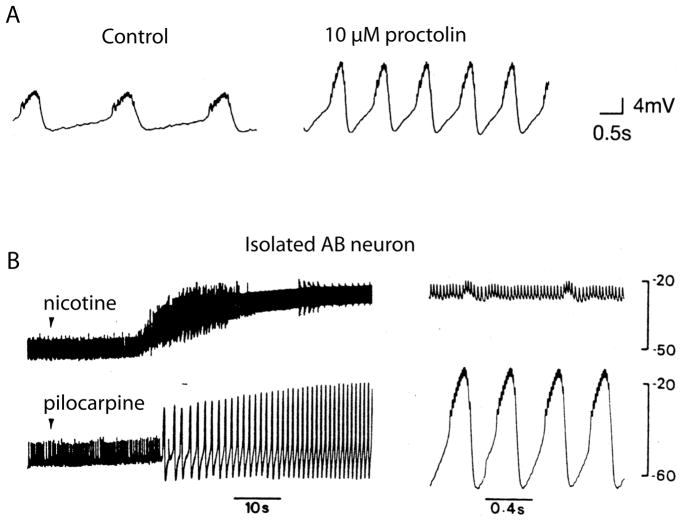
The importance of the voltage-dependence of the NMDA receptor for the induction of LTP is well-appreciated, but the ability of the NMDA receptor to induce oscillations in the spinal cord is less well-known (Sigvardt et al., 1985). The neuropeptide proctolin elicits a voltage-dependent inward current similar to that evoked by NMDA (Golowasch and Marder, 1992). This current is blocked at hyperpolarized membrane potentials by extracellular Ca2+, and has a reversal potential about 0 mV. Consequently, the peak inward current activated by proctolin is close to threshold (Golowasch and Marder, 1992).
Because of its voltage-dependence, the current activated by proctolin increases the amplitude of the oscillations generated by bursting neurons without producing a depolarization of the baseline (Fig. 5A). The same effect is seen with muscarinic agonists such as pilocarpine or oxotremorine (Marder and Paupardin-Tritsch, 1978; Swensen and Marder, 2000). In contrast, nicotine which activates a conventional nicotinic receptor (Marder and Eisen, 1984b; Marder and Paupardin-Tritsch, 1978), depolarizes the baseline of the oscillator (Fig. 5B), and can result in a depolarization block. Thus, the voltage-dependence of the current elicited by proctolin and muscarinic agonists has a built-in brake that maintains the integrity of the burst generating mechanism in the pyloric pacemaker neurons.
Stability Mechanism #3- Convergence of many modulators onto the same voltage-dependent current
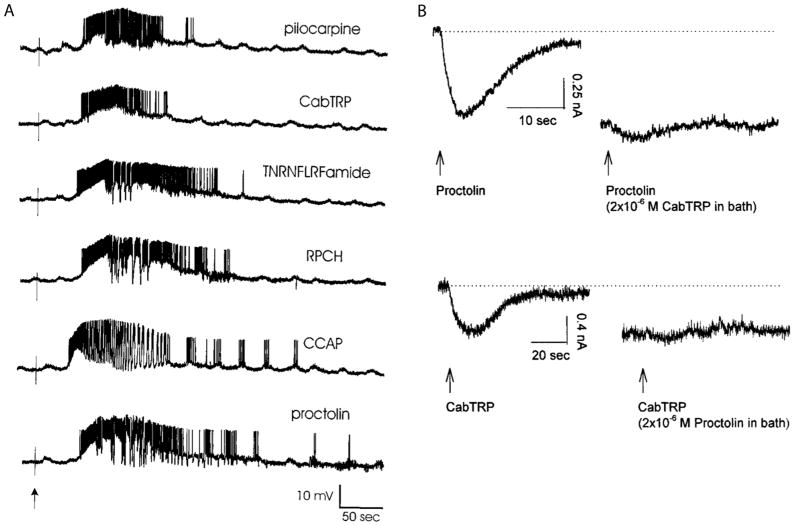
In addition to proctolin and muscarinic agonists, a large number of other peptides including Crustacean Cardioactive Peptide (CCAP), RPCH, TNRNFLRFamide, SDRNFLRFamide, Cancer borealis Tachykin-Related Peptide (CabTRP1a) activate the same voltage-dependent current (Swensen and Marder, 2000), and act on some of the same neurons (Fig. 7A). Because these modulators converge onto the same current, they occlude each other’s actions (Fig. 7B) (Swensen and Marder, 2000). Thus, if a neuron is already highly activated by one of these modulatory substances, a second of them will be relatively ineffective.
Figure 7.
Multiple modulators act on the same neuron and converge onto the same current. A) Puff applications of the modulators indicated onto intracellularly recorded LP neuron. (Swensen and Marder, 2000). B) Voltage-clamp recordings of inward currents evoked by proctolin and CabTRP1a. Top traces, puff of proctolin elicited an inward current. When CabTRP1a was placed in the bath, eliciting a steady-state inward current, a puff of proctolin produced only a very small additional inward current. Bottom traces, reverse experiment. (Swensen and Marder, 2000).
Stability Mechanism #4- Saturation of postsynaptic action: bigger synaptic inputs do necessarily produce larger effects on target neuron activity
Modulators can enhance the amplitude of synaptic currents many-fold. For example, RPCH produces several-fold increases in the amplitude of the inhibitory LP to PD synapse in the pyloric network of the lobster, Homarus americanus (Thirumalai et al., 2006). Although this synapse is the major feedback to the pacemaker of the pyloric rhythm, this increase in synaptic strength does not necessarily change the frequency of the pyloric rhythm (Thirumalai et al., 2006) because the effect of the inhibitory input to an oscillator often saturates as synaptic strength is increased (Prinz et al., 2003b). This saturation means that the network’s activity is de facto protected against over-modulation of the feedback synapse to the oscillator.
Stability Mechanism #5- Modulators act coordinately on multiple targets to keep systems functionally “matched”
In motor systems central pattern generating networks drive muscles, and it is the muscle movement that is important for behavior. Brezina and colleagues (Brezina et al., 2005; Brezina et al., 2000b; Brezina and Weiss, 2000; Zhurov and Brezina, 2006) have argued that coordinate modulation of muscles, neuromuscular junctions and the central pattern generating circuitry ensures that the presynaptic activity generated in the motor neurons is appropriately matched to their muscle targets. This general principle, of correlated and coordinated modulation of multiple sites in a sensory-motor circuit is likely to be a general principle, found in many nervous systems (Taghert and Nitabach, 2012).
Can modulator action be robust and predictable despite variability in underlying conductances?
Much computational and experimental evidence shows that there can be considerably variability across animals or across neurons in the parameters that control neuronal excitability and network function even when the circuit output is maintained (Calabrese et al., 2011; Goaillard et al., 2009; Nerbonne et al., 2008; Norris et al., 2011; Prinz et al., 2004; Roffman et al., 2011, 2012; Schulz et al., 2006; Schulz et al., 2007; Sobie, 2009; Swensen and Bean, 2005; Tobin et al., 2009). This raises the question of whether it is possible for neuromodulation to be reliable across individuals, if each of them has a nervous system with different underlying parameters.
The answer to this question is complicated. First, even for modulators that have robust actions, there can be significant differences in their responses to threshold concentrations (Weimann et al., 1997). Second, many modulators show state-dependent actions (Nusbaum and Marder, 1989b; Szabo et al., 2011), so that the activity or prior history of activity of the network determines the extent or sign (Spitzer et al., 2008) of modulator action. Third, modulator action may depend critically on other modulators (Brezina, 2010; Dickinson et al., 1997). That said, many networks with different underlying parameters can respond reliably to the same modulators (Grashow et al., 2009), although in this study, a small proportion of networks responded anomalously (Grashow et al., 2009). These data are reminiscent of what we see in the human population with pharmacological agents that produce anomalous responses in a small subset of people. Thus, although there are significant individual differences in circuit structures across individuals, the particular sets of network parameters found in the healthy population may be enriched for sets of parameters that permit reliable neuromodulatory control under most conditions.
Summary and Conclusions: Modulation and Connectomes
The discerning among you have already made the connection between the early belief that a connectivity diagram would be sufficient to bring understanding of how a circuit worked, and the some of the more lofty justifications made for the recent attempts to establish connectomes using anatomical methods (Briggman and Bock, 2012; Briggman and Denk, 2006; Briggman et al., 2011). Detailed anatomical data are invaluable. No circuit can be fully understood without a connectivity diagram. But, the experience of the small circuit community (Bargmann, 2012; Brezina, 2010; Getting, 1989; Jang et al., 2012; Marder and Bucher, 2007; Marder and Calabrese, 1996) demonstrates unambiguiously that a connectivity diagram is only a necessary beginning, but not in itself, an answer.
What then is the answer? The full answer will require a connectivity diagram that is supplemented with a complete description of all of the cotransmitters present in each neuron. It will require detailed information about the properties of the receptors to all of those substances. It will require having methods to record simultaneously the electrical activity of many circuit elements, to understand circuit dynamics. It will require systems that allow us to go back and forth from in vitro and in vivo preparations. It will require computational models that will help us to understand how behavior at one level emerges from the properties of a lower level.
But most critically, it will require a return to appreciating the benefits of working on disparate animal species. Each animal has devised extraordinary and baroque circuit mechanisms that employ neuromodulation to achieve important behavioral flexibility in the context of its environment, neuronal complement, and biomechanical constraints. Many of the circuit configurations that we will uncover may be weird and specific solutions to particular needs of that species. It will only be by looking for general principles across species that we will find the more general rules that govern the robust and stable neuromodulation needed for functional circuit activity in all animals.
Figure 6.
Effects of modulatory substances on a bursting pacemaker neuron, A) Intracellular recordings from the isolated Anterior Burster (AB) neuron in control and proctolin. Modified from Hooper and Marder (1987). Notice the increase in amplitude without change in baseline. B) AB neuron in response to nicotine and pilocarpine.
Acknowledgments
It is impossible to do justice to even a small fraction of the papers and investigators who have contributed to the changes in conceptual framework that we have seen since these beginning days of the study of circuits and their neuromodulation. I apologize to all those whose work has given us so much and yet goes unmentioned here. I thank Dr. Marie Goeritz for help with the figures. This review benefitted by support from NS17813 from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams ME, O’Shea M. Peptide cotransmitter at a neuromuscular junction. Science. 1983;221:286–288. doi: 10.1126/science.6134339. [DOI] [PubMed] [Google Scholar]
- Adams PR, Brown DA. Luteinizing hormone-releasing factor and muscarinic agonists act on the same voltage-sensitive K+-current in bullfrog sympathetic neurones. Br J Pharmacol. 1980;68:353–355. doi: 10.1111/j.1476-5381.1980.tb14547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargmann CI. Beyond the connectome: how neuromodulators shape neural circuits. Bioessays. 2012;34:458–465. doi: 10.1002/bies.201100185. [DOI] [PubMed] [Google Scholar]
- Barker DL, Molinoff PB, Kravitz EA. Octopamine in the lobster nervous system. Nature New Biol. 1972;236:61–62. doi: 10.1038/newbio236061a0. [DOI] [PubMed] [Google Scholar]
- Bishop CA, Wine JJ, Nagy F, O’Shea MR. Physiological consequences of a peptide cotransmitter in a crayfish nerve-muscle preparation. J Neurosci. 1987;7:1769–1779. doi: 10.1523/JNEUROSCI.07-06-01769.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitz DM, Beenhakker MP, Nusbaum MP. Different sensory systems share projection neurons but elicit distinct motor patterns. J Neurosci. 2004;24:11381–11390. doi: 10.1523/JNEUROSCI.3219-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitz DM, Christie AE, Coleman MJ, Norris BJ, Marder E, Nusbaum MP. Different proctolin neurons elicit distinct motor patterns from a multifunctional neuronal network. J Neurosci. 1999;19:5449–5463. doi: 10.1523/JNEUROSCI.19-13-05449.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitz DM, Christie AE, Marder E, Nusbaum MP. Distribution and effects of tachykinin-like peptides in the stomatogastric nervous system of the crab, Cancer borealis. J Comp Neurol. 1995;354:282–294. doi: 10.1002/cne.903540209. [DOI] [PubMed] [Google Scholar]
- Blitz DM, Nusbaum MP. Neural circuit flexibility in a small sensorimotor system. Curr Opin Neurobiol. 2011 doi: 10.1016/j.conb.2011.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitz DM, White RS, Saideman SR, Cook A, Christie AE, Nadim F, Nusbaum MP. A newly identified extrinsic input triggers a distinct gastric mill rhythm via activation of modulatory projection neurons. J Exp Biol. 2008;211:1000–1011. doi: 10.1242/jeb.015222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brezina V. Beyond the wiring diagram: signalling through complex neuromodulator networks. Philos Trans R Soc Lond B Biol Sci. 2010;365:2363–2374. doi: 10.1098/rstb.2010.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brezina V, Church PJ, Weiss KR. Temporal pattern dependence of neuronal peptide transmitter release: models and experiments. J Neurosci. 2000a;20:6760–6772. doi: 10.1523/JNEUROSCI.20-18-06760.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brezina V, Horn CC, Weiss KR. Modeling neuromuscular modulation in Aplysia. III. Interaction of central motor commands and peripheral modulatory state for optimal behavior. J Neurophysiol. 2005;93:1523–1556. doi: 10.1152/jn.00475.2004. [DOI] [PubMed] [Google Scholar]
- Brezina V, Orekhova IV, Weiss KR. Optimization of rhythmic behaviors by modulation of the neuromuscular transform. J Neurophysiol. 2000b;83:260–279. doi: 10.1152/jn.2000.83.1.260. [DOI] [PubMed] [Google Scholar]
- Brezina V, Weiss KR. Analyzing the functional consequences of transmitter complexity. Trends Neurosci. 1997;20:538–543. doi: 10.1016/s0166-2236(97)01120-x. [DOI] [PubMed] [Google Scholar]
- Brezina V, Weiss KR. The neuromuscular transform constrains the production of functional rhythmic behaviors. J Neurophysiol. 2000;83:232–259. doi: 10.1152/jn.2000.83.1.232. [DOI] [PubMed] [Google Scholar]
- Briggman KL, Bock DD. Volume electron microscopy for neuronal circuit reconstruction. Curr Opin Neurobiol. 2012;22:154–161. doi: 10.1016/j.conb.2011.10.022. [DOI] [PubMed] [Google Scholar]
- Briggman KL, Denk W. Towards neural circuit reconstruction with volume electron microscopy techniques. Curr Opin Neurobiol. 2006;16:562–570. doi: 10.1016/j.conb.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Briggman KL, Helmstaedter M, Denk W. Wiring specificity in the direction-selectivity circuit of the retina. Nature. 2011;471:183–188. doi: 10.1038/nature09818. [DOI] [PubMed] [Google Scholar]
- Brown DA, Adams PR. Muscarinic suppression of a novel voltage-sensitive K+ current in a vertebrate neurone. Nature. 1980;283:673–676. doi: 10.1038/283673a0. [DOI] [PubMed] [Google Scholar]
- Burrows M. Co-ordinating interneurones of the locust which convey two patterns of motor commands: their connexions with flight motoneurones. J Exp Biol. 1975a;63:713–733. doi: 10.1242/jeb.63.3.713. [DOI] [PubMed] [Google Scholar]
- Burrows M. Monosynaptic connexions between wing stretch receptors and flight motoneurones of the locust. J Exp Biol. 1975b;62:189–219. doi: 10.1242/jeb.62.1.189. [DOI] [PubMed] [Google Scholar]
- Calabrese RL, Norris BJ, Wenning A, Wright TM. Coping with variability in small neuronal networks. Integrative and comparative biology. 2011;51:845–855. doi: 10.1093/icb/icr074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese RL, Peterson E. Neural control of heartbeat in the leech, Hirudo medicinalis. Symp Soc Exp Biol. 1983;37:195–221. [PubMed] [Google Scholar]
- Camardo JS, Shuster MJ, Siegelbaum SA, Kandel ER. Modulation of a specific potassium channel in sensory neurons of Aplysia by serotonin and cAMP-dependent protein phosphorylation. Cold Spring Harb Symp Quant Biol. 1983;48(Pt 1):213–220. doi: 10.1101/sqb.1983.048.01.024. [DOI] [PubMed] [Google Scholar]
- Carraway R, Leeman SE. The isolation of a new hypotensive peptide, neurotensin, from bovine hypothalami. J Biol Chem. 1973;248:6854–6861. [PubMed] [Google Scholar]
- Chang MM, Leeman SE. Isolation of a sialogogic peptide from bovine hypothalamic tissue and its characterization as substance P. J Biol Chem. 1970;245:4784–4790. [PubMed] [Google Scholar]
- Chklovskii DB, Vitaladevuni S, Scheffer LK. Semi-automated reconstruction of neural circuits using electron microscopy. Curr Opin Neurobiol. 2010;20:667–675. doi: 10.1016/j.conb.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Christie AE, Skiebe P, Marder E. Matrix of neuromodulators in neurosecretory structures of the crab, Cancer borealis. J Exp Biol. 1995;198:2431–2439. doi: 10.1242/jeb.198.12.2431. [DOI] [PubMed] [Google Scholar]
- Clark MC, Baro DJ. Molecular cloning and characterization of crustacean type-one dopamine receptors: D1alphaPan and D1betaPan. Comp Biochem Physiol B Biochem Mol Biol. 2006;143:294–301. doi: 10.1016/j.cbpb.2005.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark MC, Baro DJ. Arthropod D2 receptors positively couple with cAMP through the Gi/o protein family. Comp Biochem Physiol B Biochem Mol Biol. 2007;146:9–19. doi: 10.1016/j.cbpb.2006.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark MC, Khan R, Baro DJ. Crustacean dopamine receptors: localization and G protein coupling in the stomatogastric ganglion. J Neurochem. 2008;104:1006–1019. doi: 10.1111/j.1471-4159.2007.05029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cropper EC, Lloyd PE, Reed W, Tenenbaum R, Kupfermann I, Weiss KR. Multiple neuropeptides in cholinergic motor neurons of Aplysia: evidence for modulation intrinsic to the motor circuit. Proc Natl Acad Sci (USA) 1987;84:3486–3490. doi: 10.1073/pnas.84.10.3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Bermudez ND, Marder E. Multiple modulators act on the cardiac ganglion of the crab, Cancer borealis. J Exp Biol. 2007;210:2873–2884. doi: 10.1242/jeb.002949. [DOI] [PubMed] [Google Scholar]
- Dale H. Pharmacology and nerve endings. Proc R Soc Med. 1935;28:319–332. [PMC free article] [PubMed] [Google Scholar]
- Dando MR, Selverston AI. Command fibres from the supra-oesophageal ganglion to the stomatogastric ganglion in Panulirus argus. J Comp Physiol. 1972;78:138–175. [Google Scholar]
- Denk W, Briggman KL, Helmstaedter M. Structural neurobiology: missing link to a mechanistic understanding of neural computation. Nat Rev Neurosci. 2012;13:351–358. doi: 10.1038/nrn3169. [DOI] [PubMed] [Google Scholar]
- DeRiemer SA, Strong JA, Albert KA, Greengard P, Kaczmarek LK. Enhancement of calcium current in Aplysia neurones by phorbol ester and protein kinase C. Nature. 1985;313:313–316. doi: 10.1038/313313a0. [DOI] [PubMed] [Google Scholar]
- Dickinson PS, Fairfield WP, Hetling JR, Hauptman J. Neurotransmitter interactions in the stomatogastric system of the spiny lobster: One peptide alters the response of a central pattern generator to a second peptide. J Neurophysiol. 1997;77:599–610. doi: 10.1152/jn.1997.77.2.599. [DOI] [PubMed] [Google Scholar]
- Dickinson PS, Hauptman J, Hetling J, Mahadevan A. RPCH modulation of a multi-oscillator network: effects on the pyloric network of the spiny lobster. J Neurophysiol. 2001;85:1424–1435. doi: 10.1152/jn.2001.85.4.1424. [DOI] [PubMed] [Google Scholar]
- Dickinson PS, Marder E. Peptidergic modulation of a multioscillator system in the lobster. I. Activation of the cardiac sac motor pattern by the neuropeptides proctolin and red pigment concentrating hormone. J Neurophysiol. 1989;61:833–844. doi: 10.1152/jn.1989.61.4.833. [DOI] [PubMed] [Google Scholar]
- Dickinson PS, Mecsas C, Marder E. Neuropeptide fusion of two motor-pattern generator circuits. Nature. 1990;344:155–158. doi: 10.1038/344155a0. [DOI] [PubMed] [Google Scholar]
- Dickinson PS, Nagy F. Control of a central pattern generator by an identified modulatory interneurone in Crustacea. II. Induction and modification of plateau properties in pyloric neurones. J Exp Biol. 1983;105:59–82. doi: 10.1242/jeb.105.1.59. [DOI] [PubMed] [Google Scholar]
- Dickinson PS, Stemmler EA, Barton EE, Cashman CR, Gardner NP, Rus S, Brennan HR, McClintock TS, Christie AE. Molecular, mass spectral, and physiological analyses of orcokinins and orcokinin precursor-related peptides in the lobster Homarus americanus and the crayfish Procambarus clarkii. Peptides. 2009;30:297–317. doi: 10.1016/j.peptides.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudel J. Facilitatory effects of 5-hydroxy-tryptamine on the crayfish neuromuscular junction. Arch exp Path u Pharmak. 1965;249:515–528. doi: 10.1007/BF00246558. [DOI] [PubMed] [Google Scholar]
- Dunlap K, Fischbach GD. Neurotransmitters decrease the calcium conductance activated by depolarization of embryonic chick sensory neurones. J Physiol (Lond) 1981;317:519–535. doi: 10.1113/jphysiol.1981.sp013841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen JS, Marder E. A mechanism for production of phase shifts in a pattern generator. J Neurophysiol. 1984;51:1375–1393. doi: 10.1152/jn.1984.51.6.1375. [DOI] [PubMed] [Google Scholar]
- Flamm RE, Harris-Warrick RM. Aminergic modulation in lobster stomatogastric ganglion. I. Effects on motor pattern and activity of neurons within the pyloric circuit. J Neurophysiol. 1986a;55:847–865. doi: 10.1152/jn.1986.55.5.847. [DOI] [PubMed] [Google Scholar]
- Flamm RE, Harris-Warrick RM. Aminergic modulation in lobster stomatogastric ganglion. II. Target neurons of dopamine, octopamine, and serotonin within the pyloric circuit. J Neurophysiol. 1986b;55:866–881. doi: 10.1152/jn.1986.55.5.866. [DOI] [PubMed] [Google Scholar]
- Furukawa Y, Nakamaru K, Sasaki K, Fujisawa Y, Minakata H, Ohta S, Morishita F, Matsushima O, Li L, Alexeeva V, et al. PRQFVamide, a novel pentapeptide identified from the CNS and gut of Aplysia. J Neurophysiol. 2003;89:3114–3127. doi: 10.1152/jn.00014.2003. [DOI] [PubMed] [Google Scholar]
- Getting PA. Mechanisms of pattern generation underlying swimming in Tritonia. I. Neuronal network formed by monosynaptic connections. J Neurophysiol. 1981;46:65–79. doi: 10.1152/jn.1981.46.1.65. [DOI] [PubMed] [Google Scholar]
- Getting PA. Emerging principles governing the operation of neural networks. Annu Rev Neurosci. 1989;12:185–204. doi: 10.1146/annurev.ne.12.030189.001153. [DOI] [PubMed] [Google Scholar]
- Glusman S, Kravitz EA. The action of serotonin on excitatory nerve terminals in lobster nerve-muscle preparations. J Physiol. 1982;325:223–241. doi: 10.1113/jphysiol.1982.sp014147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goaillard JM, Taylor AL, Schulz DJ, Marder E. Functional consequences of animal-to-animal variation in circuit parameters. Nat Neurosci. 2009;12:1424–1430. doi: 10.1038/nn.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman MS, Golowasch J, Marder E, Abbott LF. Global structure, robustness, and modulation of neuronal models. J Neurosci. 2001;21:5229–5238. doi: 10.1523/JNEUROSCI.21-14-05229.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golowasch J, Marder E. Proctolin activates an inward current whose voltage dependence is modified by extracellular Ca2+ J Neurosci. 1992;12:810–817. doi: 10.1523/JNEUROSCI.12-03-00810.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grashow R, Brookings T, Marder E. Reliable neuromodulation from circuits with variable underlying structure. Proc Natl Acad Sci U S A. 2009;106:11742–11746. doi: 10.1073/pnas.0905614106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris-Warrick RM. Neuromodulation and flexibility in Central Pattern Generator networks. Curr Opin Neurobiol. 2011 doi: 10.1016/j.conb.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris-Warrick RM, Coniglio LM, Barazangi N, Guckenheimer J, Gueron S. Dopamine modulation of transient potassium current evokes phase shifts in a central pattern generator network. J Neurosci. 1995a;15:342–358. doi: 10.1523/JNEUROSCI.15-01-00342.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris-Warrick RM, Coniglio LM, Levini RM, Gueron S, Guckenheimer J. Dopamine modulation of two subthreshold currents produces phase shifts in activity of an identified motoneuron. J Neurophysiol. 1995b;74:1404–1420. doi: 10.1152/jn.1995.74.4.1404. [DOI] [PubMed] [Google Scholar]
- Harris-Warrick RM, Johnson BR. Checks and balances in neuromodulation. Front Behav Neurosci. 2010:4. doi: 10.3389/fnbeh.2010.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris-Warrick RM, Kravitz EA. Cellular mechanisms for modulation of posture by octopamine and serotonin in the lobster. J Neurosci. 1984;4:1976–1993. doi: 10.1523/JNEUROSCI.04-08-01976.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitler WJ, Burrows M. The locust jump. II. Neural circuits of the motor programme. J Exp Biol. 1977;66:221–241. doi: 10.1242/jeb.66.1.221. [DOI] [PubMed] [Google Scholar]
- Hockberger P, Connor JA. Alteration of calcium conductances and outward current by cyclic adenosine monophosphate (cAMP) in neurons of Limax maximus. Cellular and molecular neurobiology. 1984;4:319–338. doi: 10.1007/BF00733595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper SL, Marder E. Modulation of a central pattern generator by two neuropeptides, proctolin and FMRFamide. Brain Res. 1984;305:186–191. doi: 10.1016/0006-8993(84)91138-7. [DOI] [PubMed] [Google Scholar]
- Hooper SL, Marder E. Modulation of the lobster pyloric rhythm by the peptide proctolin. J Neurosci. 1987;7:2097–2112. doi: 10.1523/JNEUROSCI.07-07-02097.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper SL, Weaver AL. Motor neuron activity is often insufficient to predict motor response. Curr Opin Neurobiol. 2000;10:676–682. doi: 10.1016/s0959-4388(00)00158-6. [DOI] [PubMed] [Google Scholar]
- Jan LY, Jan YN. Peptidergic transmission in sympathetic ganglia of the frog. J Physiol. 1982;327:219–246. doi: 10.1113/jphysiol.1982.sp014228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang H, Kim K, Neal SJ, Macosko E, Kim D, Butcher RA, Zeiger DM, Bargmann CI, Sengupta P. Neuromodulatory State and Sex Specify Alternative Behaviors through Antagonistic Synaptic Pathways in C. elegans. Neuron. 2012;75:585–592. doi: 10.1016/j.neuron.2012.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BR, Brown JM, Kvarta MD, Lu JY, Schneider LR, Nadim F, Harris-Warrick RM. Differential modulation of synaptic strength and timing regulate synaptic efficacy in a motor network. J Neurophysiol. 2011;105:293–304. doi: 10.1152/jn.00809.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BR, Harris-Warrick RM. Aminergic modulation of graded synaptic transmission in the lobster stomatogastric ganglion. J Neurosci. 1990;10:2066–2076. doi: 10.1523/JNEUROSCI.10-07-02066.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczarek LK, Levitan IB, editors. Neuromodulation: the Biochemical Control of Neuronal Excitability. New York: Oxford University Press; 1987. [Google Scholar]
- Kaczmarek LK, Strong JA, Kauer JA. The role of protein kinases in the control of prolonged changes in neuronal excitability. Prog Brain Res. 1986;69:77–90. doi: 10.1016/s0079-6123(08)61050-x. [DOI] [PubMed] [Google Scholar]
- Kandel ER, Schwartz JH. Molecular biology of learning: modulation of transmitter release. Science. 1982;218:433–443. doi: 10.1126/science.6289442. [DOI] [PubMed] [Google Scholar]
- Karhunen T, Vilim FS, Alexeeva V, Weiss KR, Church PJ. Targeting of peptidergic vesicles in cotransmitting terminals. J Neurosci. 2001;21:RC127. doi: 10.1523/JNEUROSCI.21-03-j0005.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz PS. Intrinsic and extrinsic neuromodulation of motor circuits. Curr Opin Neurobiol. 1995;5:799–808. doi: 10.1016/0959-4388(95)80109-x. [DOI] [PubMed] [Google Scholar]
- Katz PS, Frost WN. Intrinsic neuromodulation in the Tritonia swim CPG: serotonin mediates both neuromodulation and neurotransmission by the dorsal swim interneurons. J Neurophysiol. 1995a;74:2281–2294. doi: 10.1152/jn.1995.74.6.2281. [DOI] [PubMed] [Google Scholar]
- Katz PS, Frost WN. Intrinsic neuromodulation in the Tritonia swim CPG: the serotonergic dorsal swim interneurons act presynaptically to enhance transmitter release from interneuron C2. J Neurosci. 1995b;15:6035–6045. doi: 10.1523/JNEUROSCI.15-09-06035.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz PS, Frost WN. Intrinsic neuromodulation: altering neuronal circuits from within. Trends Neurosci. 1996;19:54–61. doi: 10.1016/0166-2236(96)89621-4. [DOI] [PubMed] [Google Scholar]
- Keller R. Crustacean neuropeptides: structures, functions and comparative aspects. Experientia. 1992;48:439–448. doi: 10.1007/BF01928162. [DOI] [PubMed] [Google Scholar]
- Kerkut GA, Cottrell GA. Acetylcholine and 5-Hydroxytryptamine in the Snail Brain. Comp Biochem Physiol. 1963;71:53–63. doi: 10.1016/0010-406x(63)90069-0. [DOI] [PubMed] [Google Scholar]
- Kerkut GA, Walker RJ. The effect of L-glutamate, acetylcholine and gamma-aminobutyric acid on the miniature end-plate potentials and contractures of the coxal muscles of the cockroach, Periplaneta americana. Comp Biochem Physiol. 1966;17:435–454. doi: 10.1016/0010-406x(66)90579-2. [DOI] [PubMed] [Google Scholar]
- Kim YJ, Zitnan D, Galizia CG, Cho KH, Adams ME. A command chemical triggers an innate behavior by sequential activation of multiple peptidergic ensembles. Curr Biol. 2006;16:1395–1407. doi: 10.1016/j.cub.2006.06.027. [DOI] [PubMed] [Google Scholar]
- Klein M, Camardo J, Kandel ER. Serotonin modulates a specific potassium current in the sensory neurons that show presynaptic facilitation in Aplysia. Proc Natl Acad Sci U S A. 1982;79:5713–5717. doi: 10.1073/pnas.79.18.5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein M, Kandel ER. Presynaptic modulation of voltage-dependent Ca2+ current: mechanism for behavioral sensitization in Aplysia californica. Proc Natl Acad Sci U S A. 1978;75:3512–3516. doi: 10.1073/pnas.75.7.3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloppenburg P, Zipfel WR, Webb WW, Harris-Warrick RM. Highly localized Ca2+ accumulation revealed by multiphoton microscopy in an identified motoneuron and its modulation by dopamine. J Neurosci. 2000;20:2523–2533. doi: 10.1523/JNEUROSCI.20-07-02523.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh HY, Weiss KR. Activity-dependent peptidergic modulation of the plateau-generating neuron B64 in the feeding network of Aplysia. J Neurophysiol. 2007;97:1862–1867. doi: 10.1152/jn.01230.2006. [DOI] [PubMed] [Google Scholar]
- Kristan WB, Jr, Calabrese RL. Rhythmic swimming activity in neurones of the isolated nerve cord of the leech. J Exp Biol. 1976;65:643–668. doi: 10.1242/jeb.65.3.643. [DOI] [PubMed] [Google Scholar]
- Kristan WBJ, Stent GS, Ort CA. Neuronal control of swimming in the medicinal leech. III. Impulse patterns of the motor neurons. J comp Physiol. 1974;94:155–176. [Google Scholar]
- Kupfermann I. Functional studies of cotransmission. Physiol Rev. 1991;71:683–732. doi: 10.1152/physrev.1991.71.3.683. [DOI] [PubMed] [Google Scholar]
- Levitan IB. Modulation of neuronal activity by peptides and neurotransmitters: possible role of cyclic nucleotides. J Physiol (Paris) 1978;74:521–525. [PubMed] [Google Scholar]
- Levitan IB, Harmar AJ, Adams WB. Synaptic and hormonal modulation of a neuronal oscillator: a search for molecular mechanisms. J Exp Biol. 1979;81:131–151. doi: 10.1242/jeb.81.1.131. [DOI] [PubMed] [Google Scholar]
- Li L, Floyd PD, Rubakhin SS, Romanova EV, Jing J, Alexeeva VY, Dembrow NC, Weiss KR, Vilim FS, Sweedler JV. Cerebrin prohormone processing, distribution and action in Aplysia californica. J Neurochem. 2001;77:1569–1580. doi: 10.1046/j.1471-4159.2001.00360.x. [DOI] [PubMed] [Google Scholar]
- Lichtman JW, Denk W. The big and the small: challenges of imaging the brain’s circuits. Science. 2011;334:618–623. doi: 10.1126/science.1209168. [DOI] [PubMed] [Google Scholar]
- Lingle C. The modulatory action of dopamine on crustacean foregut neuromuscular preparations. J Exp Biol. 1981;94:285–299. [Google Scholar]
- Ma M, Bors EK, Dickinson ES, Kwiatkowski MA, Sousa GL, Henry RP, Smith CM, Towle DW, Christie AE, Li L. Characterization of the Carcinus maenas neuropeptidome by mass spectrometry and functional genomics. General and comparative endocrinology. 2009a;161:320–334. doi: 10.1016/j.ygcen.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma M, Szabo TM, Jia C, Marder E, Li L. Mass spectrometric characterization and physiological actions of novel crustacean C-type allatostatins. Peptides. 2009b;30:1660–1668. doi: 10.1016/j.peptides.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma M, Wang J, Chen R, Li L. Expanding the Crustacean neuropeptidome using a multifaceted mass spectrometric approach. Journal of proteome research. 2009c;8:2426–2437. doi: 10.1021/pr801047v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder E. Mechanisms Underlying Neurotransmitter Modulation of a Neuronal Circuit. Trends in Neurosciences. 1984;7:48–53. [Google Scholar]
- Marder E, Bucher D. Understanding circuit dynamics using the stomatogastric nervous system of lobsters and crabs. Annu Rev Physiol. 2007;69:291–316. doi: 10.1146/annurev.physiol.69.031905.161516. [DOI] [PubMed] [Google Scholar]
- Marder E, Calabrese RL. Principles of rhythmic motor pattern generation. Physiol Rev. 1996;76:687–717. doi: 10.1152/physrev.1996.76.3.687. [DOI] [PubMed] [Google Scholar]
- Marder E, Eisen JS. Electrically coupled pacemaker neurons respond differently to same physiological inputs and neurotransmitters. J Neurophysiol. 1984a;51:1362–1374. doi: 10.1152/jn.1984.51.6.1362. [DOI] [PubMed] [Google Scholar]
- Marder E, Eisen JS. Transmitter identification of pyloric neurons: electrically coupled neurons use different neurotransmitters. J Neurophysiol. 1984b;51:1345–1361. doi: 10.1152/jn.1984.51.6.1345. [DOI] [PubMed] [Google Scholar]
- Marder E, Hooper SL. Neurotransmitter modulation of the stomatogastric ganglion of decapod crustaceans. In: Selverston AI, editor. Model Neural Networks and Behavior. New York: Plenum Press; 1985. pp. 319–337. [Google Scholar]
- Marder E, Paupardin-Tritsch D. The pharmacological properties of some crustacean neuronal acetylcholine, gamma-aminobutyric acid and l-glutamate responses. J Physiol. 1978;280:213–236. doi: 10.1113/jphysiol.1978.sp012381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder E, Weimann JM. Modulatory control of multiple task processing in the stomatogastric nervous system. In: Kien J, McCrohan C, Winlow B, editors. Neurobiology of Motor Progamme Selection. New York: Pergamon Press; 1992. pp. 3–19. [Google Scholar]
- McCormick DA. Cholinergic and noradrenergic modulation of thalamocortical processing. Trends Neurosci. 1989;12:215–221. doi: 10.1016/0166-2236(89)90125-2. [DOI] [PubMed] [Google Scholar]
- McCormick DA. Cellular mechanisms underlying cholinergic and noradrenergic modulation of neuronal firing mode in the cat and guinea pig dorsal lateral geniculate nucleus. J Neurosci. 1992;12:278–289. doi: 10.1523/JNEUROSCI.12-01-00278.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Bal T. Sleep and arousal: thalamocortical mechanisms. Annu Rev Neurosci. 1997;20:185–215. doi: 10.1146/annurev.neuro.20.1.185. [DOI] [PubMed] [Google Scholar]
- Miller JP, Selverston A. Rapid killing of single neurons by irradiation of intracellularly injected dye. Science. 1979;206:702–704. doi: 10.1126/science.386514. [DOI] [PubMed] [Google Scholar]
- Morgan PT, Perrins R, Lloyd PE, Weiss KR. Intrinsic and extrinsic modulation of a single central pattern generating circuit. J Neurophysiol. 2000;84:1186–1193. doi: 10.1152/jn.2000.84.3.1186. [DOI] [PubMed] [Google Scholar]
- Morris LG, Hooper SL. Muscle response to changing neuronal input in the lobster (Panulirus interruptus) stomatogastric system: slow muscle properties can transform rhythmic input into tonic output. J Neurosci. 1998;18:3433–3442. doi: 10.1523/JNEUROSCI.18-09-03433.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulloney B, Selverston AI. Organization of the stomatogastric ganglion in the spiny lobster. I. Neurons driving the lateral teeth. J Comp Physiol. 1974a;91:1–32. [Google Scholar]
- Mulloney B, Selverston AI. Organization of the stomatogastric ganglion in the spiny lobster. III. Coordination of the two subsets of the gastric system. J Comp Physiol. 1974b;91:53–78. [Google Scholar]
- Nagy F, Dickinson PS. Control of a central pattern generator by an identified modulatory interneurone in crustacea. I. Modulation of the pyloric motor output. J Exp Biol. 1983;105:33–58. doi: 10.1242/jeb.105.1.33. [DOI] [PubMed] [Google Scholar]
- Nagy F, Dickinson PS, Moulins M. Control by an identified modulatory neuron of the sequential expression of plateau properties of, and synaptic inputs to, a neuron in a central pattern generator. J Neurosci. 1988;8:2875–2886. doi: 10.1523/JNEUROSCI.08-08-02875.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nerbonne JM, Gerber BR, Norris A, Burkhalter A. Electrical remodelling maintains firing properties in cortical pyramidal neurons lacking KCND2-encoded A-type K+ currents. J Physiol. 2008;586:1565–1579. doi: 10.1113/jphysiol.2007.146597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris BJ, Wenning A, Wright TM, Calabrese RL. Constancy and variability in the output of a central pattern generator. J Neurosci. 2011;31:4663–4674. doi: 10.1523/JNEUROSCI.5072-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusbaum MP, Blitz DM, Swensen AM, Wood D, Marder E. The roles of co-transmission in neural network modulation. Trends Neurosci. 2001;24:146–154. doi: 10.1016/s0166-2236(00)01723-9. [DOI] [PubMed] [Google Scholar]
- Nusbaum MP, Marder E. A Neuronal Role for a Crustacean Red Pigment Concentrating Hormone-Like Peptide - Neuromodulation of the Pyloric Rhythm in the Crab, Cancer borealis. Journal of Experimental Biology. 1988;135:165–181. [Google Scholar]
- Nusbaum MP, Marder E. A modulatory proctolin-containing neuron (MPN). I. Identification and characterization. J Neurosci. 1989a;9:1591–1599. doi: 10.1523/JNEUROSCI.09-05-01591.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusbaum MP, Marder E. A modulatory proctolin-containing neuron (MPN). II. State-dependent modulation of rhythmic motor activity. J Neurosci. 1989b;9:1600–1607. doi: 10.1523/JNEUROSCI.09-05-01600.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oginsky MF, Rodgers EW, Clark MC, Simmons R, Krenz WD, Baro DJ. D2 receptors receive paracrine neurotransmission and are consistently targeted to a subset of synaptic structures in an identified neuron of the crustacean stomatogastric nervous system. J Comp Neurol. 2010;518:255–276. doi: 10.1002/cne.22225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka M, Kravitz EA, Potter DD. Physiological and chemical architecture of a lobster ganglion with particular reference to GABA and glutamate. J Neurophysiol. 1967;30:725–752. doi: 10.1152/jn.1967.30.4.725. [DOI] [PubMed] [Google Scholar]
- Pasztor VM, Bush BM. Peripheral modulation of mechano-sensitivity in primary afferent neurons. Nature. 1987;326:793–795. doi: 10.1038/326793a0. [DOI] [PubMed] [Google Scholar]
- Peck JH, Gaier E, Stevens E, Repicky S, Harris-Warrick RM. Amine modulation of Ih in a small neural network. J Neurophysiol. 2006;96:2931–2940. doi: 10.1152/jn.00423.2005. [DOI] [PubMed] [Google Scholar]
- Peng YY, Horn JP. Continuous repetitive stimuli are more effective than bursts for evoking LHRH release in bullfrog sympathetic ganglia. J Neurosci. 1991;11:85–95. doi: 10.1523/JNEUROSCI.11-01-00085.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y, Zucker RS. Release of LHRH is linearly related to the time integral of presynaptic Ca2+ elevation above a threshold level in bullfrog sympathetic ganglion. Neuron. 1993;10:465–473. doi: 10.1016/0896-6273(93)90334-n. [DOI] [PubMed] [Google Scholar]
- Prinz AA. Computational approaches to neuronal network analysis. Philos Trans R Soc Lond B Biol Sci. 2010;365:2397–2405. doi: 10.1098/rstb.2010.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz AA, Billimoria CP, Marder E. Alternative to hand-tuning conductance-based models: construction and analysis of databases of model neurons. J Neurophysiol. 2003a;90:3998–4015. doi: 10.1152/jn.00641.2003. [DOI] [PubMed] [Google Scholar]
- Prinz AA, Bucher D, Marder E. Similar network activity from disparate circuit parameters. Nat Neurosci. 2004;7:1345–1352. doi: 10.1038/nn1352. [DOI] [PubMed] [Google Scholar]
- Prinz AA, Thirumalai V, Marder E. The functional consequences of changes in the strength and duration of synaptic inputs to oscillatory neurons. J Neurosci. 2003b;23:943–954. doi: 10.1523/JNEUROSCI.23-03-00943.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proekt A, Vilim FS, Alexeeva V, Brezina V, Friedman A, Jing J, Li L, Zhurov Y, Sweedler JV, Weiss KR. Identification of a new neuropeptide precursor reveals a novel source of extrinsic modulation in the feeding system of Aplysia. J Neurosci. 2005;25:9637–9648. doi: 10.1523/JNEUROSCI.2932-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers EW, Fu JJ, Krenz WD, Baro DJ. Tonic nanomolar dopamine enables an activity-dependent phase recovery mechanism that persistently alters the maximal conductance of the hyperpolarization-activated current in a rhythmically active neuron. J Neurosci. 2011a;31:16387–16397. doi: 10.1523/JNEUROSCI.3770-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers EW, Krenz WD, Baro DJ. Tonic dopamine induces persistent changes in the transient potassium current through translational regulation. J Neurosci. 2011b;31:13046–13056. doi: 10.1523/JNEUROSCI.2194-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roffman RC, Norris BJ, Calabrese RL. Animal-to-animal variability of connection strength in the leech heartbeat central pattern generator. J Neurophysiol. 2011 doi: 10.1152/jn.00903.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roffman RC, Norris BJ, Calabrese RL. Animal-to-animal variability of connection strength in the leech heartbeat central pattern generator. J Neurophysiol. 2012;107:1681–1693. doi: 10.1152/jn.00903.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saideman SR, Christie AE, Torfs P, Huybrechts J, Schoofs L, Nusbaum MP. Actions of kinin peptides in the stomatogastric ganglion of the crab Cancer borealis. J Exp Biol. 2006;209:3664–3676. doi: 10.1242/jeb.02415. [DOI] [PubMed] [Google Scholar]
- Saideman SR, Ma M, Kutz-Naber KK, Cook A, Torfs P, Schoofs L, Li L, Nusbaum MP. Modulation of rhythmic motor activity by pyrokinin peptides. J Neurophysiol. 2007;97:579–595. doi: 10.1152/jn.00772.2006. [DOI] [PubMed] [Google Scholar]
- Schulz DJ, Goaillard JM, Marder E. Variable channel expression in identified single and electrically coupled neurons in different animals. Nat Neurosci. 2006;9:356– 362. doi: 10.1038/nn1639. [DOI] [PubMed] [Google Scholar]
- Schulz DJ, Goaillard JM, Marder E. Quantitative expression profiling of identified neurons reveals cell-specific constraints on highly variable levels of gene expression. Proc Natl Acad Sci U S A. 2007;104:13187–13191. doi: 10.1073/pnas.0705827104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz TL, Harris-Warrick RM, Glusman S, Kravitz EA. A peptide action in a lobster neuromuscular preparation. J Neurobiol. 1980;11:623–628. doi: 10.1002/neu.480110611. [DOI] [PubMed] [Google Scholar]
- Selverston AI. Are central pattern generators understandable? Behavioral and Brain Sciences. 1980;3:535–571. [Google Scholar]
- Seung HS. Neuroscience: Towards functional connectomics. Nature. 2011;471:170–172. doi: 10.1038/471170a. [DOI] [PubMed] [Google Scholar]
- Siegelbaum SA, Camardo JS, Kandel ER. Serotonin and cyclic AMP close single K+ channels in Aplysia sensory neurones. Nature. 1982;299:413–417. doi: 10.1038/299413a0. [DOI] [PubMed] [Google Scholar]
- Sigvardt KA, Grillner S, Wallen P, Van Dongen PA. Activation of NMDA receptors elicits fictive locomotion and bistable membrane properties in the lamprey spinal cord. Brain Res. 1985;336:390–395. doi: 10.1016/0006-8993(85)90676-6. [DOI] [PubMed] [Google Scholar]
- Siwicki KK, Beltz BS, Kravitz EA. Proctolin in identified serotonergic, dopaminergic, and cholinergic neurons in the lobster, Homarus americanus. J Neurosci. 1987;7:522–532. doi: 10.1523/JNEUROSCI.07-02-00522.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobie EA. Parameter sensitivity analysis in electrophysiological models using multivariable regression. Biophys J. 2009;96:1264–1274. doi: 10.1016/j.bpj.2008.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer N, Cymbalyuk G, Zhang H, Edwards DH, Baro DJ. Serotonin transduction cascades mediate variable changes in pyloric network cycle frequency in response to the same modulatory challenge. J Neurophysiol. 2008;99:2844–2863. doi: 10.1152/jn.00986.2007. [DOI] [PubMed] [Google Scholar]
- Stein W. Modulation of stomatogastric rhythms. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2009;195:989–1009. doi: 10.1007/s00359-009-0483-y. [DOI] [PubMed] [Google Scholar]
- Stemmler EA, Bruns EA, Cashman CR, Dickinson PS, Christie AE. Molecular and mass spectral identification of the broadly conserved decapod crustacean neuropeptide pQIRYHQCYFNPISCF: the first PISCF-allatostatin (Manduca sexta- or C-type allatostatin) from a non-insect. General and comparative endocrinology. 2010;165:1–10. doi: 10.1016/j.ygcen.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stent GS, Kristan WB, Jr, Friesen WO, Ort CA, Poon M, Calabrese RL. Neuronal generation of the leech swimming movement. Science. 1978;200:1348–1357. doi: 10.1126/science.663615. [DOI] [PubMed] [Google Scholar]
- Stent GS, Thompson WJ, Calabrese RL. Neural control of heartbeat in the leech and in some other invertebrates. Physiol Rev. 1979;59:101–136. doi: 10.1152/physrev.1979.59.1.101. [DOI] [PubMed] [Google Scholar]
- Sweedler JV, Li L, Rubakhin SS, Alexeeva V, Dembrow NC, Dowling O, Jing J, Weiss KR, Vilim FS. Identification and characterization of the feeding circuit-activating peptides, a novel neuropeptide family of aplysia. J Neurosci. 2002;22:7797–7808. doi: 10.1523/JNEUROSCI.22-17-07797.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swensen AM, Bean BP. Robustness of burst firing in dissociated purkinje neurons with acute or long-term reductions in sodium conductance. J Neurosci. 2005;25:3509–3520. doi: 10.1523/JNEUROSCI.3929-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swensen AM, Marder E. Multiple peptides converge to activate the same voltage-dependent current in a central pattern-generating circuit. J Neurosci. 2000;20:6752–6759. doi: 10.1523/JNEUROSCI.20-18-06752.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swensen AM, Marder E. Modulators with convergent cellular actions elicit distinct circuit outputs. J Neurosci. 2001;21:4050–4058. doi: 10.1523/JNEUROSCI.21-11-04050.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo TM, Chen R, Goeritz ML, Maloney RT, Tang LS, Li L, Marder E. Distribution and physiological effects of B-type allatostatins (myoinhibitory peptides, MIPs) in the stomatogastric nervous system of the crab, Cancer borealis. J Comp Neurol. 2011;519:2658–2676. doi: 10.1002/cne.22654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taghert PH, Nitabach MN. Peptide neurmodulation in invertebrate model systems. Neuron. 2012 doi: 10.1016/j.neuron.2012.08.035. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AL, Goaillard JM, Marder E. How multiple conductances determine electrophysiological properties in a multicompartment model. J Neurosci. 2009;29:5573–5586. doi: 10.1523/JNEUROSCI.4438-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thirumalai V, Prinz AA, Johnson CD, Marder E. Red pigment concentrating hormone strongly enhances the strength of the feedback to the pyloric rhythm oscillator but has little effect on pyloric rhythm period. J Neurophysiol. 2006;95:1762–1770. doi: 10.1152/jn.00764.2005. [DOI] [PubMed] [Google Scholar]
- Tobin AE, Cruz-Bermudez ND, Marder E, Schulz DJ. Correlations in ion channel mRNA in rhythmically active neurons. PLoS ONE. 2009;4:e6742. doi: 10.1371/journal.pone.0006742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilim FS, Sasaki K, Rybak J, Alexeeva V, Cropper EC, Jing J, Orekhova IV, Brezina V, Price D, Romanova EV, et al. Distinct mechanisms produce functionally complementary actions of neuropeptides that are structurally related but derived from different precursors. J Neurosci. 2010;30:131–147. doi: 10.1523/JNEUROSCI.3282-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker RJ, Woodruff GN, Glaizner B, Sedden CB, Kerkut GA. The pharmacology of Helix dopamine receptor of specific neurones in the snail, Helix aspersa. Comp Biochem Physiol. 1968;24:455–469. doi: 10.1016/0010-406x(68)90997-3. [DOI] [PubMed] [Google Scholar]
- Webster SG, Keller R, Dircksen H. The CHH-superfamily of multifunctional peptide hormones controlling crustacean metabolism, osmoregulation, moulting, and reproduction. Gen Comp Endocrinol. 2012;175:217–233. doi: 10.1016/j.ygcen.2011.11.035. [DOI] [PubMed] [Google Scholar]
- Weimann JM, Marder E. Switching neurons are integral members of multiple oscillatory networks. Curr Biol. 1994;4:896–902. doi: 10.1016/s0960-9822(00)00199-8. [DOI] [PubMed] [Google Scholar]
- Weimann JM, Meyrand P, Marder E. Neurons that form multiple pattern generators: identification and multiple activity patterns of gastric/pyloric neurons in the crab stomatogastric system. J Neurophysiol. 1991;65:111–122. doi: 10.1152/jn.1991.65.1.111. [DOI] [PubMed] [Google Scholar]
- Weimann JM, Skiebe P, Heinzel HG, Soto C, Kopell N, Jorge-Rivera JC, Marder E. Modulation of oscillator interactions in the crab stomatogastric ganglion by crustacean cardioactive peptide. J Neurosci. 1997;17:1748–1760. doi: 10.1523/JNEUROSCI.17-05-01748.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss KR, Brezina V, Cropper EC, Hooper SL, Miller MW, Probst WC, Vilim FS, Kupfermann I. Peptidergic co-transmission in Aplysia: functional implications for rhythmic behaviors. Experientia. 1992;48:456–463. doi: 10.1007/BF01928164. [DOI] [PubMed] [Google Scholar]
- Weiss KR, Cohen JL, Kupfermann I. Modulatory control of buccal musculature by a serotonergic neuron (metacerebral cell) in Aplysia. J Neurophysiol. 1978;41:181–203. doi: 10.1152/jn.1978.41.1.181. [DOI] [PubMed] [Google Scholar]
- Willows AO, Dorsett DA, Hoyle G. The neuronal basis of behavior in Tritonia. I. Functional organization of the central nervous system. J Neurobiol. 1973;4:207–237. doi: 10.1002/neu.480040306. [DOI] [PubMed] [Google Scholar]
- Wilson D. The central nervous control of locust flight. J Exp Biol. 1961;38:471–490. [Google Scholar]
- Wilson DM. Central nervous mechanisms for the generation of rhythmic behaviour in arthropods. Symp Soc Exp Biol. 1966;20:199–228. [PubMed] [Google Scholar]
- Wiwatpanit T, Powers B, Dickinson PS. Inter-animal variability in the effects of C-type allatostatin on the cardiac neuromuscular system in the lobster Homarus americanus. J Exp Biol. 2012;215:2308–2318. doi: 10.1242/jeb.069989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JS, Vilim FS, Hatcher NG, Due MR, Sweedler JV, Weiss KR, Jing J. Composite modulatory feedforward loop contributes to the establishment of a network state. J Neurophysiol. 2010;103:2174–2184. doi: 10.1152/jn.01054.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Rodgers EW, Krenz WD, Clark MC, Baro DJ. Cell specific dopamine modulation of the transient potassium current in the pyloric network by the canonical D1 receptor signal transduction cascade. J Neurophysiol. 2010;104:873–884. doi: 10.1152/jn.00195.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Sheibanie AF, Oh M, Rabbah P, Nadim F. Peptide neuromodulation of synaptic dynamics in an oscillatory network. J Neurosci. 2011;31:13991–14004. doi: 10.1523/JNEUROSCI.3624-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhurov Y, Brezina V. Variability of motor neuron spike timing maintains and shapes contractions of the accessory radula closer muscle of Aplysia. J Neurosci. 2006;26:7056–7070. doi: 10.1523/JNEUROSCI.5277-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]