Abstract
Pramipexole (PPX) is a dopamine agonist medication that has been implicated in the development of pathological gambling and other impulse control disorders. Johnson, Madden, Brewer, Pinkston, and Fowler (2011) reported that PPX increased male rats’ preference for gambling-like rewards (those arranged according to a variable-ratio schedule) over predictable rewards (those obtained from a fixed-ratio schedule). The present experiment explored the possibility that Johnson et al. underestimated the effects of PPX on gambling-like choices by constraining their rats’ daily income. In the present experiment conducted in a closed economy, PPX produced a dose-related increase in choice of the gambling-like alternative. In a control condition, PPX did not disrupt choice, suggesting the increased preference for gambling-like rewards was not due to nonspecific drug effects. Our findings are qualitatively consistent with those of Johnson et al., although the dose-related effect and larger effect size in the current study suggest that the effect of PPX on gambling-like choices is more pronounced when income was not constrained. This finding is consistent with clinical reports suggesting PPX is related to the development of problem gambling in humans.
Keywords: pramipexole, dopamine agonist, gambling, Parkinson’s disease, rat
The selective dopamine (DA) D2/D3 receptor agonist pramipexole (PPX) is commonly used in the treatment of Parkinson’s disease (PD), restless legs syndrome, and fibromyalgia. A number of clinical reports have implicated PPX in the development of impulse control disorders (ICDs) such as pathological gambling (Cornelius, Tippmann-Peikert, Slocumb, Frerichs, & Silber, 2010; Driver-Dunckley, Samanta, & Stacy, 2003; Driver-Dunckley et al., 2007; Dodd et al., 2005; Grosset et al., 2006; Holman, 2009; Molina et al., 2000), compulsive shopping (Giladi, Weitzman, Schreiber, Shabtai, & Peretz, 2007), hypersexuality (Giovannoni, O’Sullivan, Turner, Manson, & Lees, 2000; Klos, Bower, Josephs, Matsumoto, & Ahlskog, 2005; McKeon et al., 2007; Munhoz, Fabiani, Becker, & Teive, 2009), and compulsive eating (Nirenberg & Waters, 2006). One reason to suspect PPX increases impulsive decision-making is that the drug has greater affinity for the D3 receptor subtype (Kvernmo, Härtter, & Bürger, 2006) expressed predominantly in the limbic areas of the brain (Sokoloff, Giros, Martres, Bouthenet, & Schwartz, 1990). Limbic activity is widely believed to play a role in impulsive decision-making, particularly in addiction contexts (e.g., Lader, 2008).
A small experimental literature has evaluated the effects of PPX on impulsivity; where this concept is operationalized as preference for a smaller-sooner over a larger-later reward. Hamidovic, Kang, and de Wit (2008) reported PPX (0.25 or 0.5 mg) did not affect the degree to which delayed rewards were discounted in value by humans, but their sample size was small (n=8) and a trend toward steeper discounting at the 0.5 mg dose was visually apparent. Voon et al. (2010) reported PPX increased impulsive choices made by a sample of PD patients who, before the study, had developed an ICD (pathological gambling or compulsive shopping) after taking PPX or ropinirole (another DA agonist with differential affinity for the D3 receptor). Suspending the patients’ intake of these drugs decreased their impulsive decision-making on the Experiential Discounting Task (Reynolds & Schiffbauer, 2004). Choices of PD patients without an ICD were unaffected by the DA agonists.
Three experiments with rats have produced mixed results. Madden, Johnson, Brewer, Pinkston, and Fowler (2010) reported PPX (0.1–0.3 mg/kg) significantly increased impulsive choices. However, in their second experiment, which used a different choice procedure (developed by Evenden & Ryan, 1996), only trend-level increases in impulsive choice were observed, and only at the 0.03 mg/kg dose (higher doses nonspecifically disrupted choice). Koffarnus, Newman, Grundt, Rice, and Woods (2011) reported similar outcomes with this procedure.
Three experiments have evaluated the effects of PPX on gambling-related behavior. Riba, Krämer, Heldmann, Richter, and Münte (2008) reported that healthy humans placed larger bets following unexpected wins when given acute PPX (0.5 mg). Of the five other gambling measures that might have been affected by PPX (e.g., wagering more following a loss), no significant differences from placebo were detected. Voon et al. (2011) reported PD patients with ICDs made riskier choices in a gambling task when taking PPX than when not. PPX had no effect on choices made by healthy volunteers or PD patients without ICDs.
A single nonhuman experiment has evaluated the effects of PPX on gambling-related behavior. Johnson, Madden, Brewer, Pinkston, and Fowler (2011) examined the effects of PPX on rats’ allocation of a daily response budget between certain and probabilistic sources of food; when the response budget was expended no additional food could be obtained, and none was provided by the experimenter. The certain alternative was a fixed-ratio (FR) schedule where the number of responses per food reward was the same every time. The probabilistic, gambling-like alternative was a variable-ratio (VR) schedule (unpredictable number of responses per reward). In one condition, the FR schedule requirement was much lower than the VR requirement and this contrast established a stable, though non-exclusive preference for the FR. Acute PPX at doses of 0.1–0.3 mg/kg significantly increased rats’ preferences for the gambling-like VR schedule; the effect was not dose-related. The opposite effect of PPX (i.e., decrease in VR preference) was not observed in a control condition in which stable preference for the VR alternative was established prior to dosing, suggesting the increase in VR choice observed previously was not due to nonspecific disruptive effects of the drug.
The response-budget feature of the Johnson et al. (2011) experiment allowed an examination of the effects of PPX on gambling-like choices in a context where choosing the VR alternative resulted in losses of income. While rich in face validity, a shortcoming of this procedure is that the income losses associated with a VR choice were relatively larger in the low-gambling condition than in the high-gambling (control) condition. This is because in the former condition the response budget was substantially lower than in the latter, and this asymmetry may have resulted in a suppression of choosing the gambling-like alternative in the low-gambling baseline (i.e., an under-estimation of the effect of PPX on preference for a gambling-like alternative). To address this concern, the present study was conducted in a true closed economy in which income was not artificially constrained by the experimenter and no supplemental feeding was provided (e.g., Hursh, 1984). Session duration was long (4 hours) to ensure that choice was affected by the economic contingencies and PPX, rather than by constraints on response time. Under these conditions, an expanded picture of the effects of acute PPX on preference for probabilistic sources of reward may be obtained.
Method
Subjects
Eight experimentally naïve male Wistar rats were obtained from Charles River (Raleigh, NC). Rats were 14 weeks old at the start of the experiment and were housed individually. A 12 hr/12 hr light/dark cycle was programmed in the colony room and water was available ad libitum in the home cages. Rats were maintained in a closed-economy in which all food was obtained during the experimental sessions. Animal use was in accordance with the Institutional Animal Care and Use Committee of the University of Kansas.
Apparatus
Sessions took place within standard operant conditioning chambers (24.1 cm × 30.5 cm × 21.0 cm; Med Associates, Inc., St. Albans, VT). Centered on the front wall of each chamber and positioned 1 cm above the floor grid was a pellet receptacle (3 cm × 4 cm) into which a pellet dispenser (H14-23R, Coulbourn Instruments, Allentown, PA) could deliver nutritional grain-based rat pellets (45 mg; Bio-Serv, Frenchtown, NJ). An infrared pellet detector (Pinkston, Ratzlaff, Madden, & Fowler, 2008) monitored pellet deliveries. Above the receptacle (10 cm) was a non-retractable lever with retractable levers to the left and right (spaced 11 cm apart). A 28-V DC cue light was positioned 6 cm above each lever. A house light was centered 19 cm above the floor on the rear wall. Each chamber was equipped with a white noise speaker and was situated within a sound-attenuating box (ENV-018MD; Med Associates, Inc., St. Albans, VT). All experimental events were programmed using MED-PC™ IV software and were executed via a PC in an adjacent room.
Procedure
With the exception of the response budget and the fixed-VR sequence in drug sessions (discussed below), the procedures employed in the present experiment were identical to those used by Johnson et al. (2011); therefore, an abbreviated description of these procedures is provided. After preliminary training, 4-hour experimental sessions were programmed to begin with a series of four 21-trial blocks. The first 16 trials in each block were forced-choice trials, and the remaining 5 were free-choice trials. On forced-choice trials, rats completed either the FR and VR-50 schedules (8 trials of each) assigned to the left and right levers (assignment counterbalanced across rats). The FR value depended upon the baseline condition (see below). For no-injection (control) sessions, VR response requirements were selected randomly, with replacement, from the following array: [1, 33, 67, 99]. In this way, the VR schedule was conceptualized as a gambling-like source of income because the relation between response and reward was probabilistic much like a random-ratio schedule, according to which most gambling games are programmed (e.g., slot machines). To ensure choice differences between drug and saline sessions were a function of drug and dose, a fixed sequence of VR response requirements was programmed in every saline and drug session.
When the rat completed either response requirement, three food pellets were delivered to the pellet receptacle (at an approximate rate of one pellet every 0.75 s) and the next trial was initiated without an inter-trial interval. In the event of pellet-dispenser failure, sessions terminated if the pellet detector determined that all 3 pellets had not been delivered within 6 seconds of pellet-dispenser activation. The final five trials within each 21-trial block were free-choice trials on which the rat could choose between the FR and VR schedules. After four of these 21-trial blocks (84 trials), the remainder of the session was composed of free-choice trials.
Low-gambling baseline condition
Four rats (randomly assigned) completed this condition first (see Table 1). On free-choice trials, rats chose between obtaining three food pellets by either completing a VR-50 or an adjusting FR schedule. The FR value was initially set at 5 and was adjusted between sessions until VR choice was ≤ 20% for ten consecutive sessions. The FR value was decreased by 1 or 2 responses (depending on how far choice was outside the target range) if VR choice was > 20% for two consecutive sessions. The FR value was increased using the same rules if VR choice was 0%. The goal was to produce stable non-exclusive preference for the FR alternative. After stability was achieved, pre-session saline and PPX injections were initiated (see below). At the conclusion of the condition, rats that completed the low-gambling condition first completed the high-gambling condition next, and vice versa.
Table 1.
Fixed-ratio (FR) value at which stable choice was achieved and the number of sessions required to achieve stability in each baseline condition (in order of exposure) for individual rats.
| Rat | Baseline Condition | FR Value | Sessions to Stability |
|---|---|---|---|
| 1 | Low-gambling | 10 | 19 |
| High-gambling | 60 | 34 | |
| 2 | Low-gambling | 10 | 26 |
| High-gambling | 70 | 79 | |
| 3 | Low-gambling | 5 | 22 |
| High-gambling | 35 | 31 | |
| 4 | Low-gambling | 1 | 41 |
| High-gambling | 40 | 41 | |
| 5 | High-gambling | 35 | 13 |
| Low-gambling | 3 | 33 | |
| 6 | High-gambling | 30 | 12 |
| Low-gambling | 10 | 71 | |
| 7 | High-gambling | 35 | 98 |
| Low-gambling | 10 | 12 |
High-gambling baseline condition
As before, rats chose between a VR-50 and an adjusting FR schedule. The FR value was initially set at 30 and was adjusted between sessions until VR choice was ≥ 80% for ten consecutive sessions with no adjustments to the FR value. The FR value was increased by 5 responses if VR choice was < 80% for two consecutive sessions. The FR value was decreased using the same rules if VR choice was 100%. Once the stability criterion was met, the pre-session drug-administration regimen was initiated in the next session.
Drug administration
Once a stable low- or high-gambling baseline was established, an initial saline test was administered. PPX was then administered in a descending dose order. PPX hydrochloride (N′-propyl-4,5,6,7-tetrahydrobenzothiazole-2,6-diamine dihydrochloride) was synthesized and provided by Drs. Shaomeng Wang and Jianyong Chen (University of Michigan, Ann Arbor, MI). PPX was dissolved in physiological saline. Four dosages of PPX (0.03, 0.1, 0.18, 0.3 mg/kg) or saline vehicle were administered subcutaneously 10 min prior to the session at a volume of 1.0 ml/kg. Each saline or drug administration was separated by at least four no-injection sessions. No-injection sessions continued until choice returned to the baseline range for four consecutive sessions (median: 4; range: 4–20 sessions). The dosing sequence was repeated three times in each baseline condition, each time separated by a saline test.
Data Analysis
PPX effects on VR choice (i.e., “gambling”) in each baseline were analyzed using separate two-way repeated-measures analysis of variance (ANOVA; IBM SPSS Statistics 19) with “dose” (saline, 0.03, 0.1, 0.18, 0.3) and “series” (first, second, and third injection) as within-subject factors. Bonferroni-corrected post-hoc comparisons were made between doses. Generalized eta squared was used to express effects sizes (see Bakeman, 2005 for a discussion of the merits of this measure when using repeated-measures ANOVA designs).
Upon completion of the high-gambling baseline condition, one rat (R8) failed to meet the choice criterion for the low-gambling baseline at the lowest possible FR value (1) after 55 sessions and was therefore excluded from the study and subsequent analyses. In the low-gambling baseline conditions, one rat (R4) failed to complete a session in which saline was administered before the session. This missing data point was interpolated from the average of the other rats in this session. In the high-gambling baseline, one rat (R5) did not respond during the final exposure to 0.3 mg/kg and this missing data point was interpolated as above. Also in the high-gambling baseline, the VR choices of one rat (R7) fell out of the baseline range between the second and third series of injections. These missing data were interpolated in two different ways: from the mean of a) this rat’s choices in the preceding two injection series, and b) from the mean of the other rats’ choices in this session. Both methods produced the same qualitative outcome so the results of method a are reported.
Because some researchers have reported D2/D3 agonists increase perseverative responding (Boulougouris, Castañé, & Robbins, 2009; Haluk & Floresco, 2009), it is important to determine if, in the present experiment, increased selection of a non-preferred lever (e.g., the VR lever in the low-gambling baseline) was due to increased perseveration on the lever that ended the final sequence of forced-choice trials. For this analysis, conditional probabilities of same-lever choice in transitions between the last forced- and the first free-choice trial were calculated and analyzed using separate two-way repeated-measures ANOVA for each baseline.
Results
Sessions required to reach stability did not differ statistically between low-gambling (M = 32, SEM = 7.41) and high-gambling (M = 44, SEM = 12.35) baseline conditions, paired-samples t(6) = −0.67, p = .53 (Table 1). Median FR requirements in the low- and high-gambling baseline conditions were FR-10 (range: 1–10) and FR-35 (range: 30–70). Individual differences in terminal FR values at which stable preference was obtained in either baseline, an index of VR preference, were not correlated with subsequent PPX dose effects (Spearman’s r > −.75, p’s > .06).
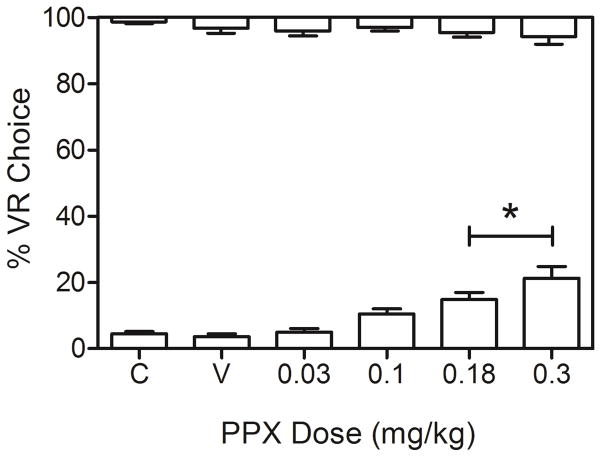
Figure 1 shows group mean percent VR choice (± SEM) as a function of PPX dose. Because there was no significant effect of series (i.e., first, second, third injection), choice was collapsed across series. Relative to saline, a significant main effect of PPX was detected in the low-gambling baseline condition (bottom x-axis), F(4, 24) = 21.15, p < .001, ηG2 = .35, but not in the high-gambling (control) condition (top axis), F(4, 24) = 0.40, p = .81. The Dose x Series interaction was not significant in either baseline (p > .6). Post-hoc comparisons revealed PPX doses of 0.18 and 0.3 mg/kg increased percent VR choice above saline levels in the low-gambling baseline (p < .02 in both cases). In addition, these same doses significantly increased VR choice above the 0.03 dose level (p < .01 in both cases). A significant linear contrast indicated PPX had a dose-related effect on percent VR choice in the low-gambling baseline condition, F(1, 6)= 45.55, p = .001, ηG2 = .52.
Figure 1. Effects of PPX on VR Choice in Low- and High-gambling Baselines.
Percent choice of the VR alternative as a function of PPX dose in low- (bottom of graph) and high-gambling (top of graph) baseline conditions. Asterisk denotes doses significantly different from saline. “C” and “V” represent control (no injection) and saline vehicle, respectively. Error bars are SEM.
In the low-gambling baseline, PPX had no effect on the probability that rats would make their first free-choice response on the same lever on which the last forced-choice trial was completed (main effect of dose, p > .31); nor did dose interact with series. In the high-gambling baseline, PPX significantly decreased the likelihood of perseveration at high PPX doses, F(4, 24) = 4.41, p < .01, ηG2 = .23, but this effect was not observed across dosing series.
Discussion
The present experiment was conducted to evaluate the effects of PPX on gambling-like preferences in a closed economy in which no budget constraints were imposed. We hypothesized that the response budgets arranged by Johnson et al. (2011) led to an underestimation of the effects of PPX on preferences for a gambling-like, VR schedule of reinforcement. The present findings offer provisional support for this hypothesis. The dose effect size produced by PPX in the present study (collapsed across series) was higher (.63) than in Johnson et al. (.36). In addition, a significant dose-response effect was detected in the present study; no such effect was reported by Johnson et al. (2011). Thus, the current findings clarify the profile of PPX effects on gambling-like choice under more economically neutral conditions than those arranged by Johnson et al.
Consistent with the findings of Johnson et al. (2011) there was no evidence that the increase in VR choice produced by PPX was due to an increase in response perseveration. Although D2/D3 agonists have been shown to induce perseveration, the finding may be limited to learning paradigms (Boulougouris et al., 2009; Haluk & Floresco, 2009) rather than the steady-state performances reported here.
Our results are in accord with contemporary hypotheses regarding the role of DA in impulsive decision-making. First, DA agonist medications like PPX disrupt humans’ abilities to learn from negative outcomes (e.g., Cools, Altamirano, & D’Esposito, 2006), a process which could have decreased the effect of having to occasionally complete a relatively larger VR response requirement (33, 66, or 99 responses) compared to the FR alternative (≤10 responses, in all cases). Second, rewards arranged on the VR alternative were occasionally immediate (i.e., delivered following a single response); PPX may have therefore increased VR choice by sensitizing rats to the prospect of immediacy (e.g., Madden et al., 2010). The latter mechanism is consistent with the hypothesis that PPX increases impulsive choice by more heavily weighting the effect of immediate rewards, which could in turn lead to increased preference for gambling with occasional immediate wins (see Madden, Francisco, Brewer, & Stein, 2011 for discussion). PPX may have also (or alternatively) increased the value of the VR alternative because of the probabilistic way in which it was scheduled to be delivered. Madden, Petry, and Johnson (2009) reported that pathological gamblers discounted the value of probabilistic monetary rewards less than matched controls. This propensity to over-value probabilistic outcomes may translate to stronger preferences for the VR alternative in the present model of nonhuman gambling.
Three shortcomings of the present study are noteworthy. First, because the behavioral procedures employed herein have not been used extensively, we do not know if the same profile of results would be produced by any drug; thus, these findings should be interpreted cautiously. Second, the procedures required considerable time and resources to reestablish stable preference between dosing series. Because the latter effect proved to be nonsignificant, future studies wishing to explore the effect of PPX against other drugs, or in combination with DA antagonists, should arrange a single session at each acute dose. Third, although reports of emergent ICDs have been most often reported in individuals with PD, we did not use Parkinsonian rats. The present findings are, therefore, qualitatively more similar to clinical reports of the development of ICDs in non-PD patients; e.g., those diagnosed with restless legs syndrome (e.g., Cornelius et al., 2010; Pourcher, Rémillard, & Cohen, 2010).
In sum, the present findings provide additional evidence that acute PPX, a D2/D3 dopamine agonist, increases preference for a gambling-like (VR schedule) over a non-gambling (FR schedule) reward source in male rats. When integrated with other nonhuman experiments examining the effects of DA modulation on choice, our findings suggest a critical role for the neurotransmitter in risk-taking and impulsivity (Hand, Fox, & Reilly, 2009; St. Onge & Floresco, 2009; van Gaalen, van Koten, Schoffelmeer, & Vanderschuren, 2006; Wade, de Wit, & Richards, 2000). The extent to which these findings have relevance in the clinical use of PPX must await further research evaluating the effects of other compounds on choice in this preparation. Future investigations may also profit from evaluating the effects of receptor-specific antagonists (see e.g., Koffarnus et al., 2011). Our findings provide support for the utility of nonhuman laboratory models of human gambling (Madden, Ewan, & Lagorio, 2007; Scarf et al., 2011; Zeeb, Robbins, & Winstanley, 2009).
Acknowledgments
The research was supported by grants from the National Institutes of Health (National Institute on Drug Abuse): R21 DA023564, R01 DA029605 awarded to the second author. Pramipexole was provided under NIH grant DA 020669 (P.I., James H. Woods, University of Michigan, Ann Arbor). The authors would like to acknowledge Adam Brewer and Jonathan Pinkston, Ph.D. for their intellectual contributions to the present study.
Footnotes
All authors contributed in a significant way and have read and approved the final manuscript.
Disclosures
None of the authors have any real or potential conflict(s) of interest, including financial, personal, or other relationships with other organizations or pharmaceutical/biomedical companies that may inappropriately impact or influence the research and interpretation of the findings.
References
- Bakeman R. Recommended effect size statistics for repeated measures designs. Behavior Research Methods. 2005;37:379–384. doi: 10.3758/bf03192707. [DOI] [PubMed] [Google Scholar]
- Boulougouris V, Castañé A, Robbins TW. Dopamine D2/D3 receptor agonist quinpirole impairs spatial reversal learning in rats: Investigation of D3 receptor involvement in persistent behavior. Psychopharmacology (Berl) 2009;202:611–620. doi: 10.1007/s00213-008-1341-2. [DOI] [PubMed] [Google Scholar]
- Cools R, Altamirano L, D’Esposito M. Reversal learning in Parkinson’s disease depends on medication status and outcome valence. Neuropsychologia. 2006;44:1663–1673. doi: 10.1016/j.neuropsychologia.2006.03.030. [DOI] [PubMed] [Google Scholar]
- Cornelius JR, Tippmann-Peikert M, Slocumb NL, Frerichs CF, Silber MH. Impulse control disorders with the use of dopaminergic agents in restless legs syndrome: A case-control study. Sleep. 2010;33:81–87. [PMC free article] [PubMed] [Google Scholar]
- Dodd ML, Klos KJ, Bower JH, Geda YE, Josephs KA, Ahlskog JE. Pathological gambling caused by drugs used to treat Parkinson’s disease. Archives of Neurology. 2005;62:1377–1381. doi: 10.1001/archneur.62.9.noc50009. [DOI] [PubMed] [Google Scholar]
- Driver-Dunckley ED, Noble BN, Hentz JG, Evidente VG, Caviness JN, Parish J, Adler CH. Gambling and increased sexual desire with dopaminergic medications in restless legs syndrome. Clinical Neuropharmacology. 2007;30:249–255. doi: 10.1097/wnf.0b013e31804c780e. [DOI] [PubMed] [Google Scholar]
- Driver-Dunckley E, Samanta J, Stacy M. Pathological gambling associated with dopamine agonist therapy in Parkinson’s disease. Neurology. 2003;61:422–423. doi: 10.1212/01.wnl.0000076478.45005.ec. [DOI] [PubMed] [Google Scholar]
- Evenden JL, Ryan CN. The pharmacology of impulsive behaviour in rats: The effects of drugs on response choice with varying delays of reinforcement. Psychopharmacology (Berl) 1996;128:161–170. doi: 10.1007/s002130050121. [DOI] [PubMed] [Google Scholar]
- Giladi N, Weitzman N, Schreiber S, Shabtai H, Peretz C. New onset heightened interest or drive for gambling, shopping, eating or sexual activity in patients with Parkinson’s disease: The role of dopamine agonist treatment and age at motor symptoms onset. Journal of Psychopharmacology. 2007;21:501–506. doi: 10.1177/0269881106073109. [DOI] [PubMed] [Google Scholar]
- Giovannoni G, O’Sullivan JD, Turner K, Manson AJ, Lees AJ. Hedonistic homeostatic dysregulation in patients with Parkinson’s disease on dopamine replacement therapies. Journal of Neurology, Neurosurgery, and Psychiatry. 2000;68:423–428. doi: 10.1136/jnnp.68.4.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosset KA, Macphee G, Pal G, Stewart D, Watt A, Davie J, Grosset DG. Problematic gambling on dopamine agonists: Not such a rarity. Movement Disorders. 2006;21:2206–2208. doi: 10.1002/mds.21110. [DOI] [PubMed] [Google Scholar]
- Haluk DM, Floresco SB. Ventral striatal dopamine modulation of different forms of behavioral flexibility. Neuropsychopharmacology. 2009;34:2041–2052. doi: 10.1038/npp.2009.21. [DOI] [PubMed] [Google Scholar]
- Hamidovic A, Kang UJ, de Wit H. Effects of low to moderate doses of pramipexole on impulsivity and cognition in healthy volunteers. Journal of Clinical Psychopharmacology. 2008;28:45–51. doi: 10.1097/jcp.0b013e3181602fab. [DOI] [PubMed] [Google Scholar]
- Hand DJ, Fox AT, Reilly MP. Differential effects of d-amphetamine on impulsive choice in spontaneously hypertensive and Wistar-Kyoto rats. Behavioural Pharmacology. 2009;20:549–553. doi: 10.1097/FBP.0b013e3283305ee1. [DOI] [PubMed] [Google Scholar]
- Holman AJ. Impulse control disorder behaviors associated with pramipexole used to treat fibromyalgia. Journal of Gambling Studies. 2009;25:425–431. doi: 10.1007/s10899-009-9123-2. [DOI] [PubMed] [Google Scholar]
- Hursh SR. Behavioral economics. Journal of the Experimental Analysis of Behavior. 1984;42:435–452. doi: 10.1901/jeab.1984.42-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PS, Madden GJ, Brewer AT, Pinkston JW, Fowler SC. Effects of acute pramipexole on preference for gambling-like schedules of reinforcement in rats. Psychopharmacology (Berl) 2011;213:11–18. doi: 10.1007/s00213-010-2006-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klos KJ, Bower JH, Josephs KA, Matsumoto JY, Ahlskog JE. Pathological hypersexuality predominantly linked to adjuvant dopamine agonist therapy in Parkinson’s disease and multiple system atrophy. Parkinsonism & Related Disorders. 2005;11:381–386. doi: 10.1016/j.parkreldis.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Koffarnus MN, Newman AH, Grundt P, Rice KC, Woods JH. Effects of selective dopaminergic compounds on a delay-discounting task. Behavioural Pharmacology. 2011;22:300–311. doi: 10.1097/FBP.0b013e3283473bcb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvernmo T, Härtter S, Bürger E. A review of the receptor-binding and pharmacokinetic properties of dopamine agonists. Clinical Therapeutics. 2006;28:1065–1078. doi: 10.1016/j.clinthera.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Lader M. Antiparkinsonian medication and pathological gambling. CNS Drugs. 2008;22:407–441. doi: 10.2165/00023210-200822050-00004. [DOI] [PubMed] [Google Scholar]
- Madden GJ, Ewan EE, Lagorio CH. Toward an animal model of gambling: Delay discounting and the allure of unpredictable outcomes. Journal of Gambling Studies. 2007;23:63–83. doi: 10.1007/s10899-006-9041-5. [DOI] [PubMed] [Google Scholar]
- Madden GJ, Francisco MT, Brewer AT, Stein JS. Delay discounting and gambling. Behavioural Processes. 2011;87:43–49. doi: 10.1016/j.beproc.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden GJ, Johnson PS, Brewer AT, Pinkston JW, Fowler SC. Effects of pramipexole on impulsive choice in male Wistar rats. Experimental and Clinical Psychopharmacology. 2010;18:267–276. doi: 10.1037/a0019244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden GJ, Petry NM, Johnson PS. Pathological gamblers discount probabilistic rewards less steeply than matched controls. Experimental and Clinical Psychopharmacology. 2009;17:283–290. doi: 10.1037/a0016806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeon A, Josephs KA, Klos KJ, Hecksel K, Bower JH, Bostwick JM, Ahlskog JE. Unusual compulsive behaviors primarily related to dopamine agonist therapy in Parkinson’s disease and multiple system atrophy. Parkinsonism & Related Disorders. 2007;13:516–519. doi: 10.1016/j.parkreldis.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Molina JA, Saínz-Artiga MJ, Fraile A, Jiménez-Jiménez FJ, Villanueva C, Ortí-Pareja M, Bermejo F. Pathologic gambling in Parkinson’s disease: A behavioral manifestation of pharmacologic treatment? Movement Disorders. 2000;15:869–872. doi: 10.1002/1531-8257(200009)15:5<869::aid-mds1016>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Munhoz RP, Fabiani G, Becker N, Teive HA. Increased frequency and range of sexual behavior in a patient with Parkinson’s disease after use of pramipexole: A case report. Journal of Sexual Medicine. 2009;6:1177–1180. doi: 10.1111/j.1743-6109.2008.00861.x. [DOI] [PubMed] [Google Scholar]
- Nirenberg MJ, Waters C. Compulsive eating and weight gain related to dopamine agonist use. Movement Disorders. 2006;21:524–529. doi: 10.1002/mds.20757. [DOI] [PubMed] [Google Scholar]
- Pinkston JW, Ratzlaff KL, Madden GJ, Fowler SC. An inexpensive infrared detector to verify the delivery of food pellets. Journal of the Experimental Analysis of Behavior. 2008;90:249–255. doi: 10.1901/jeab.2008.90-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourcher E, Rémillard S, Cohen H. Compulsive habits in restless legs syndrome patients under dopaminergic treatment. Journal of the Neurological Sciences. 2010;290:52–56. doi: 10.1016/j.jns.2009.11.010. [DOI] [PubMed] [Google Scholar]
- Reynolds B, Schiffbauer R. Measuring state changes in human delay discounting: An experiential discounting task. Behavioural Processes. 2004;67:343–356. doi: 10.1016/j.beproc.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Riba J, Krämer UM, Heldmann M, Richter S, Münte TF. Dopamine agonist increases risk taking but blunts reward-related brain activity. PLoS One. 2008;3:e247. doi: 10.1371/journal.pone.0002479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarf D, Miles K, Sloan A, Goulter N, Hegan M, Seid-Fatemi A, Colombo M. Brain cells in the avian “prefrontal cortex” code for features of slot-machine-like gambling. PLoS One. 2011;6:e14589. doi: 10.1371/journal.pone.0014589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokoloff P, Giros B, Martres MP, Bouthenet ML, Schwartz JC. Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature. 1990;347:146–151. doi: 10.1038/347146a0. [DOI] [PubMed] [Google Scholar]
- St Onge JR, Floresco SB. Dopaminergic modulation of risk-based decision making. Neuropsychopharmacology. 2009;34:681–697. doi: 10.1038/npp.2008.121. [DOI] [PubMed] [Google Scholar]
- van Gaalen MM, van Koten R, Schoffelmeer AN, Vanderschuren LJ. Critical involvement of dopaminergic neurotransmission in impulsive decision making. Biological Psychiatry. 2006;60:66–73. doi: 10.1016/j.biopsych.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Voon V, Gao J, Brezing C, Symmonds M, Ekanayake V, Fernandez H, Hallett M. Dopamine agonists and risk: Impulse control disorders in Parkinson’s disease. Brain. 2011;134:1438–1446. doi: 10.1093/brain/awr080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voon V, Reynolds B, Brezing C, Gallea C, Skaljic M, Ekanayake V, Hallett M. Impulsive choice and response in dopamine agonist-related impulse control behaviors. Psychopharmacology (Berl) 2010;207:645–659. doi: 10.1007/s00213-009-1697-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade TR, de Wit H, Richards JB. Effects of dopaminergic drugs on delayed reward as a measure of impulsive behavior in rats. Psychopharmacology (Berl) 2000;150:90–101. doi: 10.1007/s002130000402. [DOI] [PubMed] [Google Scholar]
- Zeeb FD, Robbins TW, Winstanley CA. Serotonergic and dopaminergic modulation of gambling behavior as assessed using a novel rat gambling task. Neuropsychopharmacology. 2009;34:2329–2343. doi: 10.1038/npp.2009.62. [DOI] [PubMed] [Google Scholar]