Abstract
The neural crest is a population of migratory cells that follows specific pathways during development, eventually differentiating to form parts of the face, heart, and peripheral nervous system, the latter of which includes contributions from placodal cells derived from the ectoderm. Stationary, premigratory neural crest cells acquire the capacity to migrate by undergoing an epithelial-to-mesenchymal transition that facilitates their emigration from the dorsal neural tube. This emigration involves, in part, the dismantling of cell-cell junctions, including apically localized tight junctions in the neuroepithelium. In this study, we have characterized the role of the transmembrane tight junction protein claudin-1 during neural crest and placode ontogeny. Our data indicate that claudin-1 is highly expressed in the developing neuroepithelium but is down-regulated in migratory neural crest cells, although expression persists in the ectoderm from which the placode cells arise. Depletion or overexpression of claudin-1 augments or reduces neural crest cell emigration, respectively, but does not impact the development of several cranial placodes. Taken together, our results reveal a novel function for a tight junction protein in the formation of migratory cranial neural crest cells in the developing vertebrate embryo.
1. Introduction
Neural crest cells are a migratory cell population that eventually differentiate to form pigment cells, craniofacial structures, parts of the heart, and components of the peripheral nervous system (Le Dourarin and Kalcheim, 1999). Initially found as adherent neuroepithelial cells within the dorsal neural tube, premigratory neural crest cells subsequently delaminate and acquire the capacity to migrate by undergoing an epithelial-to-mesenchymal transition (EMT) (Hay, 1995). During EMT, premigratory neural crest cells lose apicobasal polarity, dismantle cellular junctions, and rearrange their cytoskeleton to facilitate migration to their final destinations at various sites in the developing embryo.
An important change that occurs as premigratory neural crest cells undergo EMT is the loss of tight junctions located on the apical side of the neuroepithelium. These junctions maintain apicobasal polarity and form a gate to regulate the flow of molecules between both adjacent cells and the apical and basolateral compartments of a given cell (Farquhar and Palade, 1963; Gupta and Ryan, 2010; Lal-Nag and Morin, 2009). Tight junctions consist of a network of protein-based strands connecting adjacent epithelial cells and contain three major transmembrane components (claudins, occludins, and tricellulin)(Schulzke and Fromm, 2009; Tsukita et al., 2008). The claudin protein family is comprised of 24 transmembrane proteins (Angelow et al., 2008; Schulzke and Fromm, 2009) that define tight junction selectivity and affect paracellular transport in tissues throughout the body (Tsukita and Furuse, 1998). Claudins regulate many critical developmental processes in vertebrates, with their loss leading to developmental abnormalities or even death (Tsukita et al., 1996). Specifically, claudin-1 knock-out mice are able to survive until birth, eventually dying due to water loss from skin barrier defects. This is similar to the human frame-shift mutation phenotype which has been implicated in the skin barrier disease neonatal ichthyosis and sclerosing cholangitis (NISCH) syndrome (Furuse et al., 2002; Hadj-Rabia et al., 2004). During chick embryogenesis, claudin-1 is expressed in the ectoderm and neural epithelium and plays a functional role during heart looping (Simard et al., 2005; Simard et al., 2006). Lower levels of claudin-1 are also observed in the primitive streak relative to the ectoderm, indicating that down-regulation of claudin-1 is necessary for EMT during gastrulation (Simard et al., 2005). Furthermore, overexpression of the transcriptional repressor Snail2 has been shown to directly repress claudin and occludin expression in cultured cells during Snail-induced EMT (Ikenouchi et al., 2003). In keeping with this observation, recent studies reveal that claudin-1 protein levels are significantly decreased in several types of invasive breast cancer cells (Tokes et al., 2005), and that loss of claudin-1 correlates with recurrence of rectal cancer and decreased survival (Yoshida et al., 2011).
Given the importance of claudin-1 during the EMT underlying gastrulation and cancer cell metastasis and the molecular similarities between neural crest cell and cancer cell EMT, it is highly likely that regulation of this tight junction component is also crucial during neural crest cell development. It is known that tight junctions must be dismantled prior to neural crest cell emigration (Sauka-Spengler and Bronner-Fraser, 2008) and that occludin, a tight junction transmembrane protein, is down-regulated in the neuroepithelium during neural tube closure (Aaku-Saraste et al., 1996). It was also recently shown that the tight junction scaffolding protein cingulin plays a critical role in regulating neural crest cell emigration in the developing chick embryo (Wu et al., 2011). We now characterize the expression of claudin-1 in early chick embryos and suggest a functional role for claudin-1 during neural crest cell emigration. Here, we show that claudin-1 is expressed in the proper spatio-temporal pattern to play a role in neural crest cell development, and depletion or overexpression of claudin-1 enhances or impairs neural crest cell emigration, respectively. Taken together, our findings reveal a novel role for claudin-1 in neural crest cell emigration and further demonstrate the importance of dismantling tight junctions during vertebrate development.
2. Results
2.1 Expression of claudin-1 protein during early chick development
The expression of claudin-1 mRNA and protein during chick embryo development has already been characterized by (Simard et al., 2005; Simard et al., 2006). Claudin-1 is localized to the ectoderm as early as HH4 (Fig 1a), and its expression is prominent at the apical region of the neural tube (lumen) at later stages. (Fig 1b,c). To ensure consistency during our investigation, we performed all subsequent analysis at the midbrain level. Immunostaining reveals that claudin-1 protein is present in the midbrain in HH8 and HH10 embryos (Fig 1b and c, respectively), representing time points at which the cranial neural crest is transitioning from a premigratory to a migratory state. Closer examination reveals that its expression is specifically decreased within the dorsal neural folds, even at HH8 prior to neural tube closure and crest cell emigration. At later stages, claudin-1 is strongly expressed in the overlying ectodermal cells but absent in migrating mesenchymal neural crest cells, as expected (Fig 1c).
Figure 1. Expression of claudin-1 protein in the developing chick embryo.
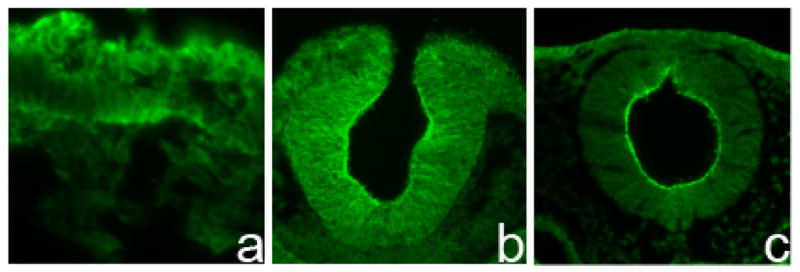
(a–c) Claudin-1 protein immunohistochemistry on transverse cross-sections of (a) HH4 embryo, (b) HH8 embryo and (c) HH10 embryo.
2.2 Loss of claudin-1 increases neural crest cell emigration
A key step in neural crest cell development is the transition from a premigratory state, where precursor cells are present in the dorsal neural tube, to a migratory state in which cells undergo EMT and delaminate from the dorsal neural tube to commence migration along defined pathways. Since claudin-1 is a critical component of tight junction complexes which form between epithelial cells, we hypothesized that claudin-1 may have a role in controlling the ability of premigratory neural crest cells to undergo EMT and exit the neural tube. To test this, a morpholino antisense oligonucleotide (MO) was designed against the translation start site of claudin-1. As a control, a second morpholino was made with a 5 base pair mismatch to the claudin-1 target sequence sufficient to ensure there was no binding and consequently, no loss of claudin-1 protein (Sauka-Spengler and Barembaum, 2008) (Fig 2a-b; yellow arrowhead in (a) points to region of reduced expression).
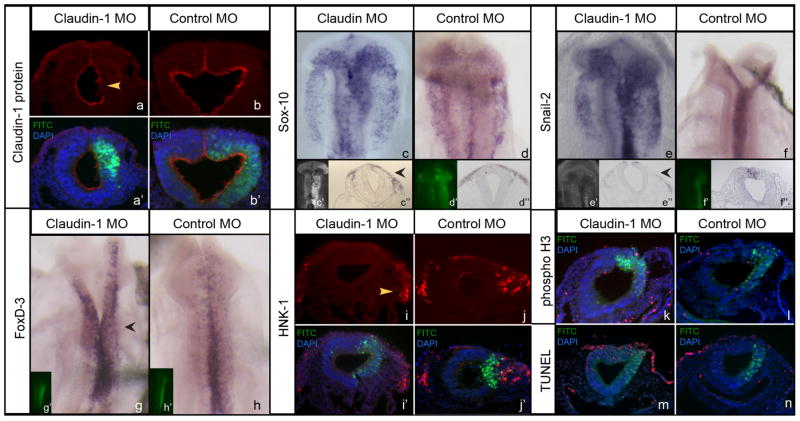
Figure 2. Depletion of claudin-1 increases neural crest cell emigration.
(a) Claudin-1 immunostaining in an embryo electroporated with Claudin-1 MO; (a’) transverse cross-section shown in (a) overlaid with FITC (morpholino tag; green) and DAPI (blue). (b) Claudin-1 immunostaining in an embryo electroporated with 5 bp mismatch control MO; (b’) transverse cross-section shown in (b) with overlay as above in (a). (c–d) Sox-10 expression in an embryo electroporated with (c) Claudin-1 MO or (d) control MO; (c’,d’) whole-mount image showing FITC (morpholino) labeling from electroporation; (c’’,d’’) transverse cross-section of (c,d). (e–f) Snail-2 expression in an embryo electroporated with (e) Claudin-1 MO or (f) control MO; (ef,d’) whole-mount image showing FITC (morpholino) labeling from electroporation; (e’’,f’’) transverse cross-section of (e,f). (g–h) Fox-D3 expression in an embryo electroporated with (g) Claudin-1 MO or (h) control MO; (g’,h’) whole-mount image showing FITC (morpholino) labeling from electroporation. (i–j) HNK-1 immunostaining in an embryo electroporated with Claudin-1 (i) or control (j) MO; (i’,j’) transverse cross-section shown in (i,j) overlaid with FITC (green) and DAPI (blue). (k,l) transverse cross-section of an embryo electroporated with Claudin-1 (k) or control (l) MO showing phospho-histone H3 immunostaining (red), FITC (green) and DAPI (blue). (m,n) transverse cross-section of an embryo electroporated with Claudin-1 (m) or control (n) MO showing TUNEL-positive cells (red), FITC (green) and DAPI (blue). Yellow arrowhead in (a) points to region of morpholino injection; black arrowheads in (c’’, e’’ and g) and yellow arrowhead in (i) point to side of embryo electroporated with Claudin-1 MO.
Claudin-1 MO was introduced via in ovo electroporation into one side of the neural folds of chick embryos at HH8, at a point just prior to neural crest cell emigration. Sox-10, a gene expressed in emigrating and migrating neural crest cells (Cheng et al., 2000), was increased on the side of the embryos electroporated with Claudin-1 MO in 22/29 embryos, but not in 4/4 embryos electroporated with control MO (Fig 2c–d; black arrowhead in c’’). Snail-2, which is similarly expressed in premigratory and migrating cranial neural crest cells (Nieto et al., 1994), was enhanced in 7/9 embryos electroporated with Claudin-1 MO but not in 7/7 controls (Fig 2e–f; black arrowhead in e’’). Fox-D3, which is expressed slightly earlier than Sox-10 in premigratory neural crest cells and is also present in migrating crest cells (Kos et al., 2001), was also augmented following Claudin-1 MO electroporation in 7/7 embryos but not in 4/4 controls (Fig 2g,h; black arrowhead in g). HNK-1, a protein found on the surface of migratory neural crest cells (Bronner-Fraser, 1986), was increased on the electroporated side of 3/4 embryos electroporated with Claudin-1 MO but not in 5/5 controls (Fig 2i,j; yellow arrowhead in i). We also observed no change in cell proliferation via phospho-histone H3 (PH3) immunostaining (Fig 2k,l, 3/3 embryos)(Coles et al., 2007; Jhingory et al., 2010; Wu et al., 2011) or in cell death through a TUNEL assay (Fig 2m,n, 4/4 embryos)(Coles et al., 2007; Jhingory et al., 2010; Wu et al., 2011) or caspase 3 immunostaining (data not shown) in the neural tube and migratory neural crest cell population upon depletion of claudin-1.
To quantify the effects of claudin-1 depletion, we counted the number of Sox-10-positive cells on both the electroporated and contralateral control sides of embryos in serial transverse sections taken through the midbrain. We observed a statistically significant 1.6-fold increase in the number of Sox-10-positive migratory neural crest cells upon claudin-1 knock-down compared to the contralateral control side, with a Student’s t test of p < 0.000001, and no change noted upon treatment with control MO (Claudin-1 MO-treated side: 106 +/− 5, contralateral control side: 65 +/− 2; control MO-treated side: 64 +/− 2, contralateral control side: 62 +/− 2). To elucidate potential effects on molecular markers associated with EMT, we examined serial transverse sections of treated embryos for changes in the premigratory neural crest cell marker Cadherin6B (Cad6-B) and laminin, which labels the basal lamina. We found no significant difference in the distribution and level of these proteins upon claudin-1 depletion (Supplemental Fig 1a,b), comparable (for Cad6-B) with our prior studies on cingulin, another tight junction component (Wu et al., 2011).
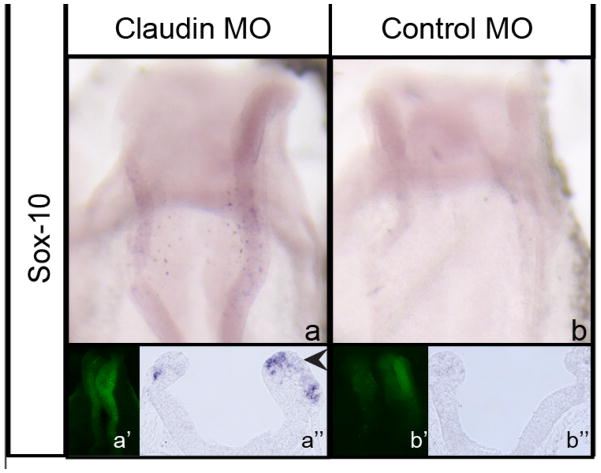
We next addressed whether the expansion in the migratory neural crest cell domain was due to premature neural crest cell emigration or due to general increases in the numbers of migratory neural crest cells. Although our PH3 results point to no difference in cell proliferation, the issue of precocious emigration was not previously addressed in our experiments. To assess the timing of emigration, we performed claudin-1 knock-down and re-incubated embryos to stages prior to neural crest cell emigration (4–5ss). We found that claudin-1 depletion leads to the premature appearance of newly emigrating Sox-10-positive neural crest cells (Fig 3a-a”, arrowhead; 4/4 embryos), with no change (little to no Sox-10 expression) observed in control MO-treated embryos (Fig 3b–b’’, 4/4 embryos). These data indicate that loss of claudin-1 impacts neural crest cell emigration in a temporal manner. Taken together, these results demonstrate that claudin-1 depletion promotes precocious midbrain neural crest cell emigration, leading to the expansion of the migratory neural crest domain in the embryo.
Figure 3. Depletion of claudin-1 results in precocious neural crest cell emigration.
(a–b) Sox-10 expression in a 4ss embryo electroporated with (a) Claudin-1 MO or (b) control MO; (a’,b’) whole-mount image showing FITC (morpholino) labeling from electroporation; (a’’,b’’) transverse cross-section of (a,b). Arrowhead in (a’’) points to precocious neural crest cell emigration.
2.3 Overexpression of claudin-1 reduces neural crest cell emigration
Since claudin-1 loss leads to increased neural crest cell emigration, we performed the converse experiment to examine whether excess claudin-1 is able to inhibit neural crest cell emigration. Overexpression of claudin-1 was shown to cause increased claudin-1 levels while overexpression of control vector did not (Fig 4a,b; yellow arrowhead in (a) indicates region of increased expression). Claudin-1 overexpression reduced Sox-10 expression on the electroporated side of 15/18 embryos, but expression was unaffected in 8/8 embryos electroporated with control (empty parent vector) (Fig 4c–d; black arrowhead in c’’). Similarly, Snail-2 expression was diminished in 10/14 embryos overexpressing claudin-1 but not in 9/9 control embryos (Fig 4e–f; black arrowhead in e’’), and Fox-D3 was reduced in 8/8 embryos but not in 5/5 controls (Fig 4g,h; black arrowhead in g). HNK-1 labeling was also decreased on the electroporated side of 5/6 embryos but not in 5/5 controls (Fig 4i–j; yellow arrowhead in i). Furthermore, we noted no change in cell proliferation (Fig 4k,l, PH3, 5/5 embryos) or in cell death (Fig 4m,n, 5/5 embryos) in the neural tube and migratory neural crest cell population upon claudin-1 overexpression.
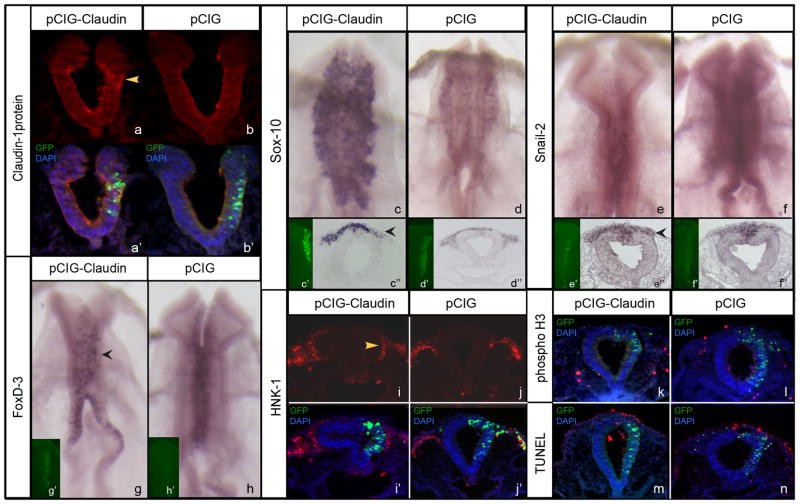
Figure 4. Overexpression of claudin-1 reduces neural crest cell emigration.
(a) Claudin-1 immunostaining in an embryo electroporated with pCIG-Claudin-1; (a’) transverse cross-section shown in (a) overlaid with GFP (green) and DAPI (blue). (b) Claudin-1 immunostaining in an embryo electroporated with empty parent vector pCIG; (b’) transverse cross-section shown in (b) with overlay as above in (a). (c-d) Sox-10 expression in an embryo electroporated with (c) pCIG-Claudin or (d) pCIG; whole-mount image showing GFP (expression-vector) labeling in (c’,d’), respectively; transverse cross-section of embryo shown in (c’’,d’’), respectively. (e–f) Snail-2 expression in an embryo electroporated with (e) pCIG-Claudin or (f) pCIG; whole-mount image showing GFP (expression-vector) labeling in (e’,f’), respectively; transverse cross-section of embryo shown in (e’’,f’’), respectively. (g–h) Fox-D3 expression in an embryo electroporated with (g) pCIG-Claudin or (h) pCIG; whole-mount image showing GFP (expression-vector) labeling in (g’,h’), respectively. (i, j) HNK-1 immunostaining in an embryo electroporated with pCIG-Claudin (i) or pCIG (j); (i’, j’) transverse cross-section shown in (i,j) overlaid with GFP and DAPI. (k,l) transverse cross-section of an embryo electroporated with pCIG-Claudin (k) or pCIG (l) showing phospho-histone H3 immunostaining (red), GFP staining (green), and DAPI (blue). (m,n) transverse cross-section of an embryo electroporated with pCIG-Claudin (m) or pCIG (n) showing TUNEL-positive cells (red), GFP staining (green), and DAPI (blue). Yellow arrowhead in (a) points to region of Claudin-1 overexpression; black arrowheads in (c’’, e’’ and g) and yellow arrowhead in (i) point to side of embryo electroporated with Claudin-1 overexpression construct.
To quantify the effects of claudin-1 overexpression, we documented the number of Sox-10-positive cells on both the electroporated and contralateral control sides of embryos in serial transverse sections taken through the midbrain. We observed a statistically significant 1.4-fold decrease in the number of Sox-10-positive migratory neural crest cells upon claudin-1 overexpression compared to the contralateral control side, with a Student’s t test of p < 0.05, and no difference observed upon treatment with the control pCIG construct (pCIG-claudin-1-treated side: 59 +/− 5, contralateral control side: 83 +/− 8; pCIG-treated side: 43 +/− 5, contralateral control side: 42 +/− 5). To uncover possible effects on molecular markers associated with EMT, we inspected serial transverse sections of treated embryos for changes in Cad6-B and laminin. We found no significant difference in the distribution and level of these proteins upon claudin-1 overexpression (Supplementary Fig 1c,d), similar to what we have observed previously (Wu et al., 2011). In summary, claudin-1 appears to be a critical component in the tight junctions linking premigratory neural crest cells, since loss of claudin-1 leads to changes in gene expression that point to premature neural crest cell emigration and an increase in the number of migratory neural crest cells, while overexpression of claudin-1 results in the opposite phenotype.
2.4 Depletion or overexpression of claudin-1 has no effect on placode development
Placode development occurs exclusively in the cranial region and is necessary for the formation of paired sensory structures (Baker and Bronner-Fraser, 2001; Schlosser, 2006). Placodes form from thickenings of the cranial ectoderm, and although development and differentiation is occurring at the same stages as early neural crest cell differentiation, gene expression is largely distinct between these systems. Since claudin-1 is expressed in the ectoderm, we asked whether claudin-1 is also necessary for placodal development.
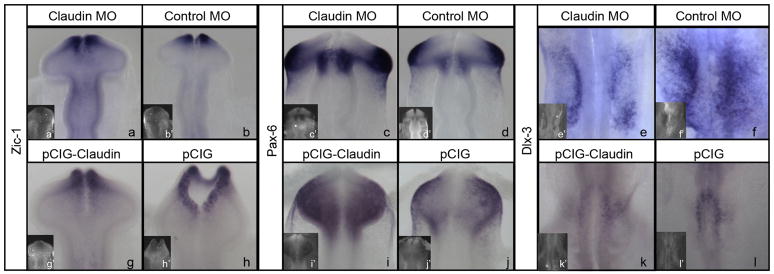
Claudin-1 or control MO was introduced via in ovo electroporation into the regions forming the anterior placodes. Expression of Zic-1 labeling the olfactory placode was unchanged following electroporation of Claudin-1 MO (6/6 embryos) or control MO (3/3 embryos) (Fig 5a,b). Pax-6 expression, which marks the lens placode, was not altered in 11/11 embryos electroporated with Claudin-1 MO or 4/4 embryos electroporated with control MO (Fig 5c,d). Finally, expression of Dlx-3 in the otic placode was also comparable in 6/6 embryos electroporated with Claudin-1 MO or 7/7 embryos electroporated with control MO (Fig 5e,f).
Figure 5. Claudin depletion or overexpression does not affect expression of placode markers.
(a–b, g-h) Zic-1 expression in embryos electroporated with (a) Claudin-1 MO, (b) 5 bp mismatch control MO, (g) pCIG-Claudin-1, or (h) pCIG; (a’,b’,g’,h’) show MO or GFP, respectively. (c–d, i–j) Pax-6 expression in embryos electroporated with (c) Claudin-1 MO, (d) 5 bp mismatch control MO (i) pCIG-Claudin-1, or (j) pCIG; (c’,d’,i’,j’) show MO or GFP, respectively. (e-f, k-l) Dlx-3 expression in embryos electroporated with (e) Claudin-1 MO, (f) 5 bp mismatch control MO, (k) pCIG-Claudin-1, or (l) pCIG; (e’,f’,k’,l’) show MO or GFP, respectively.
The lack of effect of claudin-1 on placode development was surprising given that neural crest development was sensitive to the loss or overexpression of claudin-1. In order to fully test the requirement of placode development for claudin-1, the same panel of genes was examined following claudin-1 overexpression. We observed no change in Zic-1 (13/13 embryos), Pax-6 (15/15 embryos) or Dlx-3 (13/13 embryos) expression upon claudin-1 overexpression. A control pCIG vector was also electroporated, and no alterations in gene expression were detected in 7/7 embryos for Zic-1, 9/9 embryos for Pax-6 or 6/6 embryos for Dlx-3 (Fig 5g-l). We conclude that placode development at the stages examined is not affected by perturbations in claudin-1.
3. Discussion
3.1 Claudins in development and disease
Claudin proteins regulate many critical developmental processes in vertebrates, with loss of expression leading to developmental abnormalities or even death (Furuse et al., 2002). Claudin expression is also both spatially and temporally dynamic throughout development. Recent studies have revealed that claudin-1 is expressed in the ectoderm and neural epithelium during chick embryogenesis, and that claudin-1 plays a role in the process of heart looping (Simard et al., 2005; Simard et al., 2006). Chick claudin-3 is observed early in development in the head folds, with later expression documented in areas such as the anterior intestinal portal, otic vesicle, and pharyngeal endoderm and pouches (Haworth et al., 2003). In both the mouse and chick, claudin-3 localizes to the nephric duct and ureteric bud, and additional experiments reveal that it plays an important role in regulating tubule formation from inner medullary collecting duct cells in vitro (Haddad et al., 2011). Chick claudin-5 expression is restricted to the extra-embryonic tissue at HH4 and 6, with embryonic expression not observed until HH8 and correlating with the onset of vascularization, a finding corroborated by documentation of later claudin-5 expression in the developing vasculature (Collins et al., 2012). Claudin-5 is also transiently expressed during the formation of the chick retinal pigment epithelium, with highest levels observed between embryonic days 10–14 (Kojima et al., 2002). The chick intestinal epithelium also expresses claudin-3, -5, and -16, with localization observed along the entire villus, in the crypt and lower villus, and in upper villus goblet cells, respectively (Ozden et al., 2010). In Xenopus, Xclaudin controls left-right patterning (Brizuela et al. 2001), and gain or loss of the Xenopus claudin-1 homolog, XClaudin-1, disrupts normal convergent-extension during gastrulation (Chang et al. 2010). The Xclaudin-5 genes (5a and 5b) are observed in the mesoderm and are required for heart tube formation (Yamagishi et al., 2010). Finally, claudins-1 and -11 are found during formation of the blood-testes barrier in the pheasant (Park et al., 2010), while claudins-1 through -19 are expressed in gradients along the longitudinal axis of the developing mouse gastrointestinal tract (Holmes et al., 2006). As such, claudins play diverse functional roles during the development of many organisms.
Claudin-1 has also gained attention due to its aberrant expression pattern in different types of cancer. Recent studies show that claudin-1 protein levels are significantly decreased in several types of invasive breast cancer cells (Tokes et al., 2005), suggesting that loss of claudin-1 may contribute to the invasive properties of these cells. Another study revealed that loss of claudin-1 expression in stage II and III rectal cancer patients is associated with cancer recurrence and decreased patient survival (Yoshida et al., 2011) and breast cancer recurrence (Morohashi et al. 2007). In colon cancer, claudin-1 has a regulatory role in tumor growth and metastasis. Intriguingly, claudin-1 expression increases in colon carcinoma and metastasis, and is often mislocalized to the nucleus in colon cancer cells. When claudin-1 expression was perturbed in colon cancer cell lines, structural changes in EMT markers occurred (Dhawan et al., 2005). A subset of breast cancer tumors also has high claudin-1 levels (Myal et al. 2010). The importance of claudin-1 in cancer cell EMT is still under investigation, and future studies may reveal that this protein plays a role in other types of cancers as well. Claudin-1 may therefore be useful as a prognostic indicator for various types of cancers and could serve as a therapeutic target for cancer treatment.
3.2 Claudin-1 function in the neural crest
Prior to their emigration from the dorsal neural tube, premigratory neural crest cells must dismantle both adherens and tight junctions. Our data indicate that claudin-1 is expressed in premigratory neural crest cells but is absent in migratory neural crest cells. This result indicates that claudin-1-containing tight junctions are lost as migratory neural crest cells emerge from the neural tube and are kept off during the migratory process. Although it is possible that tight junctions mediated by other transmembrane proteins are present in migratory neural crest cells, it is highly unlikely given data from other systems (Ikenouchi et al., 2003; Sauka-Spengler and Bronner-Fraser, 2008). Intriguingly, the loss of claudin-1 exclusively affects tight junctions in the premigratory neural crest cell population but does not impact tight junctions holding together neighboring neural tube cells. We hypothesize that other claudin or transmembrane tight junction molecules play a more important role in maintaining tight junction integrity throughout the remaining neuroepithelium. This idea is supported by our results herein, which show no change in neural tube architecture after claudin-1 perturbation. A good candidate for another transmembrane tight junction protein that might fulfill this role is claudin-3 (Haworth et al., 2005) (or potentially other claudins not yet identified in the neural tube) and/or other transmembrane tight junction proteins, such as junctional adhesion molecules (JAMs) (Ebnet et al., 2004), tricellulin (Ikenouchi et al., 2005), or the MarvelD family of proteins that associate with occludin (Steed et al., 2009). JAM-A expression has been documented in the chick retinal pigment epithelium (Luo et al., 2006), but it is unclear if this, and/or other JAM molecules (B, C, and 4) are present in the neuroepithelium. As their name suggests, tricellulin proteins mediate tricellular contacts, but no tricellulin genes have been annotated in the chick genome. A predicted sequence can be found in PubMed for MarvelD3, but no experimental data are available for it or any other MarvelD family member in the chick. Although occludin expression has been documented in the chick neuroepithelium, it is down-regulated during neural tube closure and is thus not a good candidate for maintaining neuroepithelial tight junctions outside of the dorsal region in later developmental stages (Aaku-Saraste et al., 1996). Collectively, these data imply that other as of yet identified tight junction transmembrane proteins play an important role in maintaining tight junction integrity throughout the neural tube. Interestingly, the chick trunk appears to be devoid of claudin-1, except in the very caudal neural tube, suggesting that another transmembrane tight junction molecule could be performing a comparable function to claudin-1 in this region of the embryo.
To investigate the effects of claudin-1 perturbation on neural crest cell emigration and migration at cranial levels, we used MO-mediated knock-down and overexpression of claudin-1 in the developing chick midbrain. We find that early depletion of claudin-1 promotes neural crest cell emigration while overexpression of claudin-1 impedes neural crest cell emigration. This occurs in the absence of any change in cell proliferation or cell death in the neural tube or migratory neural crest cell population, and with no appreciable differences noted in molecular markers of EMT such as Cad6-B and laminin. Because the latter observed effects may be directly or indirectly related to changes in claudin-1 levels, the mechanistic role of claudin-1 in EMT is still unclear. Our data, however, indicate that the premature loss of claudin-1 in midbrain premigratory neural crest cells leads to precocious neural crest cell emigration, manifesting as an expansion of the migratory neural crest cell population on the treated side of the embryo. Conversely, overexpression of claudin-1 results in the retention of tight junctions within the midbrain premigratory neural crest cell population and thus precludes the emergence of emigrating neural crest cells, reducing the migratory neural crest cell population on the treated side of the embryo. Together, our results reveal a critical role for claudin-1 in modulating cranial neural crest cell emigration.
3.3 Claudin-1 function in the placodes
Given the important role of claudin-1 in controlling neural crest cell emigration, we next assessed claudin-1 function in the cranial placodes by examining several notable placodal markers. Although claudin-1 is expressed in the proper spatio-temporal pattern within the head ectoderm to have a role in placode development, perturbation of claudin-1 did not affect the formation of the olfactory, lens and otic placodes. These data suggest that other tight junction molecule(s) besides claudin-1 play a crucial role during the development of these placodes, and perhaps during the ingression of placodal cells from the ectoderm, whereas claudin-1 function is more important to the generation of migratory neural crest cells. Other tight junction proteins expressed in the chick ectoderm include claudin-3 (Haworth et al., 2005) and occludin (Aaku-Saraste et al., 1996), which may in turn contribute to the formation of these different cranial placodes.
In summary, our data show that claudin-1 infuences the emigration of neural crest cells in the chick midbrain. As such, claudin-1 levels must be tightly modulated in order to ensure proper neural crest cell emigration and subsequent neural crest cell migration. Taken together, our results are the first to reveal a novel function for the tight junction protein claudin-1 in the neural crest and further stress the importance of dismantling cellular tight junctions to facilitate the emigration of neural crest cells from the dorsal neural tube during embryogenesis.
Experimental Procedures
Chick embryo collection
Fertilized chicken eggs were obtained from Hy-Line North America, L.L.C., and McIntyre Poultry, CA) and were incubated at 38°C in humidified chambers (Egg Cartons, MA). Embryos were staged according to the number of somites (ss) counted. (Hamburger and Hamilton, 1951).
Design and electroporation of claudin-1 antisense morpholino
A 3’ fluorescein-labeled morpholino (MO), 5’-GCGCTGTTGGTTGTGCTCCCGTGTT-3’, was designed to knock-down claudin-1 mRNA translation according to the manufacturer’s instructions (GeneTools, L.L.C.). A five base pair mismatch fluorescein-labeled claudin-1 control MO, 5’-AgACcGGAcCACAACCAAgAcCGC-3’, was used that does not target claudin-1 mRNA (mutated bases are in lower case, GeneTools, L.L.C). The MOs were introduced into chick embryos using a modified version of in ovo electroporation (Itasaki et al., 1999; Jhingory et al., 2010). Briefly, the MOs were electroporated at concentrations ranging from 0.5mM-1mM into the neural tube lumen at the midbrain axial level, and 2, 25V, 30 mSec pulses were applied across the embryo. Electroporated embryos were re-incubated for specific time periods and then collected for further processing. Confirmation of effective MO delivery was established upon examination of embryos using a fluorescent microscope. Only those embryos showing high MO (or GFP, see below) fluorescence were chosen for further analysis.
Claudin-1 cloning and in vivo overexpression
The claudin-1 cDNA was cloned directionally into the pCIG chick expression vector via PCR using a chick cDNA library (7–12ss) as the template to yield pCIG-claudin-1 and sequenced to confirm accuracy. The plasmid and the pCIG control were both used at a concentration of 2.5μg/μl during electroporation experiments (described above).
Whole-mount in situ hybridization
Whole-mount in situ hybridization was performed on electroporated embryos as described in (Wilkinson and Nieto, 1993). Processed embryos were imaged in 70% glycerol or phosphate buffered saline on a Zeiss SteREO Discovery.V8 or Zeiss Stemi SV11 microscope. Images were captured using the Zeiss Axiovision Rel 4.6 software with the Zeiss Axiocam MRc5 or MRc camera. Transverse-sections were obtained by cryostat-sectioning gelatin-embedded embryos at 14 μm in a Leico Frigocut or Fisher Microm cryostat, and coverslips were mounted on processed sections using Fluoromount G (Fisher). Sections were viewed at room temperature using a Zeiss AxioObserver.Z1 inverted microscope, and images were acquired using the Zeiss Axiovision Rel 4.6 software with the Zeiss Axiocam HRC camera. All exported images were processed in Adobe Photoshop 9.0 or CS4 (Adobe Systems). Figures were created using Adobe IIlustrator CS4 (Adobe Systems).
Immunohistochemistry
Immunohistochemical detection of various proteins was performed in whole-mount or on transverse sections following fixation of embryos and cryostat-sectioning. For analysis of claudin-1 protein distribution, embryos were at stages ranging from the 2ss to 10ss. Following fixation with 2% PFA, embryos were embedded in 20% gelatin and cryostat-sectioned at 14μm. Claudin-1 primary antibody (Invitrogen, mouse-anti claudin-1, 1:150) was applied to slides, followed by a secondary antibody conjugated to a fluorophore (Invitrogen Alexafluor goat-anti-mouse IgG, 1:300). Sections were stained with DAPI to mark cell nuclei. To identify migrating neural crest cells, sections were stained with HNK-1 (1:100 followed by 1:200 dilution of Invitrogen Alexafluor, goat anti-mouse IgM). Cad6-B (Developmental Studies Hybridoma Bank CCD6B-1, mouse-anti-Cad6-B, 1:50; secondary antibody Invitrogen Alexafluor goat-anti-mouse IgG1, 1:500) and laminin (Sigma L9393, mouse-anti-laminin, 1:200; secondary antibody from Invitrogen Alexafluor rabbit-anti-mouse, 1:500) proteins were detected on transverse midbrain sections as described (Wu et al., 2011). Quantification of Cad6-B and laminin staining was performed using ImageJ software (NIH). To assess cell proliferation, phospho-histone H3 (PH3, Millipore, 1:500) immunostaining was carried out on electroporated embryos following collection at the 8–10ss and transverse sectioning, as in (Coles et al., 2007; Jhingory et al., 2010; Wu et al., 2011). To identify apoptotic cells, a TUNEL assay (Roche) was performed on electroporated embryos following collection at the 8–10ss, transverse sectioning and processing as in (Coles et al., 2007; Jhingory et al., 2010; Wu et al., 2011); alternatively, immunostaining was carried out on transverse cross-sections of embryos using Caspase-3 (R&D systems, 1:100) followed by 1:500 Invitrogen Alexafluor goat-anti-rabbit IgG. Sections were viewed at room temperature using a Zeiss AxioObserver.Z1 inverted microscope, and images were acquired using the Zeiss Axiovision Rel 4.6 software with the Zeiss Axiocam HRC camera. All exported images were processed in Adobe Photoshop 9.0 or CS4 (Adobe Systems), and figures were created using Adobe IIlustrator or Photoshop CS4 (Adobe Systems).
Cell counts
Cell counts were carried out as described previously (Jhingory et al., 2010; Wu et al., 2011). Briefly, counts were performed on seven to nine consecutive sections through the midbrain for three representative embryos. Sections were co-stained with DAPI to allow visualization of nuclei, and every DAPI-stained nucleus surrounded by dark purple cytoplasmic Sox-10 staining was counted. Cell counts were averaged over the number of sections obtained from each embryo, and fold differences between the number of cells on the electroporated side and contralateral control side were calculated. The standard error of the mean was then calculated, and a Student’s t test was performed to establish statistical significance of results.
Supplementary Material
(a–d) Representative transverse sections taken through the midbrain of embryos treated with either Claudin-1 MO (a), control MO (b), pCIG-Claudin-1 (c), or pCIG (d) and processed for immunohistochemistry for Cad6-B (red) and laminin (blue). Green indicates MO or GFP.
Highlights.
Claudin-1 is expressed in the developing neuroepithelium and ectoderm but is absent from migratory neural crest cells.
Claudin-1 depletion augments neural crest cell emigration.
Claudin-1 depletion leads to premature neural crest cell emigration.
Claudin-1 overexpression inhibits neural crest cell emigration.
Perturbation of claudin-1 does not affect placode development.
Acknowledgments
The authors would like to thank Ms. Abigail Figat for technical assistance. This work was supported by Grants NIH-HD037105 and DE16459 (M.E.B.). Additional support for this research was provided by the University of Maryland from the Howard Hughes Medical Institute Undergraduate Science Education Program (T.E.N.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Katherine J. Fishwick, Email: kjf@caltech.edu.
Theresa Neiderer, Email: tneidere@umd.edu.
Sharon Jhingory, Email: sjhingor@umd.edu.
Marianne Bronner, Email: mbronner@caltech.edu.
References
- Aaku-Saraste E, Hellwig A, Huttner WB. Loss of occludin and functional tight junctions, but not ZO-1, during neural tube closure--remodeling of the neuroepithelium prior to neurogenesis. Dev Biol. 1996;180:664–79. doi: 10.1006/dbio.1996.0336. [DOI] [PubMed] [Google Scholar]
- Angelow S, Ahlstrom R, Yu AS. Biology of claudins. Am J Physiol Renal Physiol. 2008;295:F867–76. doi: 10.1152/ajprenal.90264.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker CV, Bronner-Fraser M. Vertebrate cranial placodes I. Embryonic induction. Dev Biol. 2001;232:1–61. doi: 10.1006/dbio.2001.0156. [DOI] [PubMed] [Google Scholar]
- Brizuela BJ, Wessely O, De Robertis EM. Overexpression of the Xenopus tight-junction protein claudin causes randomization of the left-right body axis. Dev Biol. 2001;230:217–29. doi: 10.1006/dbio.2000.0116. [DOI] [PubMed] [Google Scholar]
- Bronner-Fraser M. Analysis of the Early Stages of Trunk Neural Crest Migration in Avian Embryos Using Monoclonal Antibody HNK-1. Dev Biol. 1986;115:44–55. doi: 10.1016/0012-1606(86)90226-5. [DOI] [PubMed] [Google Scholar]
- Chang DJ, Hwang YS, Cha SW, Chae JP, Hwang SH, Hahn JH, Bae YC, Lee HS, Park MJ. Xclaudin-1 is required for proper gastrulation in Xenopus laevis. Biochem Biophys Res Commun. 2010;397:75–87. doi: 10.1016/j.bbrc.2010.05.068. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Cheung M, Abu-Elmagd MM, Orme A, Scotting PJ. Chick sox10, a transcription factor expressed in both early neural crest cells and central nervous system. Brain Res Dev Brain Res. 2000;121:233–41. doi: 10.1016/s0165-3806(00)00049-3. [DOI] [PubMed] [Google Scholar]
- Coles EG, Taneyhill LA, Bronner-Fraser M. A critical role for Cadherin6B in regulating avian neural crest emigration. Dev Biol. 2007;312:533–544. doi: 10.1016/j.ydbio.2007.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins MM, Baumholtz AI, Ryan AK. Claudin-5 expression in the vasculature of the developing chick embryo. Gene Expr Patterns. 2012 Feb 3;2012 doi: 10.1016/j.gep.2012.01.005. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Dhawan P, Singh AB, Deane NG, No Y, Shiou SR, Schmidt C, Neff J, Washington MK, Beauchamp RD. Claudin-1 regulates cellular transformation and metastatic behavior in colon cancer. J Clin Invest. 2005;115:1765–76. doi: 10.1172/JCI24543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebnet K, Suzuki A, Ohno S, Vestweber D. Junctional adhesion molecules (JAMs): More molecules with dual functions? J Cell Sci. 2004;117:19–29. doi: 10.1242/jcs.00930. [DOI] [PubMed] [Google Scholar]
- Farquhar MG, Palade GE. Junctional complexes in various epithelia. J Cell Biol. 1963;17:375–412. doi: 10.1083/jcb.17.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M, Hata M, Furuse K, Yoshida Y, Haratake A, Sugitani Y, Noda T, Kubo A, Tsukita S. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: a lesson from claudin-1-deficient mice. J Cell Biol. 2002;156:1099–111. doi: 10.1083/jcb.200110122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta IR, Ryan AK. Claudins: unlocking the code to tight junction function during embryogenesis and in disease. Clin Genet. 2010;77:314–25. doi: 10.1111/j.1399-0004.2010.01397.x. [DOI] [PubMed] [Google Scholar]
- Haddad N, El Andalousi J, Khairallah H, Yu M, Ryan AK, Gupta IR. The tight junction protein claudin-3 shows conserved expression in the nephric duct and ureteric bud and promotes tubulogenesis in vitro. Am J Physiol Renal Physiol. 2011;301:F1057–65. doi: 10.1152/ajprenal.00497.2010. [DOI] [PubMed] [Google Scholar]
- Hadj-Rabia S, Baala L, Vabres P, Hamel-Teillac D, Jacquemin E, Fabre M, Lyonnet S, De Prost Y, Munnich A, Hadchouel M, Smahi A. Claudin-1 gene mutations in neonatal sclerosing cholangitis associated with ichthyosis: a tight junction disease. Gastroenterology. 2004;127:1386–90. doi: 10.1053/j.gastro.2004.07.022. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J Morph. 1951;88:49–92. doi: 10.1002/aja.1001950404. [DOI] [PubMed] [Google Scholar]
- Haworth KE, El-Hanfy A, Prayag S, Healy C, Dietrich S, Sharpe P. Expression of Claudin-3 during chick development. Gene Expr Patterns. 2003;6:40–4. doi: 10.1016/j.modgep.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Hay ED. An overview of epithelio-mesenchymal transformation. Acta Anat (Basel) 1995;154:8–20. doi: 10.1159/000147748. [DOI] [PubMed] [Google Scholar]
- Holmes JL, Van Itallie CM, Rasmussen JE, Anderson JM. Claudin profiling in the mouse during postnatal intestinal development and along the gastrointestinal tract reveals complex expression patterns. Gene Expr Patterns. 2006;6:581–8. doi: 10.1016/j.modgep.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Ikenouchi J, Matsuda M, Furuse M, Tsukita S. Regulation of tight junctions during the epithelium-mesenchyme transition: direct repression of the gene expression of claudins/occludin by Snail. J Cell Sci. 2003;116:1959–67. doi: 10.1242/jcs.00389. [DOI] [PubMed] [Google Scholar]
- Ikenouchi J, Furuse M, Furuse K, Sasaki H, Tsukita S, Tsukita S. Tricellulin constitutes a novel barrier at tricellular contacts of epithelial cells. J Cell Biol. 2005;171:939–45. doi: 10.1083/jcb.200510043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itasaki N, Bel-Vialar S, Krumlauf R. 'Shocking' developments in chick embryology: electroporation and in ovo gene expression. Nat Cell Biol. 1999;1:E203–7. doi: 10.1038/70231. [DOI] [PubMed] [Google Scholar]
- Jhingory S, Wu CY, Taneyhill LA. Novel insight into the function and regulation of alphaN-catenin by Snail2 during chick neural crest cell migration. Dev Biol. 2010;344:896–910. doi: 10.1016/j.ydbio.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima S, Rahner C, Peng S, Rizzolo LJ. Claudin-5 is transiently expressed during the development of the retinal pigment epithelium. J Membr Biol. 2002;186:81–8. doi: 10.1007/s00232-001-0137-7. [DOI] [PubMed] [Google Scholar]
- Kos R, Reedy MV, Johnson RL, Erickson CA. The winged-helix transcription factor FoxD3 is important for establishing the neural crest lineage and repressing melanogenesis in avian embryos. Development. 2001;128:1467–79. doi: 10.1242/dev.128.8.1467. [DOI] [PubMed] [Google Scholar]
- Lal-Nag M, Morin PJ. The claudins. Genome Biol. 2009;10:235. doi: 10.1186/gb-2009-10-8-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Dourarin NM, Kalcheim C. The Neural Crest. Cambridge University Press; Cambridge, UK: 1999. [Google Scholar]
- Luo Y, Fukuhara M, Weitzman M, Rizzolo LJ. Expression of JAM-A, AF-6, PAR-3 and PAR-6 during the assembly and remodeling of RPE tight junctions. Brain Res. 2006;1110:55–63. doi: 10.1016/j.brainres.2006.06.059. [DOI] [PubMed] [Google Scholar]
- Morohashi S, Kusimi T, Sato F, Odagiri H, Yoshihara S, Hakamada K, Sasaki M, Kijima H. Decreased expression of claudin-1 correlates with recurrence status in breast cancer. Int J Mol Med. 2007;20:139–43. [PubMed] [Google Scholar]
- Myal Y, Leygue E, Blanchard AA. Claudin 1 in breast tumorigenesis: revelation of a possible novel "claudin high" subset of breast cancers. J Biomed Biotechnol. 2010;2010:956897. doi: 10.1155/2010/956897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto MA, Sargent MG, Wilkinson DG, Cooke J. Control of cell behavior during vertebrate development by Slug, a zinc finger gene. Science. 1994;264:835–9. doi: 10.1126/science.7513443. [DOI] [PubMed] [Google Scholar]
- Ozden O, Black BL, Ashwell CM, Tipsmark CK, Borski RJ, Grubb BJ. Developmental profiled of claudin-3, -5 and -16 proteins in the epithelium of the chick intestine. Anat Rec (Hoboken) 2010;293:1175–83. doi: 10.1002/ar.21163. [DOI] [PubMed] [Google Scholar]
- Park CJ, Lee JE, Oh YS, Shim S, Nah WH, Choi KJ, Gye MC. Expression of claudin-1 and -11 in immature and mature pheasant (Phasianus colchicus) testes. Theriogenology. 2010;75:445–58. doi: 10.1016/j.theriogenology.2010.09.012. [DOI] [PubMed] [Google Scholar]
- Sauka-Spengler T, Barembaum M. Gain- and Loss-of-Function Approaches in the Chick Embryo. In: Bronner-Fraser M, editor. Avian Embryology. Vol. 87. Elsevier; 2008. pp. 237–256. [DOI] [PubMed] [Google Scholar]
- Sauka-Spengler T, Bronner-Fraser M. A gene regulatory network orchestrates neural crest formation. Nat Rev Mol Cell Biol. 2008;9:557–68. doi: 10.1038/nrm2428. [DOI] [PubMed] [Google Scholar]
- Schlosser G. Induction and specification of cranial placodes. Dev Biol. 2006;294:303–51. doi: 10.1016/j.ydbio.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Schulzke JD, Fromm M. Tight junctions: molecular structure meets function. Ann N Y Acad Sci. 2009;1165:1–6. doi: 10.1111/j.1749-6632.2009.04925.x. [DOI] [PubMed] [Google Scholar]
- Simard A, Di Pietro E, Ryan AK. Gene expression pattern of Claudin-1 during chick embryogenesis. Gene Expr Patterns. 2005;5:553–60. doi: 10.1016/j.modgep.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Simard A, Di Pietro E, Young CR, Plaza S, Ryan AK. Alterations in heart looping induced by overexpression of the tight junction protein Claudin-1 are dependent on its C-terminal cytoplasmic tail. Mech Dev. 2006;123:210–27. doi: 10.1016/j.mod.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Steed E, Rodrigues NT, Balda MS, Matter K. Identification of MarvelD3 as a tight junction-associated transmembrane protein of the occludin family. BMC Cell Biol. 2009;10:95. doi: 10.1186/1471-2121-10-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokes AM, Kulka J, Paku S, Szik A, Paska C, Novak PK, Szilak L, Kiss A, Bogi K, Schaff Z. Claudin-1, -3 and -4 proteins and mRNA expression in benign and malignant breast lesions: a research study. Breast Cancer Res. 2005;7:R296–305. doi: 10.1186/bcr983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukita S, Furuse M. Overcoming barriers in the study of tight junction functions: from occludin to claudin. Genes Cells. 1998;3:569–73. doi: 10.1046/j.1365-2443.1998.00212.x. [DOI] [PubMed] [Google Scholar]
- Tsukita S, Furuse M, Itoh M. Molecular dissection of tight junctions. Cell Struct Funct. 1996;21:381–5. doi: 10.1247/csf.21.381. [DOI] [PubMed] [Google Scholar]
- Tsukita S, Yamazaki Y, Katsuno T, Tamura A, Tsukita S. Tight junction-based epithelial microenvironment and cell proliferation. Oncogene. 2008;27:6930–8. doi: 10.1038/onc.2008.344. [DOI] [PubMed] [Google Scholar]
- Wilkinson DG, Nieto MA. Detection of messenger RNA by in situ hybridization to tissue sections and whole mounts. Methods Enzymol. 1993;225:361–373. doi: 10.1016/0076-6879(93)25025-w. [DOI] [PubMed] [Google Scholar]
- Wu CY, Jhingory S, Taneyhill LA. The tight junction scaffolding protein cingulin regulates neural crest cell migration. Dev Dyn. 2011;240:2309–23. doi: 10.1002/dvdy.22735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi M, Ito Y, Arizumi T, Komazaki S, Danno H, Michiue T, Asashima M. Claudin5 genes encoding tight junction proteins are required for Xenopus heart formation. Dev Growth Differ. 2010;52:665–75. doi: 10.1111/j.1440-169X.2010.01204.x. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Kinugasa T, Akagi Y, Kawahara A, Romeo K, Shiratsuchi I, Ryu Y, Gotanda Y, Shirouzu K. Decreased expression of claudin-1 in rectal cancer: a factor for recurrence and poor prognosis. Anticancer Res. 2011;31:2517–25. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(a–d) Representative transverse sections taken through the midbrain of embryos treated with either Claudin-1 MO (a), control MO (b), pCIG-Claudin-1 (c), or pCIG (d) and processed for immunohistochemistry for Cad6-B (red) and laminin (blue). Green indicates MO or GFP.