Abstract
In recent years, methyl one-carbon metabolism has received a great deal of attention because the disruption of methyl balance in a variety of genetically modified mice is associated with the development of various forms of liver injury, namely fatty liverdisease and hepatocellular carcinoma (HCC). In addition, patients with liver disease often have an abnormal expression of key genes involved in methionine metabolism as well as elevated serum levels of methionine and homocysteine (Hcy). S-adenosylmethionine (SAMe) has rapidly moved from being a methyl donor to a key metabolite that regulates hepatocyte proliferation, necrosis and differentiation. Biosynthesis of SAMe occurs in all mammalian cells as the first step in methionine catabolism in a reaction catalyzed by methionine adenosyltransferase (MAT). Decreased hepatic SAMe biosynthesis is a consequence of numerous forms of chronic liver injury. In an animal model of chronic liver SAMe deficiency, the liver is predisposed to further injury and develops spontaneous steatohepatitis and HCC. SAMe treatment in experimental animal models of liver injury shows that its hepatoprotective properties. Meta-analyses also showed that it is effective in the treatment of patients with cholestatic liver diseases. We studied the survival of liver cells treated with SAMe and betaine using Hepa 1–6 and E47/C34 cell lines. We showed that exogenous SAMe decreased the number of Hepa 1–6 and E47/C34 cells, and increased the number of dead cells in vitro. Betaine had no significant effect on the number of surviving cells and the number of dead cells. The combination of both methyl donors significantly increased the survival of liver cells and reduced necrosis, compare to SAMe alone. This study showed the inhibition of the proliferatino and increased necrosis in response to SAMe on liver cancer cell lines Hepa 1–6 and C34.
Keywords: Liver, Methyl donors, SAMe, Betaine, cells death, necrosis
INTRODUCTION
In the early 1930s, Banting and Best, the discoverers of insulin, found that choline could prevent the development of fatty liver disease (steatosis) in pancreatectomized dogs treated with insulin (Newberne, 1986). Later work indicated that in rats and mice, diets deficient in labile methyl groups (choline, methionine, betaine, folate) produced fatty liver. Also long-term administration of diets deficient in choline and methionine caused hepatocellular carcinoma possibly by a global hypomethylation of the tumors (Cheung et al., 2009; Herath et al., 2006).
Recent progress in the understanding of the human dietary requirement for choline highlights the importance of genetic variation and epigenetics in human nutrient requirements (Henning and Swendseid, 1996; Zeisel, 2009). Choline is an essential component of all cell membranes, important in multi-cellular mechanisms, bioactive phospholipids and the neurotransmitter acetylcholine. Choline has been considered a required dietary nutrient since 1998 by the US Institute of Medicine's Food and Nutrition Board (Biagioni et al., 2000; Buchman, 2009; Institute of Medicine, 1998; Michel et al., 2006). Choline is a major dietary source of methyl-groups. One of choline's metabolites, betaine, participates in the methylation of homocysteine to form methionine (Ueland et al., 2005). A recommended dietary intake for choline in humans was set in 1998, and a portion of the choline requirement can be met via endogenous de novo synthesis of phosphatidylcholine catalyzed by phosphatidylethanolamine N-methyltransferase (PEMT) in the liver. Though many foods contain choline, many humans do not get enough in their diets.
The long-term administration of diets deficient in choline was seen to cause liver cancer in rats (Copeland and Salmon, 1946; Pogribny and Beland, 2009; Pogribny et al., 2009; Salmon and Copeland, 1954). In 1983, using an amino acid-defined diet, Mikol et al demonstrated that a severe methyl deficiency produced liver cancer in rats even in the absence of any additional carcinogen (Mikol et al., 1983). Suppressor tumor genes are hypermethylated and oncogenes are hypomethylated. However, methyl deficiency induces tumor formation only in sensitive animals. Indeed, Shivapurkar et al showed that a methionine- and choline-deficient dietary regimen that lowers the 5-methylcytosine levels in DNA and enhances liver tumor formation in male F344 rats does not do so in male C3H mice (Shivapurkar et al., 1986). For over 60 years, studies showed a preventive effect of methionine on carcinogenesis. However, this protective effect was more pronounced in animals fed methionine- and choline-deficient diets rather than methionine- and choline-adequate diets.
S-adenosylmethionine (SAMe) is synthesized from methionine, an essential amino acid for protein synthesis (Kozak, 1989). This reaction is catalyzed by methionine adenosyltransferase (MAT) (Mato et al., 1997). Various chronic liver disorders, including alcoholic, biliary and viral cirrhosis, markedly decrease the hepatic activities of methionine adenosyltransferase (MAT), the enzyme that catalyzes the synthesis of SAMe from L-methionine and ATP (Mato et al., 1997). In mammals, two genes code for MAT: one expressed exclusively in the liver and a second enzyme present in all tissues. Two MAT isoenzymes, MATI and MATIII, are expressed primarily in the adult liver. They are, respectively, tetramers and dimers of the same single subunit encoded by the gene MAT1A (Kotb et al., 1997). MAT2A is expressed in all tissues, even the liver but its over expression in the liver is associated with liver proliferation and carcinogenesis. However, MAT1A expression diminishes during liver regeneration and after partial hepatectomy and is inhibited in HCC (Mato and Lu, 2007). The change of MAT ratio leads to a decrease of SAMe content in the liver. The same results were observed in MAT1a knockout mice (Lu et al., 2001), during partial hepatectomy (Huang et al., 1998) and liver cancer (Lu and Mato, 2005). In human hepatocellular carcinoma (HCC), MAT1A is replaced by MAT2A. This is important pathogenetically because MAT2A expression is associated with lower SAMe levels and faster growth, whereas exogenous SAMe treatment inhibits growth.
In hepatocytes, SAMe levels are high in quiescent cells and low in proliferative cells (Cai et al., 1998). Low SAMe levels were observed after a partial hepatectomy when the cells were proliferating (Mato and Lu, 2007). This proliferation can be inhibited by exogenous SAMe (Cai et al., 1998). A very interesting studied done by Ansorena et al showed that the effect of SAMe induced apoptosis of rat hepatocytes (Ansorena et al., 2002). SAMe protected okadaic acid-induced apoptosis in normal hepatocytes. It induced apoptosis in liver cancer cell lines HepG2 and HuH-7 via the mitochondrial death pathway (Ansorena et al., 2002). Lu et al showed that SAMe inhibited growth of HCC but it was ineffective in the treatment of HCC when it was already established in the liver (Lu et al., 2009).
The purpose of this study is to determine the effect of SAMe and betaine on cell proliferation and cell necrosis in the cell line Hepa 1–6 and its transduction variant, which expresses CYP2E1 and is, called C34.
MATERIAL AND METHODS
Cell Culture
Hepa 1–6 (ATCC, Manassas, VA) derived cells from a mouse HCC were maintained in Dulbecco’s minimum essential medium (DMEM) with phenol red supplemented with 5% Fetal Bovine Serum (FBS medium). The in vitro HepG2 cell model, a human hepatoblastoma cell line was a gift from Dr. Cederbaum (Chen and Cederbaum, 1998). The cells were transduced to express CYP2E1 (E47 cell line). The control HepG2 cells were transduced with the vector only (C34 cell line). Both cells, C34 and E47, are clones of the HepG2 cell line. C34 and E47 cells were grown in MEM containing 10% fetal bovine serum (FBS), 0.5 mg/ml G418 supplemented with 100 U/ml penicillin and 100 ug/ml streptomycin in a humidified atmosphere of 5% CO2 at 37°C The cells were treated at 48h and 96h with or without SAMe and Betaine (Sigma-Aldrich, Saint Louis MO). The doses of SAMe used were 1, 2 and 4mM. For betaine, we used 1% (84mM), 2% (168mM) and 4% (336mM). At the different times of treatment, the cells were exposed to Trypan-Blue (Sigma-Aldrich, St Louis, MO) to estimate the necrosis effect of betaine and SAMe (Dye exclusion assay for necrotic cell detection).
Cell Staining
Tissue culture cells were fixed with 100% Ethanol and stained with Hematoxylin and Eosin. Apoptois and cell proliferation (mitosis) were accessed histologically.
Statistical analysis
P values were determined by ANOVA and Student-Newman-Keuls for multiple group comparisons (Sigma-Stat software, San Francisco, CA).
Results and Discussion
MAT1a is the major enzyme converting methionine to SAMe. An important role of MAT1a is to reduce the growth of tumor in vivo and to decrease blood vessel formation, with an increase of apoptosis occuring (Li et al., 2010). During partial hepatectomy, the expression of MAT1a and the concentration of SAMe in the liver decrease when the proliferation of hepatocytes increases (Mato et al., 2002; Mato and Lu, 2007). A similar phenomenon occurs in HCC where the expression of MAT1a and SAMe biosynthesis decreases (Lu and Mato, 2008). In previous publications, we succeeded in proving that SAMe prevents the proliferation of hepatocytes, in the DDC refed mice model (Oliva et al., 2008). Recent publications of the Lu group showed the important role of SAMe in cell growth and the apoptosis induced in tumors cell lines: HepG2 (human hepatoblastoma), HT-29 (human colorectal adenocarcinoma), RKO (human colon adenocarcinoma), Huh7 (HCC) (Li et al., 2010; Li et al., 2009; Yang et al., 2004).
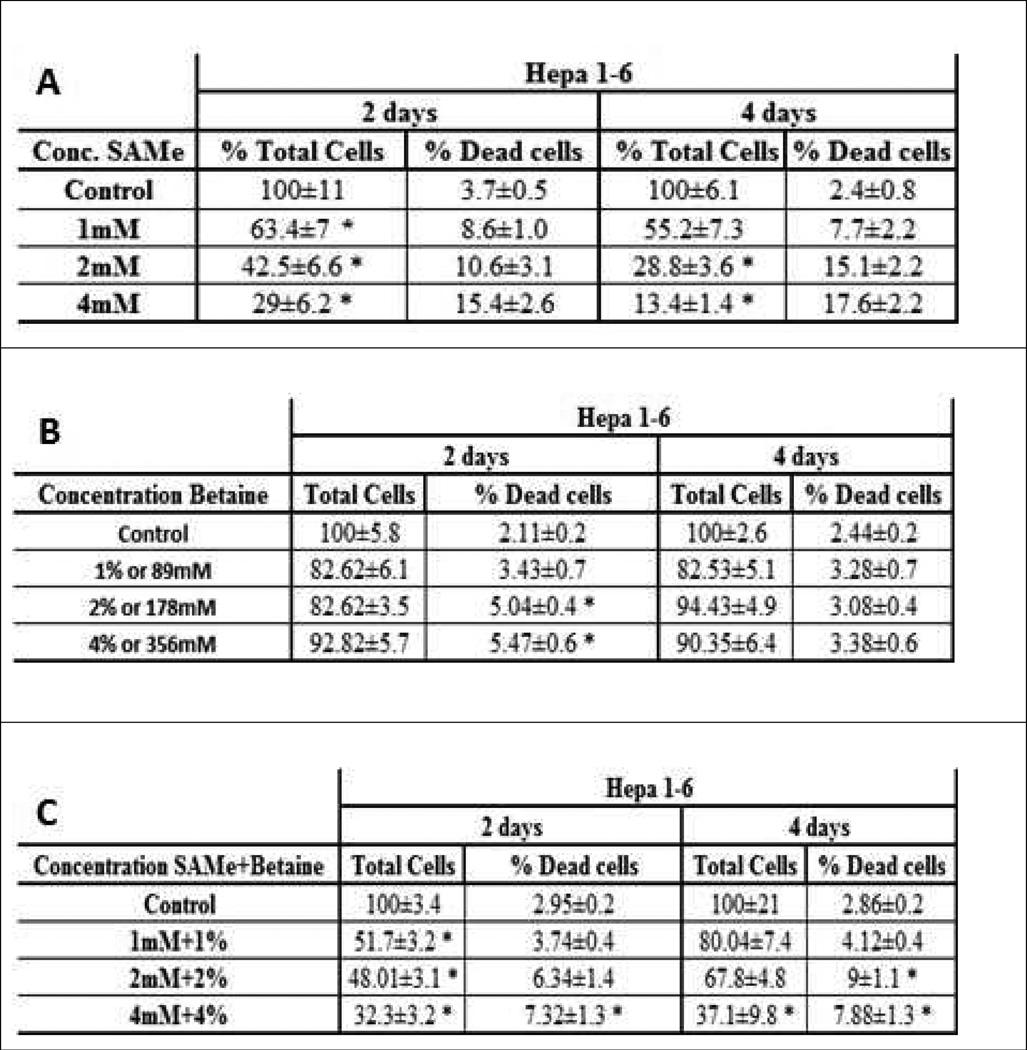
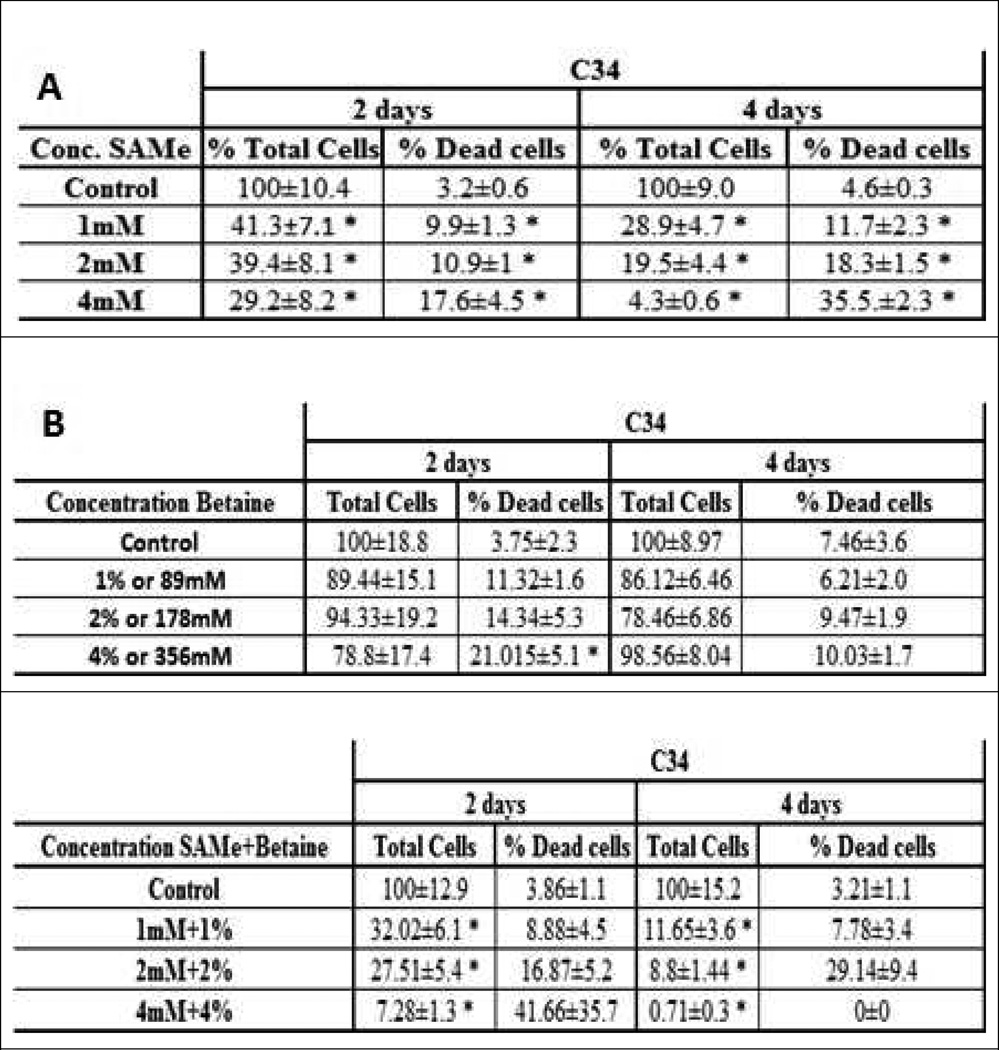
We tested the effect of SAMe and betaine, two methyl donors, on the survival of Hepa1-6 and E47/C34 cell line (HepG2 clones). We observed a decrease in the survival of the Hepa1-6 cells, when the cells were treated for 2 days and 4 days (Fig 1A). The number of necrotic hepatocytes increased and the number of cells decreased in a dose response manner (1mM to 4mM of SAMe). A similar phenomenon was observed on the C34 cell line (Fig 2A). However, the effect of SAMe seemed more efficient in the C34 cell line, indicating a different sensitivity and phenotype of the 2 cells lines. The necrotic change was also amplified when the cells were treated 4 days (Fig 1A and 2A). A similar treatment was done with betaine to both cell lines. We observed an increase of cell death in the C34 cells, but not for Hepa1–6, in a dose response (Fig 1B and 2B). However, the growth of the cells was just delayed (Fig 1B) or slightly decreased, in both cell lines (Fig 2B). With SAMe treatment, the C34 cells were more sensitive to the treatment than Hepa1–6. A co-treatment of the cells with SAMe and betaine partially prevented the necrosis caused by SAMe (Fig 1C and 2C).
Fig 1.
Effect of SAMe and betaine on the survival of Hepa 1–6. (n=6). A) Total number of cells after 2 days decreased significantly. The percentage of dead cells for 2 days increased. Total number of cells after 4 days decreased significantly. The percentage of dead cells after 4 days increased significantly. B) Total number of cells after 2 and 4 days didn't change significantly. The percentage of dead cells after 2 and 4 days increased lightly compare to the control. C) Total number of cells after 2 days decreased significantly. The percentage of dead cells after 2 days was increased significantly at the 4mM+4% only. Total number of cells after 4 days was decreased significantly decreased at the 4mM+4%only. The percentage of dead cells after 4 days was significant at 2mM+2% and 4mM+4% (*: Significant difference compare to the control p<0.05)
Fig 2.
Effect of SAMe and betaine on the survival of C34 cells. (n=6). A) Total number of cells after 2 days decreased significantly. The percentage of dead cells for 2 days increased. Total number of cells after 4 days decreased significantly. The percentage of dead cells after 4 days increased significantly. B) Total number of cells after 2 and 4 days didn't change significantly. The percentage of dead cells after 2 days increased lightly compare to the control, and significantly after 4 days. C) Total number of cells after 2 days decreased significantly. The percentage of dead cells after 2 days was increased significantly. Total number of cells after 4 days was decreased significantly decreased at the 4mM+4%only. The percentage of dead cells after 4 days was significant at 2mM+2% and 4mM+4% (*: Significant difference compare to the control p<0.05)
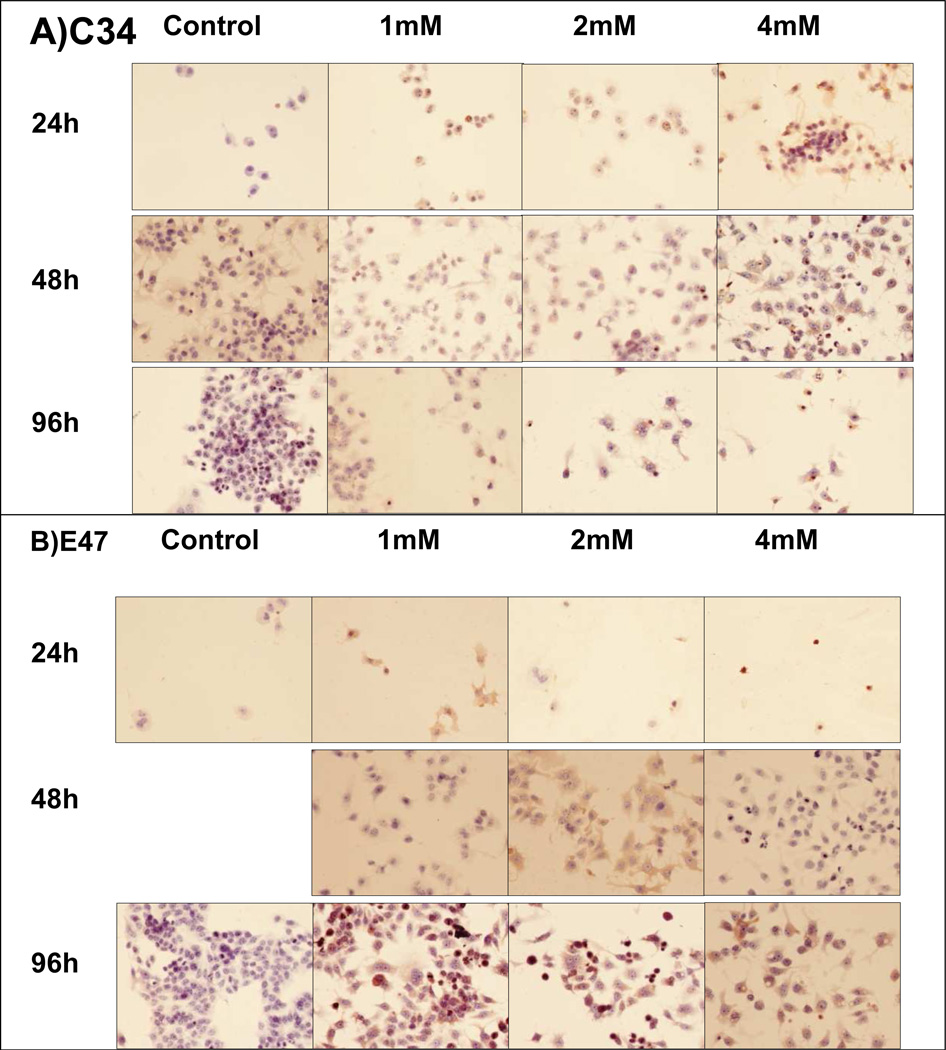
We also analyzed the growth of the C34 and E47 cell lines, 2 clones of the HepG2 cell line, to study the different sensitivity of the cells to SAMe. In absence of SAMe, the cells C34 and E47 grew well (Fig 3A/B). However, in the presence of SAMe, C34 cells did not grow but the E47 cells were able to grow more than the C34 cells. Nevertheless, we observed that SAMe slowed down the growth of the E47 cells, especially at the concentration of 2mM or higher concentrations.
Fig 3.
C34 cells were treated 24h, 48h and 96h. A) C34 cells treated 2 days with different dose of SAMe showed a decrease in the proliferation rates of cells, compared to the 24h treated. At 4 days of treatment, we observed a dramatic decrease in the cells number, when SAMe was added to the culture media. B) E47 cells treated 2 days with different dose of SAMe showed an unchanged number of cells, compare to the 24h treated. At 4 days of treatment, at 1mM, we did not observed a dramatic changes in the number of the cells. At 2mM, the numbers of cells to decrease.
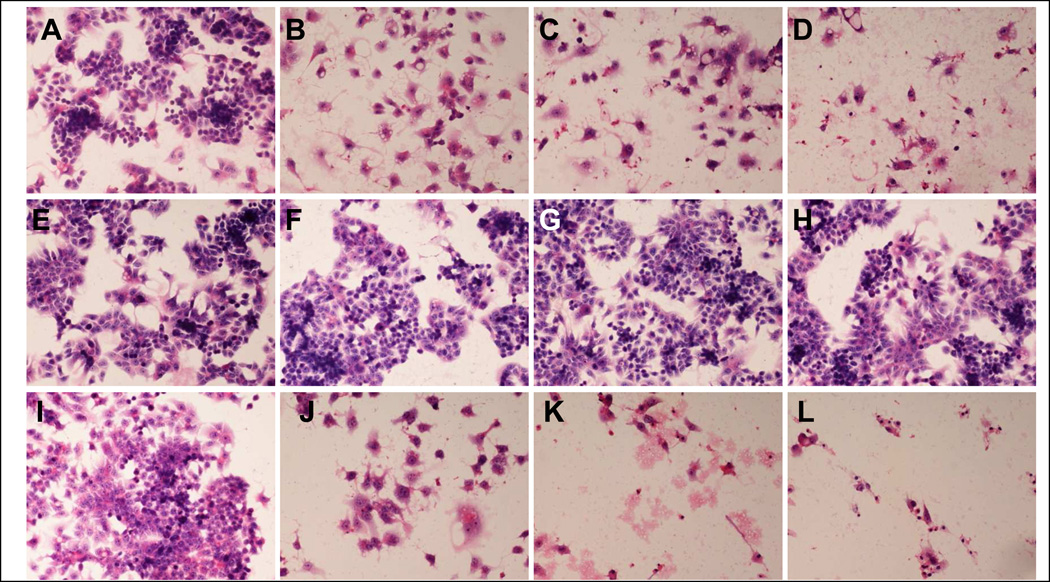
SAMe reduced cell proliferation and induced cell death in a dose dependent manner, in the C34 cell line (Fig 4). Betaine did not have a significant effect on the proliferation and the survival of the cells. However, betaine enhanced the effect of SAMe on the proliferation. SAMe and betaine had the same effect on the Hepa 1–6, except when they were combined (Fig 5). Betaine had a protective effect on the proliferation and survival effect of SAMe. The C34 cells, without treatment, are proliferating, since we observe numerous cells in mitosis (Fig 6A). However, in presence of SAMe (concentration), the C34 cells developed apoptosis (Fig 6B). We observed at 1mM of SAMe, that there were no cells in mitosis (Data not show).
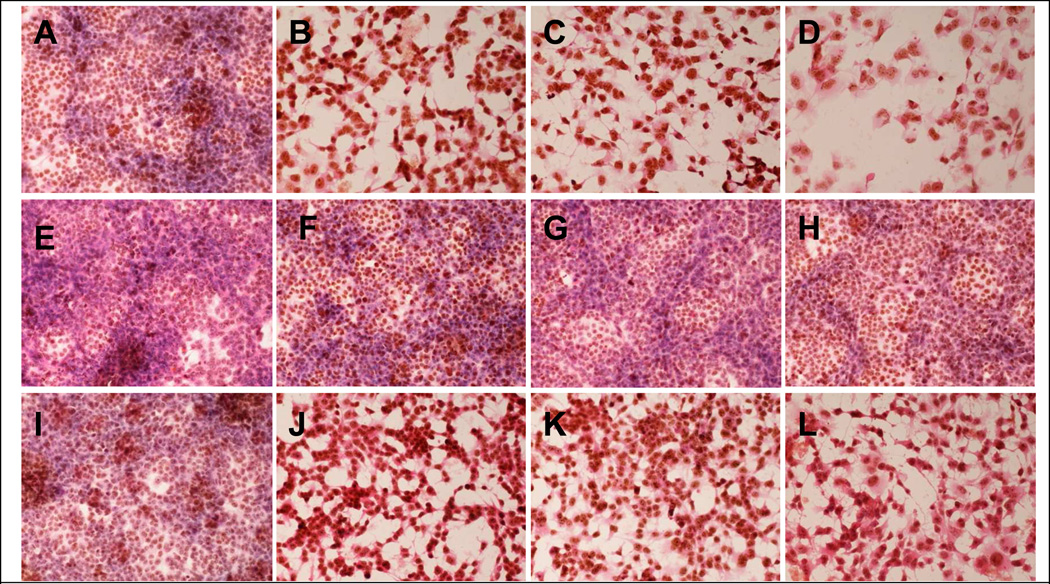
Fig 4.
C34 cells were treated with SAMe (A–D), Betaine(E–H) or combination of Betaine and SAMe(I–L). Photomicrographs were taken four days after treatment. SAMe reduced cell proliferation and induced cell death in a dose dependent manner (A: control, B: Same 1mM, C: SAMe 2mM, D : SAMe 4mM). Betaine itself had no significant effect on cell proliferation and survival (E : control, F: betaine 1%, G betaine 2%, H betaine 4%). However, it enhanced SAMe’s effect (I: control, J: SAMe 1mM and betaine 1%, K : SAMe 2mM and betaine 2%, L: SAMe 4mM and betaine 4%)
Fig 5.
Hepa1–6 cells are treated with SAMe (A–D), Betaine(E–H) or combination of Betaine and SAMe(I–L). Photomicrographs taken four days after treatment. SAMe reduced cell proliferation and induced cell death in dose dependent manner (A: control, B: SAMe 1mM, C: SAMe 2mM, D : SAMe 4mM). Betaine itself had no significant effect on cell proliferation and survival(E : control, F: betaine 1%, G betaine 2%, H betaine 4%), however, it attenuates SAMe’s effect (I :control, J:SAMe 1mM and betaine 1%, K : SAMe 2mM and betaine 2%, L: SAMe 4mM and betaine 4%)
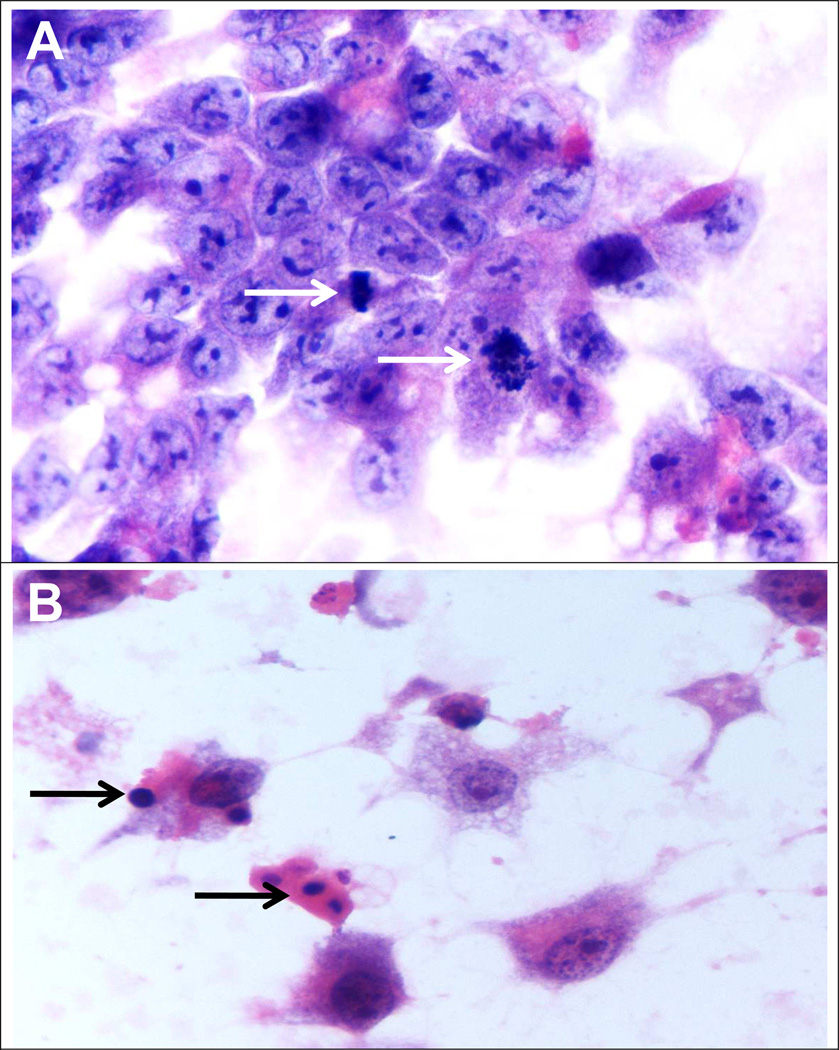
Fig 6.
A) We observed cells during cells division, without no treatment (Arrow: Metaphase and prophase) (x1560). B) Condensed DNA indicated the cells are in apoptosis, in presence of SAMe at 4mM for 4 days (x1560).
These results showed that SAMe could be used to prevent the growth of HCC. However, the phenotype of each HCC will be different and the effectiveness of SAMe to prevent growth will be different in each cell line. Lu et al showed that SAMe could prevent the tumor establishment by blocking the angiogenesis but SAMe was ineffective in the treatment of pre-establish liver tumor (Lu et al., 2009). SAMe's chemopreventive effect on cell proliferation may be related to its proapoptotic action (Lu et al., 2009). However, the difference between the proapoptotic effect between SAMe and betaine is difficult to explain because betaine increases SAMe levels in vivo. In 2002, Graf et al showed that betaine prevented the hepatocyte apoptosis induced by bile acids, in vivo and in vitro, mainly by inhibiting the mitochondrial apoptotic pathway (Graf et al., 2002). In our case, we observed a protective effect of betaine against the necrotic effect of SAMe.
Acknowledgement
The study was supported by NIH/NIAAA grants 8116 and the USC Alcohol Center Grant on Liver and Pancreatic P50-011999 Morphology Core.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ansorena E, et al. S-adenosylmethionine and methylthioadenosine are antiapoptotic in cultured rat hepatocytes but proapoptotic in human hepatoma cells. Hepatology. 2002;35:274–280. doi: 10.1053/jhep.2002.30419. [DOI] [PubMed] [Google Scholar]
- Biagioni S, et al. Acetylcholine synthesis and neuron differentiation. Int J Dev Biol. 2000;44:689–697. [PubMed] [Google Scholar]
- Buchman AL. The addition of choline to parenteral nutrition. Gastroenterology. 2009;137:S119–S128. doi: 10.1053/j.gastro.2009.08.010. [DOI] [PubMed] [Google Scholar]
- Cai J, et al. Differential expression of methionine adenosyltransferase genes influences the rate of growth of human hepatocellular carcinoma cells. Cancer Res. 1998;58:1444–1450. [PubMed] [Google Scholar]
- Chen Q, Cederbaum AI. Cytotoxicity and apoptosis produced by cytochrome P450 2E1 in Hep G2 cells. Mol Pharmacol. 1998;53:638–648. doi: 10.1124/mol.53.4.638. [DOI] [PubMed] [Google Scholar]
- Cheung HH, et al. DNA methylation of cancer genome. Birth Defects Res C Embryo Today. 2009;87:335–350. doi: 10.1002/bdrc.20163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland DH, Salmon WD. The Occurrence of Neoplasms in the Liver, Lungs, and Other Tissues of Rats as a Result of Prolonged Choline Deficiency. Am J Pathol. 1946;22:1059–1079. [PubMed] [Google Scholar]
- Graf D, et al. Prevention of bile acid-induced apoptosis by betaine in rat liver. Hepatology. 2002;36:829–839. doi: 10.1053/jhep.2002.35536. [DOI] [PubMed] [Google Scholar]
- Henning SM, Swendseid ME. The role of folate, choline, and methionine in carcinogenesis induced by methyl-deficient diets. Adv Exp Med Biol. 1996;399:143–155. doi: 10.1007/978-1-4613-1151-5_11. [DOI] [PubMed] [Google Scholar]
- Herath NI, et al. Review of genetic and epigenetic alterations in hepatocarcinogenesis. J Gastroenterol Hepatol. 2006;21:15–21. doi: 10.1111/j.1440-1746.2005.04043.x. [DOI] [PubMed] [Google Scholar]
- Huang ZZ, et al. Changes in methionine adenosyltransferase during liver regeneration in the rat. Am J Physiol. 1998;275:G14–G21. doi: 10.1152/ajpgi.1998.275.1.G14. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine, a. N. A. o. S. U. Dietary reference intakes for folate, thiamin, riboflavin, niacin, vitamin B12, panthothenic acid, biotin, and choline. Washington, DC: National Academy Press; 1998. pp. 390–422. 1998. Choline. [PubMed] [Google Scholar]
- Kotb M, et al. Consensus nomenclature for the mammalian methionine adenosyltransferase genes and gene products. Trends Genet. 1997;13:51–52. doi: 10.1016/s0168-9525(97)01013-5. [DOI] [PubMed] [Google Scholar]
- Kozak M. The scanning model for translation: an update. J Cell Biol. 1989;108:229–241. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, et al. Forced expression of methionine adenosyltransferase 1A in human hepatoma cells suppresses in vivo tumorigenicity in mice. Am J Pathol. 2010;176:2456–2466. doi: 10.2353/ajpath.2010.090810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li TW, et al. S-Adenosylmethionine and methylthioadenosine inhibit cellular FLICE inhibitory protein expression and induce apoptosis in colon cancer cells. Mol Pharmacol. 2009;76:192–200. doi: 10.1124/mol.108.054411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu SC, et al. Methionine adenosyltransferase 1A knockout mice are predisposed to liver injury and exhibit increased expression of genes involved in proliferation. Proc Natl Acad Sci U S A. 2001;98:5560–5565. doi: 10.1073/pnas.091016398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu SC, Mato JM. Role of methionine adenosyltransferase and S-adenosylmethionine in alcohol-associated liver cancer. Alcohol. 2005;35:227–234. doi: 10.1016/j.alcohol.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Lu SC, Mato JM. S-Adenosylmethionine in cell growth, apoptosis and liver cancer. J Gastroenterol Hepatol. 2008;23(Suppl 1):S73–S77. doi: 10.1111/j.1440-1746.2007.05289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu SC, et al. S-adenosylmethionine in the chemoprevention and treatment of hepatocellular carcinoma in a rat model. Hepatology. 2009;50:462–471. doi: 10.1002/hep.22990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mato JM, et al. S-adenosylmethionine synthesis: molecular mechanisms and clinical implications. Pharmacol Ther. 1997;73:265–280. doi: 10.1016/s0163-7258(96)00197-0. [DOI] [PubMed] [Google Scholar]
- Mato JM, et al. S-Adenosylmethionine: a control switch that regulates liver function. FASEB J. 2002;16:15–26. doi: 10.1096/fj.01-0401rev. [DOI] [PubMed] [Google Scholar]
- Mato JM, Lu SC. Role of S-adenosyl-L-methionine in liver health and injury. Hepatology. 2007;45:1306–1312. doi: 10.1002/hep.21650. [DOI] [PubMed] [Google Scholar]
- Michel V, et al. Choline transport for phospholipid synthesis. Exp Biol Med (Maywood) 2006;231:490–504. doi: 10.1177/153537020623100503. [DOI] [PubMed] [Google Scholar]
- Mikol YB, et al. Hepatocarcinogenesis in rats fed methyl-deficient, amino acid-defined diets. Carcinogenesis. 1983;4:1619–1629. doi: 10.1093/carcin/4.12.1619. [DOI] [PubMed] [Google Scholar]
- Newberne PM. Lipotropic factors and oncogenesis. Adv Exp Med Biol. 1986;206:223–251. doi: 10.1007/978-1-4613-1835-4_18. [DOI] [PubMed] [Google Scholar]
- Oliva J, et al. Fat10 is an epigenetic marker for liver preneoplasia in a drug-primed mouse model of tumorigenesis. Exp Mol Pathol. 2008;84:102–112. doi: 10.1016/j.yexmp.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogribny IP, Beland FA. DNA hypomethylation in the origin and pathogenesis of human diseases. Cell Mol Life Sci. 2009;66:2249–2261. doi: 10.1007/s00018-009-0015-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogribny IP, et al. Role of DNA damage and alterations in cytosine DNA methylation in rat liver carcinogenesis induced by a methyl-deficient diet. Mutat Res. 2009;669:56–62. doi: 10.1016/j.mrfmmm.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Salmon WD, Copeland DH. Liver carcinoma and related lesions in chronic choline deficiency. Ann N Y Acad Sci. 1954;57:665–677. doi: 10.1111/j.1749-6632.1954.tb36443.x. [DOI] [PubMed] [Google Scholar]
- Shivapurkar N, et al. Hepatic DNA methylation and liver tumor formation in male C3H mice fed methionine- and choline-deficient diets. J Natl Cancer Inst. 1986;77:213–217. [PubMed] [Google Scholar]
- Ueland PM, et al. Betaine: a key modulator of one-carbon metabolism and homocysteine status. Clin Chem Lab Med. 2005;43:1069–1075. doi: 10.1515/CCLM.2005.187. [DOI] [PubMed] [Google Scholar]
- Yang H, et al. S-adenosylmethionine and its metabolite induce apoptosis in HepG2 cells: Role of protein phosphatase 1 and Bcl-x(S) Hepatology. 2004;40:221–231. doi: 10.1002/hep.20274. [DOI] [PubMed] [Google Scholar]
- Zeisel SH. Epigenetic mechanisms for nutrition determinants of later health outcomes. Am J Clin Nutr. 2009;89:1488S–1493S. doi: 10.3945/ajcn.2009.27113B. [DOI] [PMC free article] [PubMed] [Google Scholar]