Abstract
The axon initial segment (AIS), with its dense clusters of voltage-gated ion channels decorating the axonal membrane, regulates action potential initiation and modulation. The AIS also functions as a barrier to maintain axodendritic polarity, and its precise axonal location contributes to the fine-tuning of neuronal excitability. Therefore, it is not surprising that mutations in AIS-related genes, disruption of the molecular organization of the AIS and altered AIS ion channel expression, function, location and/or density are emerging as key players in neurological disorders. Here, we consider the role of the AIS in nervous system disease and injury.
Keywords: action potential, axon initial segment, disease, ion channel
Introduction
Neurons are highly polarized cells with multiple distinct membrane domains. The subcellular localization and molecular compositions of these domains are essential to their functions. For example, dendritic synapses are highly enriched in neurotransmitter receptors that respond to pre-synaptic input. The axon initial segment (AIS) is another unique domain with high densities of ion channels that integrates the input from thousands of synapses to generate an action potential (AP). The AP is directionally propagated along the axon toward the axonal terminal where, at chemical synapses, neuronal output is mediated by the release of neurotransmitter. Therefore, the AIS functions at the intersection between neuronal input and axonal output. Given the importance of the AIS for a neuron’s input–output relationship, it is easy to understand how perturbations of AIS organization or integrity can have profound effects on nervous system function.
Several genes encoding proteins found at the AIS are emerging as risk factors for neurological diseases (Scheffer et al., 2007; Alarcón et al., 2008; Arking et al., 2008; Williams et al., 2011). Furthermore, calpain-mediated disassembly of the AIS cytoskeleton was recently identified as an early consequence of ischemia-induced neuronal injury (Schafer et al., 2009). Here, we provide a brief overview of the function and molecular organization of the AIS, and its role in maintaining neuronal polarity. We also discuss recent work demonstrating AIS plasticity in response to activity deprivation during nervous system development. We then consider how diseases including channelopathies, developmental neuropsychiatric conditions like autism and schizophrenia, and autoimmune disorders could impact AIS function. Finally, we speculate how AIS integrity could be compromised during aging and how these insults may contribute to cognitive decline.
Role of the axon initial segment
The AIS is a specialized membrane domain situated in the proximal axon. The protein complex assembled at the AIS is characterized by a high density of voltage-gated ion channels, cytoskeletal adaptor proteins and cell adhesion molecules (CAMs) (Grubb & Burrone, 2010a) (Fig. 1). Many of the AIS proteins implicated in human disease directly contribute to its two central functions – controlling the initiation of APs and maintaining neuronal polarity (Fig. 2).
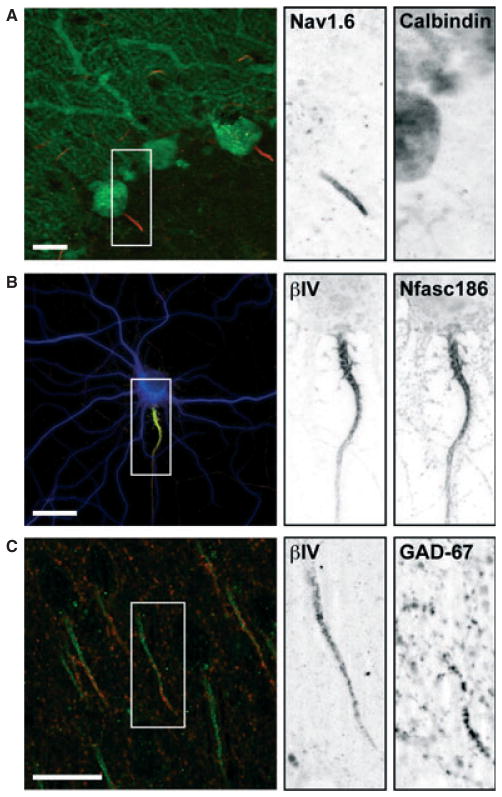
Fig. 1.
The AIS is decorated by dense clusters of voltage-gated Na+ channels and is selectively innervated by GABAergic interneurons. (A) Voltage-gated Na+ channel Nav1.6 (red) is highly enriched at the AIS of cerebellar Purkinje neurons. Purkinje cell marker calbindin (green) is restricted to the somatodendritic domain. (B) AIS proteins βIV spectrin (green) and Nfasc186 (red) assemble within the proximal axon of a cultured hippocampal neuron independently of glial interaction or extrinsic factors. (C) Glutamic acid decarboxylase (GAD-67)-positive chandelier cell axonal cartridges (red) contact the distal AIS, denoted by βIV spectrin (green), in mouse neocortex. Scale bars: 20 μm.
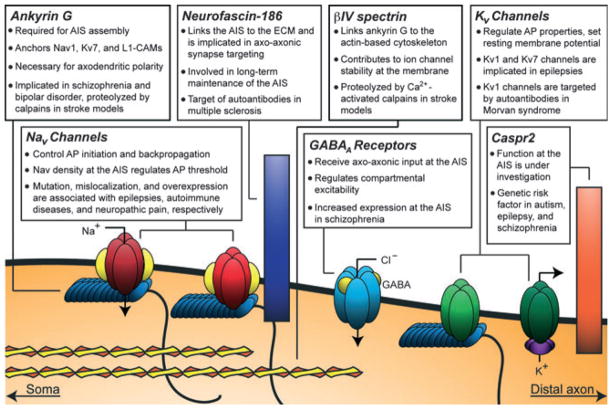
Fig. 2.
AIS protein association with nervous system pathologies and injuries. Ion channels, CAMs, neurotransmitter receptors and cytoskeletal adaptor proteins enriched at the AIS have been linked to neurological disorders including epilepsy, neuropsychiatric developmental disorders and autoimmune disorders. Nervous system injury can also cause disassembly of the AIS. ECM, extracellular matrix.
The site of action potential initiation
Early intracellular recordings in motor neurons identified the AIS as the spike initiation zone (Coombs et al., 1957). In these studies, AIS spike activity always preceded, at a predictable interval, the generation of a somatodendritic spike element. This finding was attributed to the special electrical properties of the AIS associated with its more hyperpolarized AP threshold potential relative to the somatodendritic domain (Coombs et al., 1957). In 1981, autoradiographic experiments using an Nav channel-binding neurotoxin (125I-scorpion toxin) revealed a non-uniform distribution of Nav channels in neurons with the highest densities observed at the AIS (Catterall, 1981). Elevated Nav channel density alone, based on the statistical probability of channel opening and the fraction of channels needed to elicit the all-or-none response, could explain the reduced AP threshold at the AIS relative to the soma. Several studies support the conclusion that the AIS is the site of AP initiation in a variety of central nervous system neuron types (Stuart & Sakmann, 1994; Mainen et al., 1995; Khaliq & Raman, 2006; Palmer & Stuart, 2006; Atherton et al., 2008; Kole et al., 2008; Kole & Stuart, 2008; Foust et al., 2010; Popovic et al., 2011). Nevertheless, the precise Nav channel density at the AIS remains controversial (Colbert & Johnston, 1996; Kole et al., 2008; Fleidervish et al., 2010; Lorincz & Nusser, 2010).
At one extreme, patch-clamp studies revealed no difference in Nav channel density between the AIS and the somatodendritic domain (Colbert & Johnston, 1996). Consistent with this idea, Fleidervish et al. (2010) recently reported high-resolution Na+ imaging data supporting a mere threefold increase in Nav channel density at the AIS. Instead of Nav channel density setting the low AP threshold at the AIS, the authors suggest that Nav channel gating kinetics may differ here and claim that channel density estimates based on immunofluorescent labeling may be misleading due to the detection of a non-functional pool of channels. However, it is difficult to imagine mechanisms that lead to a non-functional pool of channels on the cell membrane. In contrast to these observations, measurement of Nav channel density using freeze-fracture immunogold electron microscopy (Lorincz & Nusser, 2010), immunofluorescent labeling (Wollner & Catterall, 1986) and patch-clamp recording following depolymerization of the actin-based cytoskeleton (Kole et al., 2008) revealed an AIS Nav channel density 50-fold that of the soma and proximal dendrites.
It is likely that both channel density and specialized gating kinetics contribute to the low AP threshold at the AIS. Active regulation of either property provides a plausible mechanism for altering neuronal excitability in an activity-dependent manner. Post-translational modification of AIS Nav channels (e.g. phosphorylation) could change the local AP threshold by altering open channel probability (Cantrell & Catterall, 2001; Baek et al., 2011). If a non-functional pool of channels exists, dysregulation of the balance between functional and non-functional Nav channel populations may contribute to the pathogenesis of certain epilepsies and developmental disorders as well as hyperexcitability following injury.
Overview of the molecular organization of the axon initial segment
The AIS consists of densely clustered voltage-gated ion channels supported by a specialized cytoskeletal scaffold. However, structural and molecular diversity can be seen among neuron classes. For example, differences in ion channel composition (Nusser, 2009) and synaptic innervation of the AIS (DeFelipe et al., 1985; Huang et al., 2007) contribute to the functional diversity of neurons. Even neurons responding to similar stimuli can strategically alter the axonal location of their AIS (Kuba et al., 2006; Fried et al., 2009). Additional examples of differences at the AIS among neuronal populations are discussed at greater length below.
Ion channel localization at the axon initial segment
Action potential generation depends on the density, availability and biophysical properties of Nav and Kv channels (Bean, 2007). The distribution of these channels within the AIS and ion channel gene expression patterns underlie the variety of AP shapes observed between neuron classes (Lai & Jan, 2006; Bean, 2007; Nusser, 2009).
Sodium channels are recruited to the AIS through direct interaction with the cytoskeletal scaffolding protein ankyrin-G (ankG) (Zhou et al., 1998; Garrido et al., 2003; Lemaillet et al., 2003) and are further promoted by casein kinase 2-mediated phosphorylation of two serine residues in the AIS-targeting motif (Garrido et al., 2003) of Nav channels (Bréchet et al., 2008). A subset of Kv channels are also enriched at the AIS through interaction with ankG (Pan et al., 2006). Channel distribution within the AIS can be further divided into proximal and distal subcompartments (Van Wart & Matthews, 2006a; Hu et al., 2009). Modeling analyses performed by Hu et al. (2009) suggest that the dense clusters of Nav1.6 channels at the distal AIS contribute to AP initiation, whereas proximal Nav1.2 clusters regulate AP backpropagation to the soma and dendrites. Although Nav1.6 is expressed in almost all central nervous system neuron types, it tends to be co-expressed with Nav1.1 in inhibitory GABAergic neurons but with Nav1.2 in excitatory glutamatergic neurons (Lorincz & Nusser, 2008).
Temporal control of channel expression is also thought to be important. For example, during early development, Nav1.2 channel expression precedes that of Nav1.6 in retinal ganglion cells (Van Wart & Matthews, 2006a). This is reflected in the observation that Nav1.2 channels are the first Nav channels enriched at the developing retinal ganglion cell AIS. Nav1.6 channels cluster at later time points, just prior to eye opening between post-natal weeks 2 and 3 (Boiko et al., 2003). This developmental switch is thought to be required for the generation of high-frequency AP trains that encode visual information (Wang et al., 1997; Boiko et al., 2003). Indeed, in mice lacking Nav1.6, Nav1.1 and Nav1.2 channels are present at the retinal ganglion cell AIS and the Nav1.6-deficient retinal ganglion cells can no longer sustain high-frequency bursts (Van Wart & Matthews, 2006b). Thus, maturation of the AIS protein complex is important for proper neuronal output.
Multiple Kv channels, including Kv1 and Kv7 subtypes, are enriched at the AIS of diverse central nervous system neurons (Kole et al., 2007; Pan et al., 2006). Similar to the subcompartmental arrangement of Nav channels, the precise distribution of Kv channels within the AIS probably contributes to the diversity of AP shape and firing rates observed between neuron classes. For example, Kv1.1 and Kv1.2 immunoreactivity gradually increases toward the distal part of the AIS in cortical and hippocampal pyramidal cells, and may even extend distally beyond the Nav1.6 channels in neocortical interneurons (Lorincz & Nusser, 2008). Kv1 channels carry low voltage-activated outward currents that can depolarize the AP threshold potential, making cells less excitable (Lu et al., 2004). Interestingly, Kv1.1 and Kv1.2 are not expressed at detectable levels at the cerebellar Purkinje neuron AIS, although they are enriched in the surrounding basket cell terminals and at the cerebellar interneuron AIS (Lorincz & Nusser, 2008). Although Kv1 channels are enriched at the juxtaparanode of myelinated axons through interactions with the CAM contactin-associated protein 2 (caspr2), Kv1 channel clustering at the AIS does not require caspr2 (Horresh et al., 2008; Ogawa et al., 2008, 2010). Intriguingly, the phosphorylation state of the auxiliary subunit Kvβ2 determines the AIS targeting of Kv1 channels (Vacher et al., 2011). However, with no ankG-binding capacity, the mechanism(s) of Kv1 channel localization and stabilization at the AIS remains an active area of investigation. In contrast to Kv1 channels, AIS clustering of the Kv7 channels KCNQ2 and KCNQ3 is mediated through an ankG-binding motif analogous to the Nav1 channel AIS targeting motif (Pan et al., 2006), a similarity that is a striking example of convergent molecular evolution (Hill et al., 2008). Kv7 channels at the AIS carry a non-inactivating, subthreshold K+ current (M current) and act as key regulators of neuronal excitability by controlling the resting membrane and AP threshold potentials (Shah et al., 2008; Guan et al., 2011).
GABAA-α2 receptors are enriched along the distal AIS of cortical and hippocampal pyramidal neurons. Classically, chandelier neurons, GABAergic interneurons that exclusively synapse onto the AIS, were thought to negatively regulate AP initiation in the cortex; however, GABA release leading to depolarization of the post-synaptic cell has been reported (Szabadics et al., 2006; Khirug et al., 2008; Molnár et al., 2009). Thus, although GABA neurotransmission at the AIS is inhibitory in the hippocampus (Glickfeld et al., 2009), it may be excitatory in the neocortex (Szabadics et al., 2006; Khirug et al., 2008; Woodruff et al., 2009).
Ankyrin-G-organized assembly of the axon initial segment
In vivo and in vitro experiments suggest that ankG is the first region-defining protein to become concentrated at the AIS and is required for all subsequent protein enrichment here during development and throughout life (Jenkins & Bennett, 2001; Hedstrom et al., 2008; Sobotzik et al., 2009). The relationship between ankG and the specialized microtubule (MT) network at the AIS is the subject of several current studies. Recent work shows that some MT-related proteins, such as casein kinase 2, contribute to ankG stabilization within the proximal axon during early AIS development (Sanchez-Ponce et al., 2008; Sanchez-Ponce et al., 2011). The AIS MTs themselves, existing mostly in an acetylated-form, are highly stable; in contrast, MTs in the distal axon are in a dynamic, deacetylated (mediated by histone deacetylase-6) state during axon outgrowth (Tapia et al., 2010). This differential post-translational modification of tubulin between the proximal and distal axon is necessary for the establishment of functional neuronal polarity (Tapia et al., 2010). Interestingly, although the AIS cytoskeleton is typified by fasciculated MT bundles (Palay et al., 1968), these bundles are conspicuously absent from the Purkinje neuron AIS in a cerebellum-specific ankG knockout mouse (Sobotzik et al., 2009), suggesting a cooperative link between ankG enrichment and the post-translational modification of MTs within the proximal axon.
The local actin-based cytoskeleton also contributes to AIS function. βIV spectrin, an actin-binding cytoskeletal protein, is recruited to the AIS through interaction with ankG (Yang et al., 2007). This interaction is thought to link ankG to the local actin-based cytoskeleton. In βIV spectrin mutant mice, Nav1.6 immunoreactivity is reduced at the AIS (Yang et al., 2007). The AIS cytoskeleton is linked to the extracellular matrix through the CAM neurofascin-186 (Nfasc186) (Hedstrom et al., 2007). AIS assembly is normal in the absence of Nfasc186 expression in vitro and in vivo; however, Nfasc186 is required for the long-term maintenance and function of the AIS (Hedstrom et al., 2008; Zonta et al., 2011), and for the assembly of the brevican-based extracellular matrix at the AIS (Hedstrom et al., 2007).
The axon initial segment as a post-synaptic domain
Synaptic connections where the AIS is the post-synaptic domain are termed ‘axo-axonic’ synapses (Fig. 1C) (Fairen & Valverde, 1980; Huang et al., 2007). These inputs are strategically positioned to modulate synaptic integration and AP initiation. Axo-axonic synapse assembly is thought to be experience-independent and to depend on the expression of transmembrane CAMs (Di Cristo et al., 2004). Nfasc186 has been suggested to play an important role in regulating the assembly of GABAergic synapses. For example, Pinceau synapse formation at the Purkinje neuron AIS follows the establishment of an ankG-dependent, distally-increasing Nfasc186 gradient along the AIS (Ango et al., 2004). Similarly, in hippocampal pyramidal neurons, loss of the Nfasc186 gradient disrupts localization of gephyrin, a scaffolding protein at inhibitory synapses, and interferes with GABA receptor clustering at the post-synaptic membrane (Burkarth et al., 2007). However, work by Koticha et al. (2005) shows that the mucin-like domain of Nfasc186 inhibits cell adhesion. Investigation into axo-axonic synapse formation in newly available Nfasc186 conditional knockout mouse lines (Zonta et al., 2011) may help to clarify the role of transmembrane CAMs in axo-axonic synapse assembly.
Neuronal polarity and axon initial segment stability
Maintaining neuronal polarity
The AIS maintains the electrical and physical separation of the axon from the somatodendritic domain by functioning as a ‘selectivity filter’ to permit axonal cargoes to enter the axon while excluding somatodendritic cargoes (Winckler et al., 1999; Song et al., 2009; Rasband, 2010). Polarized transport of axonal cargoes through the AIS is thought to be facilitated by the unique properties of the local MT cytoskeleton. Nakata & Hirokawa (2003) reported that the kinesin KIF5 associates with axonal-membrane-bound cargoes; however, neuron-wide MT stabilization prevents KIF5 from distinguishing between dendritic shafts and the AIS, resulting in mislocalization of the targeted proteins. Variable post-translational modification of tubulin also aids in the targeted delivery of cargoes; by virtue of its inability to bind tyrosinated tubulin, the kinesin-1 motor domain is limited to proceeding through the AIS to deliver axonal membrane components (Konishi & Setou, 2009). The maintenance of axodendritic polarity also depends, directly or indirectly, on ankG. Silencing ankG after AIS formation results in the dispersion of the ion channels, CAMs and other cytoskeletal proteins enriched at the AIS (Hedstrom et al., 2008). Without the selectivity filter established by the AIS cytoskeleton, dendritic proteins can enter the axon leading to the acquisition of both molecular and structural features of dendrites, including spines. These results were confirmed in vivo using a cerebellum-specific ankG knockout mouse (Zhou et al., 1998; Sobotzik et al., 2009).
Neurofascin-186 also plays a critical role in maintaining polarity (Zonta et al., 2011), at least in cerebellar Purkinje neurons. Using an inducible Cre to silence the expression of Nfasc186 in mature neurons, as soon as 1 week after silencing Nfasc186 expression, Nav channels, along with ankG, βIV spectrin and neuron glia-related CAM, were completely lost from the Purkinje neuron AIS. As a consequence, the AP amplitude decreased, spontaneous firing all but ceased and the mice displayed motor deficits. These results are consistent with the idea that maintenance of neuronal polarity depends on the integrity of the AIS; however, no information was provided about the stability of the AIS of other neuron types. Silencing of NF-186 expression in vitro did not affect the molecular organization of the AIS in cultured hippocampal neurons (Hedstrom et al., 2008). It is possible that Purkinje neurons are especially sensitive to alterations in AIS function, due to their unique physiology, and axons themselves may be damaged or degenerating in this model. It will be important to look at other classes of neurons in the inducible Nfasc186 knockout mouse to establish whether the importance of Nfasc186 extends across neurons of all types.
Axon initial segment plasticity
Activity-dependent changes are the hallmark of neuronal plasticity. Long-term memory formation, facilitated by synaptic plasticity, involves dynamic restructuring of synapses supported by proteolysis (Bingol & Sheng, 2011), new protein synthesis (Klann & Sweatt, 2008) and cytoskeletal rearrangements (Kasai et al., 2010). Over 40 years ago, Palay et al. (1968) postulated that structural changes in AIS shape could be a mechanism for exercising dynamic control over neuronal excitability. Local proteolysis and the incorporation of newly synthesized AIS proteins could also contribute to structural plasticity at the AIS.
Homeostatic axon initial segment plasticity
One way in which neurons fine-tune their excitability is by controlling the position and length of the AIS (Kuba et al., 2006; Grubb & Burrone, 2010b; Kuba et al., 2010). For example, neurons in the avian nucleus laminaris can be divided into three groups based on the range of critical frequencies that they respond to – low, medium or high (Kuba et al., 2006). AIS length and localization along the axon within the same group of neurons are relatively uniform, but differ substantially between groups. Modeling analysis showed that AIS length and axonal location were optimized to reduce the AP threshold and accurately encode the interaural time differences at each critical frequency (Kuba et al., 2006). Information processing can therefore be regulated at the level of AIS morphology.
Recently, it was shown that neurons can modify the structure and/or axonal location of the AIS in response to changes in activity levels (Grubb & Burrone, 2010b; Kuba et al., 2010). For example, chronic depolarization of cultured hippocampal neurons leads to a distal shift in AIS position along the axon and reduced excitability (Grubb & Burrone, 2010b). Although voltage-gated Ca2+ channel blockers completely abolish AIS relocation during chronic depolarization (Grubb & Burrone, 2010b), little is known about how axonal translocation of the AIS occurs and whether this phenomenon occurs in vivo. Thus, it will be interesting to examine AIS length and axonal position in neurons that are chronically depolarized by disease or injury, or to determine if AIS plasticity also occurs in the normal brain.
Neurons can also change their AIS properties in response to decreases in synaptic input. For example, in response to loss of cochlear afferent innervation, neurons in the nucleus magnocellularis of birds increase their AIS length (Kuba et al., 2010). Recordings revealed a compensatory increase in the intrinsic excitability of the input-deprived cells. Thus, sensory deprivation during critical periods of development initiates adaptive mechanisms to stabilize neuronal output (Kuba et al., 2010). This phenomenon is qualitatively similar to homeostatic synaptic plasticity (Turrigiano & Nelson, 2004). Injuries or diseases that affect this adaptive response at the AIS may prevent proper integration of synaptic inputs. Furthermore, altered AIS plasticity following injury could also prove maladaptive and actually exacerbate pre-existing pathological conditions (Offord & Catterall, 1989; Waxman, 2001). Studies in animal disease models with abnormal network activity should improve our understanding of the molecular mechanisms underlying homeostatic AIS plasticity in adaptive compensation.
Ion channel expression and axon initial segment plasticity
Action potential firing properties are also controlled by the temporal regulation of ion channel expression. For example, Nav1.6 channels are present at the mature cerebellar granule cell AIS but absent from the developing granule cell AIS. Studies in Nav1.6 knockout mice revealed that the transition to an Nav1.6-dominated granule cell AIS drives an increase in persistent Na+ current and improves firing reliability during sustained depolarization (Osorio et al., 2010). It is possible that some nervous system insults could induce a reversion to early developmental expression patterns that would alter AIS composition and, consequently, neuronal excitability. The reversion in Na+ channel subtype expression has previously been observed after demyelination (Rasband et al., 2003; Craner et al., 2004). Presumably, changes in the types of channels expressed in axons would involve the control of AIS protein component expression, particularly ion channels or the cytoskeletal scaffolds that regulate their retention at the AIS; however, little is known about the transcriptional and translational events that lead to changes in ion channel expression (Waxman, 2001).
Axon initial segment channelopathies
Action potential properties are defined by the coordinated activity of the voltage-gated ion channels at the AIS (Bean, 2007 and Nusser, 2009); consequently, changes in channel kinetics can have a profound impact on neuronal output. In some cases, widespread alteration in neuronal properties can even be attributed to single nucleotide polymorphisms (Wallace et al., 1998). Several genes encoding AIS channels and channel auxiliary subunits are associated with human epilepsy, leading to the suggestion that the AIS may be a site of functional convergence in the pathogenesis of epilepsy (Wimmer et al., 2010a).
Axon initial segment Nav channels in epilepsy
Voltage-gated sodium channels consist of a single pore-forming α subunit and two auxiliary β subunits. Mutations in both α and β Nav channel subunits enriched at the AIS can cause hyperexcitability and seizure activity. Nav1.1 channels are enriched at the AIS of GABAergic interneurons (Ogiwara et al., 2007; Duflocq et al., 2008; Lorincz & Nusser, 2008; Wimmer et al., 2010b). Loss of functional Nav1.1 is associated with an early-onset form of epilepsy known as Dravet syndrome or severe myoclonic epilepsy in infancy (Oakley et al., 2011). Experiments performed on severe myoclonic epilepsy in infancy model mice (Scn1a−/−) revealed a substantial loss of Na+ current and an increase in AP firing failures in GABAergic hippocampal interneurons (Yu et al., 2006; Kalume et al., 2007). Without any accompanying decrease in excitatory hippocampal pyramidal neuron activity, the loss of inhibitory control by GABAergic interneurons may underlie hyperactivity and seizure generation in severe myoclonic epilepsy in infancy (Yu et al., 2006; Oakley et al., 2011). Mutations that cause only mild functional impairment of Nav1.1 are associated with less severe forms of epilepsy in the genetic epilepsy with febrile seizures plus spectrum (Mashimo et al., 2010). A missense mutation affecting the voltage-sensing domain of Nav1.2 also causes Dravet syndrome (Shi et al., 2009).
The Nav1.6 channels underlie AP generation in several neuron types (Royeck et al., 2008; Hu et al., 2009). Hippocampal CA3 pyramidal neurons respond to weak repetitive stimulation by increasing their Nav1.6 mRNA and protein levels (Blumenfeld et al., 2009). In this case, activity-dependent facilitation mediated by changes in Nav1.6 expression contributes to hyperexcitability and presumably epileptogenesis. Consistent with this idea, decreasing Nav1.6 expression in Scn1a mutants reduces seizure susceptibility by restoring threshold potential (Martin et al., 2007). These results suggest that channel subtype-specific changes in channel expression differentially affect network activity levels and that normal nervous system function depends on balanced Nav channel activity.
Auxiliary Nav channel β subunits are also enriched at the AIS and function in cell adhesion, α subunit gating and α subunit trafficking (Isom et al., 1992; Malhotra et al., 2000; Brackenbury et al., 2010; Patino & Isom, 2010). Similar to Nav1.1, mutations in the gene encoding the Nav channel subunit β1, SCN1B, cause Dravet syndrome and genetic epilepsy with febrile seizures plus (Wallace et al., 1998; Meadows et al., 2002; Patino et al., 2009). The regulatory role of the β subunits makes them a particularly intriguing target for antiepileptic therapies as the different β subunits can have distinct effects on Nav channel kinetics depending on the cell type in which they are expressed (Brackenbury et al., 2010).
Axon initial segment Kv channels in epilepsy
The influx of K+ through slowly activating Kv channels dampens excitability following membrane depolarization and returns the neuron to the resting membrane potential. Aberrant Kv channel activity at the AIS could lead to alterations in the shape of the AP waveform and changes in intrinsic membrane properties. Genetic loci containing AIS Kv channel genes (KCNQ2 and KCNQ3) have been identified as risk regions for benign familial neonatal seizures in humans (Leppert et al., 1989; Lewis & Faber, 1993; Browne et al., 1994; Biervert et al., 1998; Charlier et al., 1998; Singh et al., 1998, 2003).
It is important to note that channelopathies are not limited to mutations in the channels themselves; alterations in the trafficking, localization, stabilization and/or expression of ion channels may also result in pathological states. Furthermore, dysfunction of Kv or Nav channels specifically at the AIS has yet to be causally linked to seizure induction. As there are low densities of these channels located in somatodendritic regions, it is also possible that channel dysfunction in the soma, dendrites or distal axons also contributes to epilepsy. However, recent sequencing efforts have revealed that even deleterious ion channel mutations do not necessarily translate into a pathologic state. Instead, the high degree of variability in manifestation of epilepsy (or lack thereof) depends on the combined activities of multiple mutant ion channels (Klassen et al., 2011).
The axon initial segment in neuropsychiatric developmental disorders
Several AIS protein-encoding genes have been identified as risk factors for neuropsychiatric developmental disorders in multiple genome-wide association studies (Abrahams et al., 2007; Alarcón et al., 2008; Arking et al., 2008; Ferreira et al., 2008; Schulze et al., 2009; Athanasiu et al., 2010). Some of these loci are even emerging as common risk factors in conditions previously considered unrelated (Ferreira et al., 2008; Burbach & van der Zwaag, 2009; Schulze et al., 2009). Much of the work to identify the molecular basis of neuropsychiatric developmental disorders focuses exclusively on synaptopathies; however, evidence is now accumulating that the aberrant organization and function of the AIS may also contribute to the pathophysiology of neuropsychiatric diseases.
The contribution of the axon initial segment to working memory: implications in schizophrenia and bipolar disorder
Cognitive dysfunction in patients with schizophrenia has been attributed to altered cortical network activity (Van Snellenberg et al., 2006). Schizophrenia and bipolar disorder are both thought to arise in part due to deficits in working memory (Raghavachari et al., 2001; Altshuler et al., 2004; Seidman et al., 2002). Recent evidence suggests that some dysfunction may be due to aberrant axo-axonic synaptic activity at the AIS (Cruz et al., 2009; Lewis, 2011). Early efforts to determine the molecular bases of schizophrenia identified a disruption in GABAergic circuitry (Bird et al., 1978; Simpson et al., 1989; Lewis et al., 2005). Further studies revealed that a specific class of interneurons is particularly affected in schizophrenia – the parvalbumin-expressing chandelier cells that form axo-axonic synaptic connections exclusively onto the AIS of cortical pyramidal neurons (Lewis, 2011) (Fig. 1C). Post-mortem analysis of schizophrenic human brain tissue revealed abnormal axo-axonic synapse composition in layers II/III of the dorso-lateral prefrontal cortex (Cruz et al., 2009). Specifically, GABAA receptor α2 subunit immunoreactivity is increased at the post-synaptic AIS membrane of patients with schizophrenia (Volk et al., 2002). This upregulation may be compensatory in response to decreased GABA release from apposed pre-synaptic terminals. Interestingly, and somewhat paradoxically (considering the increase in GABAA-α2 receptor at the AIS membrane), ankG immunoreactivity is decreased at the schizophrenic AIS in the dorso-lateral prefrontal cortex. Presumably, decreases in ankG correspond to concomitant decreases in Nav channel density at the AIS membrane and changes in neuronal excitability; however, this has not been explored experimentally. Decreases in Nav channel availability at the AIS would be expected to impair AP initiation. This could interfere with the generation of normal cortical network oscillations and working memory (Wang, 2010). Thus, the interneuron-mediated regulation of AP generation at the cortical pyramidal neuron AIS may be a point of convergence between single-cell activity and whole network synchronization.
Schizophrenia and bipolar disorder share ANK3, the gene encoding ankG, as a common genetic risk factor (Ferreira et al., 2008; Schulze et al., 2009; Athanasiu et al., 2010). However, nothing is known about changes that occur at the AIS in patients with bipolar disorder. It may be interesting to analyze axo-axonic synapse organization in postmortem human bipolar disorder tissue and AIS function in mouse models of bipolar disorder.
Autism spectrum disorders and the axon initial segment
Autism spectrum disorders are characterized by cognitive dysfunction of variable severity. Several hundred genes have been identified as autism spectrum disorder risk factors and the majority of these code for synaptic proteins and proteins involved in regulating synaptic plasticity (Bourgeron, 2009; Boda et al., 2010). However, the gene encoding caspr2 (CNTNAP2) was recently identified as a genetic susceptibility factor in patients with autism (Alarcón et al., 2008; Arking et al., 2008; Bakkaloglu et al., 2008; Rossi et al., 2008). Interestingly, CNTNAP2 is one of several autism spectrum disorder-linked genes that is also a genetic risk factor for schizophrenia, and mutations in caspr2 have also been shown to cause epilepsy (Friedman et al., 2008; International Schizophrenia Consortium, 2008; Burbach & van der Zwaag, 2009; Strauss et al., 2006). Caspr2 is enriched within the distal AIS of cortical pyramidal neurons and along myelinated axons at juxtaparanodes flanking nodes of Ranvier (Inda et al., 2006; Ogawa et al., 2008). At the juxtaparanode, caspr2 is required for Kv channel localization and regulates axoglial interactions (Poliak et al., 2003). However, Kv channels accumulate at the AIS independently of caspr2 in CNTNAP2 knockouts (Ogawa et al., 2008). It is possible that caspr2 regulates the subcompartmental distribution of Kv channels within the AIS, strategically enriching them within the distal portion where GABAergic terminals make synaptic connections (Inda et al., 2006). Changes in Kv channel distribution within the AIS could modify the generation of both the forward- and backward-propagating AP. Thus, the haploinsufficiency and single nucleotide polymorphisms in CNTNAP2 implicated in schizophrenia and autism, respectively, may result in AIS dysfunction that contributes to the pathology of these disorders.
We speculate that primary genetic insults that impair synaptic formation, function and plasticity in autism spectrum disorder may drive secondary changes at the AIS. As described above, prolonged changes in neuronal activity can alter Nav channel expression and AIS channel density (Waxman, 2001; Kuba et al., 2010). Consistent with this idea, Kuba et al. (2010) showed that neurons can adjust the distribution of Nav channels within the proximal axon, lengthening the AIS to increase neuronal excitability in response to decreased pre-synaptic input. Mouse models of neurodevelopmental disorders associated with autism may provide valuable systems in which to observe structural and functional AIS plasticity in response to altered synaptic and network activity in vivo.
Disassembly of the axon initial segment after injury
The pathophysiology of stroke is due in part to damage induced by excitotoxicity (Choi, 1996). The large and sudden influx of Ca2+ following an insult initiates several downstream effects, including the activation of proteolytic calpains (Siman et al., 1984). Schafer et al. (2009) observed rapid, calpain-mediated degradation of the AIS ankyrin/spectrin cytoskeleton following middle cerebral artery occlusion. Importantly, disruption of the AIS cytoskeleton also resulted in loss of Nav channel clusters. AIS disassembly was blocked by the calpain inhibitor MDL28170. Although both AIS disassembly and neuronal death are consequences of stroke, they are apparently separate events. Whereas application of the N-methyl-D-aspartate receptor antagonist MK801 in the oxygen–glucose deprivation cell culture model prevented cell death, it did not preserve the AIS (Schafer et al., 2009). However, co-application of MK801 with MDL28170 both prevented AIS disassembly and significantly reduced cell death. As described above, the AIS is also essential to maintain neuronal polarity. Thus, disruption of the AIS cytoskeleton by calpain-mediated proteolysis after injury results in not only loss of ion channel clustering, but also loss of polarity. We propose that loss of neuronal polarity is a previously unappreciated consequence of nervous system injury, and that preserving the cytoskeleton would be expected to both preserve polarity and facilitate the retention of AIS membrane proteins, including Nav channels, which would preserve a neuron’s ability to generate APs. These observations suggest that neuroprotective strategies alone will not preserve nervous system function.
Autoimmune disorders and the axon initial segment
Several proteins common to both nodes of Ranvier and the AIS have been implicated in autoimmune-mediated pathology. Although the factors that lead to the immune system-mediated axonal degeneration in multiple sclerosis (MS) are not fully understood, recent evidence suggests that autoantibodies against the nodal and AIS CAM Nfasc186 are significantly increased in the serum of some patients with MS (Mathey et al., 2007). Introduction of anti-Nfasc186 antibodies into hippocampal slice cultures activated the complement cascade resulting in AP conduction block and axonal injury (Mathey et al., 2007). Similarly, antibody-mediated autoimmune channelopathies can affect neuronal excitability leading to nervous system dysfunction. For example, autoantibodies against Kv1 channels were found to be upregulated in the sera of patients with Morvan syndrome (Misawa & Mizusawa, 2010). The resulting hyperexcitability of neurons within the central nervous system is associated with epilepsy, short-term memory loss and insomnia (Misawa & Mizusawa, 2010; Cornelius et al., 2011); patients suffer concomitant peripheral nervous system hyperexcitability. Thus, immune recognition of AIS and nodal proteins is a potential pathogenic mechanism for the neurological deficits observed in MS and other antibody-mediated neurological disorders.
To date, no studies have looked at the integrity of the AIS in postmortem MS tissue or in animal models of MS or other autoimmune diseases. In addition to degeneration, we speculate that irreversible loss of the AIS and neuronal polarity may contribute to the rapid, non-remitting progression of the secondary phase of MS. As axons that lose their AIS acquire a dendritic fate, and dendrites are not myelinated, it is also interesting to speculate that one potential cause for failure of remyelination is the loss of axon identity.
The aging brain
Neuronal death was thought to be the primary cause of cognitive decline during normal aging (Brody, 1970). However, more recent studies on cortical synaptic function, spine density and dendritic arbor complexity in aged animals suggest that age-related cellular changes contribute significantly to normal cognitive decline, even in the absence of significant neuronal death (Peters, 2002; Peters et al., 2008). Although changes in neuronal excitability have been shown to occur with age (Luebke et al., 2004; Kumar & Foster, 2007), little is known about the concomitant changes in ion channel expression, trafficking, localization and stabilization. Altered neuronal excitability in aging cells could be due, in part, to changes in ion channel density, subcompartmental localization and modulation at the AIS.
Disruption of the molecular organization of the nodes of Ranvier has been shown to occur in normal aging, and this is thought to result primarily from age-related demyelination (Sugiyama et al., 2002; Sloane et al., 2003; Hinman et al., 2004, 2006). As nodes of Ranvier and the AIS share a common molecular organization, we speculate that similar disruptions in AIS ion channel distribution, CAMs and/or cytoskeletal scaffolding proteins may occur in an age-dependent fashion resulting in altered excitability. These changes may be a consequence of dysregulation of internal Ca2+ levels (Toescu & Vreugdenhil, 2010) and/or mitochondrial dysfunction (Balaban et al., 2005; Reddy & Reddy 2011). Finally, nothing is known about the AIS in age-related neurodegenerative diseases. Recently, however, tubulin deacetylation in the distal axon by histone deacetylase-6, a protein implicated in both Huntington’s disease (Iwata et al., 2005; Dompierre et al., 2007) and Parkinson’s disease pathology (Kawaguchi et al., 2003; Du et al., 2010), was shown to be required for proper AIS development (Tapia et al., 2010).
Looking forward
Basic neuroscience has revealed the identities of several voltage-gated ion channels and supporting scaffolding proteins at the AIS that facilitate AP generation and maintain neuronal polarity. Furthermore, as described in this review, there is substantial circumstantial evidence linking the AIS to numerous neurological diseases. Nevertheless, many important questions remain unanswered. For example, we still have no direct evidence for a causal link between AIS channel mutations and epilepsy. Is disruption of the AIS a common feature of many nervous system diseases and injuries? Does the AIS undergo age-related degeneration or alteration? Does AIS plasticity only occur under pathological conditions, or is it a normal occurrence in the intact brain? Similarly, does AIS plasticity alter neuronal excitability to compensate for synaptic deficiencies in diseases like autism, and what are the molecular mechanisms underlying structural plasticity at the AIS? Is it possible to restore neuronal polarity after loss of the AIS? Does autoimmune-mediated attack of AIS proteins contribute to axonal degeneration?
In conclusion, the AIS is an essential regulator of neuronal output. The identities, density and modulation of voltage-gated ion channels enriched at the AIS exert powerful control over the excitability and firing patterns of neurons. We propose that disruption of the molecular organization of the AIS, due to disease, injury or aging, is a previously unappreciated focus for nervous system dysfunction. Nevertheless, additional experiments are clearly needed to define the roles of the AIS in nervous system disease. The results of these studies will enrich our perspective on how the AIS contributes to high-fidelity signal generation and activity-dependent adaptation of neuronal excitability in the developing, mature and diseased nervous system.
Abbreviations
- AIS
axon initial segment
- ankG
ankyrin-G
- AP
action potential
- CAM
cell adhesion molecule
- caspr2
contactin-associated protein 2
- MS
multiple sclerosis
- MT
microtubule
- Nfasc186
neurofascin-186
References
- Abrahams BS, Tentler D, Perederiy JV, Oldham MC, Coppola G, Geschwind DH. Genome-wide analyses of human perisylvian cerebral cortical patterning. Proc Natl Acad Sci USA. 2007;53:1722–1730. doi: 10.1073/pnas.0706128104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarcón M, Abrahams BS, Stone JL, Duvall JA, Perederiy JV, Bomar JM, Sebat J, Wigler M, Martin CL, Ledbetter DH, Nelson SF, Cantor RM, Geschwind DH. Linkage, association, and gene-expression analyses identify CNTNAP2 as an autism-susceptibility gene. Am J Hum Genet. 2008;82:150–159. doi: 10.1016/j.ajhg.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altshuler LL, Ventura J, van Gorp WG, Green MF, Theberge DC, Mintz J. Neurocognitive function in clinically stable men with bipolar I disorder or schizophrenia and normal control subjects. Biol Psychiatry. 2004;56:560–569. doi: 10.1016/j.biopsych.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Ango F, di Cristo G, Higashiyama H, Bennett V, Wu P, Huang ZJ. Anyrin-based subcellular gradient of neurofascin, an immunoglobulin family protein, directs GABAergic innervation at purkinje axon initial segment. Cell. 2004;119:257–272. doi: 10.1016/j.cell.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Arking DE, Cutler DJ, Brune CW, Teslovich TM, West K, Ikeda M, Rea A, Guy M, Lin S, Cook EH, Chakravarti A. A common genetic variant in the neurexin superfamily member CNTNAP2 increases familial risk of autism. Am J Hum Genet. 2008;82:160–164. doi: 10.1016/j.ajhg.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athanasiu L, Mattingsdal M, Kähler AK, Brown A, Gustafsson O, Agartz I, Giegling I, Muglia P, Cichon S, Rietschel M, Pieteiläinen OP, Peltonen L, Bramon E, Collier D, Clair DS, Sigurdsson E, Petursson H, Rujescu D, Melle I, Steen VM, Djurvoic S, Andreassen OA. Gene variants associated with schizophrenia in a Norwegian genome-wide study are replicated in a large European cohort. J Psychiatr Res. 2010;44:748–753. doi: 10.1016/j.jpsychires.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atherton JF, Wokosin DL, Ramanathan S, Bevan MD. Autonomous initiation and propagation of action potentials in neurons of the subthalamic nucleus. J Physiol. 2008;586:5679–5700. doi: 10.1113/jphysiol.2008.155861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek JH, Cerda O, Trimmer JS. Mass spectrometry-based phosphoproteomics reveals multisite phosphorylation on mammalian brain voltage-gated sodium and potassium channels. Semin Cell Dev Biol. 2011;22:153–159. doi: 10.1016/j.semcdb.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakkaloglu B, O’Roark BJ, Louvi A, Gupta AR, Abelson JF, Morgan TM, Chawarka K, Klin A, Ercan-Sencicek AG, Stillman AA, Tanriover G, Abrahams BS, Duvall JA, Robbins EM, Geschwin DH, Biederer T, Gunel M, Lifton RP, State MW. Molecular cytogenetic analysis and resequencing of contactin associated protein-like 2 in autism spectrum disorders. Am J Hum Genet. 2008;82:165–173. doi: 10.1016/j.ajhg.2007.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Bean BP. The action potential in mammalian central neurons. Nat Rev Neurosci. 2007;8:451–465. doi: 10.1038/nrn2148. [DOI] [PubMed] [Google Scholar]
- Biervert C, Schroeder BC, Kubisch C, Berkovic SF, Propping P, Jentsch TJ, Steinlein OK. A potassium channel mutation in neonatal human epilepsy. Science. 1998;279:403–406. doi: 10.1126/science.279.5349.403. [DOI] [PubMed] [Google Scholar]
- Bingol B, Sheng M. Deconstruction for reconstruction: the role of proteolysis in neural plasticity and disease. Neuron. 2011;69:22–32. doi: 10.1016/j.neuron.2010.11.006. [DOI] [PubMed] [Google Scholar]
- Bird ED, Spokes EG, Barnes J, Mackay AV, Iversen LL, Shepherd M. Glutamic-acid decarboxylase in schizophrenia. Lancet. 1978;1:156. doi: 10.1016/s0140-6736(78)90455-5. [DOI] [PubMed] [Google Scholar]
- Blumenfeld H, Lampert A, Klein JP, Mission J, Chen MC, Rivera M, Dib-Hajj S, Brennan AR, Hains BC, Waxman SG. Role of hippocampal sodium channel Nav1.6 in kindling epileptogenesis. Epilepsia. 2009;50:44–55. doi: 10.1111/j.1528-1167.2008.01710.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boda B, Dubos A, Muller D. Signaling mechanisms regulating synapse formation and function in mental retardation. Curr Opin Neurobiol. 2010;20:519–527. doi: 10.1016/j.conb.2010.03.012. [DOI] [PubMed] [Google Scholar]
- Boiko T, Rasband MN, Levinson SR, Caldwell JH, Mandel G, Trimmer JS, Matthews G. Compact myelin dictates the differential targeting of two sodium channel isoforms in the same axon. Neuron. 2001;30:91–104. doi: 10.1016/s0896-6273(01)00265-3. [DOI] [PubMed] [Google Scholar]
- Boiko T, Van Wart A, Caldwell JH, Levinson SR, Trimmer JS, Matthews G. Functional specialization of the axon initial segment by isoform-specific sodium channel targeting. J Neurosci. 2003;23:2306–2313. doi: 10.1523/JNEUROSCI.23-06-02306.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeron T. A synaptic trek to autism. Curr Opin Neurobiol. 2009;19:231–234. doi: 10.1016/j.conb.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Brackenbury WJ, Calhoun JD, Chen C, Miyazaki H, Nukina N, Oyama F, Ranscht B, Isom LL. Functional reciprocity between Na+ channel Nav1.6 and beta1 subunits in the coordinated regulation of excitability and neurite outgrowth. Proc Natl Acad Sci USA. 2010;107:2283–2288. doi: 10.1073/pnas.0909434107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bréchet A, Fache MP, Brachet A, Ferracci G, Baude A, Irondelle M, Pereira S, Leterrier C, Dargent B. Protein kinase CK2 contributes to the organization of sodium channels in axonal membranes by regulating their interactions with ankyrin G. J Cell Biol. 2008;183:1101–1114. doi: 10.1083/jcb.200805169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody H. Structural changes in the aging nervous system. Interdiscipl Top Gerontol. 1970;7:9–21. [Google Scholar]
- Browne DL, Gancher ST, Nutt JG, Brunt ER, Smith EA, Kramer P, Litt M. Episodic ataxia/myokymia syndrome is associated with point mutations in the human potassium channel gene, KCNA1. Nat Genet. 1994;8:136–140. doi: 10.1038/ng1094-136. [DOI] [PubMed] [Google Scholar]
- Burbach JP, van der Zwaag B. Contact in the genetics of autism and schizophrenia. Trends Neurosci. 2009;32:69–72. doi: 10.1016/j.tins.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Burkarth N, Kriebel M, Kranz EU, Volkmer H. Neurofascin regulates the formation of gephyrin clusters and their subsequent translocation to the axon hillock of hippocampal neurons. Mol Cell Neurosci. 2007;36:59–70. doi: 10.1016/j.mcn.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Cantrell AR, Catterall WA. Neuromodulation of Na+ channels: an unexpected form of cellular plasticity. Nat Rev Neurosci. 2001;2:397–407. doi: 10.1038/35077553. [DOI] [PubMed] [Google Scholar]
- Catterall WA. Localization of sodium channels in cultured neural cells. J Neurosci. 1981;1:777–783. doi: 10.1523/JNEUROSCI.01-07-00777.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlier C, Singh NA, Ryan SG, Lewis TB, Reus BE, Leach RJ, Leppert M. A pore mutation in a novel KQT-like potassium channel gene in an idiopathic epilepsy family. Nat Genet. 1998;18:53–55. doi: 10.1038/ng0198-53. [DOI] [PubMed] [Google Scholar]
- Choi DW. Ischemia-induced neuronal apoptosis. Curr Opin Neurobiol. 1996;6:667–672. doi: 10.1016/s0959-4388(96)80101-2. [DOI] [PubMed] [Google Scholar]
- Colbert CM, Johnston D. Axonal action-potential initiation and Na+ channel densities in the soma and axon initial segment of subicular pyramidal neurons. J Neurosci. 1996;16:6676–6686. doi: 10.1523/JNEUROSCI.16-21-06676.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs JS, Curtis DR, Eccles JC. The generation of impulses in motoneurones. J Physiol. 1957;139:232–249. doi: 10.1113/jphysiol.1957.sp005888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelius JR, Pittock SJ, McKeon A, Lennon VA, Aston PA, Josephs KA, Tippmann-Peikert M, Silber MH. Sleep manifestations of voltage-gated potassium channel complex autoimmunity. Arch Neurol. 2011;68:733–738. doi: 10.1001/archneurol.2011.106. [DOI] [PubMed] [Google Scholar]
- Craner MJ, Newcombe J, Black JA, Hartle C, Cuzner ML, Waxman SG. Molecular changes in neurons in multiple sclerosis: altered axonal expression of Nav1.2 and Nav1.6 sodium channels and the Na+/Ca2+ exchanger. Proc Natl Acad Sci USA. 2004;101:8168–8173. doi: 10.1073/pnas.0402765101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz DA, Weaver CL, Lovallo EM, Melchitzky DS, Lewis DA. Selective alterations in postsynaptic markers of chandelier cell inputs to cortical pyramidal neurons in subjects with schizophrenia. Neuropsychopharmacology. 2009;34:2112–2124. doi: 10.1038/npp.2009.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFelipe J, Hendry SH, Jones EG, Schmechel D. Variability in the terminations of GABAergic chandelier cell axons on initial segments of pyramidal cell axons in the monkey sensory-motor cortex. J Comp Neurol. 1985;231:364–384. doi: 10.1002/cne.902310307. [DOI] [PubMed] [Google Scholar]
- Di Cristo G, Wu C, Chattopadhyaya B, Ango F, Knott G, Welker E, Svoboda K, Huang ZJ. Subcellular domain-restricuted GAB-Aergic innervation in primary visual cortex in the absence of sensory and thalamic inputs. Nat Neurosci. 2004;7:1184–1186. doi: 10.1038/nn1334. [DOI] [PubMed] [Google Scholar]
- Dompierre JP, Godin JD, Charrin BC, Cordelières FP, King SJ, Humbert S, Saudou F. Histone deacetylase 6 inhibition compensates for the transport deficit in Huntington’s disease by increasing tubulin acetylation. J Neurosci. 2007;27:3571–3583. doi: 10.1523/JNEUROSCI.0037-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du G, Liu X, Chen X, Song M, Yan Y, Jiao R, Wang CC. Drosophila histone deacetylase 6 protects dopaminergic neurons against {alpha}-synuclein toxicity by promoting inclusion formation. Mol Biol Cell. 2010;21:2128–2137. doi: 10.1091/mbc.E10-03-0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duflocq A, Le Bras B, Buller E, Couraud F, Davenne M. Nav1.1 is predominantly expressed in nodes of Ranvier and axon initial segments. Mol Cell Neurosci. 2008;39:180–192. doi: 10.1016/j.mcn.2008.06.008. [DOI] [PubMed] [Google Scholar]
- Fairen A, Valverde F. A specialized type of neuron in the visual cortex of cat: a Golgi and electron microscope study of chandelier cells. J Comp Neurol. 1980;194:761–779. doi: 10.1002/cne.901940405. [DOI] [PubMed] [Google Scholar]
- Ferreira MA, O’Donovan MC, Meng YA, Jones IR, Ruderfer DM, Jones L, Fan J, Kirov G, Perlis RH, Green EK, Smoller JW, Grozeva D, Stone J, Nikolov I, Chambert K, Hamshere ML, Nimgaonkar VL, Moskvina V, Thase ME, Caesar S, Sachs GS, Franklin J, Gordon-Smith K, Ardlie KG, Gabriel SB, Fraser C, Blumenstiel B, Defelice M, Breen G, Gill M, Morris DW, Elkin A, Muir WJ, McGhee KA, Williamson R, MacIntyre DJ, MacLean AW, St CD, Robinson M, Van Beck M, Pereira AC, Kandaswamy R, McQuillin A, Collier DA, Bass NJ, Young AH, Lawrence J, Ferrier IN, Anjorin A, Farmer A, Curtis D, Scolnick EM, McGuffin P, Daly MJ, Corvin AP, Holmans PA, Blackwood DH, Gurling HM, Owen MJ, Purcell SM, Sklar P, Craddock N Wellcome Trust Case Control Consortium. Collaborative genome-wide association analysis supports a role for ANK3 and CACNA1C in bipolar disorder. Nat Genet. 2008;40:1056–1058. doi: 10.1038/ng.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleidervish IA, Lasser-Ross N, Gutnick MJ, Ross WN. Na+ imaging reveals little difference in action potential-evoked Na+ influx between axon and soma. Nat Neurosci. 2010;13:852–860. doi: 10.1038/nn.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foust A, Popovic M, Zecevic D, McCormick DA. Action potentials initiate in the axon initial segment and propagate through axon collaterals reliably in cerebellar Purkinje neurons. J Neurosci. 2010;30:6891–6902. doi: 10.1523/JNEUROSCI.0552-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried SI, Lasker AC, Desai NJ, Eddington DK, Rizzo JF., 3rd Axonal sodium-channel bands shape the response to electric stimulation in retinal ganglion cells. J Neurophysiol. 2009;101:1972–1987. doi: 10.1152/jn.91081.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman JI, Vrijenhoek T, Markx S, Janssen IM, van der Vliet WA, Faas BH, Knoers NV, Cahn W, Kahn RS, Edelmann L, Davis KL, Silverman JM, Brunner HG, van Kessel AG, Wijmenga C, Ophoff RA, Veltman JA. CNTNAP2 gene dosage variation is associated with schizophrenia and epilepsy. Mol Psychiatry. 2008;13:261–266. doi: 10.1038/sj.mp.4002049. [DOI] [PubMed] [Google Scholar]
- Garrido JJ, Giraud P, Carlier E, Fernandes F, Moussif A, Fache MP, Debanne D, Dargent B. A targeting motif in sodium channel clustering at the axon initial segment. Science. 2003;300:2091–2094. doi: 10.1126/science.1085167. [DOI] [PubMed] [Google Scholar]
- Glickfeld LL, Roberts JD, Somogyi P, Scanziani M. Interneurons hyperpolarize pyramidal cells along their entire somatodendritic axis. Nat Neurosci. 2009;12:21–23. doi: 10.1038/nn.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubb MS, Burrone J. Building and maintaining the axon initial segment. Curr Opin Neurobiol. 2010a;20:1–8. doi: 10.1016/j.conb.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubb MS, Burrone J. Activity-dependent relocation of the axon initial segment fine-tunes neuronal excitability. Nature. 2010b;465:1070–1074. doi: 10.1038/nature09160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan D, Higgs MH, Horton LR, Spain WJ, Foehring RC. Contributions of Kv7-mediated potassium current to sub- and suprathreshold responses of rat layer II/III neocortical pyramidal neurons. J Neurophysiol. 2011;106:1722–1733. doi: 10.1152/jn.00211.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedstrom KL, Xu X, Ogawa Y, Frischknecht R, Seidenbecher CI, Shrager P, Rasband MN. Neurofascin assembles a specialized extracellular matrix at the axon initial segment. J Cell Biol. 2007;178:875–886. doi: 10.1083/jcb.200705119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedstrom KL, Ogawa Y, Rasband MN. AnkyrinG is required for maintenance of the axon initial segment and neuronal polarity. J Cell Biol. 2008;183:635–640. doi: 10.1083/jcb.200806112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill AS, Nishino A, Nakajo K, Zhang G, Fineman JR, Selzer ME, Okamura Y, Cooper EC. Ion channel clustering at the axon initial segment and node of Ranvier evolved sequentially in early chordates. PLoS Genet. 2008;4:e31000317. doi: 10.1371/journal.pgen.1000317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinman JD, Duce JA, Siman RA, Hollander W, Abraham CR. Activation of calpain-1 in myelin and microglia in the white matter of the aged rhesus monkey. J Neurochem. 2004;89:430–441. doi: 10.1046/j.1471-4159.2004.02348.x. [DOI] [PubMed] [Google Scholar]
- Hinman JD, Peters A, Cabral H, Rosene DL, Hollander W, Rasband MN, Abraham CR. Age-related molecular reorganization at the node of Ranvier. J Comp Neurol. 2006;495:351–362. doi: 10.1002/cne.20886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horresh I, Poliak S, Grant S, Bredt D, Rasband MN, Peles E. Multiple molecular interactions determine the clustering of Caspr2 and Kv1 channels in myelinated axons. J Neurosci. 2008;28:14213–14222. doi: 10.1523/JNEUROSCI.3398-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, Tian C, Li T, Yang M, Hou H, Shu Y. Distinct contributions of Na(v)1.6 and Na(v)1.2 in action potential initiation and propagation. Nat Neurosci. 2009;12:996–1002. doi: 10.1038/nn.2359. [DOI] [PubMed] [Google Scholar]
- Huang ZJ, Di Cristo G, Ango F. Development of GABA innervation in the cerebral and cerebellar cortices. Nat Rev Neurosci. 2007;8:673–686. doi: 10.1038/nrn2188. [DOI] [PubMed] [Google Scholar]
- Inda MC, DeFelipe J, Muñoz A. Voltage-gated ion channels in the axon initial segment of human cortical pyramidal cells and their relationship with chandelier cells. Proc Natl Acad Sci USA. 2006;103:2920–2925. doi: 10.1073/pnas.0511197103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Schizophrenia Consortium. Rare chromosomal deletions and duplications increase risk of schizophrenia. Nature. 2008;455:237–241. doi: 10.1038/nature07239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isom LL, De Jongh KS, Patton DE, Reber BF, Offord J, Charbonneau H, Walsh K, Goldin AL, Catterall WA. Primary structure and functional expression of the beta 1 subunit of the rat brain sodium channel. Science. 1992;256:839–842. doi: 10.1126/science.1375395. [DOI] [PubMed] [Google Scholar]
- Iwata A, Riley BE, Johnston JA, Kopito RR. HDAC6 and microtubules are required for autophagic degradation of aggregated huntingtin. J Biol Chem. 2005;280:40282–40292. doi: 10.1074/jbc.M508786200. [DOI] [PubMed] [Google Scholar]
- Jenkins SM, Bennett V. Ankyrin-G coordinates assembly of the spectrin-based membrane skeleton, voltage-gated sodium channels, and L1 CAMs at Purkinje neuron initial segments. J Cell Biol. 2001;155:739–746. doi: 10.1083/jcb.200109026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalume F, Yu FH, Westenbroek RE, Scheuer T, Catterall WA. Reduced sodium current in Purkinje neurons from Nav1.1 mutant mice: implications for ataxia in severe myoclonic epilepsy in infancy. J Neurosci. 2007;27:11065–11074. doi: 10.1523/JNEUROSCI.2162-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai H, Fukuda M, Watanabe S, Hayashi-Takagi A, Noguchi J. Structural dynamics of dendritic spines in memory and cognition. Trends Neurosci. 2010;33:121–129. doi: 10.1016/j.tins.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Kovacs JJ, McLaurin A, Vance JM, Ito A, Yao TP. The deacetylase HDAC6 regulates aggresome formation and cell viability in response to misfolded protein stree. Cell. 2003;115:727–738. doi: 10.1016/s0092-8674(03)00939-5. [DOI] [PubMed] [Google Scholar]
- Khaliq ZM, Raman IM. Relative contributions of axonal and somatic Na channels to action potential initiation in cerebellar Purkinje neurons. J Neurosci. 2006;26:1935–1944. doi: 10.1523/JNEUROSCI.4664-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khirug S, Yamada J, Afzalov R, Voipio J, Khiroug L, Kaila K. GABAergic depolarization of the axon initial segment in cortical principal neurons is caused by the Na-K-2Cl cotransporter NKCC1. J Neurosci. 2008;28:4635–4639. doi: 10.1523/JNEUROSCI.0908-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klann E, Sweatt JD. Altered protein synthesis is a trigger for long-term memory formation. Neurobiol Learn Mem. 2008;89:247–259. doi: 10.1016/j.nlm.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klassen T, Davis C, Goldman A, Burgess D, Chen T, Wheeler D, McPherson J, Bourquin T, Lewis L, Villasana D, Morgan M, Muzny D, Gibbs R, Noebels J. Exome sequencing of ion channel genes reveals complex profiles confounding personal risk assessment in epilepsy. Cell. 2011;145:1036–1048. doi: 10.1016/j.cell.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kole MH, Stuart GJ. Is action potential threshold lowest in the axon? Nat Neurosci. 2008;11:1253–1255. doi: 10.1038/nn.2203. [DOI] [PubMed] [Google Scholar]
- Kole MH, Letzkus JJ, Stuart GJ. Axon initial segment Kv1 channels control axonal action potential waveform and synaptic efficacy. Neuron. 2007;55:633–647. doi: 10.1016/j.neuron.2007.07.031. [DOI] [PubMed] [Google Scholar]
- Kole MH, Ilschner SU, Kampa BM, Williams SR, Ruben PC, Stuart GJ. Action potential generation requires a high sodium channel density in the axon initial segment. Nat Neurosci. 2008;11:178–186. doi: 10.1038/nn2040. [DOI] [PubMed] [Google Scholar]
- Konishi Y, Setou M. Tubulin tyrosination navigates the kinesin-1 motor domain to axons. Nat Neurosci. 2009;12:559–567. doi: 10.1038/nn.2314. [DOI] [PubMed] [Google Scholar]
- Koticha D, Babiarz J, Kane-Goldsmith N, Jacob J, Raju K, Grumet M. Cell adhesion and neurite outgrowth are promoted by neurofascin NF155 and inhibited by NF186. Mol Cell Neurosci. 2005;30:137–148. doi: 10.1016/j.mcn.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Kuba H, Ishii TM, Ohmori H. Axonal site of spike initiation enhances auditory coincidence detection. Nature. 2006;444:1069–1072. doi: 10.1038/nature05347. [DOI] [PubMed] [Google Scholar]
- Kuba H, Oichi Y, Ohmori H. Presynaptic activity regulates Na(+) channel distribution at the axon initial segment. Nature. 2010;465:1075–1078. doi: 10.1038/nature09087. [DOI] [PubMed] [Google Scholar]
- Kumar A, Foster TC. Neurophysiology of old neurons and synapses. In: Riddle DR, editor. Brain Aging: Models, Methods, and Mechanisms. CRC Press; Boca Raton, FL: 2007. pp. 229–250. [PubMed] [Google Scholar]
- Lai HC, Jan LY. The distribution and targeting of neuronal voltage-gated ion channels. Nat Rev Neurosci. 2006;7:548–562. doi: 10.1038/nrn1938. [DOI] [PubMed] [Google Scholar]
- Lemaillet G, Walker B, Lambert S. Identification of a conserved ankyrin-binding motif in the family of sodium channel alpha subunits. J Biol Chem. 2003;278:27333–27339. doi: 10.1074/jbc.M303327200. [DOI] [PubMed] [Google Scholar]
- Leppert M, Anderson VE, Quattlebaum T, Stauffer D, O’Connell P, Nakamura Y, Lalouel JM, White R. Benign familial neonatal convulsions linked to genetic markers on chromosome 20. Nature. 1989;337:647–648. doi: 10.1038/337647a0. [DOI] [PubMed] [Google Scholar]
- Lewis DA. The chandelier neuron in schizophrenia. Dev Neurobiol. 2011;71:118–127. doi: 10.1002/dneu.20825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Faber DS. GABA responses and their partial occlusion by glycine in cultured rat medullary neurons. Neuroscience. 1993;52:83–96. doi: 10.1016/0306-4522(93)90184-h. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Lorincz A, Nusser Z. Cell-type-dependent molecular composition of the axon initial segment. J Neurosci. 2008;28:14329–14340. doi: 10.1523/JNEUROSCI.4833-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorincz A, Nusser Z. Molecular identity of dendritic voltage-gated sodium channels. Science. 2010;328:906–909. doi: 10.1126/science.1187958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Monsivals P, Tempel BL, Rubel EW. Activity-dependent regulation of the potassium channel subunits Kv1.1 and Kv3.1. J Comp Neurol. 2004;470:93–106. doi: 10.1002/cne.11037. [DOI] [PubMed] [Google Scholar]
- Luebke JI, Chang YM, Moore TL, Rosene DL. Normal aging results in decreased synaptic excitation and increased synaptic inhibition of layer 2/3 pyramidal cells in the monkey prefrontal cortex. Neuroscience. 2004;125:277–288. doi: 10.1016/j.neuroscience.2004.01.035. [DOI] [PubMed] [Google Scholar]
- Mainen ZF, Joerges J, Huguenard JR, Sejnowski TJ. A model of spike initiation in neocortical pyramidal neurons. Neuron. 1995;15:1427–1439. doi: 10.1016/0896-6273(95)90020-9. [DOI] [PubMed] [Google Scholar]
- Malhotra JD, Kazen-Gillespie K, Hortsch M, Isom LL. Sodium channel beta subunits mediate homophilic cell adhesion and recruit ankyrin to points of cell-cell contact. J Biol Chem. 2000;275:11383–11388. doi: 10.1074/jbc.275.15.11383. [DOI] [PubMed] [Google Scholar]
- Martin MS, Tang B, Papale LA, Yu FH, Catterall WA, Escayg A. The voltage-gated sodium channel Scn8a is a genetic modifier of severe myoclonic epilepsy in infancy. Hum Mol Genet. 2007;16:2892–2899. doi: 10.1093/hmg/ddm248. [DOI] [PubMed] [Google Scholar]
- Mashimo T, Ohmori I, Ouchida M, Ohno Y, Tsurumi T, Miki T, Wakamori M, Ishihara S, Yoshida T, Takizawa A, Kato M, Hirabayashi M, Sasa M, Mori Y, Serikawa T. A missense mutation of the gene encoding voltage-dependent sodium channel (Nav1.1) confers susceptibility to febrile seizures in rats. J Neurosci. 2010;30:5744–5753. doi: 10.1523/JNEUROSCI.3360-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathey EK, Derfuss T, Storch MK, Williams KR, Hales K, Woolley DR, Al-Hayani A, Davies SN, Rasband MN, Olsson T, Moldenhauer A, Velhin S, Hohlfeld R, Meinl E, Linington C. Neurofascin as a novel target for autoantibody-mediated axonal injury. J Exp Med. 2007;204:2363–2372. doi: 10.1084/jem.20071053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meadows LS, Malhotra J, Loukas A, Thyagarajan V, Kazen-Gillespie KA, Koopman MC, Kriegler S, Isom LL, Ragsdale DS. Functional and biochemical analysis of sodium channel beta1 subunit mutation responsible for generalized epilepsy with febrile seizures plus type 1. J Neurosci. 2002;22:10699–10709. doi: 10.1523/JNEUROSCI.22-24-10699.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misawa T, Mizusawa H. Anti-VGKC antibody-associated limbic encephalitis/Morvan syndrome. Brain Nerve. 2010;62:339–345. [PubMed] [Google Scholar]
- Molnár T, Antal K, Nyitrai G, Emri Z. gamma-Hydroxybutyrate (GHB) induces GABA(B) receptor dependent intracellular Ca2+ transients in astrocytes, but has no effect on GHB or GABA(B) receptors of medium spiny neurons in the nucleus accumbens. Neuroscience. 2009;162:268–281. doi: 10.1016/j.neuroscience.2009.05.017. [DOI] [PubMed] [Google Scholar]
- Nakata T, Hirokawa N. Microtubules provide directional cues for polarized axonal transport through interaction with kinesin motor head. J Cell Biol. 2003;162:1045–1055. doi: 10.1083/jcb.200302175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusser Z. Variability in the subcellular distribution of ion channels increases neuronal diversity. Trends Neurosci. 2009;32:267–274. doi: 10.1016/j.tins.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Oakley JC, Kalume F, Catterall WA. Insights into pathophysiology and therapy from a mouse model of Dravet syndrome. Epilepsia. 2011;52:59–61. doi: 10.1111/j.1528-1167.2011.03004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offord J, Catterall WA. Electrical activity, camp, and cytosolic calcium regulate mRNA encoding sodium channel alpha subunits in rat muscle cells. Neuron. 1989;2:1447–1452. doi: 10.1016/0896-6273(89)90190-6. [DOI] [PubMed] [Google Scholar]
- Ogawa Y, Horresh I, Trimmer JS, Bredt DS, Peles E, Rasband MN. Postsynaptic density-93 clusters Kv1 channels at axon initial segments independently of Caspr2. J Neurosci. 2008;28:5731–5739. doi: 10.1523/JNEUROSCI.4431-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa Y, Oses-Prieto J, Kim MY, Horresh I, Peles E, Burlingame AL, Trimmer JS, Meijer D, Rasband MN. ADAM22, a Kv1 channel-interacting protein, recruits membrane-associated guanylate kinases to juxtaparanodes of myelinated axons. J Neurosci. 2010;30:1038–1048. doi: 10.1523/JNEUROSCI.4661-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogiwara I, Miyamoto H, Morita N, Atapour N, Mazaki E, Inoue I, Takeuchi T, Itohara S, Yanagawa Y, Obata K, Furuichi T, Hensch TK, Yamakawa K. Na(v)1.1 localizes to axons of parvalbumin-positive inhibitory interneurons: a circuit basis for epileptic seizures in mice carrying an Scn1a gene mutation. J Neurosci. 2007;27:5903–5914. doi: 10.1523/JNEUROSCI.5270-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorio N, Cathala L, Meisler MH, Crest M, Magistretti J, Delmas P. Persistent Nav1.6 current at axon initial segments tunes spike timing of cerebellar granule cells. J Physiol. 2010;588:651–670. doi: 10.1113/jphysiol.2010.183798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palay SL, Sotelo C, Peters A, Orkand PM. The axon hillock and the initial segment. J Cell Biol. 1968;38:193–201. doi: 10.1083/jcb.38.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer LM, Stuart GJ. Site of action potential initiation in layer 5 pyramidal neurons. J Neurosci. 2006;26:1854–1863. doi: 10.1523/JNEUROSCI.4812-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Z, Kao T, Horvath Z, Lemos J, Sul JY, Cranstoun SD, Bennett V, Scherer SS, Cooper EC. A common ankyrin-G-based mechanism retains KCNQ and NaV channels at electrically active domains of the axon. J Neurosci. 2006;26:2599–2613. doi: 10.1523/JNEUROSCI.4314-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patino GA, Isom LL. Electrophysiology and beyond: multiple roles of Na+ channel β subunits in development and disease. Neurosci Lett. 2010;486:53–59. doi: 10.1016/j.neulet.2010.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patino GA, Claes LR, Lopez-Santiago LF, Slat EA, Dondeti RS, Chen C, O’Malley HA, Gray CB, Miyazaki H, Nukina N, Oyama F, De Jonghe P, Isom LL. A functional null mutation of SCN1B in a patient with Dravet syndrome. J Neurosci. 2009;29:10764–10778. doi: 10.1523/JNEUROSCI.2475-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A. The absence of significant neuronal loss from cerebral cortex with age. Neurobiol Aging. 1993;14:657–658. doi: 10.1016/0197-4580(93)90060-o. [DOI] [PubMed] [Google Scholar]
- Peters A. Structural changes that occur during normal aging of primate cerebral hemispheres. Neurosci Biobehav Rev. 2002;26:733–741. doi: 10.1016/s0149-7634(02)00060-x. [DOI] [PubMed] [Google Scholar]
- Peters A, Sethares C, Luebke JI. Synapses are lost during aging in the primate prefrontal cortex. Neuroscience. 2008;152:970–981. doi: 10.1016/j.neuroscience.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poliak S, Salomon D, Elhanany H, Sabanay H, Kiernan B, Pevny L, Stewart CL, Xu X, Chiu SY, Shrager P, Furley AJ, Peles E. Juxtaparanodal clustering of Shaker-like K+ channels in myelinated axons depends on Caspr2 and TAG-1. J Cell Biol. 2003;162:1149–1160. doi: 10.1083/jcb.200305018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovic MA, Foust AJ, McCormick DA, Zecevic D. The spatio-temporal characteristics of action potential initiation in layer 5 pyramidal neurons: a voltage-imaging study. J Physiol. 2011;589:4167–4187. doi: 10.1113/jphysiol.2011.209015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavachari S, Kahana MJ, Rizzuto DS, Caplan JB, Kirschen MP, Bourgeois B, Madsen JR, Lisman JE. Gating of human theta oscillations by a working memory task. J Neurosci. 2001;21:3175–3183. doi: 10.1523/JNEUROSCI.21-09-03175.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman IM, Sprunger LK, Meisler MH, Bean BP. Altered subthreshold sodium currents and disrupted firing patterns in Purkinje neurons of Scn8a mutant mice. Neuron. 1997;19:881–891. doi: 10.1016/s0896-6273(00)80969-1. [DOI] [PubMed] [Google Scholar]
- Rasband MN. The axon initial segment and the maintenance of neuronal polarity. Nat Rev Neurosci. 2010;11:552–562. doi: 10.1038/nrn2852. [DOI] [PubMed] [Google Scholar]
- Rasband MN, Kagawa T, Park EW, Ikenaka K, Trimmer JS. Dyregulation of axonal sodium channel isoforms after adult-onset chronic demyelination. J Neurosci Res. 2003;73:465–470. doi: 10.1002/jnr.10675. [DOI] [PubMed] [Google Scholar]
- Reddy PH, Reddy TP. Mitochondria as a therapeutic target for aging and neurodegenerative diseases. Curr Alzheimer Res. 2011;8:393–409. doi: 10.2174/156720511795745401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi E, Verri AP, Patricelli MG, Destefani V, Ricca I, Vetro A, Ciccone R, Giorda R, Toniolo D, Maraschio P, Zuffardi O. A 12Mb deletion at 7q33–q35 associated with autism spectrum disorders and primary amenorrhea. Eur J Med Genet. 2008;51:631–638. doi: 10.1016/j.ejmg.2008.06.010. [DOI] [PubMed] [Google Scholar]
- Royeck M, Horstmann MT, Remy S, Reitze M, Yaari Y, Beck H. Role of axonal NaV1.6 sodium channels in action potential initiation of CA1 pyramidal neurons. J Neurophysiol. 2008;100:2361–2380. doi: 10.1152/jn.90332.2008. [DOI] [PubMed] [Google Scholar]
- Sanchez-Ponce D, Munoz A, Garrido JJ. Casein kinase 2 and microtubules control axon initial segment formation. Mol Cell Neurosci. 2011;46:222–234. doi: 10.1016/j.mcn.2010.09.005. [DOI] [PubMed] [Google Scholar]
- Sanchez-Ponce D, Tapia M, Munoz A, Garrido JJ. New role of IKK alpha/beta phosphorylated I kappa B alpha in axon outgrowth and axon initial segment development. Mol Cell Neurosci. 2008;37:832–844. doi: 10.1016/j.mcn.2008.01.010. [DOI] [PubMed] [Google Scholar]
- Schafer DP, Jha S, Liu F, Akella T, McCullough LD, Rasband MN. Disruption of the axon initial segment cytoskeleton is a new mechanism for neuronal injury. J Neurosci. 2009;29:13242–13254. doi: 10.1523/JNEUROSCI.3376-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffer IE, Harkin LA, Grinton BE, Dibbens LM, Turner SJ, Zielinski MA, Xu R, Jackson G, Adams J, Connellan M, Petrou S, Wellard RM, Briellman RS, Wallace RH, Mulley JC, Berkovic SF. Temporal lobe epilepsy and GEFS+ phenotypes associated with SCN1B mutations. Brain. 2007;130:100–109. doi: 10.1093/brain/awl272. [DOI] [PubMed] [Google Scholar]
- Schulze TG, Detera-Wadleigh SD, Akula N, Gupta A, Kassem L, Steele J, Pearl J, Strohmaier J, Breuer R, Schwarz M, Propping P, Nöthen MM, Cichon S, Chumacher J, Rietschei M, McMahon FJ NIMH Genetics Initiative Bipolar Disorder Consortium. Two variants in Ankyrin 3 (ANK3) are independent genetic risk factors for bipolar disorder. Mol Psychiatry. 2009;14:487–491. doi: 10.1038/mp.2008.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman LJ, Kremen WS, Koren D, Faraone SV, Goldstein JM, Tsuang MT. A comparative profile analysis of neuropsychological functioning in patients with schizophrenia and bipolar psychoses. Schizophr Res. 2002;53:31–44. doi: 10.1016/s0920-9964(01)00162-1. [DOI] [PubMed] [Google Scholar]
- Shah MM, Migliore M, Valencia I, Cooper EC, Brown DA. Functional significance of axonal Kv7 channels in hippocampal pyramidal neurons. Proc Natl Acad Sci USA. 2008;105:7869–7874. doi: 10.1073/pnas.0802805105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, Yasumoto S, Nakagawa E, Fukasawa T, Uchiya S, Hirose S. Missense mutation of the sodium channel gene SCN2A causes Dravet syndrome. Brain Dev. 2009;31:758–762. doi: 10.1016/j.braindev.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Siman R, Baudry M, Lynch G. Brain fodrin: substrate for calpain I, an endogenous calcium-activated protease. Proc Natl Acad Sci USA. 1984;81:3572–3576. doi: 10.1073/pnas.81.11.3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson MD, Slater P, Deakin JF, Royston MC, Skan WJ. Reduced GABA uptake sites in the temporal lobe in schizophrenia. Neurosci Lett. 1989;107:211–215. doi: 10.1016/0304-3940(89)90819-7. [DOI] [PubMed] [Google Scholar]
- Singh NA, Charlier C, Stauffer D, DuPont BR, Leach RJ, Melis R, Ronen GM, Bjerre I, Quatllebaum T, Murphy JV, McHarg ML, Gagnon D, Rosales TO, Peiffer A, Anderson VE, Leppert M. A novel potassium channel gene, KCNQ2, is mutated in an inherited epilepsy of newborns. Nat Genet. 1998;18:25–29. doi: 10.1038/ng0198-25. [DOI] [PubMed] [Google Scholar]
- Singh NA, Westenskow P, Charlier C, Pappas C, Leslie J, Dillon J, Anderson VE, Sanguineetti MC, Leppert MF BFNC Physician Consortium. KCNQ2 and KCNQ3 potassium channel genes in benign familial neonatal convulsions: expansion of the functional and mutation spectrum. Brain. 2003;126:2726–2737. doi: 10.1093/brain/awg286. [DOI] [PubMed] [Google Scholar]
- Sloane JA, Hinman JD, Lubonia M, Hollander W, Abraham CR. Age-dependent myelin degeneration and proteolysis of oligodendrocyte proteins is associated with the activation of calpain-1 in the rhesus monkey. J Neurochem. 2003;84:157–168. doi: 10.1046/j.1471-4159.2003.01541.x. [DOI] [PubMed] [Google Scholar]
- Sobotzik JM, Sie JM, Politi C, Del Turco D, Bennett V, Deller T, Schulz C. AnkyrinG is required to maintain axodendritic polarity in vivo. Proc Natl Acad Sci USA. 2009;106:17564–17569. doi: 10.1073/pnas.0909267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song AH, Wang D, Chen G, Li Y, Luo J, Duan S, Poo MM. A selective filter for cytoplasmic transport at the axon initial segment. Cell. 2009;136:1148–1160. doi: 10.1016/j.cell.2009.01.016. [DOI] [PubMed] [Google Scholar]
- Strauss KA, Puffenberger EG, Huentelman MJ, Gottlieb S, Dobrin SE, Parod JM, Stephan DA, Morton DH. Recessive symptomatic focal epilepsy and mutant contactin-associated protein-like 2. N Eng J Med. 2006;354:1370–1377. doi: 10.1056/NEJMoa052773. [DOI] [PubMed] [Google Scholar]
- Stuart GJ, Sakmann B. Active propagation of somatic action potentials into neocortical pyramidal cell dendrites. Nature. 1994;367:69–72. doi: 10.1038/367069a0. [DOI] [PubMed] [Google Scholar]
- Sugiyama I, Tanaka K, Akita M, Yoshida K, Kawase T, Asou H. Ultrastructural analysis of the paranodal junction of myelinated fibers in 31-month-old rats. J Neurosci Res. 2002;70:309–317. doi: 10.1002/jnr.10386. [DOI] [PubMed] [Google Scholar]
- Szabadics J, Varga C, Molnár G, Oláh S, Barzó P, Tamás G. Excitatory effect of GABAergic axo-axonic cells in cortical microcircuits. Science. 2006;311:233–235. doi: 10.1126/science.1121325. [DOI] [PubMed] [Google Scholar]
- Tapia M, Wandosell F, Garrido JJ. Impaired function of HDAC6 slows down axonal growth and interferes with axon initial segment development. PLoS ONE. 2010;5:1–13. doi: 10.1371/journal.pone.0012908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toescu EC, Vreugdenhil M. Calcium and normal brain ageing. Cell Calcium. 2010;47:158–164. doi: 10.1016/j.ceca.2009.11.013. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci. 2004;5:97–107. doi: 10.1038/nrn1327. [DOI] [PubMed] [Google Scholar]
- Vacher H, Yang JW, Cerda O, Autillo-Touati A, Dargent B, Trimmer JS. Cdk-mediated phosphorylation of the Kvβ2 auxiliary subunit regulates Kv1 channel axonal targeting. J Cell Biol. 2011;192:813–824. doi: 10.1083/jcb.201007113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Snellenberg JX, Torres IJ, Thornton AE. Functional neuroimaging of working memory in schizophrenia: task performance as a moderating variable. Neuropsychopharmacology. 2006;20:497–510. doi: 10.1037/0894-4105.20.5.497. [DOI] [PubMed] [Google Scholar]
- Van Wart A, Matthews G. Expression of sodium channels Nav1.2 and Nav1.6 during postnatal development of the retina. Neurosci Lett. 2006a;403:315–317. doi: 10.1016/j.neulet.2006.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wart A, Matthews G. Impaired firing and cell-specific compensation in neuron cells nav1.6 sodium channels. J Neurosci. 2006b;26:7172–7180. doi: 10.1523/JNEUROSCI.1101-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk DW, Pierri JN, Fritschy JM, Auh S, Sampson AR, Lewis DA. Reciprocal alterations in pre- and postsynaptic inhibitory markers at chandelier cell inputs to pyramidal neurons in schizophrenia. Cereb Cortex. 2002;12:1063–1070. doi: 10.1093/cercor/12.10.1063. [DOI] [PubMed] [Google Scholar]
- Wallace RH, Wang DW, Singh R, Scheer IE, George AL, Jr, Phillips HA, Saar K, Reis A, Johnson EW, Sutherland GR, Berkovic SF, Mulley JC. Febrile seizures and generalized epilepsy associated with a mutation in the Na+-channel beta1 subunit gene SCN1B. Nat Genet. 1998;19:366–370. doi: 10.1038/1252. [DOI] [PubMed] [Google Scholar]
- Wang XJ. Neurophysiological and computational principles of cortical rhythms in cognition. Physiol Rev. 2010;90:1195–1268. doi: 10.1152/physrev.00035.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GY, Ratto GM, Bisti S, Chalupa LM. Functional development of intrinsic properties in ganglia cells of the mammalian retina. J Neurophysiol. 1997;78:2895–2903. doi: 10.1152/jn.1997.78.6.2895. [DOI] [PubMed] [Google Scholar]
- Waxman SG. Transcriptional channelopathies: an emerging class of disorders. Nat Rev Neurosci. 2001;2:652–659. doi: 10.1038/35090026. [DOI] [PubMed] [Google Scholar]
- Waxman SG, Dib-Hajj SD, Cummins TR, Black JA. Sodium channels and their genes: dynamic expression in the normal nervous system, dysregulation in disease states. Brain Res. 2000;886:5–14. doi: 10.1016/s0006-8993(00)02774-8. [DOI] [PubMed] [Google Scholar]
- Williams HJ, Craddock N, Russo G, Hamshere ML, Moskvina V, Dwyer S, Smith RL, Green E, Grozeva D, Holmans P, Owen MJ, O’Donovan MC. Most genome-wide significant susceptibility loci for schizophrenia and bipolar disorder reported to date cross-traditional diagnostic boundaries. Hum Mol Genet. 2011;20:387–391. doi: 10.1093/hmg/ddq471. [DOI] [PubMed] [Google Scholar]
- Wimmer VC, Reid CA, So EY, Berkovic SF, Petrou S. Axon initial segment dysfunction in epilepsy. J Physiol. 2010a;588:1829–1840. doi: 10.1113/jphysiol.2010.188417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmer VC, Reid CA, Mitchell S, Richards KL, Scaf BB, Leaw BT, Hill EL, Royeck M, Horstmann MT, Cromer BA, Davies PJ, Xu R, Lerche H, Berkovic SF, Beck H, Petrou S. Axon initial segment dysfunction in a mouse model of genetic epilepsy with febrile seizures plus. J Clin Invest. 2010b;120:2661–2671. doi: 10.1172/JCI42219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winckler B, Forscher P, Mellman I. A diffusion barrier maintains distribution of membrane proteins in polarized neurons. Nature. 1999;397:698–701. doi: 10.1038/17806. [DOI] [PubMed] [Google Scholar]
- Wollner DA, Catterall WA. Localization of sodium channels in axon hillocks and initial segments of retinal ganglion cells. Proc Natl Acad Sci USA. 1986;83:8424–8428. doi: 10.1073/pnas.83.21.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff A, Xu Q, Anderson SA, Yuste R. Depolarizing effect of neocortical chandelier neurons. Front Neural Circuits. 2009;3:15. doi: 10.3389/neuro.04.015.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Ogawa Y, Hedstrom KL, Rasband MN. betaIV spectrin is recruited to axon initial segments and nodes of Ranvier by ankyrinG. J Cell Biol. 2007;176:509–519. doi: 10.1083/jcb.200610128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu FH, Mantegazza M, Westenbroek RE, Robbins CA, Kalume F, Burton KA, Spain WJ, McKnight GS, Scheuer T, Catterall WA. Reduced sodium current in GABAergic interneurons in a mouse model of severe myoclonic epilepsy in infancy. Nat Neurosci. 2006;9:1142–1149. doi: 10.1038/nn1754. [DOI] [PubMed] [Google Scholar]
- Zhou D, Lambert S, Malen PL, Carpenter S, Boland LM, Bennett V. AnkyrinG is required for clustering of voltage-gated Na channels at axon initial segments and for normal potential firing. J Cell Biol. 1998;143:1295–1304. doi: 10.1083/jcb.143.5.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zonta B, Dsmazieres A, Rinaldi A, Tait S, Sherman DL, Nolan MF, Brophy PJ. A critical role for Neurofascin in regulating action potential initiation through maintenance of the axon initial segment. Neuron. 2011;69:945–956. doi: 10.1016/j.neuron.2011.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]