Abstract
Introduction
Poxviral vaccines have been given to over 1 billion people in the successful global eradication of smallpox. Since then, recombinant poxviruses have been investigated extensively as a novel immunotherapy for cancer, undergoing several iterations to optimize their immunogenicity and efficacy. The current platform expressing multiple costimulatory molecules plus a tumor-associated antigen such as PSA, i.e., PSA-TRICOM (PROSTVAC-V/F), is promising and is currently in a phase III randomized, placebo-controlled clinical trial in metastatic castration-resistant prostate cancer.
Areas covered
This review discusses the clinical development of poxviral-based cancer vaccines, with a particular focus on the rationale for combining vaccines with other treatment modalities, including radiotherapy, chemotherapy, hormonal therapy, other immune-based therapies, and molecularly targeted therapy. We also discuss the importance of appropriate patient selection in clinical trial design.
Expert Opinion
Preclinical and early clinical studies with poxviral vector vaccines have shown promising results with this novel immunologic approach both as vaccine alone and combined with other therapies. The challenges of translating the science of immunotherapy to clinical practice include clinical trial design that includes appropriate patient selection, appropriate endpoints, and identification of meaningful surrogate biomarkers.
Keywords: combination approach, immune-related response criteria, PANVAC-V/F, PROSTVAC-V/F, therapeutic cancer vaccine, tumor growth rate
1. Introduction
Several vector-based cancer vaccines have been evaluated in various stages of clinical trials. Among them, poxviral-based vaccines have made major advances in development and in clinical trials. Poxviruses have been studied as vaccine vectors for decades. This review will discuss the background and biology of poxviral-based vaccines, including the development of first-generation therapeutic cancer vaccines and newer generations being tested in late-phase clinical trials.
2. Historical background
Centuries ago it was recognized that people who survived smallpox, though scarred, were protected from recurrent disease [1]. In 10th-century China and India, this observation led to a procedure known as variolation, which involved inoculating susceptible individuals with smallpox scab material to induce a mild infection and, as a consequence, prevent more severe disease [1, 2]. Although early variolation practices had a mortality rate as high as 1%, and risked the development of severe infection and spread of disease, variolation was used beneficially in many parts of the world.
In 18th-century England, it was observed that cowpox lesions resembled those of smallpox, and that milkmaids who contracted cowpox appeared to be protected against smallpox infection. Dr. Edward Jenner tested these observations by inoculating individuals with cowpox material and rechallenging them with variolation. When smallpox pustules did not appear in these individuals, Jenner was convinced that inoculating with cowpox material could protect against naturally transmitted smallpox. This was the inception of the science of immunology, and it led to deeper understanding of poxviruses, the development of live vaccinia virus vaccine, the successful global eradication of smallpox, and research into the use of Poxviridae for gene therapy and cancer vaccines.
3. Biology of Poxviridae
Poxviridae comprises viruses that can infect vertebrates and invertebrates. They are composed of linear double-stranded DNA containing about 200,000 base pairs. Unlike other DNA viruses, poxviruses have their own transcription machinery, a viral DNA-dependent RNA polymerase, and post-transcriptional modifying enzymes, allowing self-sufficient cytoplasmic replication [1]. After infecting a cell, the viral core is released into the cytoplasm. The viral transcription system is activated to express approximately 100 early gene products, including DNA polymerase and other factors needed for replication of the vaccinia genome. Replication then signals the expression of intermediate and late genes, the products of which are mostly structural proteins. Pre-virion particles are assembled into intracellular mature viruses (IMVs), some of which are targeted to the Golgi network. Following envelopment, IMVs form intracellular enveloped viruses that are propelled to the cell surface by the polymerization of actin filaments. The viruses may remain attached to the membrane as cell-associated enveloped viruses or be released into the medium as extracellular enveloped viruses [3].
4. Advantages of poxviral vectors in cancer vaccines
Several unique features of poxviral vectors make them effective vehicles for cancer vaccine delivery. A poxvirus has a large genome that can integrate more than 25,000 base pairs of foreign DNA without compromising infectivity or other essential functions. It replicates within the cytoplasm and its genome does not integrate into host DNA, so it poses no risk of mutation. Most importantly, vaccinia has proven safe and effective in over a billion people in the worldwide smallpox eradication campaign.
5. First-generation recombinant poxviral vector in cancer immunotherapy
One of the first recombinant poxviral vectors used in cancer immunotherapy was the vaccinia virus. Preclinical studies were conducted with recombinant vaccinia (rV) expressing various tumor antigens, such as the extracellular domain of the rat neu oncogene-encoded protein, p185 [4], polyoma virus-specific tumor-specific antigens [5], epithelial tumor antigen [6], early bovine papilloma virus proteins [7], and carcinoembryonic antigen (CEA) [8–10]. Most of these constructs were produced via homologous recombination of a plasmid containing the transgene into the thymidine kinase gene of the vaccinia virus [11].
The first rV vector tested in human subjects with cancer was rV-CEA [12]. CEA is a 180 k-Da glycoprotein expressed on most GI, breast, thyroid, ovarian, lung, and other cancers. A 2.4-kb cDNA clone containing the complete coding sequence was isolated from a human colon tumor cell library and inserted into the vaccinia genome. The resulting vaccine was administered intradermally monthly for 3 months at doses up to 107 plaque-forming units (pfu) without serious toxicity. CEA-specific cytotoxic T-cell lines were derived by prolonged in vitro culture of HLA-A2-restricted peripheral blood lymphocytes [13]. Table 1 lists studies of CEA-targeted poxviral-vector vaccines.
Table 1.
Clinical trials with CEA-targeted poxviral vaccines
| Ref. | Type of trial | N | Vaccine | Dose/schedule | Results |
|---|---|---|---|---|---|
| [12, 13] | Phase I in advanced carcinoma | 26 | rV-CEA | I.d. rV-CEA 107 pfu monthly ×3 | Class I HLA-A2-restricted CTL response. |
| [94] | Phase I in metastatic CEA-expressing adenocarcinoma | 20 | rV-CEA | Arm A: i.d. rV-CEA 107 or 108 pfu q4 wks ×2. Arm B: s.c. rV-CEA 107 or 108 pfu q4 wks ×2. |
No CEA-specific immune response. Both techniques induced vaccinia-specific immune response. |
| [18] | Phase I in advanced CEA-expressing tumors | 18 | rV-CEA (V) and rA-CEA (A) | Arm A: VAAA AA… Arm B: AAAV VV…. GM-CSF and low-dose IL-2 given as adjuvant. |
VAAA superior in generation of CEA-specific T-cell response. Optimal prime-boost strategy: rV prime with rA boosts. |
| [95] | Phase I in advanced CEA-expressing cancers | 20 | ALVAC-CEA | Arm A: 0.25M pfu q28dx3 Arm B: 2.5M pfu q28dx3 Arm C: 25M pfu q28dx3 |
A: well tolerated. B: no objective response C: CEA-specific immune response |
| [26] | Phase I in CEA-expressing adenocarcinoma | 18 | ALVAC-CEA-B7.1 | Arm A: 4.5×106 pfu i.m. q4 wks ×3 Arm B: 4.5×107 pfu i.m. q4 wks ×3 Arm C: 4.5×108 pfu i.m. q4 wks ×3 |
3 pts had clinically stable disease that correlated with increasing CEA-specific precursor T cells. Repeated vaccinations resulted in enhanced CEA-specific T-cell responses. |
| [27, 96] | Pilot/phase I in advanced CEA-expressing cancers | 60 | ALVAC-CEA-B7.1 | 4.5×108 pfu i.d. every 2 wks ×4, followed by monthly boosts if stable or response. Arm A: vaccine alone Arm B: vaccine plus GM-CSF ×5 days |
Some clinical response. CEA-specific T-cell responses in pts treated with vaccine alone; none with vaccine plus GM-CSF. |
| [39] | Phase I in advanced CEA-expressing tumors | 58 | rV-CEA-TRICOM (V) and rF-CEA-TRICOM (F) | Cohorts 1–3: sequential FF… Cohorts 4–6: sequential V-FF… Cohorts 7 & 8: sequential V-FF… with GM-CSF. |
40% of pts had stable disease for ≥4 mos. One patient had pathologic CR. CEA-specific T cells enhanced in majority of pts. |
| [71] | Pilot study in CEA- or MUC1-expressing metastatic cancers | 25 | PANVAC-V/F | PANVAC-V 2×108 pfu s.c. on D1. PANVAC-F on D15, 29, and 43, then monthly. GM-CSF 100 mcg given as adjuvant. | CEA- and/or MUC1-specific immune response in 9/16 pts. CR in pts with metastatic breast cancer with liver metastasis, gastric cancer, clear cell ovarian cancer. |
| [97] | Phase I in advanced pancreatic cancer | 22 | PANVAC-V/F | PANVAC-V prime. PANVAC-F biweekly for 3 doses. | Median OS in 2 studies: 7.9 and 6.3 mos. One-year survival rate: 33% and 30%. |
| [41, 98] | Phase III in advanced pancreatic cancer | 255 | PANVAC-V/F | Arm A: rV-PANVAC prime with rF-PANVAC boost biweekly for 3 doses, then monthly. Arm B: palliative chemotherapy. Arm C: best supportive care. |
No difference in OS. |
| [70] | Pilot study in cohorts with ovarian (n = 14) and breast (n = 12) cancers | 26 | PANVAC-V/F | PANVAC V/F monthly | Evidence of clinical benefit in patients with limited tumor burden and minimal prior chemotherapy. In breast cancer: 4 pts with SD; 1 with CR. One ovarian cancer patient had time to progression of 38 months on vaccine alone. |
| [99] | Phase I in locally advanced or metastatic pancreatic cancer | 18 (est) | PANVAC-F, PANVAC-V/F | I.t. rF-PANVAC; s.c. PANVAC-V/F | Ongoing |
CR = complete response; D = day; ets = estimated; i.d. = intradermal; i.m. = intramuscular; i.t. = intratumoral; mos = months; OS = overall survival; pfu = plaque-forming units; pts = patients; s.c. = subcutaneous; SD = stable disease; wks = weeks
ALVAC: recombinant canarypox vector
PANVAC: recombinant poxviral vector expressing CEA, MUC1, and TRICOM
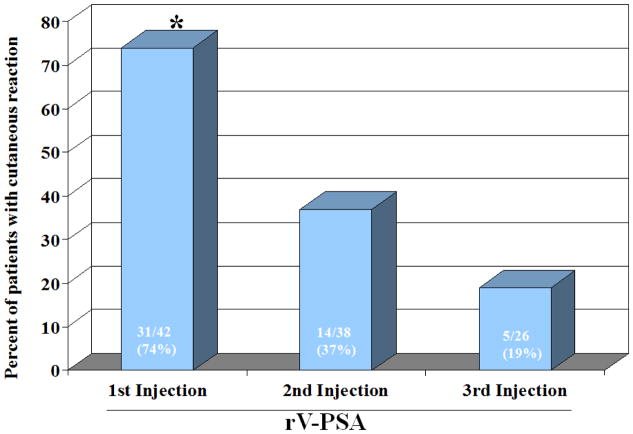
The same rV platform with a PSA transgene (rV-PSA) has also been evaluated in 3 phase I trials [14–16]. A trial in 6 patients with rising PSA after radical prostatectomy demonstrated that 3 monthly injections of rV-PSA induced durable PSA responses. One patient on study had undetectable PSA for > 8 months [14]. In another trial with 42 patients with metastatic castration-resistant prostate cancer (mCRPC), rV-PSA vaccine was able to overcome tolerance to the self-antigen and induce an immune response to PSA [16]. T cells taken from this group of patients were able to lyse PSA-expressing tumor cells in vitro. Interestingly, although the initial vaccination with rV-PSA induced a robust immune response, even in patients who had been vaccinated against smallpox during childhood, the cutaneous response to subsequent immunization was significantly diminished, thought to be due to the development of neutralizing antibodies against the viral coat proteins induced by subsequent immunization (Figure 1). Several approaches have been undertaken to overcome this limitation and to optimize the immunization strategy. Table 2 lists selected clinical trials of poxviral vaccines containing PSA.
Figure 1.
Bars show diminished cutaneous reaction to subsequent vaccination with rV-PSA, suggesting the development of neutralizing antibodies to vaccinia. P<0.005 between first injection and 2nd or 3rd injections (McNemar’s exact test) [16].
Table 2.
Clinical trials with PSA-targeted poxviral vaccines
| Ref. | Type of trial | N | Vaccine | Dose/schedule | Results |
|---|---|---|---|---|---|
| [14] | Phase I in pts with rising PSA after radical prostatectomy | 6 | rV-PSA | rV-PSA | Minimal toxicity. Durable PSA response. |
| [15] | Phase I in pts with rising PSA after definitive primary treatment or metastatic disease | 33 | rV-PSA | rV-PSA (2.65×106) rV-PSA (2.65×107) rV-PSA (2.65×108) 10 pts received GM-CSF |
14/33 pts had stable PSA for ≥ 6 mos. 9 pts stable for 11–25 mos. 6 pts progression-free with stable PSA. PSA-specific T-cell response to PSA-3. |
| [16] | Phase I in mCRPC | 42 | rV-PSA | rV-PSA monthly ×3 | Increase in proportion of PSA-specific T cells after vaccination. In vitro assay showed these T cells could lyse PSA-expressing tumor cells. |
| [19, 20] | Phase II in pts with biochemical progression after local therapy | 64 | rV-PSA (V) rF-PSA (F) |
Sequential F monthly, then every 3 mos. Arm A: FFFF Arm B: FFFV Arm C: VFFF |
At 19.1 mos: 45.3% PSA progression-free. 78.1% clinically progression-free. Trend favoring VFFF treatment schedule. 47% had increase in PSA-reactive T cells. Median time to PSA progression: 9.2 and 9.1 mos for arms A and B respectively; 18.2 mos for arm C (rV-PSA prime and rF-PSA boost; p = 0.15 by logrank test). |
| [23] | Phase II in localized disease | 30 | rV-PSA + rV-B7.1, followed by monthly rF-PSA boost ×7 (vaccine) | Arm A: vaccine + RT Arm B: RT only Adjuvants: GM-CSF and low-dose IL-2. RT given between 4th and 6th vaccinations. |
13/17 pts had ≥3-fold increase in PSA-specific T cells. Evidence of de novo generation of T cells to other prostate-associated antigens not found in vaccine (antigen spreading). |
| [24, 73] | Phase II in nonmetastatic CRPC | 42 | rV-PSA + rV-B7.1, followed by rF-PSA monthly boost (vaccine) | Arm A: vaccine Arm B: nilutamide Cross-over to both treatments allowed at progression. Adjuvants: GM-CSF 100 mcg s.c., D1-4 and low-dose IL-2 (6 MIU/M2). |
Median OS 4.4 years from date of enrollment. Trend toward improved OS for pts initially randomized to vaccine arm (median 5.1 vs. 3.4 years; p = 0.13). |
| [69, 100] | Randomized phase II in mCRPC | 28 | rV-PSA + rV-B7.1, followed by rF-PSA monthly boost (vaccine) | Arm A: vaccine + docetaxel Arm B: vaccine alone (cross-over allowed) Adjuvant: GM-CSF |
Equivalent immune responses for vaccine and vaccine + docetaxel. Median PFS on docetaxel was 6.1 mos after receiving vaccine vs. 3.7 mos with same regimen in historic control. Median OS: 21.6 mos vs. 15.5 mos Halabi-predicted. |
| [25] | Pilot study in localized disease | 18 | rV-PSA + rV-B7.1, followed by rF-PSA monthly boost ×7 | rV-PSA + rV-B7.1 followed by monthly boosts with rF-PSA ×8 cycles. GM-CSF 100 mcg on D1-4, IL-2 0.6 MIU/M2 on D8-21 s.c. | 5/8 HLA-A2+ pts had increased PSA-specific T cells. |
| [43] | Phase I in mCRPC | 15 | PROSTVAC-V/F | Cohort 1: FFF Cohort 2: VFFF Cohort 3: VFFF + recombinant GM-CSF Cohorts 4 & 5: VFFF + rF-GM-CSF Adjuvant: rF-GM-CSF |
Time to clinical progression on vaccine: 20.5 weeks. No measurable response seen. PSA-specific immune response in 4/6 HLA-A2+ pts. Decrease in PSA velocity in 9/15 pts. |
| [44] | Phase II in mCRPC | 32 | PROSTVAC-V/F | PROSTVAC-V followed by PROSTVAC-F boosts until progression. Adjuvant: GM-CSF |
Median OS: 26.6 mos (vaccine) vs. 17.4 mos (Halabi-predicted). |
| [88] | Randomized phase II in mCRPC | 125 | PROSTVAC-V/F | Arm A: PROSTVAC-V prime on D1 with PROSTVAC-F on weeks 3, 5, 9, 13, 17, and 21. Adjuvant: GM-CSF Arm B: control empty vector |
No difference in PFS (p = 0.6). OS: 25.1 mos vs. 16.6 mos (HR 0.56; p = 0.0061). |
| [74] | Randomized phase II in nonmetastatic CRPC | 26 | PROSTVAC-V/F + flutamide | Arm A: flutamide alone Arm B: PROSTVAC-V followed by PROSTVAC-F boosts until progression + flutamide. Adjuvant: GM-CSF |
Median time to progression: 223 days for vaccine vs. 85 days for flutamide combination. |
| [101] | Pilot study in locally recurrent disease after radiation | 21 | PROSTVAC-V/F | rV-PSA-TRICOM prime. I.p. rF-PSA-TRICOM boost with i.p. rF-GM-CSF. Cohorts 3–5: i.p. rF-GM-CSF Cohort 5: concurrent s.c. and i.p. boosts. Prime: D1. Boosts: D29, 57, and 85. |
18/21 pts had stable or improved PSA on study. 16/21 pts had stable or improved PSA DT. 4/8 evaluable pts had immune response by ELISPOT. 11/15 pts had decreased or stable Treg function. Biopsies pre- and post-vaccination showed increases in immunologic tumor infiltrates. |
| [75] | Phase I in mCRPC | 30 | PROSTVAC-V/F + ipilimumab | rV-PSA-TRICOM D1. rF-PSA-TRICOM D15, 29, then monthly. Ipilimumab 1, 3, 5, or 10 mg/kg in sequential dose levels monthly. | Median time to radiographic progression for CN pts: 5.9 mos. OS 31.8 mos vs. Halabi-predicted 18.5 mos. 2-yr survival rate: 74%. Median OS for CN pts: 30.9 mos. No significant difference in OS based on dose level. 4/6 evaluable pts at higher dose levels had > 2-fold increase in antigen-specific T-cell responses. |
| [102] | Phase II in pts with PSA progression after local therapy | 50 to step 1 19 to step 2 |
PROSTVAC-V/F | PROSTVAC-V/F with GM-CSF monthly for 3 cycles, then every 12 weeks. | Pre-treatment PSADT: 4.4 mos vs 7.7 mos on-treatment PSADT. Overall biochemical response rate: 2%. 25 pts had stable disease. |
CN = chemotherapy-naïve; CR = complete response; i.p. = intraprostatic; mos = months; OS = overall survival; PR = partial response; PSADT = PSA doubling time; pts = patients; RT = radiotherapy; s.c. = subcutaneous; Treg = T regulatory cell
PROSTVAC: poxviral vector expressing PSA and TRICOM
6. Strategies for optimizing poxviral cancer vaccines
6.1 Prime and boost
One approach to optimizing poxviral vaccines is to expose the immune system to the same epitope with a different vector that can infect cells and express transgenes, but cannot replicate, thus minimizing the amount of viral protein to which the immune system is exposed. In a preclinical model, Hodge et al. demonstrated that priming with rV and boosting with a replication-incompetent avipox such as canarypox (ALVAC™) or recombinant fowlpox (rF) was superior to either viral vector alone in generating an immune response [17]. Marshall et al. validated this diversified prime-boost strategy in a randomized clinical trial, showing that priming with rV-CEA followed by multiple avipox-CEA boosts (VAAA) was significantly more potent in inducing CEA-specific T-cell precursor frequency than the converse strategy (AAAV) [18]. This V/F strategy (rV-PSA priming with rF-PSA boosts) was validated by the ECOG E7897 study [19], where it was associated with a trend toward improved progression-free survival (PFS) (9.1 months for FFFF, 9.2 months for FFFV, 18.2 months for VFFF; p = 0.15 by logrank test) [20]. Interestingly, concurrent vaccination with a V/F vaccine and a vaccine with a yeast vector expressing the same antigen induced a greater immune response than either strategy alone [21].
Modified vaccinia Ankara (MVA), long used as a smallpox vaccine, is a potentially safer, replication-incompetent form of vaccinia virus with unique immunostimulatory properties that could make it a superior priming vaccine. Compared to an rV counterpart, a single immunization with recombinant MVA expressing CEA induced greater expression of several serum cytokines associated with enhanced T-cell immunity [22]. On the theory that this effect preconditioned the vaccination site for a more effective boost, a strategy was devised using a recombinant MVA prime followed 7 days later by rF boosts at the same injection site. In a murine model, this approach demonstrated more potent tumor antigen-specific T-cell response and superior antitumor activity than an rV priming strategy [22]. The MVA priming strategy has yet to be evaluated in a clinical trial.
6.2 Costimulation
Another approach to optimizing immunization strategies is T-cell costimulation. T-cell activation depends on 2 major interactions: (a) the interaction of MHC peptide complex with T-cell receptors and (b) the interaction of costimulatory molecules on antigen-presenting cells (APCs) with corresponding receptors on T cells. Costimulation plays a particularly important role in eliciting an immune response to a cancer vaccine because tumor antigens are, by definition, weakly immunogenic.
One of the most extensively studied costimulatory molecules is B7.1, which is the ligand for T-cell surface antigens CD28 and CTLA-4. Several preclinical studies and early clinical studies used rV expressing B7.1 (rV-B7.1) as a vaccine platform. A strategy of priming with rV-PSA admixed with rV-B7.1, followed by rF-PSA boosts, has been shown to augment the immune response in several clinical trials in prostate cancer [23–25]. Another platform used in clinical trials, a nonreplicating canarypox virus constructed to express both CEA and B7.1 (ALVAC-CEA-B7.1), induced an augmented CEA-specific T-cell response as well as a clinical response in patients with CEA-expressing tumors [25, 27].
In the early 1990s, the costimulatory molecules lymphocyte function-associated antigen (LFA)-3 and intercellular adhesion molecule (ICAM)-1 were first described [28, 29] and studied extensively as a way to augment the immune response to a tumor antigen. These studies eventually led to the development of a recombinant vector expressing a TRiad of human T-cell COstimulatory Molecules (B7.1, LFA-3, and ICAM-1), designated TRICOM [30–31]. Several preclinical studies have demonstrated that T-cell activation by TRICOM is significantly greater than that produced by only 1 or 2 costimulatory molecules [30–33].
One of the mechanisms by which APCs infected with TRICOM-containing vectors activate naïve T cells is thought to be via the T-cell acquisition of costimulatory molecules and peptide-MHC complex from APCs [34, 35]. The data suggest that acquisition of costimulatory molecules/MHC molecules leads to sustained activation and signaling in T cells. Further studies have demonstrated that multiple costimulation increases CTL avidity, which may contribute to effective lysis of tumor cells [36, 37]. It also enhances the production of multiple cytokines and the expansion of tumor antigen-specific memory CD8+ T cells [38].
The first-generation poxviral vaccine containing TRICOM—rV and rF vectors expressing both CEA and TRICOM (rV/F-CEA-TRICOM)—was evaluated in 58 patients with CEA-expressing tumors. Vaccination with rV/F-CEA-TRICOM in a prime-boost strategy demonstrated durable clinical response, including one pathologic complete response and enhanced CEA-specific T-cell response in a majority of patients [39]. Furthermore, a different platform, rV-TRICOM, showed evidence of clinical activity in a pilot study of 13 patients with advanced melanoma. Four of 13 (31%) had an objective clinical response, including one patient who achieved a durable complete response [40]. Successful incorporation of additional transgenes of tumor-associated antigens into the TRICOM platform led to the development of rV/F-PSA-TRICOM (PROSTVAC®-V/F) and rV/F-MUC1-CEA-TRICOM (PANVAC™-V/F). These vaccines have been reviewed in detail [41, 42].
6.3 Immune adjuvants
The rationale for using immune adjuvants is to enhance the immune response to vaccine through the adjuvants’ direct effect on APCs and T cells. The cytokine GM-CSF has been studied extensively in both preclinical and clinical studies. GM-CSF recruits dendritic cells (DCs) to the site of vaccination, enhances antigen presentation, and promotes DC maturation and migration to draining lymph nodes, where they can activate T cells. In clinical trials, GM-CSF has been used in several forms: a recombinant protein (GM-CSF), a recombinant vector expressing GM-CSF (e.g., rF-GM-CSF) [43, 44], a tumor cell vaccine modified to secrete GM-CSF [45], or as APCs pulsed with a fusion protein (GM-CSF-PAP) [46]. Some of these trials suggested an enhanced immune response. The clinical utility of GM-CSF as a vaccine adjuvant will be prospectively evaluated in a phase III randomized controlled trial of PROSTVAC-V/F in mCRPC. Other immune adjuvants that primarily affect APCs include CpG oligonucleotides [47, 48], FLT3 ligand [49], resiquimod [50], TLR3 agonist, and poly I:C [51].
Interleukin (IL)-2 has been used in several preclinical and clinical studies as an immune adjuvant. IL-2 is a T-cell growth factor which, at high doses, has demonstrated clinically significant antitumor effects against advanced melanoma and renal cell carcinoma (RCC). The major drawback of IL-2, however, is significant toxicity. Several clinical trials have thus used low-dose IL-2 as an immune adjuvant. In a phase II trial in localized prostate cancer, 30 patients were randomized 2:1 to receive vaccine plus radiotherapy or radiotherapy alone. Patients in the combination arm received a prime of rV-PSA admixed with rV-B7.1, followed by 7 monthly rF-PSA boosts. Each vaccine was given with 4 daily doses of GM-CSF, along with IL-2 at 4 MIU/m2 given subcutaneously in the abdomen for 5 daily doses in week 2. This regimen was reasonably well tolerated, with no grade 3 toxicity attributable to vaccine. However, many patients had symptoms related to IL-2 that required dose reduction. While none of the patients in the radiotherapy-alone arm showed evidence of PSA-specific T-cell responses, the majority of vaccinated patients had an increase in PSA-specific T cells, as well as evidence of de novo generation of T cells specific to other prostate-associated antigens that were not present in the vaccine. An even lower, metronomic dose of IL-2 (0.6 MIU/m2) was used in a follow-up, single-arm study in an attempt to avoid the toxicities of low-dose IL-2 [25]. In this trial, the majority of evaluable patients had a > 3-fold increase in PSA-specific T cells, with fewer toxicities compared to the previous trial [23].
TG4010 (recombinant MVA expressing both IL-2 and MUC1, a high molecular-weight mucin overexpressed in many carcinomas) was evaluated in a phase II study in 40 prostate cancer patients with biochemical failure. Although the primary endpoint of the study was not met, 13 of 40 patients showed a > 2-fold improvement in PSA doubling time, and 10 patients had stable PSA levels for > 8 months, with minimal vaccine-related toxicity [52]. In a phase IIB trial in 148 patients with stage IIIB/IV MUC1-expressing non-small cell lung cancer (NSCLC), the addition of TG4010 to first-line chemotherapy showed a trend toward improved 6-month PFS (43.2% vs 35.1%; p = 0.307) and response rate (31% vs 21%; p = 0.082) compared with chemotherapy alone [53]. Other immune adjuvants that primarily affect T cells include IL-7 [54], IL-12 [55], and IL-15 [56].
7. Poxviral vaccines combined with other treatment modalities
Results of numerous clinical trials have suggested that therapeutic cancer vaccines have little efficacy as monotherapy for patients with large tumor burdens or rapidly growing tumors [57]. There are several reasons for this limitation: (a) large tumor architecture may impede T-cell penetration; (b) tumor cells outnumber antigen-specific T cells; (c) tumor cells produce several immunoregulatory molecules that can anergize T cells and induce myeloid-derived suppressor cells and regulatory T cells (Tregs); and (d) tumor cells down-regulate MHC class I molecules [58]. Combining vaccine with other treatment modalities such as debulking surgery, cytotoxic chemotherapy, or radiotherapy may help to overcome these limitations.
7.1 Poxviral vaccine plus radiotherapy
Radiotherapy is a very effective cytotoxic modality commonly used for the treatment of many types of cancer. Radiation causes cell death by creating free radicals that damage DNA, and also by directly damaging DNA in ways that make it unamenable to repair mechanisms, leading to cell death. Several preclinical studies have shown that low-dose radiation causes phenotypic changes in tumor cells, such as increased expression of Fas, tumor antigens, MHC class I, and ICAM-1 [59, 60]. In clinical trials, it was shown that radiotherapy and hormonal therapy induce antigen-specific immune responses in prostate cancer patients [61] and that vaccines can have a synergistic antitumor effect in combination with local tumor radiotherapy [62]. In early clinical trials in patients with localized prostate cancer, rV-PSA/rV-B7.1 priming vaccine followed by monthly boosts with rF-PSA, along with standard radiotherapy, generated significant immune responses, including antigen cascade, and suggested some clinical benefit [23, 25]. However, in a small pilot study, where patients with hepatic metastases of GI malignancies were treated with rV/F-CEA-TRICOM plus radiotherapy to hepatic metastases, no significant clinical benefit was observed [63], primarily because most of the patients were heavily pretreated with ≥3 prior systemic therapies and liver-targeted chemotherapy perfusion.
7.2 Poxviral vaccine plus chemotherapy
It was long believed that chemotherapy would compromise the host immune system. Although this may hold true for patients who have received several lines of cytotoxic chemotherapy at therapeutic doses, some chemotherapy drugs render tumor cells more susceptible to immune-mediated cytolysis. A number of chemotherapy drugs up-regulate MHC class I molecules, costimulatory molecules, and several tumor antigens on the surface of tumor cells [64, 65], enhance macrophage antitumor activity and apoptosis (doxorubicin) [66], increase pro-inflammatory cytokine production (docetaxel) [67], and decrease Tregs (cyclophosphamide) [68].
In a phase II trial, 28 mCRPC patients were randomized to receive a poxviral-based PSA vaccine either alone or with docetaxel [69]. Patients in the vaccine-alone arm were allowed to cross over to receive docetaxel alone at disease progression. After 3 months of therapy, patients in the combination arm had similar PSA-specific T-cell responses, suggesting that docetaxel, even with corticosteroids, did not attenuate T-cell response to vaccine. Interestingly, evidence of antigen cascade was also observed. The study also suggested that those who received docetaxel following progression on vaccine had longer PFS than the historical control (6.1 vs 3.7 months). It was unclear whether initial immune response to the vaccine potentiated or enhanced the efficacy of the subsequent therapy. The ECOG E1809 study is prospectively evaluating the benefit of vaccine followed by chemotherapy vs chemotherapy alone in a randomized controlled trial (NCT01145508).
PANVAC-V/F has demonstrated early evidence of clinical activity in patients with metastatic breast, ovarian, and gastric cancers [70, 71]. A randomized phase II trial is currently evaluating PANVAC-V/F plus docetaxel vs docetaxel alone in patients with metastatic breast cancer (NCT00179309).
7.3 Poxviral vaccine plus hormonal therapy
Hormonal therapy is a mainstay of treatment for prostate and hormone receptor-positive breast cancers. Mercader et al. prospectively studied 33 patients with biopsy-proven, stage T1 to T2b prostate cancer to evaluate the immunological effect of androgen-ablative therapy on prostate tissue. Patients (n = 33) were randomized to receive no pre-operative therapy (n = 7), or 1 week (n = 7), 2 weeks (n = 7), 3 weeks (n = 5), or 4 weeks of pre-operative androgen ablation before undergoing radical prostatectomy. Results showed that hormonal therapy triggered vigorous T cell-mediated inflammation within the prostate. T-cell infiltration into prostate tissue, consisting predominantly of CD4+ T cells and relatively fewer CD8+ T cells, was readily apparent after 1 to 4 weeks of therapy. T cells within the treated prostate showed restricted T-cell receptor gene usage, suggesting a local oligoclonal response [72].
In a phase II trial, 42 patients with non-metastatic CRPC were randomized to receive either a poxviral-based PSA vaccine or nilutamide (an FDA-approved androgen receptor antagonist). Patients in either arm who developed rising PSA could cross over to receive the combined therapies. After a median follow-up of 4.4 years, there was a trend toward improved survival in the vaccine arm. A subgroup analysis of patients randomized to the vaccine arm showed substantially improved survival in patients who had indolent disease characteristics at baseline (PSA < 20 ng/dl, Gleason score ≤7, prior radiotherapy) [73]. Another phase II trial randomized 26 patients with non-metastatic CRPC to receive flutamide alone or with PROSTVAC-V/F. A preliminary report showed that patients in the combination arm had improved time to progression compared with patients who received flutamide alone (223 vs 85 days, respectively) [74].
7.4 Poxviral vaccine plus other immunotherapies
Combining a therapeutic cancer vaccine with another immune-based therapy is an attractive strategy for enhancing immunologic response and generating synergistic antitumor effects. Ipilimumab (anti-CTLA-4 antibody), an immune checkpoint inhibitor, was recently approved by the FDA for treatment of metastatic melanoma. A preclinical study showed that an anti-CTLA-4 monoclonal antibody combined with a poxviral vaccine resulted in higher-avidity antigen-specific T cells [37]. In a phase I clinical trial, 30 patients with mCRPC were treated with PSA-TRICOM in combination with ipilimumab. The 2-year survival rate in this trial was 74%; median overall survival (OS) was 31.8 months vs the 18.5 months predicted by the Halabi nomogram [75].
In a phase II trial in metastatic RCC, MVA expressing the tumor antigen 5T4 (MVA-5T4) was tested in combination with high-dose IL-2 [76]. Patients (n = 25) received 3 vaccinations every 3 weeks followed by high-dose IL-2 (600,000 IU/kg) after the second and third vaccinations. Three patients (12%) were disease-free after nephrectomy or resection of residual metastasis; 12 patients (48%) had stable disease in association with a trend toward increased effector CD8+ T cells and a decrease in Tregs. In a later randomized, placebo-controlled phase III trial, 733 patients with metastatic RCC were treated with standard of care (interferon, sunitinib, or IL-2) alone or in combination with MVA-5T4 [77]. The study did not show a significant difference in OS between the 2 arms. However, in an exploratory analysis, a subset of patients with good prognosis (MSKCC 0), who were treated with the vaccine followed by high-dose IL-2, had a significant survival advantage over patients receiving IL-2 alone (HR 0.54; p = 0.046).
7.5 Poxviral vaccine plus small molecule targeted therapy
Cancer therapeutics have made landmark advances in the last decade, particularly with the development of molecularly targeted therapies such as imatinib and vemurafenib, and passive immunotherapeutics such as rituximab and trastuzumab. Intense investigations in cancer genomics have led to deeper understanding of the driving mutations of individual cancers. Several molecularly targeted therapies have demonstrated clinical response that translated into prolonged survival, demonstrating the proof of concept. However, the complexities of cancer genomics are unlikely to be overcome by a single therapeutic approach. The rationale for combining a molecularly targeted therapy with a therapeutic cancer vaccine is several-fold. Both classes of agents are well tolerated. Some targeted agents, such as imatinib, have been shown to potentiate an antitumor T-cell response in a murine GIST model [78]. BRAF kinase inhibitor was shown to enhance T-cell recognition of melanoma without affecting lymphocyte function [79, 80]. Treatment with BCL-2 inhibitors has been associated with decreased Treg function. In a murine model, sequential treatment with BCL-2 inhibitors following vaccination with CEA-TRICOM showed an increased activated CD8+ cell:Treg ratio at the tumor site and a significant reduction in pulmonary nodules [81]. Vaccines have been used concurrently with imatinib in patients with chronic myelogenous leukemia [82, 83]. There is currently intense interest in combining a poxviral vaccine with a molecularly targeted small molecule inhibitor.
7.6 Poxviral vaccine following surgery
Reducing tumor burden by resecting primary tumors or metastases creates an ideal setting for therapeutic cancer vaccines. Recently, Morse et al. reported that patients who received PANVAC vaccine following resection of colorectal cancer metastases had dramatically superior survival outcomes compared with concurrent unvaccinated controls with 97% 2 year survival compared with 75%, a number more consistent with other contemporary series [84]. Several non-poxviral vaccines have also demonstrated clinical benefit in this disease setting. In a randomized controlled trial, OncoVAX®, an irradiated autologous tumor cell vaccine, demonstrated a 5-year survival advantage of 9.8% in patients with resected stage II colon cancer [85]. This vaccine was granted fast-track designation by the FDA and will be studied in a phase III confirmatory trial in patients with stage II colon cancer following surgery [86]. Likewise, a randomized, placebo-controlled phase II trial in 182 patients with completely resected, MAGE-A3-expressing, stage IB/II NSCLC, the protein-based vaccine MAGE-A3 demonstrated a trend toward enhanced disease-free interval and OS [87]. This vaccine is being evaluated in the phase III MAGRIT trial (MAGE-A3 as Adjuvant, Non-Small Cell Lung Cancer Immunotherapy).
8. Expert Opinion
Major developments in poxviral-vector cancer vaccines include (a) recombinant vectors that can express multiple genes for costimulatory molecules along with multiple tumor antigens, (b) replication-incompetent poxviral vectors such as MVA, canarypox (ALVAC), or avipox that do not induce a host neutralizing response, and (c) the strategy of priming with an immunogenic vaccinia vector, followed by boosting with a replication-incompetent avipox or fowlpox vector to heighten the immune response. This strategy has become standard for many poxviral vaccine platforms.
Despite sound preclinical science and promising early clinical trial data, some clinical trials of therapeutic cancer vaccines were terminated early due to negative results. An example is the industry-sponsored phase III trial of PANVAC-V/F in advanced pancreatic cancer patients who had failed front-line therapy [41]. It is well known that patients with advanced pancreatic cancer have a life expectancy of about 3 months. Cytotoxic chemotherapy, radiotherapy, biologics and targeted therapies, and combinations thereof have failed to produce meaningful clinical benefit in this patient population. Given the aggressive biology of pancreatic cancer and its significant accompanying morbidity, stimulating the immune system to produce a clinical response in this patient population would be challenging and difficult at best. In contrast, colorectal cancer patients with low tumor burden, such as those rendered disease-free after resection of metastases, who were treated with PANVAC had superior survival outcomes compared with historical controls [84]. Thus, it is likely that the failure of the earlier trial was due to inappropriate patient selection rather than failure of the vaccine itself.
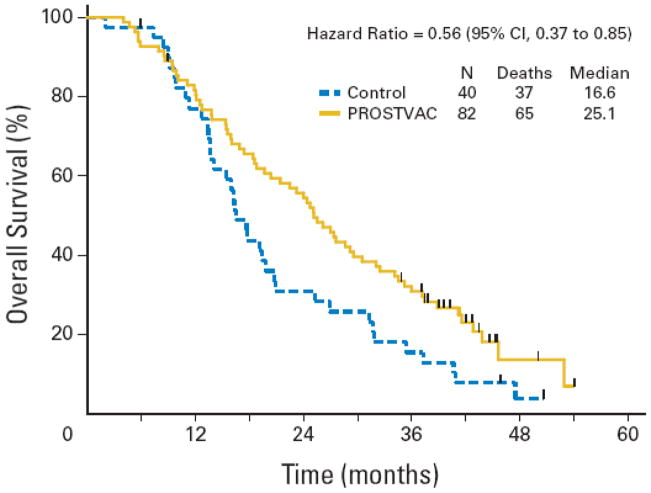
Similarly, PROSTVAC-VF administered in asymptomatic or minimally symptomatic mCRPC patients showed a statistically significant survival difference of 8.5 months (25.1 vs 16.6 months; estimated HR 0.56; 95% CI, 0.37 to 0.85; stratified logrank p = 0.0061) in a randomized, placebo-controlled trial (Figure 2) [88]. Another single-arm phase II trial of PROSTVAC in mCRPC patients showed a similar survival outcome, with a median OS of 26.6 months. A subgroup analysis showed that the survival benefit was more pronounced in patients with a predicted survival of > 18 months than in those with a predicted survival of < 18 months [44]. In stark contrast, GVAX, an allogeneic whole tumor cell vaccine modified to secrete GM-CSF, despite promising results in phase II trials, failed to confirm its efficacy in 2 large phase III trials (VITAL-1 and VITAL-2) in patients with mCRPC. In the VITAL-2 trial in 408 symptomatic mCRPC patients, increased deaths in the vaccine plus docetaxel arm vs docetaxel alone led to early termination. In this study, the median predicted survival was 13 months in both arms, and patients in the combination arm received fewer cycles of docetaxel.
Figure 2.
Kaplan-Meier estimator of overall survival. Solid gold line = PROSTVAC arm. Dashed blue line = control arm. Vertical ticks = censoring times. Estimated median overall survival is 25.1 months for the PROSTVAC arm and 16.6 months for the control arm. (Reproduced with permission from Journal of Clinical Oncology.)
Results of clinical trials have repeatedly shown that the ideal candidates for cancer vaccines are patients with low tumor burden or indolent tumor biology [89]. At present, there is a need for a reliable biomarker to select for ideal candidates for vaccine. Although several clinical parameters such as Halabi-predicted survival, circulating tumor cells, and PSA doubling time are used in clinical practice to gauge tumor biology, none has been validated in prospective trials. As the field of cancer genomics expands, a tool such as quantitative gene expression may be very useful in predicting the biology of prostate cancer, making it a potentially useful predictive marker in vaccine therapy [90].
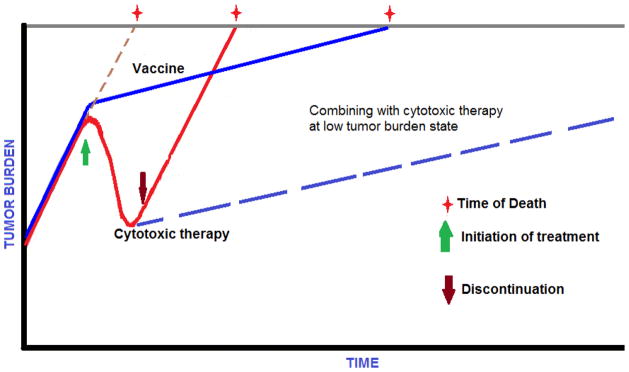
PROSTVAC appears to prolong OS without affecting median PFS. This pattern of clinical benefit has also been observed with other immunotherapeutic agents, such as sipuleucel-T [46] and ipilimumab [91]. It is possible that this finding may be due to the fact that conventional methods of efficacy assessment (RECIST, WHO Response Criteria, or Prostate Cancer Clinical Trials Working Group Guidelines) are not able to account for a delayed slowing of the tumor growth rate [92]. Because it is essential to understand the impact of new immunotherapies on tumors, a mathematical concept of the tumor growth rate constant has been proposed to account for the phenomenon of prolonged OS without changes in PFS. This model has been evaluated retrospectively in several clinical trials at the National Cancer Institute [93]. As depicted in Figure 3, although cytotoxic chemotherapy effectively decreased tumor volume while patients were on treatment, off treatment, the pre-treatment tumor growth rate resumed appeared to be unchanged until death. On the other hand, vaccine therapy did not have an immediate effect on the tumor growth rate while patients were on treatment (about 3 months). However, over time, it appeared to substantially decrease and this may have led to improved survival.
Figure 3.
Tumor growth rate is a dynamic biological process that is the combined result of cells dying and dividing over time. Cytotoxic therapy reduces tumor burden as long as it is being administered. However, when treatment is terminated, the pretreatment tumor growth rate resumes and the patient succumbs to disease in a predictable manner. A therapeutic cancer vaccine does not decrease tumor burden immediately; however, it can prolong survival by decreasing the tumor growth rate. Combining vaccine with other therapeutics in low tumor burden states can maximize the survival benefit. (Adapted from Madan et al 2010 [92])
Clinical development of poxviral cancer vaccines is ongoing and highly promising, particularly the further investigation of PROSTVAC-V/F in prostate cancer. Based on emerging data from multiple clinical trials, we believe there are a few important point to consider as we move forward with optimizing the clinical benefit of therapeutic vaccines. First, appropriate clinical trial design that includes patients with indolent disease characteristics and low tumor burden. Second, combining cancer vaccines with other therapies may optimize clinical benefit. Finally, identifying predictive biomarkers and appropriate immune-related efficacy assessment tools for evaluating cancer vaccines should allow for more efficient proof of concept studies.
Table 3.
Clinical trial with TRICOM-expressing poxviral vaccine
| Ref. | Type of trial | N | Vaccine | Dose/schedule | Results |
|---|---|---|---|---|---|
| [40] | Pilot study in metastatic melanoma | 13 | rV-TRICOM | 3 monthly intralesional injections of rV-TRICOM at doses ranging from 5.1×106 pfu to 5.1×108 pfu | 30.7% objective clinical response. One patient had CR for > 22 mos. |
CR = complete response; mos = months; pfu = plaque-forming units
Highlights.
Poxviral vectors have proven immunogenicity, safety, and preliminary evidence of efficacy.
Recombinant poxviruses have been engineered to enhance immune response by incorporating the transgenes of costimulatory molecules along with tumor-associated antigens, for use in a diversified prime-boost vaccination strategy.
The benefit of vaccine may be maximized by combination with other cancer therapeutics that reduce tumor burden, modulate regulatory immune cells, or alter tumor phenotype, making tumors more susceptible to immune-mediated killing.
Proper patient selection is a critical factor in clinical trials of therapeutic cancer vaccines. Patients with indolent disease characteristics and/or low tumor burden appear to be optimal candidates for vaccine therapy.
A phase II randomized controlled trial of PROSTVAC-V/F in mCRPC has demonstrated prolonged survival. This vaccine is being evaluated in a phase III, randomized, placebo-controlled trial in mCRPC patients with indolent disease characteristics, with overall survival as the primary endpoint.
Immune-related markers of clinical benefit need to be identified and developed. In the absence of reliable surrogate makers, overall survival is the ultimate primary endpoint for clinical trials of poxviral-based cancer vaccines as single modalities.
Footnotes
Declaration of interest
Authors have no conflict of interest.
Bibliography
- 1.Moss B, Flexner C. Vaccinia virus expression vectors. Annu Rev Immunol. 1987;5:305–24. doi: 10.1146/annurev.iy.05.040187.001513. [DOI] [PubMed] [Google Scholar]
- 2.Moss B. Smallpox vaccines: targets of protective immunity. Immunol Rev. 2011;239:8–26. doi: 10.1111/j.1600-065X.2010.00975.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grosenbach DW, Hruby DE. Biology of vaccinia virus acylproteins. Front Biosci. 1998;3:d354–64. doi: 10.2741/a280. [DOI] [PubMed] [Google Scholar]
- 4.Bernards R, Destree A, McKenzie S, et al. Effective tumor immunotherapy directed against an oncogene-encoded product using a vaccinia virus vector. Proc Natl Acad Sci U S A. 1987;84:6854–8. doi: 10.1073/pnas.84.19.6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lathe R, Kieny MP, Gerlinger P, et al. Tumour prevention and rejection with recombinant vaccinia. Nature. 1987;326:878–80. doi: 10.1038/326878a0. [DOI] [PubMed] [Google Scholar]
- 6.Hareuveni M, Gautier C, Kieny MP, et al. Vaccination against tumor cells expressing breast cancer epithelial tumor antigen. Proc Natl Acad Sci U S A. 1990;87:9498–502. doi: 10.1073/pnas.87.23.9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meneguzzi G, Kieny MP, Lecocq JP, et al. Vaccinia recombinants expressing early bovine papilloma virus (BPV1) proteins: retardation of BPV1 tumour development. Vaccine. 1990;8:199–204. doi: 10.1016/0264-410x(90)90045-n. [DOI] [PubMed] [Google Scholar]
- 8.Kaufman H, Schlom J, Kantor J. A recombinant vaccinia virus expressing human carcinoembryonic antigen (CEA) Int J Cancer. 1991;48:900–7. doi: 10.1002/ijc.2910480618. [DOI] [PubMed] [Google Scholar]
- 9.Kantor J, Irvine K, Abrams S, et al. Immunogenicity and safety of a recombinant vaccinia virus vaccine expressing the carcinoembryonic antigen gene in a nonhuman primate. Cancer Res. 1992;52:6917–25. [PubMed] [Google Scholar]
- 10.Kantor J, Irvine K, Abrams S, et al. Antitumor activity and immune responses induced by a recombinant carcinoembryonic antigen-vaccinia virus vaccine. J Natl Cancer Inst. 1992;84:1084–91. doi: 10.1093/jnci/84.14.1084. [DOI] [PubMed] [Google Scholar]
- 11.Mackett M, Smith GL, Moss B. General method for production and selection of infectious vaccinia virus recombinants expressing foreign genes. J Virol. 1984;49:857–64. doi: 10.1128/jvi.49.3.857-864.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamilton JM, Chen A, Nguyen B. Phase I study of recombinant vaccinia virus (rV) that expresses human carcinoembryonic antigen (CEA) in adult patients with adenocarcinomas. Proc Am Soc Clin Oncol. 1994;13:961. [Google Scholar]
- 13.Tsang KY, Zaremba S, Nieroda CA, et al. Generation of human cytotoxic T cells specific for human carcinoembryonic antigen epitopes from patients immunized with recombinant vaccinia-CEA vaccine. J Natl Cancer Inst. 1995;87:982–90. doi: 10.1093/jnci/87.13.982. [DOI] [PubMed] [Google Scholar]
- 14.Sanda MG, Smith DC, Charles LG, et al. Recombinant vaccinia-PSA (PROSTVAC) can induce a prostate-specific immune response in androgen-modulated human prostate cancer. Urology. 1999;53:260–6. doi: 10.1016/s0090-4295(98)00539-1. [DOI] [PubMed] [Google Scholar]
- 15.Eder JP, Kantoff PW, Roper K, et al. A phase I trial of a recombinant vaccinia virus expressing prostate-specific antigen in advanced prostate cancer. Clin Cancer Res. 2000;6:1632–8. [PubMed] [Google Scholar]
- 16.Gulley J, Chen AP, Dahut W, et al. Phase I study of a vaccine using recombinant vaccinia virus expressing PSA (rV-PSA) in patients with metastatic androgen-independent prostate cancer. Prostate. 2002;53:109–17. doi: 10.1002/pros.10130. [DOI] [PubMed] [Google Scholar]
- 17.Hodge JW, McLaughlin JP, Kantor JA, Schlom J. Diversified prime and boost protocols using recombinant vaccinia virus and recombinant non-replicating avian pox virus to enhance T-cell immunity and antitumor responses. Vaccine. 1997;15:759–68. doi: 10.1016/s0264-410x(96)00238-1. [DOI] [PubMed] [Google Scholar]
- 18.Marshall JL, Hoyer RJ, Toomey MA, et al. Phase I study in advanced cancer patients of a diversified prime-and-boost vaccination protocol using recombinant vaccinia virus and recombinant nonreplicating avipox virus to elicit anti-carcinoembryonic antigen immune responses. J Clin Oncol. 2000;18:3964–73. doi: 10.1200/JCO.2000.18.23.3964. [DOI] [PubMed] [Google Scholar]
- 19.Kaufman HL, Wang W, Manola J, et al. Phase II randomized study of vaccine treatment of advanced prostate cancer (E7897): a trial of the Eastern Cooperative Oncology Group. J Clin Oncol. 2004;22:2122–32. doi: 10.1200/JCO.2004.08.083. [DOI] [PubMed] [Google Scholar]
- 20.Kaufman H, Wang W, Manola J, et al. Phase II prime/boost vaccination using poxviruses expressing PSA in hormone dependent prostate cancer: Follow-up clinical results from ECOG 7897. J Clin Oncol. 2005;23(16S):abs 4501. [Google Scholar]
- 21.Boehm AL, Higgins J, Franzusoff A, et al. Concurrent vaccination with two distinct vaccine platforms targeting the same antigen generates phenotypically and functionally distinct T-cell populations. Cancer Immunol Immunother. 2010;59:397–408. doi: 10.1007/s00262-009-0759-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hodge JW, Higgins J, Schlom J. Harnessing the unique local immunostimulatory properties of modified vaccinia Ankara (MVA) virus to generate superior tumor-specific immune responses and antitumor activity in a diversified prime and boost vaccine regimen. Vaccine. 2009;27:4475–82. doi: 10.1016/j.vaccine.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gulley JL, Arlen PM, Bastian A, et al. Combining a recombinant cancer vaccine with standard definitive radiotherapy in patients with localized prostate cancer. Clin Cancer Res. 2005;11:3353–62. doi: 10.1158/1078-0432.CCR-04-2062. [DOI] [PubMed] [Google Scholar]
- 24.Arlen PM, Gulley JL, Todd N, et al. Antiandrogen, vaccine and combination therapy in patients with nonmetastatic hormone refractory prostate cancer. J Urol. 2005;174:539–46. doi: 10.1097/01.ju.0000165159.33772.5b. [DOI] [PubMed] [Google Scholar]
- 25.Lechleider RJ, Arlen PM, Tsang KY, et al. Safety and immunologic response of a viral vaccine to prostate-specific antigen in combination with radiation therapy when metronomic-dose interleukin 2 is used as an adjuvant. Clin Cancer Res. 2008;14:5284–91. doi: 10.1158/1078-0432.CCR-07-5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horig H, Lee DS, Conkright W, et al. Phase I clinical trial of a recombinant canarypoxvirus (ALVAC) vaccine expressing human carcinoembryonic antigen and the B7. 1 co-stimulatory molecule. Cancer Immunol Immunother. 2000;49:504–14. doi: 10.1007/s002620000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.von Mehren M, Arlen P, Tsang KY, et al. Pilot study of a dual gene recombinant avipox vaccine containing both carcinoembryonic antigen (CEA) and B7. 1 transgenes in patients with recurrent CEA-expressing adenocarcinomas. Clin Cancer Res. 2000;6:2219–28. [PubMed] [Google Scholar]
- 28.Savage CO, Hughes CC, Pepinsky RB, et al. Endothelial cell lymphocyte function-associated antigen-3 and an unidentified ligand act in concert to provide costimulation to human peripheral blood CD4+ T cells. Cell Immunol. 1991;137:150–63. doi: 10.1016/0008-8749(91)90065-j. [DOI] [PubMed] [Google Scholar]
- 29.Damle NK, Klussman K, Linsley PS, Aruffo A. Differential costimulatory effects of adhesion molecules B7, ICAM-1, LFA-3, and VCAM-1 on resting and antigen-primed CD4+ T lymphocytes. J Immunol. 1992;148:1985–92. [PubMed] [Google Scholar]
- 30.Hodge JW, Sabzevari H, Yafal AG, et al. A triad of costimulatory molecules synergize to amplify T-cell activation. Cancer Res. 1999;59:5800–7. [PubMed] [Google Scholar]
- 31.Zhu M, Terasawa H, Gulley J, et al. Enhanced activation of human T cells via avipox vector-mediated hyperexpression of a triad of costimulatory molecules in human dendritic cells. Cancer Res. 2001;61:3725–34. [PubMed] [Google Scholar]
- 32.Grosenbach DW, Barrientos JC, Schlom J, Hodge JW. Synergy of vaccine strategies to amplify antigen-specific immune responses and antitumor effects. Cancer Res. 2001;61:4497–505. [PubMed] [Google Scholar]
- 33.Aarts WM, Schlom J, Hodge JW. Vector-based vaccine/cytokine combination therapy to enhance induction of immune responses to a self-antigen and antitumor activity. Cancer Res. 2002;62:5770–7. [PubMed] [Google Scholar]
- 34.Tatari-Calderone Z, Semnani RT, Nutman TB, et al. Acquisition of CD80 by human T cells at early stages of activation: functional involvement of CD80 acquisition in T cell to T cell interaction. J Immunol. 2002;169:6162–9. doi: 10.4049/jimmunol.169.11.6162. [DOI] [PubMed] [Google Scholar]
- 35.Zhou J, Tagaya Y, Tolouei-Semnani R, et al. Physiological relevance of antigen presentasome (APS), an acquired MHC/costimulatory complex, in the sustained activation of CD4+ T cells in the absence of APCs. Blood. 2005;105:3238–46. doi: 10.1182/blood-2004-08-3236. [DOI] [PubMed] [Google Scholar]
- 36.Oh S, Hodge JW, Ahlers JD, et al. Selective induction of high avidity CTL by altering the balance of signals from APC. J Immunol. 2003;170:2523–30. doi: 10.4049/jimmunol.170.5.2523. [DOI] [PubMed] [Google Scholar]
- 37.Hodge JW, Chakraborty M, Kudo-Saito C, et al. Multiple costimulatory modalities enhance CTL avidity. J Immunol. 2005;174:5994–6004. doi: 10.4049/jimmunol.174.10.5994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang S, Schlom J. Antigen-presenting cells containing multiple costimulatory molecules promote activation and expansion of human antigen-specific memory CD8+ T cells. Cancer Immunol Immunother. 2009;58:503–15. doi: 10.1007/s00262-008-0572-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marshall JL, Gulley JL, Arlen PM, et al. Phase I study of sequential vaccinations with fowlpox-CEA(6D)-TRICOM alone and sequentially with vaccinia-CEA(6D)-TRICOM, with and without granulocyte-macrophage colony-stimulating factor, in patients with carcinoembryonic antigen-expressing carcinomas. J Clin Oncol. 2005;23:720–31. doi: 10.1200/JCO.2005.10.206. [DOI] [PubMed] [Google Scholar]
- 40.Kaufman HL, Cohen S, Cheung K, et al. Local delivery of vaccinia virus expressing multiple costimulatory molecules for the treatment of established tumors. Hum Gene Ther. 2006;17:239–44. doi: 10.1089/hum.2006.17.239. [DOI] [PubMed] [Google Scholar]
- 41.Madan RA, Arlen PM, Gulley JL. PANVAC-VF: poxviral-based vaccine therapy targeting CEA and MUC1 in carcinoma. Expert Opin Biol Ther. 2007;7:543–54. doi: 10.1517/14712598.7.4.543. [DOI] [PubMed] [Google Scholar]
- 42.Madan RA, Arlen PM, Mohebtash M, et al. Prostvac-VF: a vector-based vaccine targeting PSA in prostate cancer. Expert Opin Investig Drugs. 2009;18:1001–11. doi: 10.1517/13543780902997928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arlen PM, Skarupa L, Pazdur M, et al. Clinical safety of a viral vector based prostate cancer vaccine strategy. J Urol. 2007;178:1515–20. doi: 10.1016/j.juro.2007.05.117. [DOI] [PubMed] [Google Scholar]
- 44.Gulley JL, Arlen PM, Madan RA, et al. Immunologic and prognostic factors associated with overall survival employing a poxviral-based PSA vaccine in metastatic castrate-resistant prostate cancer. Cancer Immunol Immunother. 2010;59:663–74. doi: 10.1007/s00262-009-0782-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lutz E, Yeo CJ, Lillemoe KD, et al. A lethally irradiated allogeneic granulocyte-macrophage colony stimulating factor-secreting tumor vaccine for pancreatic adenocarcinoma. A Phase II trial of safety, efficacy, and immune activation. Ann Surg. 2011;253:328–35. doi: 10.1097/SLA.0b013e3181fd271c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–22. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 47.Haining WN, Davies J, Kanzler H, et al. CpG oligodeoxynucleotides alter lymphocyte and dendritic cell trafficking in humans. Clin Cancer Res. 2008;14:5626–34. doi: 10.1158/1078-0432.CCR-08-0526. [DOI] [PubMed] [Google Scholar]
- 48.Kass E, Panicali DL, Mazzara G, et al. Granulocyte/macrophage-colony stimulating factor produced by recombinant avian poxviruses enriches the regional lymph nodes with antigen-presenting cells and acts as an immunoadjuvant. Cancer Res. 2001;61:206–14. [PubMed] [Google Scholar]
- 49.Davis ID, Chen Q, Morris L, et al. Blood dendritic cells generated with Flt3 ligand and CD40 ligand prime CD8+ T cells efficiently in cancer patients. J Immunother. 2006;29:499–511. doi: 10.1097/01.cji.0000211299.29632.8c. [DOI] [PubMed] [Google Scholar]
- 50.Igartua M, Pedraz JL. Topical resiquimod: a promising adjuvant for vaccine development? Expert Rev Vaccines. 2010;9:23–7. doi: 10.1586/erv.09.135. [DOI] [PubMed] [Google Scholar]
- 51.Jasani B, Navabi H, Adams M. Ampligen: a potential toll-like 3 receptor adjuvant for immunotherapy of cancer. Vaccine. 2009;27:3401–4. doi: 10.1016/j.vaccine.2009.01.071. [DOI] [PubMed] [Google Scholar]
- 52.Dreicer R, Stadler WM, Ahmann FR, et al. MVA-MUC1-IL2 vaccine immunotherapy (TG4010) improves PSA doubling time in patients with prostate cancer with biochemical failure. Invest New Drugs. 2009;27:379–86. doi: 10.1007/s10637-008-9187-3. [DOI] [PubMed] [Google Scholar]
- 53.Quoix E, Ramlau R, Westeel V, et al. Therapeutic vaccination with TG4010 and first-line chemotherapy in advanced non-small-cell lung cancer: a controlled phase 2B trial. Lancet Oncol. 2011 Oct 21; doi: 10.1016/S1470-2045(11)70259-5. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 54.Melchionda F, Fry TJ, Milliron MJ, et al. Adjuvant IL-7 or IL-15 overcomes immunodominance and improves survival of the CD8+ memory cell pool. J Clin Invest. 2005;115:1177–87. doi: 10.1172/JCI23134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cheever MA. Twelve immunotherapy drugs that could cure cancers. Immunol Rev. 2008;222:357–68. doi: 10.1111/j.1600-065X.2008.00604.x. [DOI] [PubMed] [Google Scholar]
- 56.Sato N, Patel HJ, Waldmann TA, Tagaya Y. The IL-15/IL-15Ralpha on cell surfaces enables sustained IL-15 activity and contributes to the long survival of CD8 memory T cells. Proc Natl Acad Sci U S A. 2007;104:588–93. doi: 10.1073/pnas.0610115104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Higano CS, Saad F, Somer B, et al. A phase III trial of GVAX immunotherapy for prostate cancer versus docetaxel plus prednisone in asymptomatic, castration-resistant prostate cancer (CRPC). ASCO Genitourinary Cancers Symposium; 2009. p. abs LBA150. [Google Scholar]
- 58.Schwartz RH. Models of T cell anergy: is there a common molecular mechanism? J Exp Med. 1996;184:1–8. doi: 10.1084/jem.184.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chakraborty M, Abrams SI, Camphausen K, et al. Irradiation of tumor cells up-regulates Fas and enhances CTL lytic activity and CTL adoptive immunotherapy. J Immunol. 2003;170:6338–47. doi: 10.4049/jimmunol.170.12.6338. [DOI] [PubMed] [Google Scholar]
- 60.Garnett CT, Palena C, Chakraborty M, et al. Sublethal irradiation of human tumor cells modulates phenotype resulting in enhanced killing by cytotoxic T lymphocytes. Cancer Res. 2004;64:7985–94. doi: 10.1158/0008-5472.CAN-04-1525. [DOI] [PubMed] [Google Scholar]
- 61.Nesslinger NJ, Sahota RA, Stone B, et al. Standard treatments induce antigen-specific immune responses in prostate cancer. Clin Cancer Res. 2007;13:1493–502. doi: 10.1158/1078-0432.CCR-06-1772. [DOI] [PubMed] [Google Scholar]
- 62.Chakraborty M, Abrams SI, Coleman CN, et al. External beam radiation of tumors alters phenotype of tumor cells to render them susceptible to vaccine-mediated T-cell killing. Cancer Res. 2004;64:4328–37. doi: 10.1158/0008-5472.CAN-04-0073. [DOI] [PubMed] [Google Scholar]
- 63.Gulley JL, Madan RA, Tsang KY, et al. A pilot safety trial investigating a vector-based vaccine targeting carcinoembryonic antigen in combination with radiotherapy in patients with gastrointestinal malignancies metastatic to the liver. Expert Opin Biol Ther. 2011;11:1409–18. doi: 10.1517/14712598.2011.615741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Matsuzaki I, Suzuki H, Kitamura M, et al. Cisplatin induces fas expression in esophageal cancer cell lines and enhanced cytotoxicity in combination with LAK cells. Oncology. 2000;59:336–43. doi: 10.1159/000012192. [DOI] [PubMed] [Google Scholar]
- 65.Maas IW, Boven E, Pinedo HM, et al. The effects of gamma-interferon combined with 5-fluorouracil or 5-fluoro-2′-deoxyuridine on proliferation and antigen expression in a panel of human colorectal cancer cell lines. Int J Cancer. 1991;48:749–56. doi: 10.1002/ijc.2910480520. [DOI] [PubMed] [Google Scholar]
- 66.Maccubbin DL, Wing KR, Mace KF, et al. Adriamycin-induced modulation of host defenses in tumor-bearing mice. Cancer Res. 1992;52:3572–6. [PubMed] [Google Scholar]
- 67.Chan OT, Yang LX. The immunological effects of taxanes. Cancer Immunol Immunother. 2000;49:181–5. doi: 10.1007/s002620000122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lutsiak ME, Semnani RT, De Pascalis R, et al. Inhibition of CD4(+)25+ T regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide. Blood. 2005;105:2862–8. doi: 10.1182/blood-2004-06-2410. [DOI] [PubMed] [Google Scholar]
- 69.Arlen PM, Gulley JL, Parker C, et al. A randomized phase II study of concurrent docetaxel plus vaccine versus vaccine alone in metastatic androgen-independent prostate cancer. Clin Cancer Res. 2006;12:1260–9. doi: 10.1158/1078-0432.CCR-05-2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mohebtash M, Tsang KY, Madan RA, Huen NY, et al. A pilot study of MUC-1/CEA/TRICOM poxviral-based vaccine in patients with metastatic breast and ovarian cancer. Clin Cancer Res. 2011 Nov 8; doi: 10.1158/1078-0432.CCR-11-0649. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gulley JL, Arlen PM, Tsang KY, et al. Pilot study of vaccination with recombinant CEA-MUC-1-TRICOM poxviral-based vaccines in patients with metastatic carcinoma. Clin Cancer Res. 2008;14:3060–9. doi: 10.1158/1078-0432.CCR-08-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mercader M, Bodner BK, Moser MT, et al. T cell infiltration of the prostate induced by androgen withdrawal in patients with prostate cancer. Proc Natl Acad Sci U S A. 2001;98:14565–70. doi: 10.1073/pnas.251140998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Madan RA, Gulley JL, Schlom J, et al. Analysis of overall survival in patients with nonmetastatic castration-resistant prostate cancer treated with vaccine, nilutamide, and combination therapy. Clin Cancer Res. 2008;14:4526–31. doi: 10.1158/1078-0432.CCR-07-5048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bilusic M, Gulley J, Heery C, et al. A randomized phase II study of flutamide with or without PSA-TRICOM in nonmetastatic castration-resistant prostate cancer (CRPC) J Clin Oncol. 2011;29(7S):abs 163. [Google Scholar]
- 75.Madan R, Mohebtash M, Arlen P, et al. Overall survival (OS) analysis of a phase l trial of a vector-based vaccine (PSA-TRICOM) and ipilimumab (Ipi) in the treatment of metastatic castration-resistant prostate cancer (mCRPC) J Clin Oncol. 2010;28(15S):abs 2550. [Google Scholar]
- 76.Kaufman HL, Taback B, Sherman W, et al. Phase II trial of Modified Vaccinia Ankara (MVA) virus expressing 5T4 and high dose Interleukin-2 (IL-2) in patients with metastatic renal cell carcinoma. J Transl Med. 2009;7:2. doi: 10.1186/1479-5876-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Amato RJ, Hawkins RE, Kaufman HL, et al. Vaccination of metastatic renal cancer patients with MVA-5T4: a randomized, double-blind, placebo-controlled phase III study. Clin Cancer Res. 2010;16:5539–47. doi: 10.1158/1078-0432.CCR-10-2082. [DOI] [PubMed] [Google Scholar]
- 78.Balachandran VP, Cavnar MJ, Zeng S, et al. Imatinib potentiates antitumor T cell responses in gastrointestinal stromal tumor through the inhibition of Ido. Nat Med. 2011;17:1094–100. doi: 10.1038/nm.2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Boni A, Cogdill AP, Dang P, et al. Selective BRAFV600E inhibition enhances T-cell recognition of melanoma without affecting lymphocyte function. Cancer Res. 2010;70:5213–9. doi: 10.1158/0008-5472.CAN-10-0118. [DOI] [PubMed] [Google Scholar]
- 80.Comin-Anduix B, Chodon T, Sazegar H, et al. The oncogenic BRAF kinase inhibitor PLX4032/RG7204 does not affect the viability or function of human lymphocytes across a wide range of concentrations. Clin Cancer Res. 2010;16:6040–8. doi: 10.1158/1078-0432.CCR-10-1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Farsaci B, Sabzevari H, Higgins JP, et al. Effect of a small molecule BCL-2 inhibitor on immune function and use with a recombinant vaccine. Int J Cancer. 2010;127:1603–13. doi: 10.1002/ijc.25177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li Z, Qiao Y, Liu B, et al. Combination of imatinib mesylate with autologous leukocyte-derived heat shock protein and chronic myelogenous leukemia. Clin Cancer Res. 2005;11:4460–8. doi: 10.1158/1078-0432.CCR-05-0250. [DOI] [PubMed] [Google Scholar]
- 83.Smith BD, Kasamon YL, Kowalski J, et al. K562/GM-CSF immunotherapy reduces tumor burden in chronic myeloid leukemia patients with residual disease on imatinib mesylate. Clin Cancer Res. 2010;16:338–47. doi: 10.1158/1078-0432.CCR-09-2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Morse M, Niedzwiecki D, Marshall JL, et al. Survival rates among patients vaccinated following resection of colorectal cancer metastases in a phase II randomized study compared with contemporary controls. J Clin Oncol. 2011;29:abs 3557. [Google Scholar]
- 85.Uyl-de Groot CA, Vermorken JB, Hanna MG, Jr, et al. Immunotherapy with autologous tumor cell-BCG vaccine in patients with colon cancer: a prospective study of medical and economic benefits. Vaccine. 2005;23:2379–87. doi: 10.1016/j.vaccine.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 86.Vaccinogen Clinical Trials: Proof of Efficacy. [cited October 2011]; Available from: http://www.vaccinogeninc.com/vaccinogen/clinical-trials/
- 87.Vansteenkiste J, Zielinski M, Linder A, et al. Final results of a multi-center, double-blind, randomized, placebo-controlled phase II study to assess the efficacy of MAGE-A3 immunotherapeutic as adjuvant therapy in stage IB/II non-small cell lung cancer (NSCLC) J Clin Oncol. 2007;25(18S):abs 7554. [Google Scholar]
- 88.Kantoff PW, Schuetz TJ, Blumenstein BA, et al. Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol. 2010;28:1099–105. doi: 10.1200/JCO.2009.25.0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gulley JL, Madan RA, Schlom J. Impact of tumour volume on the potential efficacy of therapeutic vaccines. Curr Oncol. 2011;18:e150–7. doi: 10.3747/co.v18i3.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Klein E, Falzarano S, Maddala T, et al. Use of quantitative gene expression in primary and highest Gleason pattern cancers to identify genes associated with clinical recurrence after radical prostatectomy. J Clin Oncol. 2011;29(7S):abs 39. [Google Scholar]
- 91.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Madan RA, Gulley JL, Fojo T, Dahut WL. Therapeutic cancer vaccines in prostate cancer: the paradox of improved survival without changes in time to progression. Oncologist. 2010;15:969–75. doi: 10.1634/theoncologist.2010-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Stein WD, Gulley JL, Schlom J, et al. Tumor regression and growth rates determined in five intramural NCI prostate cancer trials: the growth rate constant as an indicator of therapeutic efficacy. Clin Cancer Res. 2011;17:907–17. doi: 10.1158/1078-0432.CCR-10-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Conry RM, Khazaeli MB, Saleh MN, et al. Phase I trial of a recombinant vaccinia virus encoding carcinoembryonic antigen in metastatic adenocarcinoma: comparison of intradermal versus subcutaneous administration. Clin Cancer Res. 1999;5:2330–7. [PubMed] [Google Scholar]
- 95.Marshall JL, Hawkins MJ, Tsang KY, et al. Phase I study in cancer patients of a replication-defective avipox recombinant vaccine that expresses human carcinoembryonic antigen. J Clin Oncol. 1999;17:332–7. doi: 10.1200/JCO.1999.17.1.332. [DOI] [PubMed] [Google Scholar]
- 96.von Mehren M, Arlen P, Gulley J, et al. The influence of granulocyte macrophage colony-stimulating factor and prior chemotherapy on the immunological response to a vaccine (ALVAC-CEA B7. 1) in patients with metastatic carcinoma. Clin Cancer Res. 2001;7:1181–91. [PubMed] [Google Scholar]
- 97.Schuetz T, Kaufman H, JL M, Safran H. Extended survival in second-line pancreatic cancer after therapeutic vaccination. J Clin Oncol. 2005;23(16S):abs 2576. [Google Scholar]
- 98.Therion’s Panvac-VF Misses Endpoint In Pancreatic Cancer. [cited October 2011]; Available from: http://www.allbusiness.com/pharmaceuticals-biotechnology/pharmaceutical/13399572-1.html.
- 99.Vaccine Therapy and GM-CSF in Treating Patients With Locally Advanced or Metastatic Pancreatic Cancer That Cannot Be Removed By Surgery. [cited October 2011]; Available from: http://clinicaltrials.gov/ct2/show/NCT00669734.
- 100.Madan RA, Dahut W, Figg WD, et al. Comparing the overall survival of metastatic castration resistant prostate cancer patients treated with docetaxel, a vaccine admixed with one costimulatory molecule, and a vaccine with three costimulatory molecules. ASCO Genitourinary Cancers Symposium; 2009. p. abs 210. [Google Scholar]
- 101.Heery C, Pinto P, Schlom J, et al. Intraprostatic PSA-TRICOM vaccine administration in patients with locally recurrent prostate cancer. J Clin Oncol. 2011;29:abs 2530. [Google Scholar]
- 102.DiPaola RS, Chen Y, Bubley G, et al. A phase II study of PROSTVAC-V (vaccinia)/TRICOM and PROSTVAC-F (fowlpox)/TRICOM with GM-CSF in patients with PSA progression after local therapy for prostate cancer: Results of ECOG 9802. ASCO Genitourinary Cancers Symposium; 2009. p. abs 108. [Google Scholar]