Abstract
Poplars (Populus tremula × Populus alba) were transformed to overexpress Escherichia coli γ-glutamylcysteine synthetase (γ-ECS) or glutathione synthetase in the chloroplast. Five independent lines of each transformant strongly expressed the introduced gene and possessed markedly enhanced activity of the gene product. Glutathione (GSH) contents were unaffected by high chloroplastic glutathione synthetase activity. Enhanced chloroplastic γ-ECS activity markedly increased γ-glutamylcysteine and GSH levels. These effects are similar to those previously observed in poplars overexpressing these enzymes in the cytosol. Similar to cytosolic γ-ECS overexpression, chloroplastic overexpression did not deplete foliar cysteine or methionine pools and did not lead to morphological changes. Light was required for maximal accumulation of GSH in poplars overexpressing γ-ECS in the chloroplast. High chloroplastic, but not cytosolic, γ-ECS activities were accompanied by increases in amino acids synthesized in the chloroplast. We conclude that (a) GSH synthesis can occur in the chloroplast and the cytosol and may be up-regulated in both compartments by increased γ-ECS activity, (b) interactions between GSH synthesis and the pathways supplying the necessary substrates are similar in both compartments, and (c) chloroplastic up-regulation of GSH synthesis is associated with an activating effect on the synthesis of specific amino acids formed in the chloroplast.
The predominant low-molecular-mass peptide in many cells is the tripeptide glutathione (γ-Glu-Cys-Gly). Existing in reduced (GSH) and oxidized (GSSG) forms, glutathione plays a crucial role in controlling and maintaining the intracellular redox state (for review, see Noctor and Foyer, 1998a). Efficient regulation of the glutathione pool is thought to be particularly important in chloroplast metabolism, in which it provides the redox-buffering capacity vital for efficient photosynthesis and is involved in processing the oxidizing species that are inevitably formed as a result of light capture and subsequent electron transport events (Foyer and Halliwell, 1976; Kunert and Foyer, 1993). The size and redox state of the glutathione pool change in response to various environmental factors, including low temperature (Esterbauer and Grill, 1978; Wise and Naylor, 1987; Walker and McKersie, 1993), atmospheric pollutants (Madamanchi and Alscher, 1991; Sen Gupta et al., 1991; Ranieri et al., 1993), light intensity (Buwalda et al., 1990; Grace and Logan, 1996; Noctor et al., 1997a), herbicides (Foyer et al., 1995), and heavy metals (Scheller et al., 1987; Rüegsegger et al., 1990; Rüegsegger and Brunold, 1992; Schneider and Bergmann, 1995). Only comparatively recently, however, has attention been focused on the biochemical mechanisms through which these external factors exert their effects on glutathione content.
GSH is synthesized from Glu, Cys, and Gly in two ATP-dependent steps catalyzed by γ-ECS and GS. Apart from localization studies with tobacco cells, in which γ-ECS and GS activities were detected in chloroplastic and extrachloroplastic fractions (Hell and Bergmann, 1988, 1990), little is known about the intracellular compartmentalization of GSH biosynthesis. The recent cloning of the genes for GS and γ-ECS from Arabidopsis (May and Leaver, 1994; Rawlins et al., 1995; Ullmann et al., 1996) has yielded little information regarding the likelihood of compartment-specific isoforms, although a putative transit peptide sequence is present in the γ-ECS cDNA clones (May and Leaver, 1994).
It was first shown for the animal enzyme that the activity of γ-ECS is inhibited by GSH in vitro (Richman and Meister, 1975). Subsequently, γ-ECS from tobacco was also shown to be sensitive to GSH, and several authors considered this inhibition to be a feedback control mechanism governing GSH levels in planta (Hell and Bergmann, 1990; Sen Gupta et al., 1991; Schneider and Bergmann, 1995). Other studies, however, have demonstrated correlations between GSH levels and the extractable activity of γ-ECS or GS (Rüegsegger et al., 1990; Rüegsegger and Brunold, 1992; Chen and Goldsbrough, 1994), implicating enzyme synthesis/turnover in the regulation of GSH synthesis. The importance of the amount of γ-ECS in determining GSH content is supported by work with an Arabidopsis mutant possessing decreased γ-ECS activity (Cobbett et al., 1998).
In addition to these putative controls, it is clear that GSH levels also respond to the availability of amino acid substrates, particularly Cys and, in some circumstances, Gly (Buwalda et al., 1990; Strohm et al., 1995; Noctor et al., 1996, 1997b). The influence of Cys availability on GSH levels reflects the importance of GSH as a reservoir of reduced sulfur (Buwalda et al., 1990) and as the principal form in which organic sulfur is transported in many plants (Bergmann and Rennenberg, 1993). Evidence has also been presented that GSH homeostatically regulates sulfur nutrition through its effects on sulfate uptake and assimilation (Herschbach and Rennenberg, 1994; Lappartient and Touraine, 1996).
The techniques of plant transformation offer a powerful tool with which to perturb and elucidate interactions between metabolic processes. The availability of cDNAs for γ-ECS and GS from Escherichia coli (Gushima et al., 1984; Watanabe et al., 1986) provided us with a means through which the capacity for GSH synthesis might be enhanced in planta to explore such interactions and to obtain plants with constitutively increased GSH titers. Overproduction of GSH is of interest for several reasons: (a) increased levels of antioxidants may contribute to the physiological robustness of plants (Noctor and Foyer, 1998a); (b) GSH represents a convenient model for peptide manufacture by biological systems; and (c) industrial interest in high-yield biological production of GSH is fueled by its anticarcinogenic properties (Jones et al., 1992), its potential as a flavor enhancer (Ho et al., 1992), and by the high cost of its chemical synthesis.
Our previous studies involved overexpression of bacterial γ-ECS or GS in the poplar (Populus tremula × Populus alba) cytosol (Foyer et al., 1995; Strohm et al., 1995; Noctor et al., 1996, 1997a, 1997b; Arisi et al., 1997). Potential theoretical obstacles to the overproduction of GSH through overexpression of these enzymes are evident from a consideration of the complex network of regulatory mechanisms discussed above (end-product feedback control, the necessity of sufficient availability of substrates, and the homeostatic influence of GSH on sulfate assimilation). In apparent support of this notion, strong overexpression of GS in the poplar cytosol failed to induce increased GSH levels, even though synthetic capacity was shown to be enhanced (Foyer et al., 1995; Strohm et al., 1995). However, in contrast to GS, overexpression of γ-ECS led to marked increases in foliar GSH, suggesting that increased activity of this enzyme in the poplar cytosol is able to overcome the homeostatic restrictions on GSH synthesis (Noctor et al., 1996; Arisi et al., 1997). Because GSH plays important roles in chloroplast metabolism, and because the synthetic enzymes have been detected in chloroplastic fractions, we investigated the effect of targeting the bacterial γ-ECS and GS to the poplar chloroplast.
MATERIALS AND METHODS
Plant Material and Growth
Transformed and untransformed hydrid poplars (Populus tremula × Populus alba; Institut National de la Recherche Agronomique no. 717–1-B4, Versailles, France) were introduced into the greenhouse following micropropagation in vitro. Apart from material for chloroplast isolation, for which the youngest opened leaves were used, all analyses were conducted using laminar leaf material taken from mature leaves between the 6th and 11th positions from the apex of plants grown for 3 months in the greenhouse (plants 1.0–1.5 m tall).
Gene Cloning and Plant Transformation
For transformation of poplar to overexpress the Escherichia coli γ-ECS in the chloroplast, the SstI/BamHI fragment containing the gshI-coding sequence (1.7 kb; Watanabe et al., 1986) with the original start codon TTG changed to ATG (Noctor et al., 1996) was inserted into the SphI/BamHI sites of the plasmid pJIT 117 (Guerineau et al., 1988), which contains the cauliflower mosaic virus 35S promoter with a double-enhancer sequence (P70), the pea rbcS sequence coding for the chloroplast transit peptide of the rbcS (L), and a cauliflower mosaic virus poly(A) sequence. To create a translational fusion of the gshI-coding sequence with the rbcS sequence coding for the transit peptide, a synthetic oligonucleotide of 17 bases was inserted upstream of the gshI-coding sequence between the SphI and SstI sites of the created plasmid. The translational fusion obtained was 5′-TGC-ATG-CTT-GGA-CCG-CGC-GAG-CTC-GGT-ACG-GAG-GTC-AAT-ATG. The KpnI fragment comprising P70-L-gshI-poly(A) was cloned into the binary vector pBIN19 (Bevan, 1984) to create p70LgshI.
To overexpress the E. coli GS in the chloroplast, the MslI/BamHI fragment containing the E. coli-coding sequence (gshII, 1.1 kb; Gushima et al., 1984) was inserted into the SphI/BamHI sites of the plasmid pJIT 117 (Guerineau et al., 1988). To create a translational fusion of the gshII-coding sequence with the rbcS sequence coding for the transit peptide, a synthetic oligonucleotide of 25 bases was inserted upstream of the gshII-coding sequence between the SphI and MslI sites of the created plasmid. The translational fusion obtained was 5′-TGC-ATG-CCT-ATA-ATG-ATC-AAG-CTC-GGC-ATC-GTG-ATG-GAC. The SstI/XhoI fragment comprising P70-L-gshII-poly(A) was cloned into the SstI/SalI sites of the binary vector pBIN19 (Bevan, 1984) to create p70LgshII.
The binary vectors were introduced into the disarmed Agrobacterium tumefaciens strain C58 pMP90 (Koncz and Schell, 1986) by triparental mating, and transformation of poplar was carried out as described by Leplé et al. (1992). RNA gel blots and immunoblots were performed as described by Arisi et al. (1997).
Chloroplast Isolation
Approximately 15 g of finely chopped, young leaf tissue from poplars predarkened for 20 h were ground in 200 mL of semifrozen isolation medium (0.35 m sorbitol, 50 mm Mops-KOH, pH 7.4, 2.5 mm MgCl2, 1.25 mm EDTA, 3 mm DTE, and 0.3% soluble PVP). The homogenate was filtered through two layers of muslin and then four layers of muslin containing a layer of cotton wool, to give the unfractionated chloroplast filtrate. Aliquots were retained for immunoblots. The remainder was centrifuged at 4500g for 60 s and the pellet was resuspended in resuspension medium (0.35 m sorbitol, 50 mm Mops-KOH, pH 7.6, 4 mm MgCl2, 2 mm EDTA, 3 mm DTE, and 0.3% soluble PVP). The suspension was loaded on top of 10 mL of resuspension medium containing 30% (v/v) Percoll. Chloroplasts were pelleted by centrifugation at 2500g for 5 min and resuspended in resuspension medium without DTE and PVP. Immunoblots of chloroplast fractions were compared with extracts made as described for enzyme assays (see “Enzyme Assays”). These were prepared from samples taken from the same leaf material used for chloroplast extraction immediately prior to chloroplast isolation.
Enzyme Assays
Leaf extractions were performed by grinding 20 to 50 mg of leaf lamina in liquid nitrogen and then in ice-cold 0.1 m Tris-HCl, pH 8.0, 10 mm MgCl2, and 1 mm EDTA. Enzyme activities were assayed in the supernatant obtained by centrifugation at 10,000g for 15 min at 4°C or in chloroplast fractions. γ-ECS and GS activities were measured at 30°C as the rate of γ-EC or GSH formation, as previously described in detail (Arisi et al., 1997). Both compounds were quantified fluorimetrically as their monobromobimane derivatives following separation by HPLC. GR activity was measured at A340 and 25°C as the rate of NADPH oxidation in the presence of leaf extracts and GSSG, according to the method of Foyer and Halliwell (1976). Enzyme activities were undetectable in the absence of extract or any of the substrates. Soluble protein was measured in centrifuged leaf extracts or chloroplast fractions using the standard Coomassie blue dye technique (Bio-Rad) (Bradford, 1976).
Analysis of Thiols and Amino Acids
Foliar thiols were determined fluorimetrically following derivatization with monobromobimane and separation by HPLC, as described in detail by Noctor and Foyer (1998b). Simultaneous measurement of amino acids, γ-EC, and GSH was carried out by fluorimetric analysis following pre-column derivatization with o-phthalaldehyde and separation by HPLC (Noctor and Foyer, 1998b). These two methods give similar values of foliar contents of γ-EC and GSH (Noctor and Foyer, 1998b).
RESULTS
Poplar was transformed with constructs containing the E. coli genes for γ-ECS or GS fused to the peptide sequence for the rbcS transit peptide (Fig. 1). The effects of these transformations were compared with those already reported, in which the bacterial enzymes were overexpressed in the cytosol (Foyer et al., 1995; Strohm et al., 1995; Noctor et al., 1996; Arisi et al., 1997). In addition to the three ggs transformants previously obtained (ggs5, ggs11, and ggs28; Arisi et al., 1997), we obtained another ggs transformant (ggs7) with enzyme activities and foliar thiol contents similar to the lines previously produced. Six gshI (Watanabe et al., 1986) and five gshII (Gushima et al., 1984; Fig. 1) transformants were obtained.
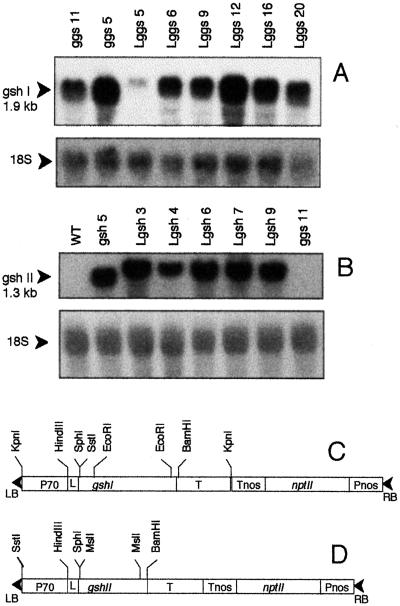
Figure 1.
RNA gel blots of total leaf RNA prepared from Lggs and Lgsh, which were transformed using the T-DNA constructs shown below the blots. A, Lggs. The probe was an EcoRI internal, 1.4-kb fragment of the gshI-coding sequence. All lanes were loaded with 20 μg of total foliar RNA. The 18S probe corresponded to a 0.5-kb fragment of a cDNA for the 18S radish rRNA. B, Lgsh. The probe was an MslI internal, 0.9-kb fragment of the gshII-coding sequence. RNA loaded and loading control are as in A. WT, Wild type. C, T-DNA construct for overexpression of γ-ECS in the chloroplast. P70, Cauliflower mosaic virus 35S promoter with double-enhancer sequence; L, pea rbcS sequence coding for the transit peptide; T, cauliflower mosaic virus poly(A) sequence; LB, left border; RB, right border; nos, nopaline synthase; nptII, neomycin phosphotransferase. D, T-DNA construct for overexpression of GS in the chloroplast. Other abbreviations are as in C.
Southern analysis following digestion of genomic DNA using two different restriction enzymes showed that these lines contained between one and four copies of the introduced gene (data not shown). Northern analysis demonstrated that, like ggs11 and ggs5, five of the six Lggs lines strongly expressed the introduced gene, whereas one chloroplastic line (Lggs5) expressed the bacterial gene more weakly (Fig. 1A). All five Lgsh lines showed strong expression of the introduced construct (Fig. 1B). mRNA expression was comparable to that of gsh (Fig. 1B, compare Lgsh transformants and gsh5).
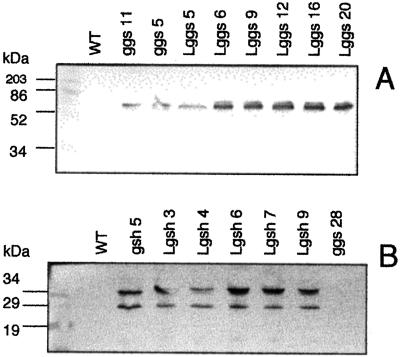
Antibodies produced against the bacterial proteins (Arisi et al., 1997) enabled immunoblots of leaf extracts from the transformed poplars (Fig. 2). Five Lggs transformants showed an intense band at 58 kD, the predicted size of the E. coli enzyme (Watanabe et al., 1986). Lggs5 showed a less intense band, similar to ggs5 and ggs11 (Fig. 2A). These differences were in agreement with extractable foliar activities of γ-ECS (Fig. 3C). It is interesting that Lggs5 had amounts of γ-ECS protein (Fig. 2A) and extractable γ-ECS activity (Fig. 3C) similar to ggs11 and ggs5, and yet it had significantly weaker expression of the bacterial gene (Fig. 1A). One explanation could be greater stability of the bacterial protein in the chloroplast than in the cytosol, as was previously observed for poplars overexpressing GR (Foyer et al., 1995).
Figure 2.
Immunoblot analysis of extracts of total soluble leaf protein from Lggs and Lgsh. All lanes were loaded with 20 μg of total soluble leaf protein. A, Rabbit antiserum against the E. coli γ-ECS (leaf extracts resolved on a 12% gel). B, Rabbit antiserum against the E. coli GS (leaf extracts resolved on a 20% gel). WT, Wild type.
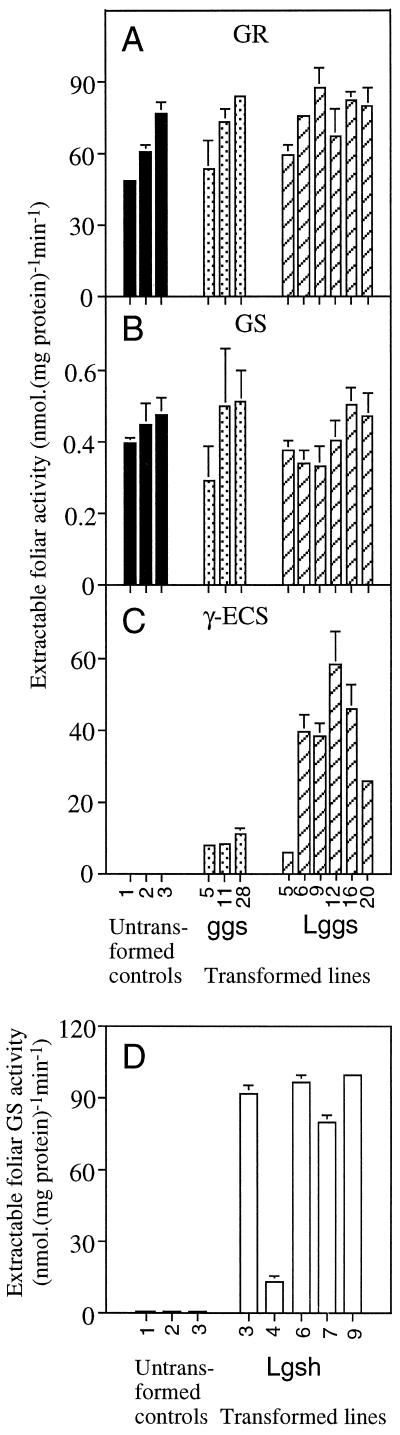
Figure 3.
Extractable activities of glutathione-associated enzymes in leaves from untransformed poplars and poplars transformed to overexpress the enzymes of glutathione synthesis. Data are means ± sd of three independent leaf extractions. Absent error bars are too insignificant to be visible. Absent columns indicate undetected activity. A, GR activity in untransformed poplars (black columns) and ggs (dotted columns) or Lggs (hatched columns). Numbers along the abscissa refer to different plants (untransformed) or plants of different transformed lines (ggs, Lggs). B, GS activity in the same plants as in A. C, γ-ECS activity in the same plants as in A. D, GS activity in untransformed poplars (three different plants) and Lgsh (five different transformed lines). GS activities in untransformed controls were 0.16 ± 0.01 (1), 0.21 ± 0.03 (2), and 0.15 ± 0.01 (3) nmol mg−1 protein min−1.
Immunoblots of leaf extracts from GS transformants detected two bands in both gsh and Lgsh (Fig. 2B; compare Arisi et al., 1997). The band of higher molecular mass corresponds to the predicted size of the bacterial GS subunit (35.6 kD; Gushima et al., 1984). The presence of these bands in Lgsh extracts correlated with markedly enhanced extractable GS activities in Lgsh (Fig. 3D). The degree of enhancement of GS activity was between 60-fold (Lgsh4) and 500-fold (Lgsh9), which is similar to that produced by cytosolic overexpression of GS (Foyer et al., 1995; Strohm et al., 1995). The extent of the increase in γ-ECS activity in poplars overexpressing γ-ECS cannot be directly calculated, because, as previously reported, γ-ECS activity is below the level of detection in untransformed poplars (Fig. 3C; Noctor et al., 1996; Arisi et al., 1997).
Because data for untransformed plants suggest that γ-ECS is rarely in excess of GS activity (Hell and Bergmann, 1990; Chen and Goldsbrough, 1994; Schneider and Bergmann, 1995), we can estimate a minimum increase in γ-ECS activity by comparison with GS activities in the same plants (Fig. 3, compare B and C). This means that Lggs possessed γ-ECS activities that were enhanced between 12-fold (Lggs5) and 150-fold (Lggs12). It should be noted that the increased γ-ECS activity in Lggs was specific: no associated changes in the extractable activities of either GS (Fig. 3B) or GR (Fig. 3A) were observed.
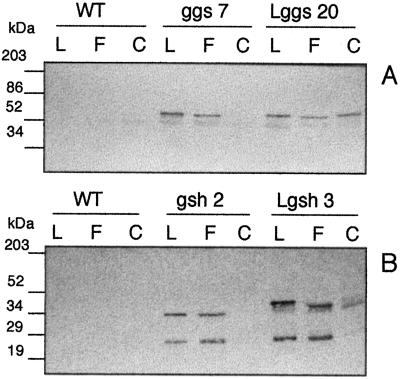
The chloroplastic localization of the bacterial gene products was checked by immunoblotting of isolated chloroplast fractions. Poplars overexpressing the respective enzymes in the cytosol were used as controls. A pronounced band observed in chloroplasts prepared from Lggs20 was absent from chloroplasts isolated from ggs7 (Fig. 4A). For Lgsh3 the band of higher molecular mass corresponding to the bacterial subunit was found in the chloroplast fraction (Fig. 4B). The band of lower molecular mass in leaf extracts from gsh and Lgsh was not located in the chloroplast (Fig. 4B; Lgsh3, lanes F and C). Neither band was detected in chloroplasts from gsh2 (Fig. 4B).
Figure 4.
Confirmation of the intracellular location of the bacterial gene products in ggs, Lggs, gsh, and Lgsh. Lanes L, Leaf extract prepared as for assay of extractable foliar enzyme activities; lanes F, chloroplast filtrate prior to centrifugation; lanes C, resuspended chloroplast pellet. WT, Untransformed (wild-type) poplar. Gels were 12% acrylamide. All lanes were loaded with 5 μg of total soluble protein. A, Immunoblot using antiserum raised against the E. coli γ-ECS. B, Immunoblot using antiserum raised against the E. coli GS.
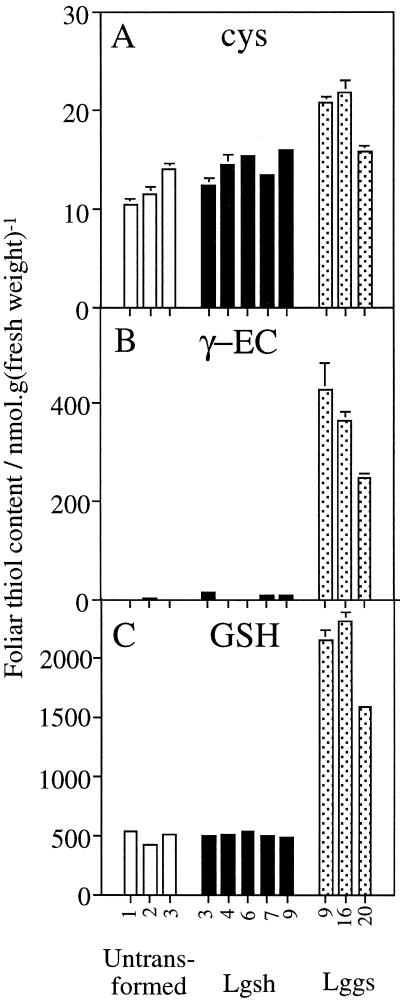
Lgsh and untransformed poplars had similar foliar contents of Cys, γ-EC, and GSH (Fig. 5). In contrast, up to 4-fold higher GSH contents were observed in Lggs (Fig. 5C). These poplars also showed very marked elevations in foliar γ-EC content, which reached levels far in excess of those observed in untransformed poplars (Fig. 5B). All Lggs lines except Lggs5, which overexpressed γ-ECS most weakly (Fig. 3C), possessed these enhanced thiol contents (see below). Increased contents of γ-EC and GSH did not cause decreased foliar Cys levels (Fig. 5A) and were not accompanied by changes in the reduction state of the glutathione pool, which in terms of GSH equivalents was approximately 90% GSH and 10% GSSG in all poplar lines (data not shown). Likewise, no change was observed in the size or reduction state of the foliar ascorbate pool (Noctor et al., 1998).
Figure 5.
Foliar glutathione content is enhanced by chloroplastic overexpression of γ-ECS but not GS. Data are means ± sd of three independent leaf extractions. Data were obtained from material sampled simultaneously. Absent error bars are too insignificant to be visible. Absent columns indicate thiol below the level of detection. Numbers beneath the graph refer to different untransformed plants or different transformed lines. A, Cys content. B, γ-EC content. C, GSH content.
Foliar thiol contents show both seasonal and diurnal changes in poplar leaves (Noctor et al., 1997a; see below). Comparisons between untransformed and transformed plants were therefore always carried out using material taken at the same time from illuminated leaves at equivalent positions on plants of comparable age. Numerous direct comparisons of this type showed that the increased thiol contents observed in Figure 5 were stable and consistent: seven separate experiments conducted throughout the year using five different Lggs lines gave a mean ± sd relative increase in GSH content of 3.10 ± 0.75 (n = 21 values, each derived from division of the mean values [n = at least three independent leaf extractions] for Lggs lines by those for untransformed poplars). Overexpression of γ-ECS in the cytosol brought about a mean ± sd relative increase in GSH of 2.81 ± 0.84 (n = 21 values, each derived from division of the mean values [n = at least three independent leaf extractions] for four ggs lines by those for untransformed poplars in 13 separate experiments conducted throughout a 2-year period).
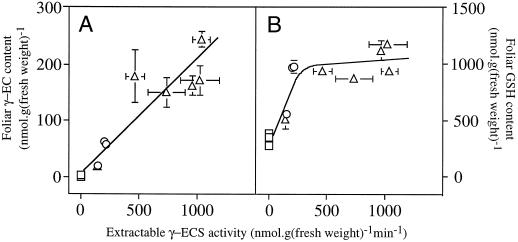
Figure 6 shows the results of one experiment in which γ-EC and GSH contents were compared with extractable γ-ECS activity in untransformed control poplars and γ-ECS transformants using material sampled from equivalent leaves on the same day. Thiol contents and γ-ECS activity were determined using two halves of the same leaf and both are expressed relative to fresh weight of leaf material (Fig. 6). An apparent ceiling effect was observed for GSH content (Fig. 6B), whereas γ-EC content showed a relatively linear dependence on γ-ECS activity (Fig. 6A).
Figure 6.
Relationships between foliar thiol contents and extractable γ-ECS activity. Data were obtained from material sampled simultaneously from illuminated leaves for thiol determinations or enzyme assays. □, Untransformed poplars; ○, ggs; ▵, Lggs. Each point is the mean ± sd of three separate extractions of a different untransformed plant or plant of a different transformed line. Absent error bars are contained within the symbol. A, Foliar γ-EC content. B, Foliar GSH content.
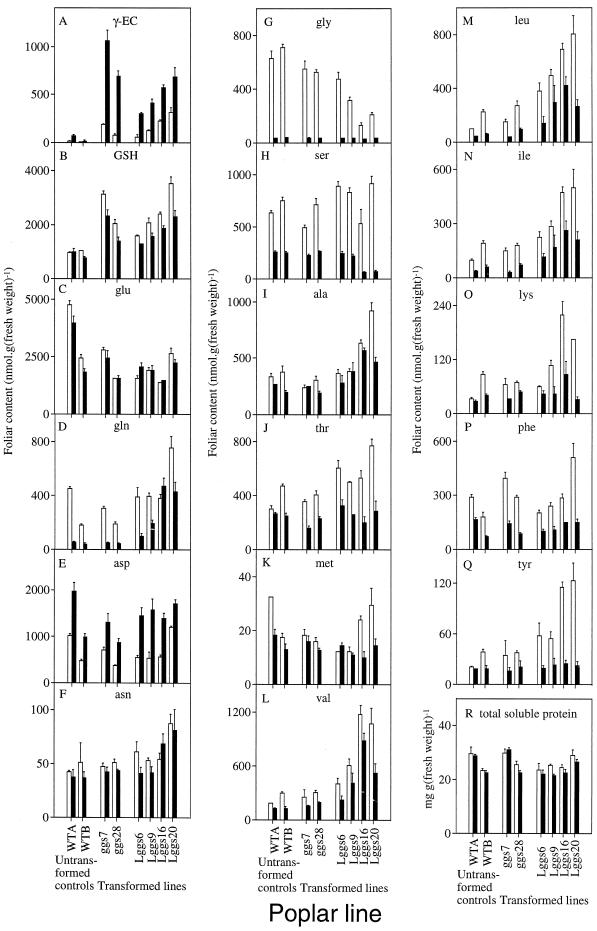
The pronounced increases in foliar thiols in the transformants overexpressing γ-ECS were not associated with visible effects on growth or morphology (Fig. 7). To investigate interactions between the enhanced capacity for GSH synthesis in ggs and Lggs poplars and other products of nitrogen assimilation, foliar GSH, γ-EC, and free amino acids were simultaneously measured in extracts from darkened and illuminated leaves. In all lines the most abundant amino acid was Glu (Fig. 8C). In Lggs as in ggs, GSH content was higher in the light and γ-EC was higher in the dark (Fig. 8, A and B). Foliar γ-EC content was also higher in the dark in untransformed poplars, but GSH content was less affected by light than in the transformants (Fig. 8, A and B). With the exception of Asp (Fig. 8E), amino acid contents were generally higher in the light (Fig. 8). The most marked light-induced increases were observed in the foliar pools of Gln (apart from Lggs16), Gly, and Ser (Fig. 8, D, G, and H). The chloroplastic transformants showed enhanced foliar contents of Val (Fig. 8L), Leu (Fig. 8M), and Ile (Fig. 8N). These increases were particularly evident in illuminated leaves from Lggs16 and Lggs20, which also had relatively high contents of Lys (Fig. 8O) and Tyr (Fig. 8Q). Total soluble leaf protein was not markedly affected by light in any of the plants (Fig. 8R).
Figure 7.
Photograph showing that ggs and Lggs are morphologically indistinguishable from untransformed poplars.
Figure 8.
Analysis of foliar contents of GSH, γ-EC, and free amino acids in illuminated and darkened leaves from untransformed poplars and from ggs and Lggs. The eighth leaf from the apex was excised from each of two untransformed (wild-type) poplars (WTA and WTB), two ggs lines (ggs7 and ggs28), and four Lggs lines (Lggs6, Lggs9, Lggs16, and Lggs20). Leaves were illuminated for 150 min (350–500 μmol m−2 s−1 at 23°C) with their cut petioles in deionized water and triplicate samples taken for analysis (open columns). The light was turned off and “dark” samples were taken following 300 min of darkness at 20°C (filled columns). All data are means ± sd of three independent leaf extractions. Absent error bars are too insignificant to be visible. Similar results were obtained in two independent experiments. His, Trp, and Pro were not measured. Small quantities of Arg were detected in all extracts but could not be quantified correctly. A, γ-EC content; B, GSH content; C, Glu content; D, Gln content; E, Asp content; F, Asn content; G, Gly content; H, Ser content; I, Ala content; J, Thr content; K, Met content; L, Val content; M, Leu content; N, Ile content; O, Lys content; P, Phe content; Q, Tyr content; R, Total soluble protein content.
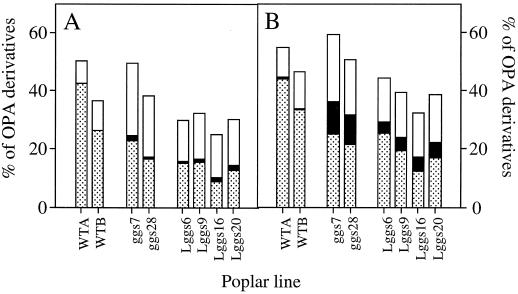
Figure 9 shows how increased γ-ECS activity in poplar leaves affects the distribution of amino acids between the most abundant free amino acid, Glu, and the pools of GSH and γ-EC. Untransformed poplars contained approximately 3 to 5 times more Glu than GSH on a molar basis (Fig. 9). This ratio was not appreciably changed by illumination (Fig. 9, compare A and B). In contrast, in ggs and Lggs, the summed pools of γ-EC and GSH exceeded Glu content on a molar basis (Fig. 9). This relationship was independent of illumination, even though the relative quantities of γ-EC and GSH were modified by light. The decrease in the proportion of amino acids accounted for by Glu in the γ-ECS overexpressors reflected the increases in γ-EC and GSH but, in illuminated leaves from the Lggs, was also partly due to enhancement of other amino acid pools: the percentage of acid-soluble primary amines accounted for by γ-EC, GSH, and Glu together was decreased from 40% to 50% to approximately 30% in leaves from Lggs in the light (Fig. 9A).
Figure 9.
Glutathione and γ-EC account for a high proportion of acid-soluble primary amines in ggs and Lggs. WTA and WTB, Untransformed (wild-type) controls. Dotted sections, Percentages of OPA-reactive compounds present as Glu; black sections, percentages of OPA-reactive compounds present as γ-EC; white sections, percentages of OPA-reactive compounds present as glutathione. All proportions were calculated on a molar basis. Data are the means of three leaf extractions. A, Illuminated leaves, conditions as described for Figure 8. B, Darkened leaves, conditions as described for Figure 8.
DISCUSSION
This work demonstrates that glutathione can be synthesized in both chloroplastic and cytosolic compartments and thus confirms data relating to the localization of γ-ECS and GS in cultured tobacco cells (Hell and Bergmann, 1988, 1990). In either location, enhanced γ-ECS activity increased foliar γ-EC contents and, consequently, foliar GSH contents. GR activity in poplar leaves is evidently sufficient to accommodate large increases in the capacity for GSH synthesis in both the chloroplast and cytosol, since the GSH:GSSG ratios are maintained when γ-ECS is overexpressed in either compartment (discussed further in Noctor et al., 1998).
The plasticity of the foliar content of amino acids has been revealed by studies overexpressing enzymes involved in amino acid synthesis (for review, see Temple and Sengupta-Gopalan, 1997). In tobacco overexpression of Asn synthetase resulted in marked increases in tissue Asn contents (Brears et al., 1993), whereas introduction of E. coli genes encoding dihydropicolinate synthase (Shaul and Galili, 1992a) or Asp kinase (Shaul and Galili, 1992b) led to enhanced contents of Lys and Thr, respectively. In the present study, increased GSH was the result of an even larger relative increase in γ-EC, which was elevated from the ranks of a low-level intermediate to a major cellular component in darkened leaves of ggs or Lggs. Nevertheless, no visible phenotypic effects were observed (Fig. 7). Likewise, measurements of growth and photosynthesis failed to find any evidence of resulting physiological disruption (G. Noctor, A.-C.M. Arisi, L. Jouanin, and C.H. Foyer, unpublished results) underlying the flexibility of plant metabolism.
The redox-active functions of GSH are linked to the Cys residue, which is maintained in the sulfhydryl form by GR. Analogous oxidoreductases that are able to use γ-EC have not been reported in plants, although such an enzyme is found in halobacteria, which synthesize γ-EC but not GSH (Newton and Javor, 1985). There are recent indications that γ-EC may be able to substitute for some of the antioxidant functions of GSH in yeast, although no evidence of a γ-EC oxidoreductase activity has been presented (Grant et al., 1997).
Although considerable attention has been paid to the significance of GSH in sulfur nutrition (Buwalda et al., 1990; Bergmann and Rennenberg, 1993; Herschbach and Rennenberg, 1994; Lappartient and Touraine, 1996), little consideration has been given to the interactions between GSH synthesis and nitrogen assimilation in plants. In yeast GSH functions as a readily mobilized store of organic nitrogen and may constitute up to 1% of cellular dry weight (Mehdi and Penninckx, 1997). In poplars overexpressing γ-ECS, maximum glutathione contents of 3 μmol g−1 fresh weight (Fig. 8) convert to approximately 0.4% of foliar dry weight, given a relative water content of 0.75 (Noctor et al., 1996). In ggs7, GSH accounted for about 25% of acid-soluble primary amines in leaf extracts (Fig. 9). Because no major OPA-reactive compounds other than those shown in Figure 8 were detected in poplar leaves, this means that 50% of acid-soluble amino acid equivalents were bound up in glutathione, compared with 20% to 25% of nonprotein amino acid equivalents in untransformed poplars, in which glutathione accounted for 8% to 10% of acid-soluble primary amines (Fig. 9).
In terms of absolute contents, glutathione was just as abundant in Lggs as in ggs (Fig. 8), but the lower proportion of primary amines accounted for by GSH, γ-EC, and Glu in the chloroplastic overexpressors (Fig. 9) reflects a general increase in amino acids such as Val, Leu, Ile, Lys, and Tyr, all of which are present in relatively low amounts in untransformed poplars (Fig. 8). Val, Ile, and Leu constitute the “branched chain” synthetic family of amino acids (Singh and Shaner, 1995), whereas Tyr and Phe are synthesized by the shikimate pathway (Hermann, 1995). Foliar contents of Lys and, to a lesser degree, Thr were also relatively high in Lggs. Available data suggest that the synthetic pathways of all of these amino acids are localized predominantly, if not exclusively, in the chloroplast (Shaul and Galili, 1992a; Hermann, 1995; Singh and Shaner, 1995). Therefore, it is interesting that enhancement of these amino acid pools was specific to chloroplastic overexpression of γ-ECS and that it appeared to occur in almost direct proportion to γ-EC and GSH contents (Fig. 8).
Transgenic introduction of enzymes into plants can lead to diminished levels of reaction substrates (Brears et al., 1993; Chavadej et al., 1994), sometimes entailing severe phenotypic effects (Fray et al., 1995). In contrast, overexpression of γ-ECS up-regulates GSH synthesis without leading to substrate depletion. We previously reported that Cys pools are unchanged or even enhanced in ggs (Noctor et al., 1996; Arisi et al., 1997). In the present study neither Cys (Fig. 5) nor Met (Fig. 8) levels were diminished in Lggs. Presumably, sulfur assimilation and Cys synthesis are adjusted to provide the Cys required for GSH synthesis without compromising sulfur-containing amino acid pools. The increased use of reduced sulfur would seem to be in conflict with the homeostatic effects reported from other studies, in which GSH translocated in the phloem inhibited sulfur uptake and assimilation at the root level (Herschbach and Rennenberg, 1994; Lappartient and Touraine, 1996). Apparently, an elevated capacity for GSH synthesis in the chloroplast or cytosol can influence metabolically upstream events to satisfy the increased substrate demand. Whether this effect has physiological significance is unclear. It is, however, interesting to note that decreased γ-ECS activity in an Arabidopsis mutant is associated with elevated Cys content (Cobbett et al., 1998). Taken together with the present data, this may implicate γ-ECS activity as important in regulating reduced sulfur status and cellular Cys levels.
As previously reported for ggs (Noctor et al., 1997a, 1997b), the enhanced chloroplastic capacity for γ-EC synthesis reveals that a light-associated factor is required for efficient conversion of γ-EC to GSH (Fig. 8). The accumulation of γ-EC in the dark is linked to low levels of Gly (Fig. 8). Other experiments revealed that the Gly used for GSH synthesis is of photorespiratory origin in both Lggs and ggs (discussed further in Noctor et al., 1998). Thus, like GSH synthesis in the cytosol, chloroplastic GSH synthesis also uses Gly produced in the peroxisomes following the oxygenation of ribulose 1,5-bisphosphate in the chloroplast.
The enhanced thiol contents in the Lggs transformants demonstrate that, as for cytosolic γ-ECS overexpression, feedback control of GSH synthesis in the chloroplast can be overcome by high γ-ECS activity. The increased foliar GSH contents do not reflect the true increase in synthetic capacity brought about by chloroplastic overexpression, as shown by the proportionally larger increases in γ-EC (Fig. 5). This is reflected in the data of Figure 6, which suggest that GSH contents reach a ceiling above which increases in γ-ECS activity have no effect. The simplest explanation for this observation is that, when γ-ECS activity is increased, GSH synthesis becomes limited by GS activity. This appears to be the case for GSH synthesis both in the chloroplast and in the cytosol and is supported by the data of Table I, which show how overexpression of γ-ECS affects the substrate-to-product ratios for the two reactions of GSH formation.
Table I.
Effect of overexpression of γ-ECS in the chloroplast or cytosol on measured product to substrate ratios for synthesis of γ-EC (reaction 1) and glutathione (reaction 2)
| Reaction | Untransformed | ggs | Lggs |
|---|---|---|---|
| 1. γ-EC/(Glu × Cys)a | |||
| Light | 0.7 ± 0.2 | 18.7 ± 5.1 | 60.7 ± 19.9 |
| Dark | 6.6 ± 5.0 | 162.8 ± 46.4 | 112.5 ± 26.3 |
| 2. GSH/(γ-EC × Gly)b | |||
| Light | 6.4 ± 4.9 | 0.6 ± 0.2 | 0.7 ± 0.1 |
| Dark | 6.8 ± 4.3 | 0.6 ± 0.1 | 1.0 ± 0.3 |
Data shown are actual values ×104 and are means ± sd of four mean values obtained in two independent experiments. Original mean values were obtained from three independent leaf extractions. Plant material was four separate plants (untransformed), two plants each of ggs7 and ggs28 and two plants each of Lggs9 and Lggs20. Light values were obtained for leaves illuminated at 300 to 450 μmol m−2 s−1 for 2.5 h; dark values were obtained for leaves darkened for 5 h.
Data shown are actual values ×10. Plant material, number of extractions, and illumination regime were as for reaction 1, except that data for Lggs are means of six separate plants (two each of Lggs9 and Lggs20 and one of Lggs6 and Lggs16).
Overexpression of γ-ECS increases the product-to-substrate ratio for the first reaction (γ-EC synthesis) between about 26-fold (ggs) and 90-fold (Lggs), suggesting that increased γ-ECS activity allows γ-EC synthesis to come closer to thermodynamic equilibrium. Even though this effect leads to increased GSH contents, it also causes the product-to-substrate ratio for the second reaction to decrease about 10-fold in both types of transformant (Table I). Because changes in Gly are accounted for in the calculated ratio for the second reaction, this effect presumably reflects limitations of GSH synthesis by GS activity (Table I). Indeed, it is noteworthy that the ratio for the second reaction is independent of light, i.e. in all poplar types, changes in the GSH-to-γ-EC ratio are offset by changes in Gly content (Table I).
The failure to induce higher GSH contents through chloroplastic GS overexpression presumably reflects limitation of the second reaction by γ-EC availability, similar to cytosolic GS overexpression (Strohm et al., 1995). This limitation, however, is removed by enhanced γ-ECS activity, and the data of Table I indicate that introduction of both enzymes together would lead to even greater increases in foliar GSH contents than those produced by γ-ECS overexpression alone. Furthermore, even if GS does not significantly restrict GSH synthesis in untransformed poplars, this activity appears not to be present in great excess. This is underlined by comparison of the present data with those obtained with tobacco overexpressing dihydropicolinate synthase, the first enzyme of a multistep pathway leading to Lys (Shaul and Galili, 1992a). In the latter study, chloroplastic overexpression of the E. coli enzyme enhanced the final product, Lys, by up to 15-fold (Shaul and Galili, 1992a). In the current study, chloroplastic overexpression of E. coli γ-ECS, the first enzyme of a two-step synthetic pathway, resulted in maximal increases in the final product (GSH) of only about 4-fold in the light (Figs. 5, 6, and 8).
Other studies of overexpressing enzymes involved in amino acid synthesis have allowed assignment of specific biosynthetic sequences to specific organelles (e.g. chloroplastic location of Lys and Thr synthesis; Shaul and Galili, 1992a, 1992b). In contrast, it is noteworthy that chloroplastic overexpression of γ-ECS brings about similar changes in GSH content to overexpression of the enzyme in the poplar cytosol, demonstrating the intracellular flexibility of GSH synthesis. The exact subcellular location of the increased amounts of GSH synthesized in the two types of transformant is difficult to ascertain, notably because of the problems of GSH exchange between compartments during subcellular fractionation (Klapheck et al., 1987).
Transport of GSH between the chloroplast and cytosol would at least partly explain the similarity of the effects of overexpression of γ-ECS in the two compartments. Nothing is known regarding transport of either γ-EC or GSH across the chloroplast envelope. However, our observation that GSH synthesis can be up-regulated in both the cytosol and the chloroplast indicates that intercompartmental transport of GSH is not essential to cellular function. Definitive localization studies are hampered in poplar by the difficulty of obtaining protoplasts or chloroplasts of high yield and intactness (G. Noctor, A.-C.M. Arisi, L. Jouanin, and C.H. Foyer, unpublished results). Nevertheless, GS activity in untransformed poplars is distributed equally between the chloroplast and the remainder of the leaf cell (G. Noctor, A.-C.M. Arisi, L. Jouanin, and C.H. Foyer, unpublished results).
Further information relevant to the fate of GSH synthesized in the chloroplast and cytosol will be provided by an examination of GSH export from the leaf in the Lggs transformants. In ggs the increased GSH contents are systemic and the transformants export considerably higher amounts of GSH in the phloem (Arisi, 1997; Herschbach et al., 1998). Whether GSH export from the leaf is also increased in Lggs or whether chloroplastically produced GSH is preferentially retained at the site of production is under investigation.
CONCLUSIONS
Chloroplastic overexpression of enzymes catalyzing the synthesis of GSH shows that (a) foliar GSH levels are unchanged by markedly enhanced chloroplastic GS activities but are elevated by chloroplastic overexpression of γ-ECS; (b) the enhancement of chloroplastic γ-ECS activity produces similar constitutive increases in foliar GSH levels to cytosolic overexpression of the enzyme; (c) maximal up-regulation of chloroplastic GSH synthesis requires the light-dependent production of Gly; (d) the increased GSH contents in Lggs do not cause detriment to plant growth or depletion of Cys contents; and (e) high γ-ECS activities in the chloroplast, but not the cytosol, are associated with increased levels of specific amino acids synthesized in the chloroplast. The data confirm that GSH can be synthesized in both the cytosol and the chloroplast and suggest that interactions among GSH synthesis, Cys synthesis, and photorespiration operate in similar ways in the two compartments.
ACKNOWLEDGMENT
We thank Akira Suzuki for generously making HPLC facilities available.
Abbreviations:
- DTE
dithioerythritol
- γ-EC
γ-glutamylcysteine
- γ-ECS
γ-glutamylcysteine synthetase
- ggs
poplar lines overexpressing γ-ECS in the cytosol
- GR
glutathione reductase
- GS
glutathione synthetase
- gsh
poplar lines overexpressing GS in the cytosol
- gshI
gene sequence for the Escherichia coli γ-ECS
- gshII
gene sequence for the Escherichia coli GS
- GSSG
glutathione disulfide
- Lggs
poplar lines overexpressing γ-ECS in the chloroplast
- Lgsh
poplar lines overexpressing GS in the chloroplast
- OPA
o-phthalaldehyde
- rbcS
Rubisco small subunit
Footnotes
A.-C.M.A. was the recipient of a fellowship from Coordenaçao de Aperfeiçoamento de Pessoal de Ensino Superior, Ministry of Education, Brazil.
LITERATURE CITED
- Arisi ACM (1997) Tolérance au stress de peupliers transformés surexprimant la superoxyde dismutase, la γ-glutamylcystéine synthétase ou la glutathion synthétase. PhD thesis. University of Paris XI, France
- Arisi ACM, Noctor G, Foyer C, Jouanin L. Modifications of thiol contents in poplars (Populus tremula × P. alba) overexpressing enzymes involved in glutathione synthesis. Planta. 1997;203:362–372. doi: 10.1007/s004250050202. [DOI] [PubMed] [Google Scholar]
- Bergmann L, Rennenberg H (1993) Glutathione metabolism in plants. In LJ De Kok, I Stulen, H Rennenberg, C Brunold, WE Rauser, eds, Sulfur Nutrition and Assimilation in Higher Plants. Regulatory, Agricultural and Environmental Aspects. SPB Academic Publishers, The Hague, The Netherlands, pp 102–123
- Bevan M. Binary Agrobacterium vectors for plant transformation. Nucleic Acids Res. 1984;12:8711–8721. doi: 10.1093/nar/12.22.8711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brears T, Liu C, Knight TJ, Coruzzi GM. Ectopic overexpression of asparagine synthetase in transgenic tobacco. Plant Physiol. 1993;103:1285–1290. doi: 10.1104/pp.103.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buwalda F, Stulen I, De Kok LJ, Kuiper PJC. Cysteine, γ-glutamylcysteine and glutathione contents of spinach leaves as affected by darkness and application of excess sulfur. II. Glutathione accumulation in detached leaves exposed to H2S in the absence of light is stimulated by the supply of glycine to the petiole. Physiol Plant. 1990;80:196–204. [Google Scholar]
- Chavadej S, Brisson N, McNeil JN, De Luca V. Redirection of tryptophan leads to production of low indole glucosinolate canola. Proc Natl Acad Sci USA. 1994;91:2166–2170. doi: 10.1073/pnas.91.6.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Goldsbrough PB. Increased activity of γ-glutamylcysteine synthetase in tomato cells selected for cadmium tolerance. Plant Physiol. 1994;106:233–239. doi: 10.1104/pp.106.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbett CS, May MJ, Howden R, Rolls B (1998) The glutathione-deficient, cadmium-sensitive mutant, cad2–1, of Arabidopsis thaliana is deficient in γ-glutamylcysteine synthetase. EMBO J (in press) [DOI] [PubMed]
- Esterbauer H, Grill D. Seasonal variation of glutathione and glutathione reductase in needles of Picea abies L. Plant Physiol. 1978;61:119–121. doi: 10.1104/pp.61.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer C, Halliwell B. The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta. 1976;133:21–25. doi: 10.1007/BF00386001. [DOI] [PubMed] [Google Scholar]
- Foyer CH, Souriau N, Perret S, Lelandais M, Kunert KJ, Pruvost C, Jouanin L. Overexpression of glutathione reductase but not glutathione synthetase leads to increases in antioxidant capacity and resistance to photoinhibition in poplar trees. Plant Physiol. 1995;109:1047–1057. doi: 10.1104/pp.109.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fray RG, Wallace A, Fraser PD, Valero D, Hedden P, Bramley PD, Grierson D. Constitutive expression of a fruit phytoene synthase gene in transgenic tomatoes causes dwarfism by redirecting metabolites from the gibberellin pathway. Plant J. 1995;8:693–701. [Google Scholar]
- Grace SC, Logan BA. Acclimation of foliar antioxidant systems to growth irradiance in three broad-leaved evergreen species. Plant Physiol. 1996;112:1631–1640. doi: 10.1104/pp.112.4.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant CM, MacIver FH, Dawes IW. Glutathione synthetase is dispensable for growth under both normal and oxidative stress conditions in the yeast Saccharomyces cerevisiae due to an accumulation of the dipeptide γ-glutamylcysteine. Mol Biol Cell. 1997;8:1699–1707. doi: 10.1091/mbc.8.9.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerineau F, Woolston S, Brooks L, Mullineaux PM. An expression cassette for targeting foreign genes into chloroplasts. Nucleic Acids Res. 1988;16:11380. doi: 10.1093/nar/16.23.11380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gushima H, Yasda S, Soeda E, Yokata M, Kondo M, Kimura A. Complete nucleotide sequence of the E. coli glutathione synthetase gsh-II. Nucleic Acids Res. 1984;12:9299–9307. doi: 10.1093/nar/12.24.9299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hell R, Bergmann L. Glutathione synthetase in tobacco suspension cultures: catalytic properties and localization. Physiol Plant. 1988;72:70–76. [Google Scholar]
- Hell R, Bergmann L. γ-Glutamylcysteine synthetase in higher plants: catalytic properties and subcellular localization. Planta. 1990;180:603–612. doi: 10.1007/BF02411460. [DOI] [PubMed] [Google Scholar]
- Hermann KM. The shikimate pathway: early steps in the biosynthesis of aromatic compounds. Plant Cell. 1995;7:907–919. doi: 10.1105/tpc.7.7.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herschbach C, Jouanin L, Rennenberg H. Overexpression of γ-glutamylcysteine synthetase, but not of glutathione synthetase, elevates glutathione allocation in the phloem of transgenic poplar (Populus tremula × P. alba) trees. Plant Cell Physiol. 1998;39:447–451. [Google Scholar]
- Herschbach C, Rennenberg H. Influence of glutathione (GSH) on net uptake of sulphate and sulphate transport in tobacco plants. J Exp Bot. 1994;45:1069–1076. [Google Scholar]
- Ho CT, Oh YC, Zhang Y, Shu CK (1992) Peptides as flavor precursors in model maillard reactions. In R Teranishi, GR Takeoka, M Güntert, eds. Flavor Precursors. Thermal and Enzymatic Conversions. American Chemical Society, Washington DC, pp 193–203
- Jones DP, Coates RJ, Flagg EW, Eley JW, Block G, Greenberg RS, Gunter EW, Jackson B. Glutathione in foods listed in the National Cancer Institute's health habits and history food frequency questionnaire. Nutr Cancer. 1992;17:57–75. doi: 10.1080/01635589209514173. [DOI] [PubMed] [Google Scholar]
- Klapheck S, Latus C, Bergmann L. Localization of glutathione synthetase and distribution of glutathione in leaf cells of Pisum sativum L. J Plant Physiol. 1987;131:123–131. [Google Scholar]
- Koncz C, Schell J. The promoter of the TL-DNA gene 5 controls the tissue-specific expression of chimaeric genes carried by a novel type of Agrobacterium primary vector. Mol Gen Genet. 1986;204:383–396. [Google Scholar]
- Kunert KJ, Foyer CH (1993) Thiol/disulfide exchange in plants. In LJ De Kok, I Stulen, H Rennenberg, C Brunold, WE Rauser, eds, Sulfur nutrition and assimilation in higher plants. Regulatory, agricultural and environmental aspects. SPB Academic Publishers, The Hague, The Netherlands, pp 139–151
- Lappartient AG, Touraine B. Demand-driven control of root ATP sulfurylase activity and sulfate uptake in intact canola. Plant Physiol. 1996;111:147–157. doi: 10.1104/pp.111.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leplé JC, Brasiliero ACM, Michel MF, Delmotte F, Jouanin L. Transformed poplars: expression of chimaeric genes using four different constructs. Plant Cell Rep. 1992;11:137–141. doi: 10.1007/BF00232166. [DOI] [PubMed] [Google Scholar]
- Madamanchi NR, Alscher RG. Metabolic bases for differences in sensitivity of two pea cultivars to sulfur dioxide. Plant Physiol. 1991;97:88–93. doi: 10.1104/pp.97.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May MJ, Leaver CJ. Arabidopsis thaliana γ-glutamylcysteine synthetase is structurally unrelated to mammalian, yeast and Escherichia coli homologs. Proc Natl Acad Sci USA. 1994;91:10059–10063. doi: 10.1073/pnas.91.21.10059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehdi K, Penninckx MJ. Microbiology. 1997;143:1885–1889. doi: 10.1099/00221287-143-6-1885. [DOI] [PubMed] [Google Scholar]
- Newton GL, Javor B. γ-Glutamylcysteine and thiosulfate are the major low-molecular-weight thiols in halobacteria. J Bacteriol. 1985;161:438–441. doi: 10.1128/jb.161.1.438-441.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor G, Arisi ACM, Jouanin L, Valadier MH, Roux Y, Foyer CH. Light-dependent modulation of foliar glutathione synthesis and associated amino acid metabolism in transformed poplar. Planta. 1997a;202:357–369. [Google Scholar]
- Noctor G, Arisi ACM, Jouanin L, Valadier MH, Roux Y, Foyer CH. The role of glycine in determining the rate of glutathione synthesis in poplars. Possible implications for glutathione production during stress. Physiol Plant. 1997b;100:255–263. [Google Scholar]
- Noctor G, Arisi ACM, Jouanin L, Kunert KJ, Rennenberg H, Foyer CH. Glutathione: biosynthesis, metabolism and relationship to stress tolerance explored in transformed plants. J Exp Bot. 1998;49:623–647. [Google Scholar]
- Noctor G, Foyer CH. Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol. 1998a;49:249–279. doi: 10.1146/annurev.arplant.49.1.249. [DOI] [PubMed] [Google Scholar]
- Noctor G, Foyer CH (1998b) Simultaneous measurement of foliar glutathione, γ-glutamylcysteine and amino acids by high-performance liquid chromatography: comparison with two other assay methods for glutathione. Anal Biochem (in press) [DOI] [PubMed]
- Noctor G, Strohm M, Jouanin L, Kunert KJ, Foyer CH, Rennenberg H. Synthesis of glutathione in leaves of transgenic poplar (Populus tremula × P. alba) overexpressing γ-glutamylcysteine synthetase. Plant Physiol. 1996;112:1071–1078. doi: 10.1104/pp.112.3.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranieri A, Lencioni L, Schenone G, Soldatini GF. Glutathione-ascorbic acid cycle in pumpkin plants grown under polluted air in open-top chambers. J Plant Physiol. 1993;142:286–290. [Google Scholar]
- Rawlins MR, Leaver CJ, May MJ. Characterisation of an Arabidopsis thaliana cDNA encoding glutathione synthetase. FEBS Lett. 1995;376:81–86. doi: 10.1016/0014-5793(95)01253-1. [DOI] [PubMed] [Google Scholar]
- Richman PG, Meister A. Regulation of γ-glutamylcysteine synthetase by nonallosteric feedback inhibition by glutathione. J Biol Chem. 1975;250:1422–1426. [PubMed] [Google Scholar]
- Rüegsegger A, Brunold C. Effect of cadmium on γ-glutamylcysteine synthesis in maize seedlings. Plant Physiol. 1992;99:428–433. doi: 10.1104/pp.99.2.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüegsegger A, Schmutz D, Brunold C. Regulation of glutathione synthesis by cadmium in Pisum sativum L. Plant Physiol. 1990;93:1579–1584. doi: 10.1104/pp.93.4.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheller HV, Huang B, Hatch E, Goldsbrough PB. Phytochelatin synthesis and glutathione levels in response to heavy metals in tomato cells. Plant Physiol. 1987;85:1031–1035. doi: 10.1104/pp.85.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider S, Bergmann L. Regulation of glutathione synthesis in suspension cultures of parsley and tobacco. Bot Acta. 1995;108:34–40. [Google Scholar]
- Sen Gupta A, Alscher RG, McCune D. Response of photosynthesis and cellular antioxidants to ozone in Populus leaves. Plant Physiol. 1991;96:650–655. doi: 10.1104/pp.96.2.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaul O, Galili G. Increased lysine synthesis in tobacco plants that express high levels of bacterial dihydropicolinate synthase in their chloroplasts. Plant J. 1992a;2:203–209. [Google Scholar]
- Shaul O, Galili G. Plant Physiol. 1992b;100:1157–1163. doi: 10.1104/pp.100.3.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh BK, Shaner DL. Biosynthesis of branched chain amino acids: from test tube to field. Plant Cell. 1995;7:935–944. doi: 10.1105/tpc.7.7.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strohm M, Jouanin L, Kunert KJ, Pruvost C, Polle A, Foyer CH, Rennenberg H. Regulation of glutathione synthesis in leaves of transgenic poplar (Populus tremula × P. alba) overexpressing glutathione synthetase. Plant J. 1995;7:141–145. [Google Scholar]
- Temple SJ, Sengupta-Gopalan C (1997) Manipulating amino acid biosynthesis. In CH Foyer, WP Quick, eds, A Molecular Approach to Primary Metabolism in Higher Plants. Taylor and Francis, London, UK, pp 155–177
- Ullman P, Gondet L, Potier S, Bach TJ. Cloning of Arabidopsis thaliana glutathione synthetase (GSH2) by functional complementation of a yeast gsh2 mutant. Eur J Biochem. 1996;236:662–669. doi: 10.1111/j.1432-1033.1996.00662.x. [DOI] [PubMed] [Google Scholar]
- Walker MA, McKersie BD. Role of the ascorbate-glutathione antioxidant system in chilling resistance of tomato. J Plant Physiol. 1993;141:234–239. [Google Scholar]
- Watanabe K, Yamano Y, Murata K, Kimura A. Nucleic Acids Res. 1986;14:4393–4400. doi: 10.1093/nar/14.11.4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RR, Naylor AW. Chilling-enhanced photooxidation. Evidence for the role of singlet oxygen and superoxide in the breakdown of pigments and endogenous antioxidants. Plant Physiol. 1987;83:278–282. doi: 10.1104/pp.83.2.278. [DOI] [PMC free article] [PubMed] [Google Scholar]