Abstract
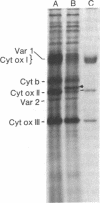
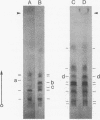
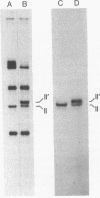
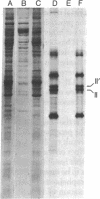
Many of the polypeptides made on endogenous ribosomes inside of yeast mitochondria are hydrophobic "integral polypeptides" which are subunits of at least three oligomeric enzyme complexes (cytochrome c oxidase, rutamycin-sensitive ATPase, and coenzyme QH2-cytochrome c reductase) of the inner mitochondrial membrane. In order to elucidate the pathway(s) followed by these polypeptides into the inner membrane we have used an in vitro mitochondrial translation system from yeast. By inhibiting this system with aurintricarboxylic acid, we have been able to demonstrate and accumulate a transient precursor to subunit II of cytochrome c oxidase. This precursor, designated II', is approximately 1,500 daltons larger than mature subunit II and most likely is a form of subunit II with an NH2-terminal extension. Although this precursor appears to be processed cotranslationally under normal conditions, it does associate in unprocessed form with mitochondrial membranes when allowed to accumulate in the presence of aurintricarboxylic acid, and it can be processed postranslationally upon removal of the drug. None of the other mitochondrial translation products made in this system exhibits larger precursors. These results indicate that at least one mitochondrial translation product has a transient "leader sequence" a,d is inserted into the inner mitochondrial membrane and processed cotranslationally, but they suggest that other pathways may be followed by the other translation products.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgese N., Mok W., Kreibich G., Sabatini D. D. Ribosomal-membrane interaction: in vitro binding of ribosomes to microsomal membranes. J Mol Biol. 1974 Sep 25;88(3):559–580. doi: 10.1016/0022-2836(74)90408-2. [DOI] [PubMed] [Google Scholar]
- Borst P., Grivell L. A. The mitochondrial genome of yeast. Cell. 1978 Nov;15(3):705–723. doi: 10.1016/0092-8674(78)90257-x. [DOI] [PubMed] [Google Scholar]
- Bretscher M. S. Membrane structure: some general principles. Science. 1973 Aug 17;181(4100):622–629. doi: 10.1126/science.181.4100.622. [DOI] [PubMed] [Google Scholar]
- Chyn T. L., Martonosi A. N., Morimoto T., Sabatini D. D. In vitro synthesis of the Ca2+ transport ATPase by ribosomes bound to sarcoplasmic reticulum membranes. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1241–1245. doi: 10.1073/pnas.76.3.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePierre J. W., Ernster L. Enzyme topology of intracellular membranes. Annu Rev Biochem. 1977;46:201–262. doi: 10.1146/annurev.bi.46.070177.001221. [DOI] [PubMed] [Google Scholar]
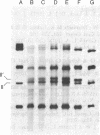
- Douglas M. G., Butow R. A. Variant forms of mitochondrial translation products in yeast: evidence for location of determinants on mitochondrial DNA. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1083–1086. doi: 10.1073/pnas.73.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder J. H., Pickett R. A., 2nd, Hampton J., Lerner R. A. Radioiodination of proteins in single polyacrylamide gel slices. Tryptic peptide analysis of all the major members of complex multicomponent systems using microgram quantities of total protein. J Biol Chem. 1977 Sep 25;252(18):6510–6515. [PubMed] [Google Scholar]
- Eytan G. D., Carroll R. C., Schatz G., Racker E. Arrangement of the subunits in solubilized and membrane-bound cytochrome c oxidase from bovine heart. J Biol Chem. 1975 Nov 25;250(22):8598–8603. [PubMed] [Google Scholar]
- Eytan G. D., Schatz G. Cytochrome c oxidase from bakers' yeast. V. Arrangement of the subunits in the isolated and membrane-bound enzyme. J Biol Chem. 1975 Jan 25;250(2):767–774. [PubMed] [Google Scholar]
- Feldman F., Mahler H. R. Mitochondrial biogenesis. Retention of terminal formylmethionine in membrane proteins and regulation of their synthesis. J Biol Chem. 1974 Jun 25;249(12):3702–3709. [PubMed] [Google Scholar]
- Goldberg I. H., Stewart M. L., Ayuso M., Kappen L. S. On the mechanisms of inhibition of polypeptide synthesis by the antibiotics sparsomycin and pactamycin. Fed Proc. 1973 Jun;32(6):1688–1697. [PubMed] [Google Scholar]
- Grollman A. P., Huang M. T. Inhibitors of protein synthesis in eukaryotes: tools in cell research. Fed Proc. 1973 Jun;32(6):1673–1678. [PubMed] [Google Scholar]
- Inouye H., Beckwith J. Synthesis and processing of an Escherichia coli alkaline phosphatase precursor in vitro. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1440–1444. doi: 10.1073/pnas.74.4.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K., Mandel G., Wickner W. Soluble precursor of an integral membrane protein: synthesis of procoat protein in Escherichia coli infected with bacteriophage M13. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1199–1203. doi: 10.1073/pnas.76.3.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katan M. B., Pool L., Groot G. S. The cytochrome bc1 complex of yeast mitochondria. Isolation and partial characterization of the cytochrome bc1 complex and cytochrome b. Eur J Biochem. 1976 May 17;65(1):95–105. doi: 10.1111/j.1432-1033.1976.tb10393.x. [DOI] [PubMed] [Google Scholar]
- Katz F. N., Rothman J. E., Lingappa V. R., Blobel G., Lodish H. F. Membrane assembly in vitro: synthesis, glycosylation, and asymmetric insertion of a transmembrane protein. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3278–3282. doi: 10.1073/pnas.74.8.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama Y., Luck D. J. Membrane-associated ribosomes in mitochondria of Neurospora crassa. J Cell Biol. 1973 Dec;59(3):776–784. doi: 10.1083/jcb.59.3.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler H. R., Dawidowicz K., Feldman F. Formate as a specific label for mitochondrial translational products. J Biol Chem. 1972 Nov 25;247(22):7439–7442. [PubMed] [Google Scholar]
- Moorman A. F., Van Ommen G. J., Grivell L. A. Transcription in yeast mitochondria: isolation and physical mapping of messenger RNAs for subunits of cytochrome c oxidase and ATPase. Mol Gen Genet. 1978 Mar 20;160(1):13–24. doi: 10.1007/BF00275114. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D., Gagnon J., Ericsson L. H., Walsh K. A. Precursor of egg white lysozyme. Amino acid sequence of an NH2-terminal extension. J Biol Chem. 1977 Sep 25;252(18):6386–6393. [PubMed] [Google Scholar]
- Poyton R. O. A versatile apparatus for polyacrylamide and agarose gel electrophoresis in plexiglas slab gel molds. Anal Biochem. 1978 Oct 15;90(2):624–632. doi: 10.1016/0003-2697(78)90155-0. [DOI] [PubMed] [Google Scholar]
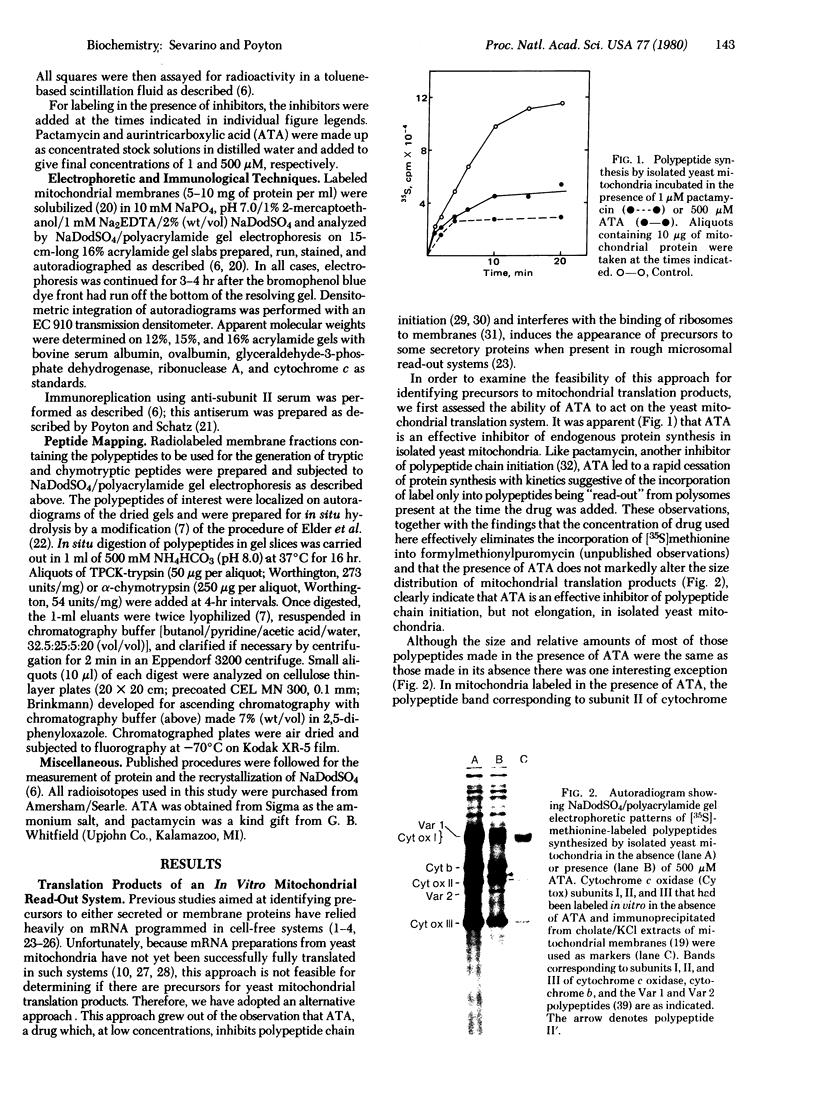
- Poyton R. O., Groot G. S. Biosynthesis of polypeptides of cytochrome c oxidase by isolated mitochondria. Proc Natl Acad Sci U S A. 1975 Jan;72(1):172–176. doi: 10.1073/pnas.72.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poyton R. O., Kavanagh J. Regulation of mitochondrial protein synthesis by cytoplasmic proteins. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3947–3951. doi: 10.1073/pnas.73.11.3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poyton R. O., McKemmie E. A polyprotein precursor to all four cytoplasmically translated subunits of cytochrome c oxidase from Saccharomyces cerevisiae. J Biol Chem. 1979 Jul 25;254(14):6763–6771. [PubMed] [Google Scholar]
- Poyton R. O., McKemmie E. Post-translational processing and transport of the polyprotein precursor to subunits IV to VII of yeast cytochrome c oxidase. J Biol Chem. 1979 Jul 25;254(14):6772–6780. [PubMed] [Google Scholar]
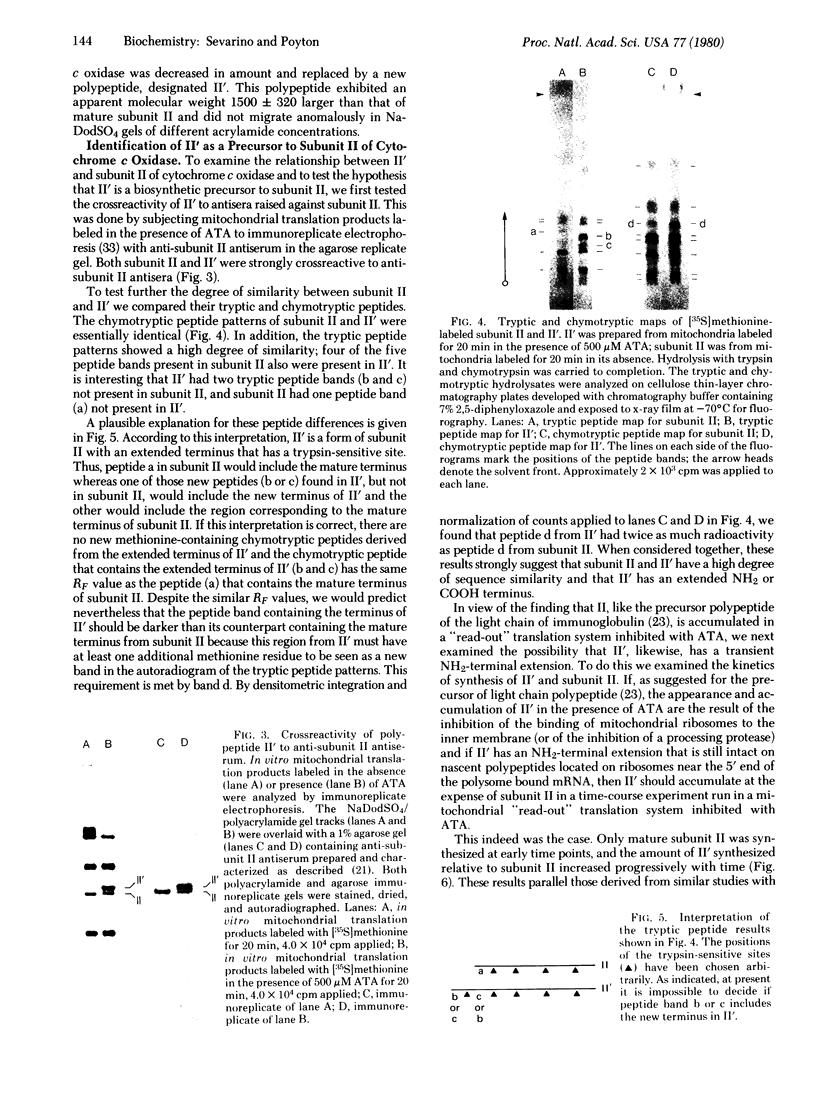
- Poyton R. O., Schatz G. Cytochrome c oxidase from bakers' yeast. III. Physical characterization of isolated subunits and chemical evidence for two different classes of polypeptides. J Biol Chem. 1975 Jan 25;250(2):752–761. [PubMed] [Google Scholar]
- Poyton R. O., Schatz G. Cytochrome c oxidase from bakers' yeast. IV. Immunological evidence for the participation of a mitochondrially synthesized subunit in enzymatic activity. J Biol Chem. 1975 Jan 25;250(2):762–766. [PubMed] [Google Scholar]
- Sebald W. Biogenesis of mitochondrial ATPase. Biochim Biophys Acta. 1977 Jun 21;463(1):1–27. doi: 10.1016/0304-4173(77)90002-7. [DOI] [PubMed] [Google Scholar]
- Showe M. K., Isobe E., Onorato L. Bacteriophage T4 prehead proteinase. II. Its cleavage from the product of gene 21 and regulation in phage-infected cells. J Mol Biol. 1976 Oct 15;107(1):55–69. doi: 10.1016/s0022-2836(76)80017-4. [DOI] [PubMed] [Google Scholar]
- Spithill T. W., Trembath M. K., Lukins H. B., Linnane A. W. Mutations of the mitochondrial DNA of Saccharomyces cerevisiae which affect the interaction between mitochondrial ribosomes and the inner mitochondrial membrane. Mol Gen Genet. 1978 Aug 17;164(2):155–162. doi: 10.1007/BF00267380. [DOI] [PubMed] [Google Scholar]
- Steffens G., Buse G. Studien an Cytochrom-c-Oxidase, I. Reinigung und Charakterisierung des Enzyms aus Rinderherzen und Identifizierung der im Komplex enthaltenen Peptidketten. Hoppe Seylers Z Physiol Chem. 1976 Aug;357(8):1125–1137. [PubMed] [Google Scholar]
- Strauss A. W., Bennett C. D., Donohue A. M., Rodkey J. A., Alberts A. W. Rat liver pre-proalbumin: complete amino acid sequence of the pre-piece. Analysis of the direct translation product of albumin messenger RNA. J Biol Chem. 1977 Oct 10;252(19):6846–6855. [PubMed] [Google Scholar]
- Toneguzzo F., Ghosh H. P. In vitro synthesis of vesicular stomatitis virus membrane glycoprotein and insertion into membranes. Proc Natl Acad Sci U S A. 1978 Feb;75(2):715–719. doi: 10.1073/pnas.75.2.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner W., Mandel G., Zwizinski C., Bates M., Killick T. Synthesis of phage M13 coat protein and its assembly into membranes in vitro. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1754–1758. doi: 10.1073/pnas.75.4.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]