Abstract
From the plasma membrane of Thermus thermophilus HB8 we have partially purified a detergent-solubilized complex of cytochromes a and c1 that actively catalyzes the transfer of electrons from ascorbate via a redox dye to oxygen. The complex is composed of two types of polypeptides, with molecular weights of approximately 55,000 and 33,000. Quantitative analysis revealed the presence of heme a, heme c, and copper in a ratio of 2:1:2, with the heme a being present at 10 +/- 1.3 nmol/mg of protein. The heme c was shown to be associated with the molecular weight 33,000 peptide and is suggested to be of the c1 type. The optical and electron paramagnetic resonance properties of this complex were found to be similar to those of eukaryotic cytochrome oxidase, suggesting the following arrangement of chromophores: a magnetically isolated cytochrome c1 and an oxygen-reducing functional unit consisting of two heme a groups and two copper ions associated with one or more larger peptides.
Full text
PDF
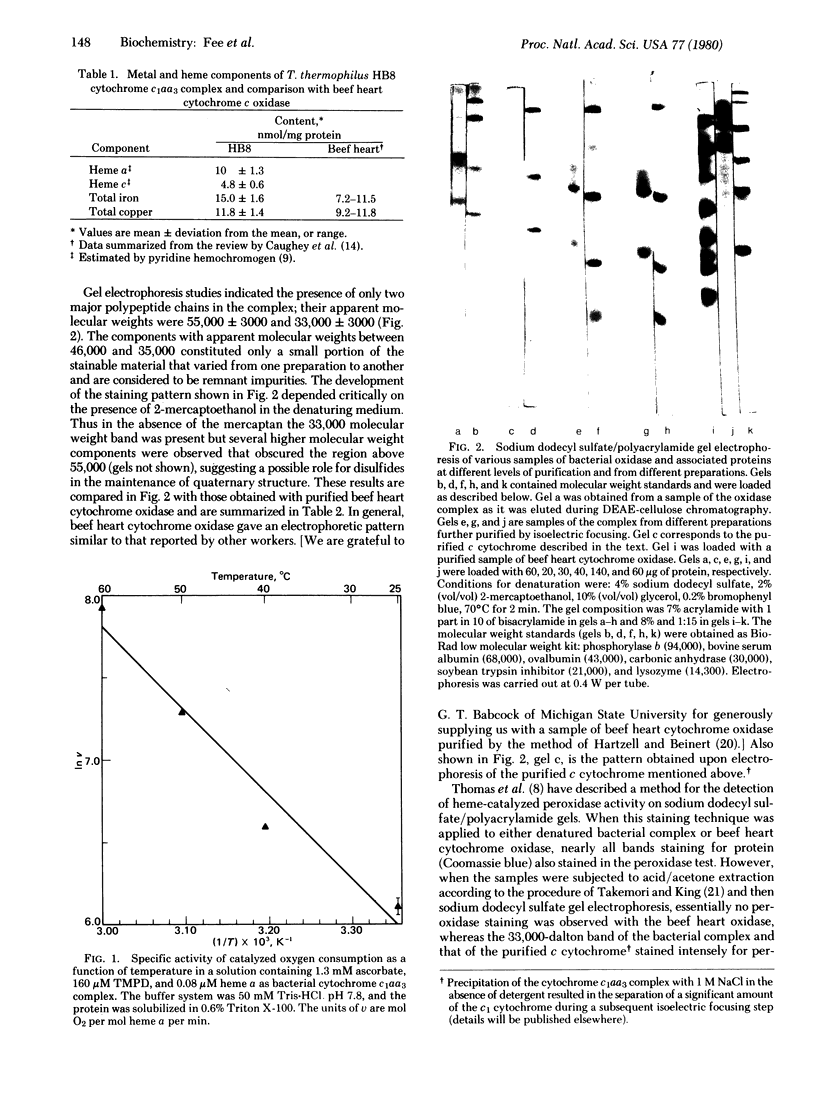
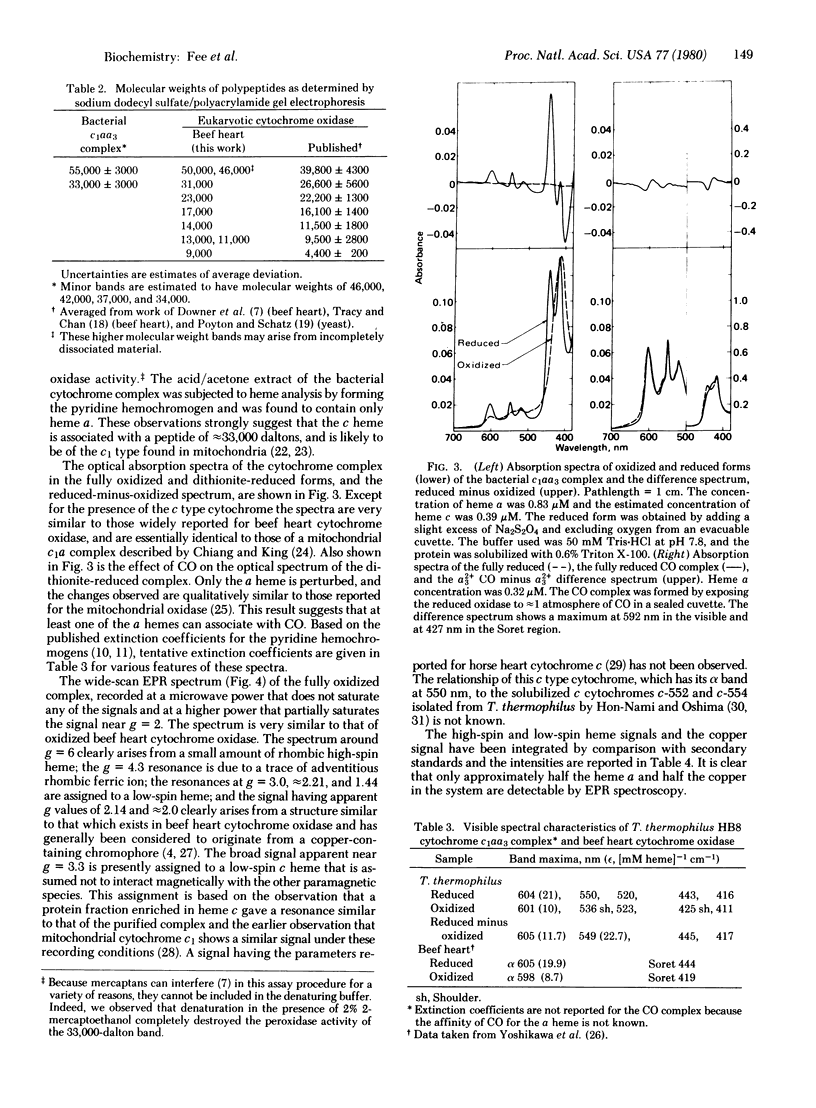
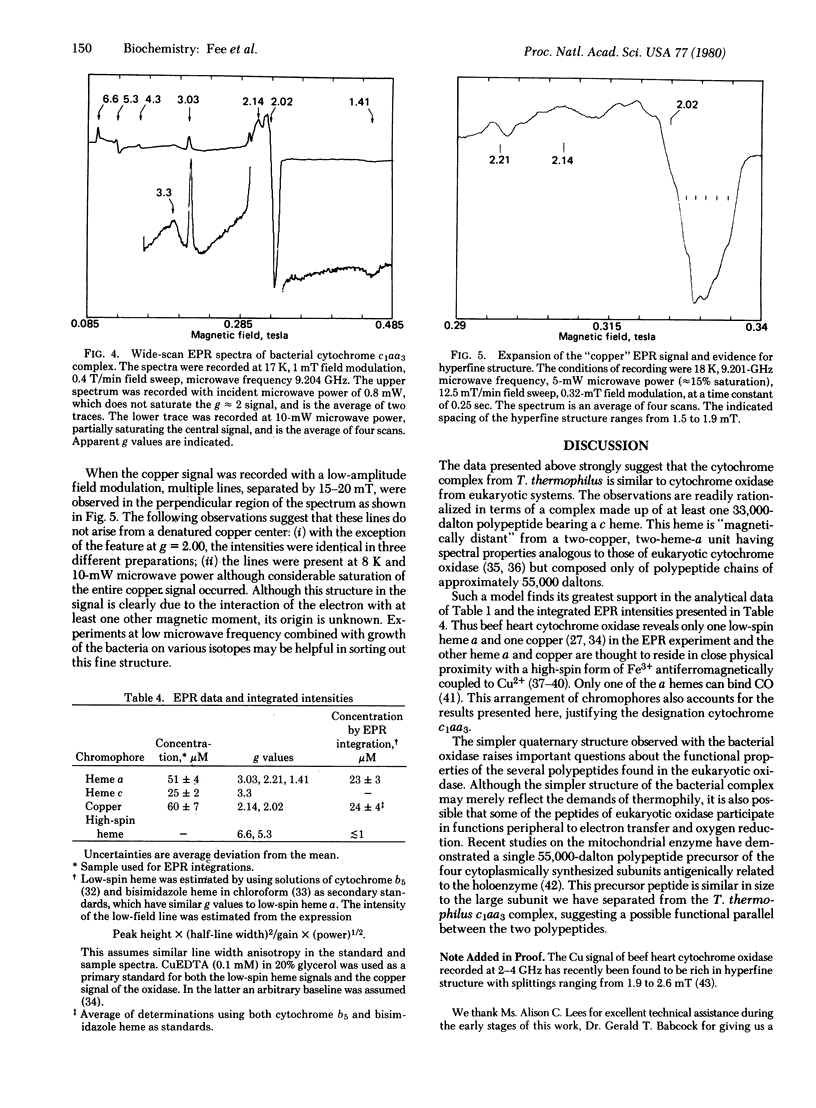

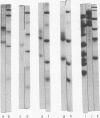
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aasa R., Albracht P. J., Falk K. E., Lanne B., Vänngard T. EPR signals from cytochrome c oxidase. Biochim Biophys Acta. 1976 Feb 13;422(2):260–272. doi: 10.1016/0005-2744(76)90137-6. [DOI] [PubMed] [Google Scholar]
- BEINERT H., GRIFFITHS D. E., WHARTON D. C., SANDS R. H. Properties of the copper associated with cytochrome oxidase as studied by paramagnetic resonance spectroscopy. J Biol Chem. 1962 Jul;237:2337–2346. [PubMed] [Google Scholar]
- BEINERT H., PALMER G. OXIDATION-REDUCTION OF THE COPPER COMPONENT OF CYTOCHROME OXIDASE. KINETIC STUDIES WITH A RAPID FREEZING TECHNIQUE. J Biol Chem. 1964 Apr;239:1221–1227. [PubMed] [Google Scholar]
- Babcock G. T., Vickery L. E., Palmer G. Electronic state of heme in cytochrome oxidase. I. Magnetic circular dichroism of the isolated enzyme and its derivatives. J Biol Chem. 1976 Dec 25;251(24):7907–7919. [PubMed] [Google Scholar]
- Bois-Poltoratsky R., Ehrenberg A. Magnetic and spectrophotometric investigations of cytochrome b5. Eur J Biochem. 1967 Oct;2(3):361–365. doi: 10.1111/j.1432-1033.1967.tb00146.x. [DOI] [PubMed] [Google Scholar]
- Capaldi R. A., Hayashi H. The polypeptide composition of cytochrome oxidase from beef heart mitochondria. FEBS Lett. 1972 Oct 1;26(1):261–263. doi: 10.1016/0014-5793(72)80587-8. [DOI] [PubMed] [Google Scholar]
- Chiang Y. L., King T. E. Cytochrome c1 complexes. J Biol Chem. 1979 Mar 25;254(6):1845–1853. [PubMed] [Google Scholar]
- Downer N. W., Robinson N. C. Characterization of a seventh different subunit of beef heart cytochrome c oxidase. Similarities between the beef heart enzyme and that from other species. Biochemistry. 1976 Jun 29;15(13):2930–2936. doi: 10.1021/bi00658a036. [DOI] [PubMed] [Google Scholar]
- Erecińska M., Wilson D. F. Cytochrome c oxidase: a synopsis. Arch Biochem Biophys. 1978 May;188(1):1–14. doi: 10.1016/0003-9861(78)90348-x. [DOI] [PubMed] [Google Scholar]
- Froncisz W., Scholes C. P., Hyde J. S., Wei Y. H., King T. E., Shaw R. W., Beiner H. Hyperfine structure resolved by 2 to 4 GHz EPR of cytochrome c oxidase. J Biol Chem. 1979 Aug 25;254(16):7482–7484. [PubMed] [Google Scholar]
- Hartzell C. R., Beinert H. Components of cytochrome c oxidase detectable by EPR spectroscopy. Biochim Biophys Acta. 1974 Dec 19;368(3):318–338. doi: 10.1016/0005-2728(74)90178-9. [DOI] [PubMed] [Google Scholar]
- Hon-Nami K., Oshima T. C-type cytochromes isloated from an extreme thermophile, Thermus thermophilus HB8. J Biochem. 1978 Feb;83(2):629–631. doi: 10.1093/oxfordjournals.jbchem.a131951. [DOI] [PubMed] [Google Scholar]
- Hon-Nami K., Oshima T. Purification and some properties of cytochrome c-552 from an extreme thermophile, Thermus thermophilus HB8. J Biochem. 1977 Sep;82(3):769–776. doi: 10.1093/oxfordjournals.jbchem.a131753. [DOI] [PubMed] [Google Scholar]
- Katan M. B., Pool L., Groot G. S. The cytochrome bc1 complex of yeast mitochondria. Isolation and partial characterization of the cytochrome bc1 complex and cytochrome b. Eur J Biochem. 1976 May 17;65(1):95–105. doi: 10.1111/j.1432-1033.1976.tb10393.x. [DOI] [PubMed] [Google Scholar]
- Kimelberg H. K., Nicholls P. Kinetic studies on the interaction of TMPD with cytochrome c and cytochrome c oxidase. Arch Biochem Biophys. 1969 Sep;133(2):327–335. doi: 10.1016/0003-9861(69)90461-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Malmström B. G. Cytochrome c oxidase: some current biochemical and biophysical problems. Q Rev Biophys. 1973 Nov;6(4):389–431. doi: 10.1017/s0033583500001578. [DOI] [PubMed] [Google Scholar]
- Moss T. H., Shapiro E., King T. E., Beinert H., Hartzell C. The magnetic susceptibility of cytochrome oxidase in the 4.2-1.5 K range. J Biol Chem. 1978 Nov 25;253(22):8072–8073. [PubMed] [Google Scholar]
- Orme-Johnson N. R., Hansen R. E., Beinert H. EPR studies of the cytochrome b-c 1 segment of the mitochondrial electron transfer system. Biochem Biophys Res Commun. 1971 Nov;45(4):871–878. doi: 10.1016/0006-291x(71)90419-0. [DOI] [PubMed] [Google Scholar]
- Peisach J., Mims W. B. Linear electric field effect in electron paramagnetic resonance for two bisimidazole--heme complexes, model compounds for B and H hemichromes of hemoglobin and for cytochrome b5. Biochemistry. 1977 Jun 14;16(12):2795–2799. doi: 10.1021/bi00631a033. [DOI] [PubMed] [Google Scholar]
- Poyton R. O., McKemmie E. A polyprotein precursor to all four cytoplasmically translated subunits of cytochrome c oxidase from Saccharomyces cerevisiae. J Biol Chem. 1979 Jul 25;254(14):6763–6771. [PubMed] [Google Scholar]
- Poyton R. O., Schatz G. Cytochrome c oxidase from bakers' yeast. III. Physical characterization of isolated subunits and chemical evidence for two different classes of polypeptides. J Biol Chem. 1975 Jan 25;250(2):752–761. [PubMed] [Google Scholar]
- RAWLINSON W. A., HALE J. H. Prosthetic groups of the cytochromes present in Corynebacterium diphtheriae with especial reference to cytochrome a. Biochem J. 1949;45(3):247-55, pl. doi: 10.1042/bj0450247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmeen I., Palmer G. Electron paramagnetic resonance of beef-heart ferricytochrome c. J Chem Phys. 1968 Mar 1;48(5):2049–2052. doi: 10.1063/1.1669014. [DOI] [PubMed] [Google Scholar]
- Sun F. F., Prezbindowski K. S., Crane F. L., Jacobs E. E. Physical state of cytochrome oxidase. Relationship between membrane formation and ionic strength. Biochim Biophys Acta. 1968 May 28;153(4):804–818. doi: 10.1016/0005-2728(68)90008-x. [DOI] [PubMed] [Google Scholar]
- TAKEMORI S., KING T. E. EFFECT OF ALKALI AND BOROHYDRIDE ON CARDIAC CYTOCHROME OXIDASE. FORMATION OF SCHIFF BASE. J Biol Chem. 1965 Jan;240:504–513. [PubMed] [Google Scholar]
- Thomas P. E., Ryan D., Levin W. An improved staining procedure for the detection of the peroxidase activity of cytochrome P-450 on sodium dodecyl sulfate polyacrylamide gels. Anal Biochem. 1976 Sep;75(1):168–176. doi: 10.1016/0003-2697(76)90067-1. [DOI] [PubMed] [Google Scholar]
- Tracy R. P., Chan S. H. Analysis of electrophoretic behavior of cytochrome c oxidase subunits. Retardation coefficient versus molecular weight in dodecyl sulfate urea gels. Biochim Biophys Acta. 1979 Jan 25;576(1):109–117. doi: 10.1016/0005-2795(79)90489-6. [DOI] [PubMed] [Google Scholar]
- Tweedle M. F., Wilson L. J. Electronic state of heme in cytochrome oxidase III. The magnetic susceptibility of beef heart cytochrome oxidase and some of its derivatives from 7-200 K. Direct evidence for an antiferromagnetically coupled Fe (III)/Cu (II) pair. J Biol Chem. 1978 Nov 25;253(22):8065–8071. [PubMed] [Google Scholar]
- Van Gelder B. F., Beinert H. Studies of the heme components of cytochrome c oxidase by EPR spectroscopy. Biochim Biophys Acta. 1969 Sep 16;189(1):1–24. doi: 10.1016/0005-2728(69)90219-9. [DOI] [PubMed] [Google Scholar]
- Wharton D. C., Gibson Q. H. Stoichiometry of carbon monoxide binding by cytochrome c oxidase. J Biol Chem. 1976 May 10;251(9):2861–2862. [PubMed] [Google Scholar]
- YONETANI T. Studies on cytochrome oxidase. III. Improved preparation and some properties. J Biol Chem. 1961 Jun;236:1680–1688. [PubMed] [Google Scholar]
- Yoshikawa S., Choc M. G., O'Toole M. C., Caughey W. S. An infrared study of CO binding to heart cytochrome c oxidase and hemoglobin A. Implications re O2 reactions. J Biol Chem. 1977 Aug 10;252(15):5498–5508. [PubMed] [Google Scholar]