Abstract
Since the endoplasmic reticulum (ER) plays a vital role in hepatocyte function, it is not surprising that a variety of liver-related diseases are associated with ER stress. As in other tissues, ER stress in the liver leads to generation of the unfolded-protein response resulting in activation of a transcriptional program that promotes restoration of homeostasis within the lumen of the ER. Previous studies using cells in culture demonstrated that ER stress induces expression of REDD1 (regulated in development and DNA damage responses), a potent repressor of signaling through the protein kinase referred to as the mechanistic target of rapamycin in complex 1 (mTORC1). In the present study, the results from the cell culture experiments were extended to show that tunicamycin-mediated ER stress in the liver in vivo also induces REDD1 gene expression. Moreover, the induction of REDD1 gene expression was shown to require the protein kinase PERK and enhanced phosphorylation of its substrate, the α-subunit of eukaryotic initiation factor 2.
Keywords: REDD1, Ddit4, eIF2, ER stress, unfolded protein response, PERK
Introduction
The protein referred to as regulated in development and DNA damage responses (REDD1; aka DNA-damage-inducible transcript 4, DDIT4) is rapidly induced in response to a wide variety of conditions including hypoxia [1], oxidative stress [2], glucocorticoid treatment [3], food deprivation [4], and conditions that deplete intracellular ATP [5] or cause DNA damage [2]. More recent studies [e.g. 6,7] have shown that REDD1 expression is also induced under conditions that promote endoplasmic reticulum (ER) stress in cells in culture through increased expression of the transcription factor, ATF4 (activating transcription factor 4). Although its exact mechanism of action is unclear, REDD1 has been shown to repress signaling through a pathway involving the protein kinase known as the mechanistic target of rapamycin (mTOR) in complex 1 (mTORC1) [1]. Because activation of mTORC1 can promote ER stress [8], induction of REDD1 expression is thought to represent a feed-back mechanism to limit mTORC1 signaling, and thereby constrain the ER stress response.
The ER plays a vital role in liver function not only because of the large number and quantity of proteins and lipoproteins synthesized in and secreted from the organ, but also because lipid metabolism and detoxification of chemicals depend upon it [9]. Thus, it is not surprising that ER stress in the liver has been linked to a variety of diseases including viral hepatitis B and C infection, alcohol-induced injury, and acetaminophen-induced toxicity [10,11]. Animal models of obesity [12] and obese humans [13] also display ER stress and this is attenuated by the selective mTORC1 inhibitor rapamycin [14]. The latter observation suggests a possible role for mTORC1 in the generation of ER stress in the liver. However, whether or not ER stress might modulate mTORC1 signaling in the liver through alterations in REDD1 expression is unknown.
The generation of ER stress leads to enhanced signaling through three independent pathways originating at the ER membrane: activation of the eukaryotic initiation factor 2α (eIF2α) kinase PKR-like ER-localized kinase (PERK, aka eIF2AK3), transport of activating transcription factor 6 (ATF6) to the Golgi where it is cleaved to release the transcriptionally active 50 kDa cytosolic N-terminal domain, and activation of the inositol-requiring enzyme 1 (IRE1, aka ER to nucleus signaling 1) [15]. Activated IRE1 removes a 26-base domain from the mRNA encoding X-box binding protein 1 (XBP1), generating the active form of the transcription factor [16]. Phosphorylation of eIF2α by PERK results in repression of global rates of protein synthesis, but also leads to enhanced translation of a few mRNAs containing upstream open reading frames in their 5’-non-coding regions; one such mRNA encodes the transcription factor ATF4 [17]. Combined, activation of the three transcription factors, ATF4, ATF6, and XBP1, induces the expression of proteins that function to relieve the stress in the ER. One such protein is GADD34 (growth arrest and DNA damage-inducible protein 34), a regulatory subunit of protein phosphatase 1 (PP1) that targets the phosphatase to eIF2α leading to its dephosphorylation and consequently to restoration of protein synthesis [18,19].
In the present study, we assessed the effect of ER stress on REDD1 expression in the liver by treating mice with a well-characterized initiator of ER stress, tunicamycin [20]. We show that tunicamycin treatment generates ER stress in the liver as assessed by enhanced phosphorylation of the α-subunit of eIF2 and enhanced splicing of the mRNA encoding XBP1, concomitant with induction of REDD1 mRNA expression. The effects of tunicamycin on eIF2α phosphorylation and REDD1 expression were attenuated in mice ectopically expressing the active domain of GADD34 in the liver as well as in mice lacking the eIF2α kinase, PERK, indicating that ER stress acts through a mechanism involving PERK-mediated eIF2α phosphorylation to induce REDD1 gene expression.
Materials and Methods
Materials
Tunicamycin was purchased from Sigma-Aldrich (St. Louis, MO). Primers for quantitative real-time PCR (qRT-PCR) were purchased from Integrated DNA Technologies, Inc. (Coralville, IA). Anti-phosphorylated eIF2α(Ser51) antibody was obtained from Cell Signaling Technology Inc. (Danvers, MA) and the anti-eIF2α monoclonal antibody was purified in house from cell culture medium obtained from dishes containing hybridoma cells provided by the late Dr. Edgar C. Henshaw [21].
Animals
All studies involving animals were reviewed and approved by the Institutional Animal Care and Use Committee of The Pennsylvania State University College of Medicine. GADD34 transgenic mice and PERK knockout mice and their corresponding wild-type littermates were a kind gift from Dr. David Ron (University of Cambridge Metabolic Research Laboratories, Cambridge, UK) and Dr. Douglas R. Cavener (Department of Biology, The Pennsylvania State University, PA), respectively. Mice weighing approximately 20–25 g were administered 1 µg tunicamycin (50 µg/ml in 5% dextrose in sterile water) / g body weight intraperitoneally [22]. Two or four hours later, mice were deeply anesthetized using isoflurane and livers were quickly removed. A portion of the liver was frozen between aluminum blocks pre-cooled to the temperature of liquid nitrogen and the remainder was homogenized as described previously [23].
Western blot analysis
Phosphorylation of eIF2α on Ser51 was assessed by a slight modification of the method described previously [7]. Briefly, liver supernatants were resolved by SDS-polyacrylamide gel electrophoresis on Bio-Rad Criterion 4–15% gels, and proteins in the gel were subsequently transferred to PVDF membrane. Blots were incubated with an anti-phospho-eIF2α(Ser51) antibody, and after development, the blots were stripped and re-probed using an antibody that recognize the protein regardless of phosphorylation state. Results using the anti-phospho-eIF2α antibody was normalized for the total expression of the protein.
RNA isolation and quantitative real-time PCR (qRT-PCR) analysis
RNA was extracted from liver using TRIzol reagent according to the manufacture’s protocol (Invitrogen). qRT-PCR for REDD1 [7], GAPDH [7], and ASNS [24] mRNA quantitation was performed as described previously.
PCR analysis of XBP1 mRNA splicing
Analysis of XBP1 mRNA splicing was performed as described previously [25]. The PCR products were resolved on a 3% agarose gel containing Sybr Safe DNA Gel Stain (Life Technologies, Grand Island, NY) along with a 1 Kb Plus DNA Ladder (Life Technologies).
Results
In order to reproducibly generate ER stress in the liver, mice were administered tunicamycin, a nucleoside antibiotic that inhibits the first step in glycoprotein synthesis, leading to generation of ER stress [20]. To assess the effectiveness of the antibiotic in generating ER stress in the liver in vivo, eIF2α phosphorylation on Ser51 was assessed by Western blot analysis. Compared to wild-type mice, basal phosphorylation of eIF2α was reduced in transgenic mice ectopically expressing the active domain of GADD34 in the liver (Fig. 1A), consistent with its role in targeting PP1 to eIF2 [18,19]. In contrast, the relative phosphorylation of eIF2α on Ser51 was more than 3.5-fold greater in the liver of wild-type mice administered tunicamycin compared to mice administered vehicle alone. Moreover, in GADD34 transgenic mice, tunicamycin-mediated eIF2α phosphorylation was significantly reduced compared to wild-type mice. One possible explanation for the attenuated response of eIF2α phosphorylation in the transgenic mice is that tunicamycin was less effective in generating ER stress in transgenic compared to wild-type animals. To assess this possibility, a second biomarker of ER stress, splicing of XBP1 mRNA [16], was assessed. As shown in Fig. 1B, in the absence of tunicamycin treatment, the majority of the XBP1 mRNA was in the inactive, unspliced form in the liver of both wild-type and GADD34 transgenic mice. Importantly, tunicamycin treatment was equally effective in promoting a shift from the unspliced to the spliced form in wild-type and transgenic animals, demonstrating that the response to the antibiotic was equivalent in the two genotypes.
Fig. 1.
Tunicamycin-generated ER stress is attenuated in GADD34 transgenic compared to wild-type mice. Mice were treated with tunicamycin (grey bars) or vehicle (black bars) for 2 hr and the liver was then removed and analyzed for (A) eIF2α phosphorylation and (B) XBP1 mRNA splicing by Western blot and PCR analysis, respectively, as described under Materials and Methods. A typical Western blot is depicted in an insert in panel A. All samples were analyzed on the same gel, but not in contiguous lanes; white lines separate non-contiguous lanes. n=9–10 per condition. Bars not sharing the same letter are significantly different, p<0.05
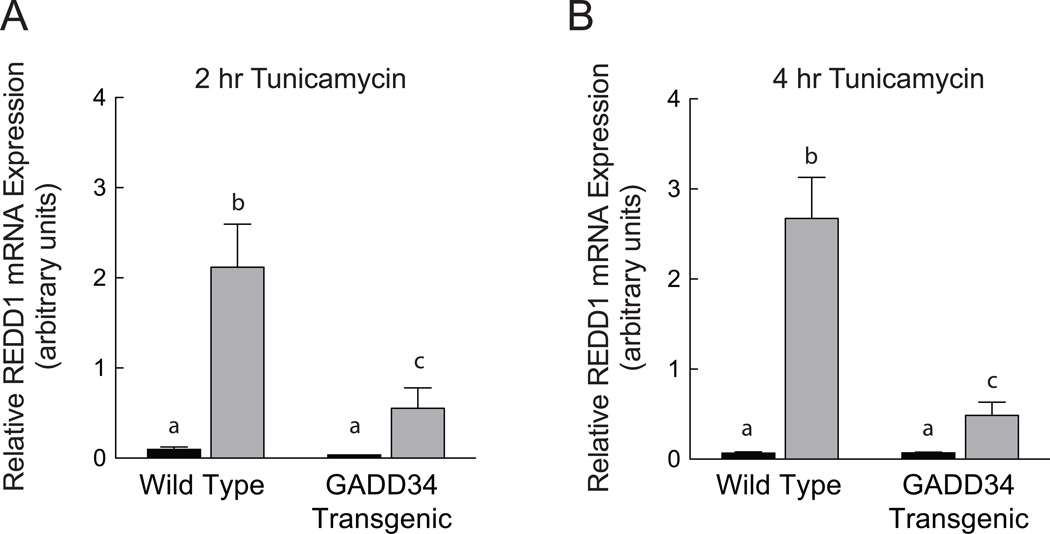
In the liver of wild-type mice, tunicamycin treatment produced an 11-fold induction in REDD1 mRNA expression (Fig. 2A). In contrast, in GADD34 transgenic mice the response was greatly attenuated, suggesting that the induction was a consequence of eIF2α phosphorylation. Qualitatively similar results were observed four hr after tunicamycin administration (Fig. 2B). Thus, the tunicamycin-induced increase in REDD1 mRNA expression was significantly greater in wild-type compared to GADD34 transgenic time points after both two and four hours of treatment.
Fig. 2.
ER stress-mediated induction of REDD1 mRNA expression is attenuated in GADD34 transgenic compared to wild-type mice. Mice were treated with tunicamycin (grey bars) or vehicle (black bars) for (A) 2 hr or (B) 4 hr and the liver was then removed and analyzed for REDD1 mRNA expression as described under Materials and Methods. N=7–8 per condition. Bars not sharing the same letter are significantly different, p<0.05
In cells in culture, ER stress induction of REDD1 mRNA expression has been linked to the transcription factor ATF4 [6,7]. Unfortunately, we have been unable to find an antibody that reliably detects the ATF4 protein in mouse liver supernatants, so possible changes in its expression could not be assessed. Instead, as a surrogate marker for ATF4 upregulation, expression of the well-characterized ATF4 target, asparagine synthetase [26], was assessed. As shown in Fig. 3, tunicamycin treatment led to a four-fold induction in asparagine synthetase mRNA expression compared to control mice. Moreover, no change in expression was observed in GADD34 transgenic mice.
Fig. 3.
ER stress- mediated induction of asparagine synthetase mRNA expression is attenuated in GADD34 transgenic compared to wild-type mice. Mice were treated with tunicamycin (grey bars) or vehicle (black bars) for 2 hr and the liver was then removed and analyzed for asparagine synthetase (ASNS) mRNA expression as described under Materials and Methods. N=4–5 per condition. Bars not sharing the same letter are significantly different, p<0.005
The results described above are consistent with the idea that the induction of REDD1 expression is a consequence of eIF2α phosphorylation. However, an alternative explanation is that GADD34 is targeting PP1 to a protein other than eIF2 that is able to modulate REDD1 gene transcription. To confirm that the induction of REDD1 expression was a result of elevated eIF2α phosphorylation, the effect of tunicamycin was assessed in mice lacking the ER-resident eIF2α kinase, PERK. In wild-type mice, tunicamycin treatment caused a significant elevation in eIF2α phosphorylation, and the effect was almost completely eliminated in PERK−/− mice (Fig. 4A). Similarly, REDD1 mRNA expression was dramatically induced in the liver of wild-type mice in response to tunicamycin treatment, but little change was observed in mice lacking the kinase (Fig. 4B). Moreover, in contrast to wild-type mice, tunicamycin had no effect on asparagine synthetase expression in PERK−/− mice (Fig. 4C). Overall, the results strongly suggest that ER stress mediates upregulation of REDD1 expression in the liver through a mechanism involving activation of PERK and elevated eIF2α phosphorylation.
Fig. 4.
ER stress- mediated induction of eIF2α phosphorylation and induction of REDD1 and asparagine synthetase mRNA expression is attenuated in PERK−/− compared to wild-type mice. Mice were treated with tunicamycin (grey bars) or vehicle (black bars) for 2 hr and the liver was then removed and analyzed for (A) eIF2α phosphorylation, (B) REDD1 mRNA expression, and (C) asparagine synthetase (ASNS) mRNA expression, as described under Materials and Methods. A typical Western blot is depicted in an insert in panel A. All samples were analyzed on the same gel, but not in contiguous lanes; white lines separate non-contiguous lanes. N=14–15 (eIF2α), N=13–14 (REDD1), or N=13 (ASNS) per condition. Bars not sharing the same letter are significantly different, p<0.05
Discussion
In cells in culture, treatment with tunicamycin or thapsigargin leads to the generation of ER stress, as assessed by elevated eIF2α phosphorylation and splicing of the XBP1 mRNA [7]. Generation of ER stress also results in induction of REDD1 mRNA expression, an effect that is not observed in mouse embryo fibroblasts lacking ATF4 [7] or in HeLa cells in which ATF4 expression is silenced using siRNA [6]. Moreover, exogenous overexpression of ATF4 in mouse embryo fibroblasts leads to induction of REDD1 mRNA expression [7]. Thus, since ATF4 binds to the REDD1 promoter [6], it is likely to mediate induction of REDD1 gene transcription.
ATF4 expression is rapidly induced following generation of ER stress due to phosphorylation of eIF2α by PERK [17]. Phosphorylation of eIF2α represses the translation of most mRNAs, but paradoxically stimulates the translation of mRNAs, such as the one encoding ATF4, that have multiple upstream open reading frames in the 5’-non-coding region [27]. In the present study, REDD1 mRNA expression was induced in wild-type mice in response to treatment with tunicamycin. However, the effect of the drug on REDD1 expression was greatly attenuated in the liver of mice exogenously expressing GADD34 or in mice lacking PERK, such that the reduction in REDD1 expression was directly proportional to the diminution in eIF2α phosphorylation. The possibility that increased eIF2α phosphorylation resulted in induction of ATF4 expression could not be directly tested in this study because of the lack of an antibody that reliably detects the mouse protein. However, ER stress-mediated induction of asparagine synthetase expression, a well-characterized target of ATF4, was increased in proportion to changes in eIF2α phosphorylation. Thus, the results are consistent with a model in which ER stress induces a cascade of events that includes activation of PERK, elevated phosphorylation of eIF2α, enhanced ATF4 mRNA translation, and ATF4-mediated induction of REDD1 gene transcription.
Overall, the results of the present study extend earlier ones using cells in culture to show that ER stress leads to induction of REDD1 gene expression in the liver in vivo. They also extend the earlier studies to show that the ER stress-mediated induction of REDD1 requires both activation of PERK as well as elevated phosphorylation of eIF2α.
Highlights.
Endoplasmic reticulum stress was generated in liver by in vivo using tunicamycin
Expression of the mRNA encoding the mTORC1 repressor REDD1 was induced
Induction of REDD1 gene expression required the protein kinase PERK
Induction of REDD1 expression was mediated through eIF2α phosphorylation / ATF4
Acknowledgments
The authors wish to thank Lydia Kutzler and Gina Dieter for help in performing the studies described herein. The studies were supported by NIH grants GM39277 (SRK) and DK13499 (LSJ). The funding agencies had no role in the design of the study, in the collection, analysis, or interpretation of the data, in the writing of the report, or in the decision to submit the article for publication.
Abbreviations
- ASNS
asparagine synthetase
- ATF
activating transcription factor
- DDIT4
DNA-damage-inducible transcript 4
- ER
endoplasmic reticulum
- eIF2
eukaryotic initiation factor 2
- eIF2α
α-subunit of eIF2
- GADD34
growth arrest and DNA damage-inducible protein
- IRE1
inositol-requiring enzyme 1
- mTOR
mechanistic target of rapamycin
- mTORC1
mTOR in complex 1
- PERK
PKR-like ER-localized kinase
- REDD
regulated in development and DNA damage responses
- XBP1
X-box binding protein 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brugarolas J, Lei K, Hurley RL, Manning BD, Reiling JH, Hafen E, Witters LA, Ellisen LW, Kaelin WG., Jr. Regulation of mTOR function in response to hypoxia by REDD1 and the TSC1/TSC2 tumor suppressor complex. Genes Develop. 2004;18:2893–2904. doi: 10.1101/gad.1256804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ellisen LW, Ramsayer KD, Johannessen CM, Yang A, Beppu H, Minda K, Oliner JD, McKeon F, Haber DA. REDD1, a developmentally regulated transcriptional target of p63 and p53, links p63 to regulation of reactive oxygen species. Molec. Cell. 2002;10:995–1005. doi: 10.1016/s1097-2765(02)00706-2. [DOI] [PubMed] [Google Scholar]
- 3.Wang H, Kubica N, Ellisen LW, Jefferson LS, Kimball SR. Dexamethasone represses signaling through the mammalian target of rapamycin in muscle cells by enhancing expression of REDD1. J. Biol. Chem. 2006;281:39128–39134. doi: 10.1074/jbc.M610023200. [DOI] [PubMed] [Google Scholar]
- 4.McGhee NK, Jefferson LS, Kimball SR. Elevated corticosterone associated with food deprivation upregulates expression in rat skeletal muscle of the mTORC1 repressor REDD1. J. Nutr. 2009;139:828–834. doi: 10.3945/jn.108.099846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sofer A, Lei K, Johannessen CM, Ellisen LW. Regulation of mTOR and cell growth in response to energy stress by REDD1. Molec. Cell. Biol. 2005;25:5834–5845. doi: 10.1128/MCB.25.14.5834-5845.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jin H-O, Seo S-K, Woo S-H, Kim E-S, Lee H-C, Yoo D-H, An S, Choe T-B, Lee S-J, Hong S-I, Rhee C-H, Kim J-I, Park I-C. Activating transcription factor 4 and CCAAT/enhancer-binding protein-[beta] negatively regulate the mammalian target of rapamycin via Redd1 expression in response to oxidative and endoplasmic reticulum stress. Free Radical Biol. Med. 2009;46:1158–1167. doi: 10.1016/j.freeradbiomed.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 7.Whitney ML, Jefferson LS, Kimball SR. ATF4 is necessary and sufficient for ER stress-induced upregulation of REDD1 expression. Biochem. Biophys. Res. Commun. 2009;379:451–455. doi: 10.1016/j.bbrc.2008.12.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ozcan U, Ozcan L, Yilmaz E, vel DK, Sahin M, Manning BD, Hotamisligil GS. Loss of the tuberous sclerosis complex tumor suppressors triggers the unfolded protein response to regulate insulin signaling and apoptosis. Molec. Cell. 2008;29:541–551. doi: 10.1016/j.molcel.2007.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fu S, Watkins SM, Hotamisligil GS. The role of endoplasmic reticulum in hepatic lipid homeostasis and stress signaling. Cell Metab. 2012;15:623–634. doi: 10.1016/j.cmet.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 10.Ji C, Kaplowitz N. ER stress: Can the liver cope? Journal of Hepatology. 2006;45:321–333. doi: 10.1016/j.jhep.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 11.Malhi H, Kaufman RJ. Endoplasmic reticulum stress in liver disease. Journal of Hepatology. 2011;54:795–809. doi: 10.1016/j.jhep.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ozcan U, Cao Q, Yilmaz E, Lee A-H, Iwakoshi NN, Ozdelen E, Tuncman G, Gorgun C, Glimcher LH, Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 13.Gregor MF, Yang L, Fabbrini E, Mohammed BS, Eagon JC, Hotamisligil GS, Klein S. Endoplasmic reticulum stress is reduced in tissues of obese subjects after weight loss. Diabetes. 2009;58:693–700. doi: 10.2337/db08-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ai D, Baez JM, Jiang H, Conlon DM, Hernandez-Ono A, Frank-Kamenetsky M, Milstein S, Fitzgerald K, Murphy AJ, Woo CW, Strong A, Ginsberg HN, Tabas I, Rader DJ, Tall AR. Activation of ER stress and mTORC1 suppresses hepatic sortilin-1 levels in obese mice. The Journal of Clinical Investigation. 2012;122:1677–1687. doi: 10.1172/JCI61248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang S, Kaufman RJ. The impact of the unfolded protein response on human disease. The Journal of cell biology. 2012;197:857–867. doi: 10.1083/jcb.201110131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001;107:881–891. doi: 10.1016/s0092-8674(01)00611-0. [DOI] [PubMed] [Google Scholar]
- 17.Harding HP, Novoa I, Zhang Y, Zeng H, Wek RC, Schapira M, Ron D. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Molec. Cell. 2000;6:1099–1108. doi: 10.1016/s1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- 18.Connor JH, Weiser DC, Li S, Hallenbeck JM, Shenolikar S. Growth arrest and DNA damage-inducible protein GADD34 assembles a novel signaling complex containing protein phosphatase 1 and inhibitor 1. Molec. Cell. Biol. 2001;21:6841–6850. doi: 10.1128/MCB.21.20.6841-6850.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Novoa I, Zeng H, Harding HP, Ron D. Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2α. J. Cell Biol. 2001;153:1011–1021. doi: 10.1083/jcb.153.5.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bassik MC, Kampmann M. Knocking out the door to tunicamycin entry. Proc. Natl. Acad. Sci. USA. 2011;108:11731–11732. doi: 10.1073/pnas.1109035108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scorsone KA, Panniers R, Rowlands AG, Henshaw EC. Phosphorylation of eukaryotic initiation factor 2 during physiological stresses which affect protein synthesis. J. Biol. Chem. 1987;262:14538–14543. [PubMed] [Google Scholar]
- 22.Zinszner H, Kuroda M, Wang X, Batchvarova N, Lightfoot RT, Remotti H, Stevens JL, Ron D. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Develop. 1998;12:982–995. doi: 10.1101/gad.12.7.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dennis MD, Schrufer TL, Bronson SK, Kimball SR, Jefferson LS. Hyperglycemia-induced O-GlcNAcylation and truncation of 4E-BP1 protein in liver of a mouse model of type 1 diabetes. J. Biol. Chem. 2011;286:34286–34297. doi: 10.1074/jbc.M111.259457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shan J, Lopez M-C, Baker HV, Kilberg MS. Expression profiling after activation of amino acid deprivation response in HepG2 human hepatoma cells. Physiological Genomics. 2010;41:315–327. doi: 10.1152/physiolgenomics.00217.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iwakoshi NN, Lee AH, Vallabhajosyula P, Otipoby KL, Rajewsky K, Glimcher LH. Plasma cell differentiation and the unfolded protein response intersect at the transcription factor XBP-1. Nat Immunol. 2003;4:321–329. doi: 10.1038/ni907. [DOI] [PubMed] [Google Scholar]
- 26.Chen H, Pan Y-X, Dudenhausen EE, Kilberg MS. Amino acid deprivation induces the transcription rate of the human asparagine synthetase gene through a timed program of expression and promoter binding of nutrient-responsive basic region/leucine zipper transcription factors as well as localized histone acetylation. J. Biol. Chem. 2004;279:50829–50839. doi: 10.1074/jbc.M409173200. [DOI] [PubMed] [Google Scholar]
- 27.Jackson RJ, Hellen CUT, Pestova TV. The mechanism of eukaryotic translation initiation and principles of its regulation. Nature Rev. Mol. Cell Biol. 2010;11:113–127. doi: 10.1038/nrm2838. [DOI] [PMC free article] [PubMed] [Google Scholar]