Abstract
The hypothalamic neuropeptide orexin (ORX) has been implicated in anxiety, and anxiety-like behaviors. The purpose of these studies was to determine the role of ORX, specifically orexin-A (ORX-A) in the bed nucleus of the stria terminalis (BNST) on anxiety-like behaviors in rats. Rats injected with ORX-A into the BNST displayed greater anxiety-like measures in the social interaction and elevated plus maze tests compared to vehicle treated controls. Such anxiety-like behaviors were not observed when the ORX-A injections were adjacent to the BNST, in the medial septum. The anxiety-inducing effects of direct infusions of ORX-A into the BNST may be a consequence of increased activation of BNST neurons. In BNST slice preparations using patch-clamp techniques, ORX-A induced membrane depolarization and generation of action potentials in a subset of BNST neurons. The anxiety-inducing effects of ORX-A in the BNST also appear to be dependent on NMDA-type glutamate receptor activity, as pre-injecting the NMDA antagonist AP5 into the BNST blocked anxiogenic effects of local ORX-A injections. Injections of AMPA-type receptor antagonists into the BNST prior to ORX-A resulted in only a partial attenuation of anxiety-like behaviors.
Keywords: Anxiety, Neuropeptide, Bed Nucleus of the Stria Terminalis
1. Introduction
The neuropeptide orexin (ORX: also known as hypocretin) is synthesized by neurons located exclusively in the hypothalamus, specifically in the perifornical, dorsomedial and lateral hypothalamus [1, 2]. Despite this circumscribed locus of ORX producing neurons, ORX projections are found throughout the central nervous system, but have particularly dense projections to certain structures such as the bed nucleus of the stria terminalis (BNST), paraventricular nucleus of the thalamus, and brainstem monoaminergic systems [3, 4]. Through these widespread projections, ORX is involved in many complex physiological, behavioral and emotional responses [for review [5–9]]. ORX’s role in emotional responses has recently become a focal point with emerging data demonstrating clinical correlates of the ORX system and depression [10–13] and more recently anxiety disorders [14, 15] and addiction (for review [16, 17]).
The role of ORX in eliciting anxiety-like behaviors has been demonstrated within multiple species including the hamster [18], mouse [19, 20], and rat [15, 21], although not in all model systems (see [22]). Activation of ORX neurons, and increased ORX gene expression has been demonstrated to occur after exposure to anxiogenic stimuli [20, 23, 24] and anxiolytic drugs block the increased activation of ORX neurons in response to anxiogenic stimuli [23]. Additionally, anxiety-like behaviors and activation of ORX-neurons in response to anxiogenic stimuli are reduced by ORX 1 receptor (ORX1r) antagonists, ORX gene silencing and in ORX deficient animals [15, 20]. Although, very little is known about the specific efferent targets of the ORX system that regulate anxiety-like behaviors, we have recently determined that systemically pretreating rats with an ORX1r antagonist blocks anxiogenic drug induced increases in cellular responses in the extended amygdala (i.e., BNST and central amygdala) [25].
The BNST receives ORX projections from the dorsomedial-perifornical and lateral hypothalamus [26] and has a dense population of Orexin fibers [3] and high expression of the ORX1r with little to no expression of the ORX2r [27, 28]. The BNST is also an important site for regulating anxiety-like responses [29–33]. Lesioning the BNST as well as blocking excitatory inputs to the BNST reduces anxiety-like measures in rats [34, 35]. Furthermore, pre-injecting an ORX1r antagonist into the BNST was effective as systemic administration in attenuating anxiety-like responses to an anxiogenic challenge [15]. Thus, the BNST represents a potential efferent target site for ORX to increase anxiety states.
Increased anxiety-like responses occur with pharmacological manipulations in the BNST that produce postsynaptic excitation/depolarization [36, 37]. Previous reports show that ORX induces postsynaptic depolarization responses in multiple brain regions [38–49]. However, this has not been demonstrated to be the case with BNST neurons. Therefore, studying the effects of ORX-A on neuronal excitability in the BNST would be informative. A likely mechanism of ORX-induced depolarization and anxiety involves an interaction between ORX and glutamate. Glutamate is reportedly co-localized and is co-released with ORX in terminals of ORX neurons [50]. ORX reportedly potentiates glutamate’s excitatory postsynaptic responses elsewhere in the CNS, and this potentiation has been demonstrated to be necessary for ORX’s induction of behavior changes [51, 52]. Thus the objectives of this study were to determine the effects of infusing ORX-A into the BNST of rats on anxiety-like behaviors, demonstrate the electrophysiological effects of direct application of ORX-A onto BNST neurons and to determine if the anxiogenic effects of ORX-A in the BNST are mediated via activation of ionotropic glutamate receptors.
2. Material and methods
2.1 Animals
Adult male Sprague-Dawley rats (Harlan Laboratories, Indianapolis, IN) between 300–350 g were used for all behavioral experiments. All animals used for the electrophysiology were male Wistar rats (100–150 g) obtained from Harlan Laboratories (Indianapolis, IN). The animals were individually housed in a temperature-controlled room (22°C), and kept on a 12-hr light-dark cycle (lights on at 07:00) with free access to food and water. All procedures were performed according to NIH Guidelines for the Care and Use of Laboratory Animals (NIH Publication no. 80–23, revised in 1996) and according to the guidelines of the Indiana University Purdue University at Indianapolis Institutional Animal Care and Use Committee.
2.2 Surgical Techniques
Rats were anesthetized by placing them in a Plexiglas box connected to an isoflurane system (MGX Research Machine, Vetamac, Rossville, IN). The animals were then placed on a stereotaxic instrument (Kopf Instruments, Tujunga, CA) with the incisor bar set at −3.3 mm and kept under a constant flow of 3–4%v isoflurane through a Plexiglas nose cone. Rats were implanted unilaterally as described previously [36] with a microinjection guide cannula (26 gage) directed towards the BNST (AP −0.24 mm, ML ±1.4, DV −6.8) or septum (AP−0.24 mm, ML ±0.8, DV −5.2) according to the brain atlas of Paxinos and Watson [53]. All rats were given a minimum of 4 days recovery prior to behavioral testing.
2.3 Drugs/compounds
Drugs were delivered bilaterally through an injector cannula (33 gauge) fitted to extend 1 mm beyond the guide cannula. Infusions into the BNST were a total volume of 100 nl delivered over 1 min and injectors were left in an additional 1 min to allow for diffusion. The drugs used were Orexin A (catalog #1455; Tocris) 300 pmoles/100 nl (within the dose range used in Orx-A measures of anxiety) [21], where Vehicle is 1% BSA, which increases the efficiency of peptide delivery [54], AP5 (catalog #A8054; Sigma), 10 pmol/100 nl with Vehicle 0.9% saline, CNQX (catalog #C239; Sigma), 250 pmol/100 nl with Vehicle 0.9% saline (Doses used to block anxiety/panic like behaviors in rats) [55], and DNQX (catalog #D0540; Sigma), 250 pmol/100 nl with Vehicle 0.9% saline.
2.4 Behavioral tests
Elevated plus maze test (EPM)
The EPM, as described previously [36] was another validated test of anxiety used in this study. It is made of a black Plexiglas apparatus (Hamilton Kinder, San Diego, CA) that consists of two open arms and two closed arms each 50.17 cm long and 10.8 cm wide. The closed arms have walls that are 40.01 cm high. The entire apparatus is elevated 100 cm above the ground on a square aluminum base. Testing time is 5 min and anxiety is measured by the amount of time the rat spends in the closed versus open arms. Test sessions were video recorded from above and scored by two independent scorers.
The Social Interaction (SI) test
The SI test originally described by File [56], has been modified by our lab and was utilized to measure anxiety-like responses [57]. The SI box itself is a blue painted Plexiglas open top box with dimensions 91.44 cm L × 91.44 cm W × 30.48 cm H. Prior to any behavioral SI testing, all rats underwent a 5 min individual habituation to the SI box. A baseline testing with an unfamiliar partner rat was obtained at least 48 hours prior to initiation of treatment. The SI procedure consists of placing the experimental rat in the SI box with another unfamiliar (age, weight, sex matched) conspecific for a 5 min session. SI tests were performed 30 minutes following the last drug injection (ORX-A). The partner rats are untreated and did not undergo surgery, and so the test rat was clearly distinguished from the partner rat by the visible presence of the guide cannula. Baseline and test sessions were done under a low light (40 watt red light) and video recorded from above. The videotaped sessions were scored at a later time by an investigator blinded to treatment conditions (PM). SI time was measured as the amount of time, in seconds, that the experimental rat spends engaging in non-aggressive physical investigation (sniffing) of the partner rat. Aggression, climbing over and non-physical contact behaviors were not scored as social interaction time. Repeated SI test sessions were always separated by at least 48 hrs and always involved a novel partner.
2.5 Histology
After the conclusion of each experiment rats were sacrificed and the location of injection sites was determined using Nissl-stained 30 μm coronal sections through the BNST at 5x magnification. Data from rats with injection sites outside of the intended region of interest were not included in behavioral analysis, except when the medial septum was targeted. See Figure 1 for parameters of accepted injection sites.
Figure 1. Location of injections and recoding electrodes.
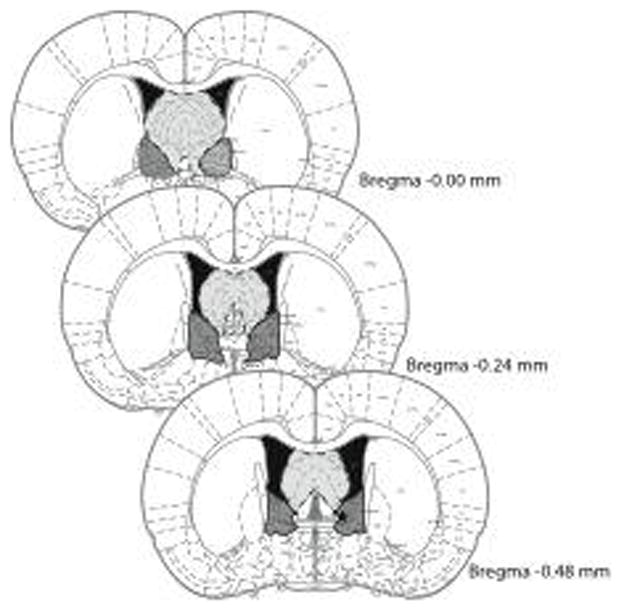
Presented here are the parameters of acceptable injection sites for the BNST (black border with shaded dark grey fill) and the medial septum (dotted boarders and light grey fill). Stars indicate location of recording electrodes used in the patch-clamp study. Images modified from [53]
2.6 Patch-clamp procedures
The animals were decapitated after isoflurane anesthesia (Abbott Laboratories, North Chicago, IL). The brains were rapidly removed and placed in ice-cold, oxygenated (95% O2/5% CO2) artificial cerebrospinal fluid (ACSF) with the following composition (in mM): 130 NaCl, 3.5 KCl, 1.1 KH2PO4, 1.3 MgCl2, 2.5 CaCl2, 10 Glucose, 30 NaHCO3. Coronal brain slices (350 μM) containing the BNST were obtained using standard procedures [58]. Slices were incubated in oxygenated ACSF at room temperature for at least 1 hr prior to recording. Then, they were transferred to a submersion-type slice chamber mounted on the stage of a Nikon E600FN Eclipse microscope (Nikon Instruments Inc., Melville, NY) and perfused at a rate of 1–2 ml per min with ACSF heated to 30°C. Whole-cell patch-clamp recordings were obtained using standard techniques with borosilicate glass electrodes (resistance 3–6 MΩ, WPI, Sarasota, FL) filled with a potassium gluconate based recording solution with the following composition (in mM): 140 K-gluconate, 2 KCl, 3 MgCl2, 10 HEPES, 5 phosphocreatine, 2 K-ATP, 0.2 Na-GTP adjusted to pH 7.3 with KOH, and having an osmolarity of 290–295 mOsm.
Whole cell recordings were made with a Multiclamp 700B amplifier (Molecular Devices, Sunnyvale, CA) using pClamp 10.2 software and a Digidata 1322A interface (Molecular Devices, Sunnyvale, CA). The holding potential for experiments was −60 mV. ORX-A was applied directly into the ACSF at a final concentration of 200 nM, aconcentration of Orx-A reported to induce depolarization [40, 45]. The following drugs were obtained from (1) Sigma-Aldrich (St. Louis, MO): K-gluconate, KCl, MgCl2, HEPES, NaCl, KH2PO4, CaCl2, glucose, NaHCO3, KOH, phosphocreatine, K-ATP, Na-GTP, (2) R&D Systems Inc (Minneapolis, MN): Orexin A.
2.8 Data analysis and statistics
2.8.1 Statistical analysis of behavioral data
The dependent variables (behaviors) analyzed for the EPM were duration and distance traveled in each area of the maze. These values were compared independently between vehicle-injected rats and ORX-A injected rats using a Student’s t test. The dependent variable for SI tests was duration of interaction. These values were compared (e.g. comparison to baseline) using a paired t test (when only baseline and treatment were compared within a single group), repeated measures one-way ANOVA or a repeated measures two-way ANOVA when warranted. In the presence of significant main effects, post-hoc pairwise comparisons were conducted using Dunnett’s to compare back to baseline values and Tukey’s HSD tests between groups. The confidence level for significance in all tests was set at p<0.05.
2.8.2 Statistical analysis of patch-clamp study
Data from patch-clamp experiments were acquired and analyzed using pClamp 10.2 (Molecular Devices, Sunnyvale, CA). Statistics and graphs were produced using GraphPad Prism 5.0 (GraphPad Software, La Jolla, CA). Statistical significance for mean comparisons before, during and after drug application within groups was determined by the repeated one-way ANOVA with Dunnett’s post-hoc analysis. The confidence level for significance in all tests was set at p<0.05.
3. Results
3.1. ORX-A injections into the BNST on anxiety-like behaviors
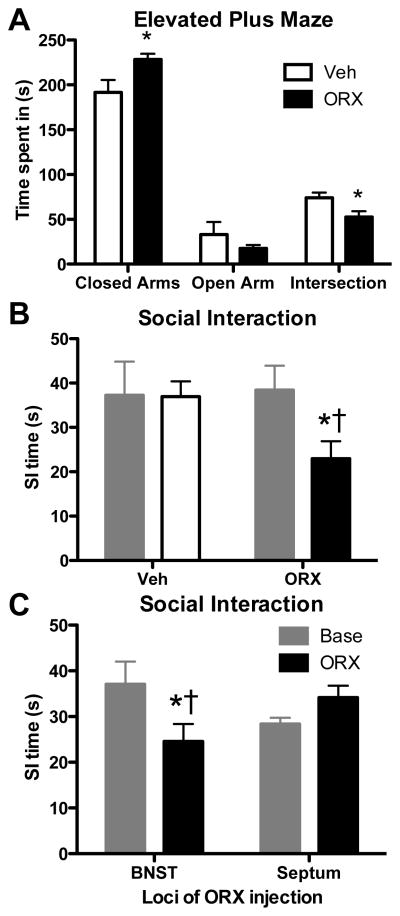
The first set of experiments was designed to determine if increasing ORX-A in the BNST is sufficient to induce anxiety-like behaviors. 30 minutes following a unilateral injection of ORX-A rats spent significantly more time in the closed arms and significantly less time in the intersection or decision area of the of EPM than when injected unilaterally with vehicle (t=2.42, p=0.032 and t=2.46, p=0.030 respectively, Fig 2A). No significant differences between injection groups were observed for time spent in open arms (t=1.08, p=0.303) or distance traveled in the closed arms [vehicle (mean ± SEM) 1144 ± 55 vs. ORX-A 989 ± 114 mm; t=1.22 p= 0.246]. Rats receiving unilateral BNST injections of ORX-A (n=7, 300 pmol/100 nL), compared to rats receiving unilateral BNST injections of vehicle (n=5, 1% BSA 100 nL), demonstrated anxiogenic-like responses in the SI test. The ORX-A, but not vehicle injections into the BNST significantly reduced SI time compared to baseline (t=4.20, p=0.006, Fig 2B). Furthermore, ORX-A injected rats had significantly lower SI compared to vehicle injected rats (t=2.54, p=0.030).
Figure 2. ORX-A into the BNST increases anxiety-like behaviors.
Presented in (A) are the mean (±SEM) times spent in each area of the elevated plus maze in rats 30 min after unilateral injections of Veh (1% BSA 100 nl, n=7, open bars) or ORX-A (300 pmol/100 nl, n=7, solid bars) into the BNST. Here ORX-A rats spent significantly more time in the closed arm and significantly less time in the decision or intersection area (* indicates difference from Veh, p ≤ 0.032). (B) Similar anxiety-like responses were observed in the SI test. SI times were significantly reduced, compared to baseline, in rats injected with ORX-A (n=7) into the BNST (*, p=0.0057), but not rats injected with Veh (n=5). SI times of rats injected with ORX-A were also significantly reduced compared to SI times of Veh injected rats (†, p=0.030). (C) Effects of ORX-A on SI time are selective to the BNST and not the adjacent medial septum. Compared to baseline, ORX-A injections into BNST (n=8) but not the medial septum (n=9) significantly reduced SI times (*, p=0.025). This ORX-A BNST induced reduction in SI time was also significantly lower than SI times of rats injected with ORX-A into the medial septum (†, p=0.049).
The reduction in SI time induced by ORX-A injections into the BNST was also selective to the BNST and not the medial septum. In a separate group of rats, ORX-A injections into the BNST (n=8, 300 pmol/100 nL) but not the medial septum (n=9, 300 pmol/100 nL) significantly reduced SI time compared to baseline (t=2.84, p=0.025, Fig 2C). Additionally, rats injected with ORX-A into the BNST had significantly lower SI compared to rats injected with ORX-A into the medial septum (t=2.14, p=0.049). Thus in three separate groups of rats and across 2 different anxiety tests, ORX-A injections into the BNST is sufficient to increase anxiety-like behavior in rats.
3.2 Effect of ORX-A on BNST neurons
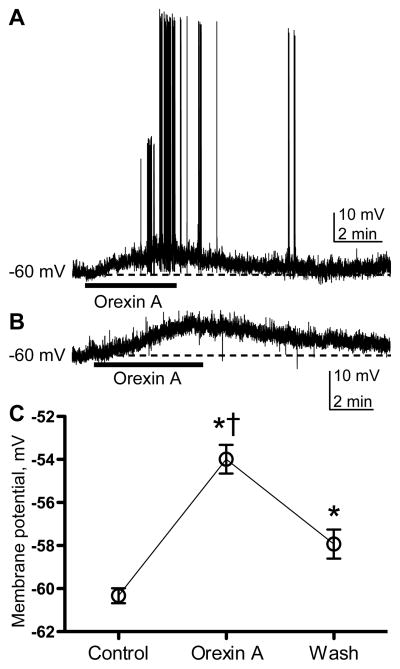
The effect of direct application of ORX-A on the membrane potential of BNST neurons was investigated using whole-cell patch-clamp in current clamp mode. ORX-A (200 nM), when applied to BNST slice was sufficient to induce depolarization and in some BNST neurons this lead to generation of action potentials (Fig 3A and B). In the presence of ORX-A and during the washout, membrane potential of BNST neurons were significantly different compared to control (baseline) values [n=6, repeated measures ANOVA F2,10=67.79 p=0.0001, Dunnett’s (ORX-A) q=11.53, p<0.001, (Wash) q=4.37, p<0.01; Fig 3C]. This depolarization of the membrane potential peaked during ORX-A application (increase of 6.05±0.67mv, mean±SEM) and remained elevated during the wash (increase of 2.07±0.67mv, mean±SEM), with the membrane potential being significantly greater during the ORX-A application than during the wash (Tukey’s q=10.13, p<0.001).
Figure 3. ORX-A induces depolarization in BNST neurons.
Presented in (A and B) are representative traces from whole-cell patch-clamp recordings in current-clamp mode before, during and after ORX-A application. These recordings clearly depict induction of action potentials (A) and membrane depolarization (A and B). Presented in (C) are mean (±SEM) membrane potentials from BNST neurons (n=6) prior to application of ORX-A (Control), during application of ORX-A and after application of ORX-A (Wash). * indicates significantly greater than Control (p≤0.001) and † indicates significantly greater than Wash (p=0.001).
3.3 Role of ORX-A and glutamate receptor interactions in the BNST on anxiety-like behavior
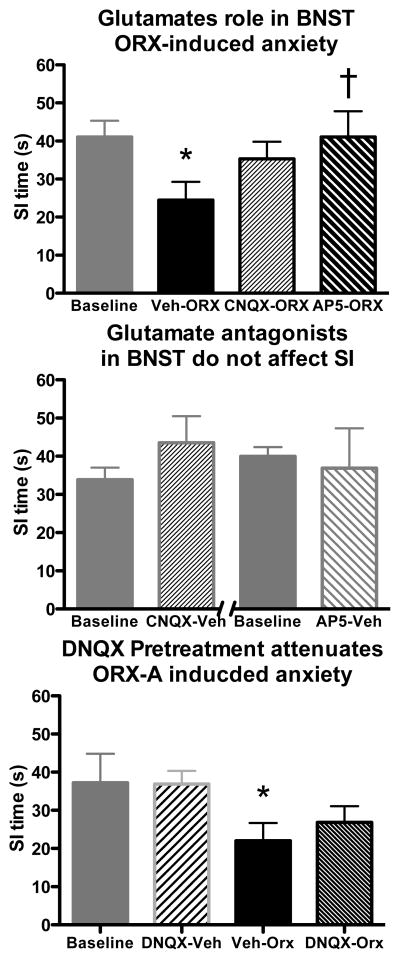
Considering ORX has been demonstrated to potentiate glutamate responses in other brain regions via interactions with glutamate receptors [N-methyl-D-aspartate-type receptors (NMDAr) and AMPA-type receptors (AMPAr)], the extent to which anxiety-like behaviors induced by BNST injections of ORX-A requires interaction with NMDA or AMPArs was investigated. Rats (n=11) were injected unilaterally into the BNST with AP5 (10 pmol/100 nl), CNQX (250 pmol/100 nl) or Vehicle (0.9% saline, 100 nL), 10 min prior to a unilateral injection of ORX-A (300 pmol/100 nL) through the same guide cannula, and SI times were observed 30 min after ORX-A injection. Between injections and before the SI test, rats were replaced into their home cages and kept in a separate staging room from where the SI testing occurred. This process was repeated every 48 hr until all rats received all three pre-ORX treatment conditions; the order of the pre-ORX treatments were counterbalanced. As observed previously (Fig 2B), ORX-A injections into the BNST significantly reduced SI times, compared to baseline, when rats were injected with Veh prior to ORX-A (RM ANOVA F3,30=3.05, p=0.044; Dunnett’s q=2.62 p<0.05, Fig 4A). Pre-injection of CNQX prior to ORX-A, attenuated the ORX-A induced reduction in SI time compared to baseline (Fig 4A). Furthermore, injection of AP5 completely blocked the ORX-A induced reduction in SI time; with SI times being significantly greater in AP5-ORX-A injected rats compared to Veh-ORX-A injected rats (Tukey’s q=3.70, p<0.05). In 2 subsets of the above rats, the consequence of injection of CNQX (n=5) or AP5 (n=5) alone 10 min prior to Veh (1% BSA) on SI time was analyzed. Here neither CNQX-Veh nor AP5-Veh altered SI times compared to baseline (p≥0.294, Fig 4B). To further clarify if the attenuation of BNST ORX-A injections-induced reductions in SI time was a result of CNQX action at the AMPA receptor and not a non-selective NMDA receptor effect, the above experiments were repeated in a new set of rats (n=9) using unilateral BNST injections of DNQX (250 pmol/100 nL). Again, Veh-ORX-A injections into the BNST significantly reduced SI times compared back to baseline or DNQX-Veh (RM ANOVA F3,18=4.05, p=0.033; Dunnett’s q≥2.80 p<0.05, Fig 4C). DNQX (250 pmol/100 nL) into the BNST 10 min prior to ORX-A injections minimally attenuated this reduction since SI times in DNQX-ORX rats were no longer significantly reduced compared to baseline, even though they were not significantly increased compared to Veh-ORX rats either.
Figure 4. In the BNST NMDAr are necessary for ORX-A induced anxiety-like behavior.
Presented in (A) are mean (±SEM) SI times (n=11, repeated measures, counter balanced design) at baseline (grey bar), and following ORX-A injections into the BNST that were preceded by Veh (Veh-ORX, solid bar), AMPAr antagonist (CNQX-ORX, thin striped bar) and NMDA antagonist, AP5 (AP5-ORX, thick striped bar). Here ORX-A injections into the BNST significantly reduced SI times compared to baseline when pre-injected with Veh. Pre-injections with CNQX or AP5 attenuated the ORX-A induced reduction in SI time. Pre-injection with AP5 completely reversed the ORX-A induced anxiety-like response; as SI times in AP5-ORX rats were significantly greater than Veh-ORX rats (* indicates significantly different from baseline p<0.05; † indicates significantly different from Veh-ORX p<0.05). Presented in (B) are mean (±SEM) SI times from baseline, or following CNQX-Veh (n=6) or AP5-veh (n=5) intra-BNST injections. Neither CNQX nor AP5 altered SI times compared to baseline. In (C) the ability to block the anxiety-like responses of BNST ORX-A injections, by an AMPAr antagonist, DNQX, with less affinity for the NMDAr than CNQX, was investigated. Presented in (C) are mean (±SEM) SI times (n=5, repeated measures, counter balanced design) at baseline (grey bar), and following BNST injection of the AMPAr antagonist DNQX followed by Veh (DNQX-Veh, grey striped bar), Veh followed by ORX-A (Veh-ORX, solid bar), and DNQX followed by ORX-A (DNQX-ORX, thin black striped bar). Again, Veh-ORX rats, but not DNQX-Veh or DNQX-ORX, had significantly reduced SI times compared to baseline (* p<0.05).
4. Discussion
ORX has been demonstrated to play a role in anxiety in multiple species including the rat, mouse, hamster and human [15, 18–21]. The anxiety-modulating effects of benzodiazepines and selective serotonin reuptake inhibitors have recently been suggested to have influence over the ORX system that correlate with anxiety-like behaviors [23, 59]. Yet few studies have investigated the potential sites through which ORX targets may induce anxiety. Previous work from our laboratory demonstrated that anxiety-like responses to a systemically delivered panicogenic stimulus could be blocked by placing an ORX1r antagonist directly into the BNST, suggesting that one of the sites responsible for regulating panic/anxiety-like behavior is the BNST and that ORX is a key neural substrate in this process [15]. Here we further support ORX’s regulation of anxiety occurring through the BNST by demonstrating that placing ORX-A into the BNST is sufficient to induce anxiety-like behavior in two different validated anxiety tests; the EPM and SI tests. In regards to the SI test, increased anxiety-like behavior by injections of ORX-A into the BNST was replicated multiple times within the current study, and this anxiogenic effect of ORX-A appears to be regionally selective as injections that were dorsal and medial to the BNST (within the medial septum) failed to induce anxiety-like responses. Similar anxiogenic responses to ORX are reported following other site-directed injections of ORX. Injections of ORX into the paraventricular nucleus of the thalamus in rats and the central amygdala of hamsters induced anxiety-like behavior in the elevated plus maze [18, 21]. Interestingly, both of these loci are related via either proximity (paraventricular nucleus of the thalamus, caudal/medial border of caudal BNST) or function (central amygdala and BNST collectively form the extended amygdala) to the BNST. So, there could well be a network of sites modulated by ORX input that are capable of enhancing anxiety-like responses.
ORX-A has affinity for both the ORX1r and ORX2r, and in this study, selectivity of action for these receptors was not systematically investigated. However, it is likely that the anxiogenic effect of ORX-A, within the BNST, is primarily via activation of ORX1r. As stated above, antagonizing ORX1r specifically in the BNST completely blocked the anxiety-like behaviors induced by systemic anxiogenic challenges [15]. Furthermore, the ORX1r has been shown, via gene expression profiles, to be the predominant ORX receptor [28] or the only ORX receptor expressed in the BNST [27]. However, the involvement of the ORX2r in BNST regulation of anxiety has not been systematically tested and thus the involvement of ORX2r cannot be ruled out at this time.
In the BNST, ORX-A also likely induces anxiety-like behaviors via a direct enhancement of excitatory mechanisms on BNST neurons. Here we demonstrate for the first time, to our knowledge, that ORX-A produced depolarization and in some cases action potentials in BNST neurons. These effects were long-lasting as the depolarization continued for several minutes after the ORX-A was removed. Similar depolarizing effects of ORX-A have been reported in multiple brain regions including, brain stem, midbrain, diencephalic and telencephalic structures [38–49]. ORX-A induced depolarization has been linked to increased protein kinase C activity [41, 47, 52, 60], phospholipase C activity [41, 52, 61], cation influx [47, 49, 62, 63], as well as increased intracellular Ca2+ [40, 41, 64]. In some cases ORX-A induced depolarization may be mediated via indirect action and is likely a result of increased glutamate release [39].
The present data suggest that glutamate and ORX interaction may be necessary for ORX-A’s anxiogenic effects in the BNST. Induction of anxiety by ORX-A into the BNST was completely blocked by pre-treating the BNST with the NMDAr antagonist AP5, while pretreatment with AMPAr antagonists produced an attenuation, but not complete blockade of the ORX-A induced anxiety, suggesting an interaction between ORX-A and the glutamatergic system in the BNST with a stronger role for NMDAr and an inconclusive role of the AMPAr. Similarly, ORX-A in the ventral tegmental area has been demonstrated to potentiate neuronal responses to endogenous glutamate release, and ORX1r antagonism reduced VTA neuronal activity [51, 52]. Glutamate and ORX interactions in the VTA were also demonstrated by Borgland and colleagues [52], where they reported ORX-A rapidly potentiated NMDAr-mediated neurotransmission, via ORX1r, PLC/PKC dependent insertion into synapses. In this same study, ORX-A application had no effect on AMPAr-mediated neurotransmission 15 min after application, but did result in potentiation of AMPAr-mediated currents 3–4 hours after application [52]. The AMPAr antagonists’ partial blockade of BNST-ORX-A induced anxiety may suggest that in the BNST ORX-A induced changes in AMPAr mediated events may start as early as 30 min after application.
At this point the source of the glutamate that interacts with ORX-A in the BNST to produce anxiety-like effects is unclear. One potential source of glutamate for this interaction is from the ORX neurons themselves. Evidence for glutamate and ORX co-localization and co-release from ORX terminals has been reported in the Locus Coeruleus [50]. Thus it is possible that ORX neurons originating from the hypothalamus and projecting to the BNST also contain both ORX and glutamate. These BNST projecting ORX neurons may be activated at a low frequency by cues for increased vigilance, possibly leading only to release of glutamate. However, when the cue signals something beyond increased vigilance and becomes anxiogenic, the BNST projecting ORX neurons may respond with high frequency activation, required for release of neuropeptides (for review see [65]), then both glutamate and ORX could be co-released onto BNST neurons. The result on the post-synaptic BNST neurons would be ORX induced potentiation of glutamate responses to produce a potent depolarization leading to the expression of anxiety-like behaviors.
Highlights.
Orexin A into the Bed Nucleus of the Stia terminalis is sufficient to induce anxiety-like behaviors in male rats
Orexin A induces depolarization and action potentials in a subset of BNST neurons
Orexin A interaction with NMDA-type glutamate receptors is necessary for anxiety-like behaviors induced by orexin A injections into the BNST
Acknowledgments
This research was supported by RO1s MH52619 and MH065702.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:322–7. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–85. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 3.Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, et al. Neurons containing hypocretin (orexin) project to multiple neuronal systems. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nambu T, Sakurai T, Mizukami K, Hosoya Y, Yanagisawa M, Goto K. Distribution of orexin neurons in the adult rat brain. Brain research. 1999;827:243–60. doi: 10.1016/s0006-8993(99)01336-0. [DOI] [PubMed] [Google Scholar]
- 5.Sakurai T. Orexin: a link between energy homeostasis and adaptive behaviour. Curr Opin Clin Nutr Metab Care. 2003;6:353–60. doi: 10.1097/01.mco.0000078995.96795.91. [DOI] [PubMed] [Google Scholar]
- 6.Thompson JL, Borgland SL. A role for hypocretin/orexin in motivation. Behavioural brain research. 2011;217:446–53. doi: 10.1016/j.bbr.2010.09.028. [DOI] [PubMed] [Google Scholar]
- 7.Sakurai T, Mieda M. Connectomics of orexin-producing neurons: interface of systems of emotion, energy homeostasis and arousal. Trends in pharmacological sciences. 2011;32:451–62. doi: 10.1016/j.tips.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 8.Kuwaki T. Orexin links emotional stress to autonomic functions. Autonomic neuroscience : basic & clinical. 2011;161:20–7. doi: 10.1016/j.autneu.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Boutrel B, Cannella N, de Lecea L. The role of hypocretin in driving arousal and goal-oriented behaviors. Brain research. 2010;1314:103–11. doi: 10.1016/j.brainres.2009.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brundin L, Bjorkqvist M, Petersen A, Traskman-Bendz L. Reduced orexin levels in the cerebrospinal fluid of suicidal patients with major depressive disorder. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 2007;17:573–9. doi: 10.1016/j.euroneuro.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Brundin L, Petersen A, Bjorkqvist M, Traskman-Bendz L. Orexin and psychiatric symptoms in suicide attempters. Journal of affective disorders. 2007;100:259–63. doi: 10.1016/j.jad.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 12.Rotter A, Asemann R, Decker A, Kornhuber J, Biermann T. Orexin expression and promoter-methylation in peripheral blood of patients suffering from major depressive disorder. Journal of affective disorders. 2011;131:186–92. doi: 10.1016/j.jad.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt FM, Brugel M, Kratzsch J, Strauss M, Sander C, Baum P, et al. Cerebrospinal fluid hypocretin-1 (orexin A) levels in mania compared to unipolar depression and healthy controls. Neuroscience letters. 2010;483:20–2. doi: 10.1016/j.neulet.2010.07.038. [DOI] [PubMed] [Google Scholar]
- 14.Strawn JR, Pyne-Geithman GJ, Ekhator NN, Horn PS, Uhde TW, Shutter LA, et al. Low cerebrospinal fluid and plasma orexin-A (hypocretin-1) concentrations in combat-related posttraumatic stress disorder. Psychoneuroendocrinology. 2010;35:1001–7. doi: 10.1016/j.psyneuen.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Johnson PL, Truitt W, Fitz SD, Minick PE, Dietrich A, Sanghani S, et al. A key role for orexin in panic anxiety. Nat Med. 2010;16:111–5. doi: 10.1038/nm.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perna G, Guerriero G, Caldirola D. Emerging drugs for panic disorder. Expert Opin Emerg Drugs. 2011;16:631–45. doi: 10.1517/14728214.2011.628313. [DOI] [PubMed] [Google Scholar]
- 17.Borgland SL, Labouebe G. Orexin/hypocretin in psychiatric disorders: present state of knowledge and future potential. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35:353–4. doi: 10.1038/npp.2009.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Avolio E, Alo R, Carelli A, Canonaco M. Amygdalar orexinergic-GABAergic interactions regulate anxiety behaviors of the Syrian golden hamster. Behavioural brain research. 2011;218:288–95. doi: 10.1016/j.bbr.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki M, Beuckmann CT, Shikata K, Ogura H, Sawai T. Orexin-A (hypocretin-1) is possibly involved in generation of anxiety-like behavior. Brain research. 2005;1044:116–21. doi: 10.1016/j.brainres.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 20.Plaza-Zabala A, Martin-Garcia E, de Lecea L, Maldonado R, Berrendero F. Hypocretins regulate the anxiogenic-like effects of nicotine and induce reinstatement of nicotine-seeking behavior. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:2300–10. doi: 10.1523/JNEUROSCI.5724-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Y, Li S, Wei C, Wang H, Sui N, Kirouac GJ. Orexins in the paraventricular nucleus of the thalamus mediate anxiety-like responses in rats. Psychopharmacology. 2010;212:251–65. doi: 10.1007/s00213-010-1948-y. [DOI] [PubMed] [Google Scholar]
- 22.Singareddy R, Uhde T, Commissaris R. Differential effects of hypocretins on noise-alone versus potentiated startle responses. Physiology & behavior. 2006;89:650–5. doi: 10.1016/j.physbeh.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 23.Panhelainen AE, Korpi ER. Evidence for a role of inhibition of orexinergic neurons in the anxiolytic and sedative effects of diazepam: A c-Fos study. Pharmacology, biochemistry, and behavior. 2011;101:115–24. doi: 10.1016/j.pbb.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 24.Ida T, Nakahara K, Murakami T, Hanada R, Nakazato M, Murakami N. Possible involvement of orexin in the stress reaction in rats. Biochemical and biophysical research communications. 2000;270:318–23. doi: 10.1006/bbrc.2000.2412. [DOI] [PubMed] [Google Scholar]
- 25.Johnson PL, Samuels BC, Fitz SD, Federici L, Hammes N, Early MC, et al. Orexin 1 receptors are a novel target to modulate panic responses and the panic brain network. Physiology & behavior. doi: 10.1016/j.physbeh.2012.04.016. In this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sakurai T. The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness. Nat Rev Neurosci. 2007;8:171–81. doi: 10.1038/nrn2092. [DOI] [PubMed] [Google Scholar]
- 27.Trivedi P, Yu H, MacNeil DJ, Van der Ploeg LH, Guan XM. Distribution of orexin receptor mRNA in the rat brain. FEBS letters. 1998;438:71–5. doi: 10.1016/s0014-5793(98)01266-6. [DOI] [PubMed] [Google Scholar]
- 28.Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, et al. Differential expression of orexin receptors 1 and 2 in the rat brain. The Journal of comparative neurology. 2001;435:6–25. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- 29.Lee Y, Davis M. Role of the hippocampus, the bed nucleus of the stria terminalis, and the amygdala in the excitatory effect of corticotropin-releasing hormone on the acoustic startle reflex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1997;17:6434–46. doi: 10.1523/JNEUROSCI.17-16-06434.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Treit D, Aujla H, Menard J. Does the bed nucleus of the stria terminalis mediate fear behaviors? Behav Neurosci. 1998;112:379–86. doi: 10.1037//0735-7044.112.2.379. [DOI] [PubMed] [Google Scholar]
- 31.Sullivan GM, Apergis J, Bush DE, Johnson LR, Hou M, Ledoux JE. Lesions in the bed nucleus of the stria terminalis disrupt corticosterone and freezing responses elicited by a contextual but not by a specific cue-conditioned fear stimulus. Neuroscience. 2004;128:7–14. doi: 10.1016/j.neuroscience.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 32.Davis M. Neural systems involved in fear and anxiety measured with fear-potentiated startle. Am Psychol. 2006;61:741–56. doi: 10.1037/0003-066X.61.8.741. [DOI] [PubMed] [Google Scholar]
- 33.Sink KS, Walker DL, Yang Y, Davis M. Calcitonin gene-related peptide in the bed nucleus of the stria terminalis produces an anxiety-like pattern of behavior and increases neural activation in anxiety-related structures. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:1802–10. doi: 10.1523/JNEUROSCI.5274-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Waddell J, Morris RW, Bouton ME. Effects of bed nucleus of the stria terminalis lesions on conditioned anxiety: aversive conditioning with long-duration conditional stimuli and reinstatement of extinguished fear. Behavioral neuroscience. 2006;120:324–36. doi: 10.1037/0735-7044.120.2.324. [DOI] [PubMed] [Google Scholar]
- 35.Walker DL, Davis M. Double dissociation between the involvement of the bed nucleus of the stria terminalis and the central nucleus of the amygdala in startle increases produced by conditioned versus unconditioned fear. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1997;17:9375–83. doi: 10.1523/JNEUROSCI.17-23-09375.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee Y, Fitz S, Johnson PL, Shekhar A. Repeated stimulation of CRF receptors in the BNST of rats selectively induces social but not panic-like anxiety. Neuropsychopharmacology. 2008;33:2586–94. doi: 10.1038/sj.npp.1301674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sajdyk T, Johnson P, Fitz S, Shekhar A. Chronic inhibition of GABA synthesis in the bed nucleus of the stria terminalis elicits anxiety-like behavior. Journal of psychopharmacology. 2008;22:633–41. doi: 10.1177/0269881107082902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eriksson KS, Sergeeva O, Brown RE, Haas HL. Orexin/hypocretin excites the histaminergic neurons of the tuberomammillary nucleus. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21:9273–9. doi: 10.1523/JNEUROSCI.21-23-09273.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Follwell MJ, Ferguson AV. Cellular mechanisms of orexin actions on paraventricular nucleus neurones in rat hypothalamus. The Journal of physiology. 2002;545:855–67. doi: 10.1113/jphysiol.2002.030049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burdakov D, Liss B, Ashcroft FM. Orexin excites GABAergic neurons of the arcuate nucleus by activating the sodium--calcium exchanger. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:4951–7. doi: 10.1523/JNEUROSCI.23-12-04951.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang B, Samson WK, Ferguson AV. Excitatory effects of orexin-A on nucleus tractus solitarius neurons are mediated by phospholipase C and protein kinase C. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:6215–22. doi: 10.1523/JNEUROSCI.23-15-06215.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu M, Zaborszky L, Hajszan T, van den Pol AN, Alreja M. Hypocretin/orexin innervation and excitation of identified septohippocampal cholinergic neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:3527–36. doi: 10.1523/JNEUROSCI.5364-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murai Y, Akaike T. Orexins cause depolarization via nonselective cationic and K+ channels in isolated locus coeruleus neurons. Neuroscience research. 2005;51:55–65. doi: 10.1016/j.neures.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 44.Brown RE, Winston S, Basheer R, Thakkar MM, McCarley RW. Electrophysiological characterization of neurons in the dorsolateral pontine rapid-eye-movement sleep induction zone of the rat: Intrinsic membrane properties and responses to carbachol and orexins. Neuroscience. 2006;143:739–55. doi: 10.1016/j.neuroscience.2006.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Govindaiah G, Cox CL. Modulation of thalamic neuron excitability by orexins. Neuropharmacology. 2006;51:414–25. doi: 10.1016/j.neuropharm.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 46.Kolaj M, Doroshenko P, Yan Cao X, Coderre E, Renaud LP. Orexin-induced modulation of state-dependent intrinsic properties in thalamic paraventricular nucleus neurons attenuates action potential patterning and frequency. Neuroscience. 2007;147:1066–75. doi: 10.1016/j.neuroscience.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 47.Kohlmeier KA, Watanabe S, Tyler CJ, Burlet S, Leonard CS. Dual orexin actions on dorsal raphe and laterodorsal tegmentum neurons: noisy cation current activation and selective enhancement of Ca2+ transients mediated by L-type calcium channels. Journal of neurophysiology. 2008;100:2265–81. doi: 10.1152/jn.01388.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kolaj M, Coderre E, Renaud LP. Orexin peptides enhance median preoptic nucleus neuronal excitability via postsynaptic membrane depolarization and enhancement of glutamatergic afferents. Neuroscience. 2008;155:1212–20. doi: 10.1016/j.neuroscience.2008.06.059. [DOI] [PubMed] [Google Scholar]
- 49.Kim J, Nakajima K, Oomura Y, Wayner MJ, Sasaki K. Electrophysiological effects of orexins/hypocretins on pedunculopontine tegmental neurons in rats: an in vitro study. Peptides. 2009;30:191–209. doi: 10.1016/j.peptides.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 50.Henny P, Brischoux F, Mainville L, Stroh T, Jones BE. Immunohistochemical evidence for synaptic release of glutamate from orexin terminals in the locus coeruleus. Neuroscience. 2010;169:1150–7. doi: 10.1016/j.neuroscience.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moorman DE, Aston-Jones G. Orexin/hypocretin modulates response of ventral tegmental dopamine neurons to prefrontal activation: diurnal influences. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:15585–99. doi: 10.1523/JNEUROSCI.2871-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron. 2006;49:589–601. doi: 10.1016/j.neuron.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 53.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 5. Amsterdam ; Boston: Elsevier Academic Press; 2005. [Google Scholar]
- 54.Sajdyk TJ, Schober DA, Gehlert DR, Shekhar A. Role of corticotropin-releasing factor and urocortin within the basolateral amygdala of rats in anxiety and panic responses. Behavioural brain research. 1999;100:207–15. doi: 10.1016/s0166-4328(98)00132-6. [DOI] [PubMed] [Google Scholar]
- 55.Johnson PL, Shekhar A. Panic-prone state induced in rats with GABA dysfunction in the dorsomedial hypothalamus is mediated by NMDA receptors. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:7093–104. doi: 10.1523/JNEUROSCI.0408-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.File SE. The use of social interaction as a method for detecting anxiolytic activity of chlordiazepoxide-like drugs. J Neurosci Methods. 1980;2:219–38. doi: 10.1016/0165-0270(80)90012-6. [DOI] [PubMed] [Google Scholar]
- 57.Shekhar A, Keim SR. The circumventricular organs form a potential neural pathway for lactate sensitivity: implications for panic disorder. J Neurosci. 1997;17:9726–35. doi: 10.1523/JNEUROSCI.17-24-09726.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Molosh AI, Johnson PL, Fitz SD, Dimicco JA, Herman JP, Shekhar A. Changes in central sodium and not osmolarity or lactate induce panic-like responses in a model of panic disorder. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35:1333–47. doi: 10.1038/npp.2010.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nollet M, Gaillard P, Minier F, Tanti A, Belzung C, Leman S. Activation of orexin neurons in dorsomedial/perifornical hypothalamus and antidepressant reversal in a rodent model of depression. Neuropharmacology. 2011;61:336–46. doi: 10.1016/j.neuropharm.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 60.Louhivuori LM, Jansson L, Nordstrom T, Bart G, Nasman J, Akerman KE. Selective interference with TRPC3/6 channels disrupts OX1 receptor signalling via NCX and reveals a distinct calcium influx pathway. Cell Calcium. 2010;48:114–23. doi: 10.1016/j.ceca.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 61.Lund PE, Shariatmadari R, Uustare A, Detheux M, Parmentier M, Kukkonen JP, et al. The orexin OX1 receptor activates a novel Ca2+ influx pathway necessary for coupling to phospholipase C. The Journal of biological chemistry. 2000;275:30806–12. doi: 10.1074/jbc.M002603200. [DOI] [PubMed] [Google Scholar]
- 62.Van Den Pol AN, Patrylo PR, Ghosh PK, Gao XB. Lateral hypothalamus: early developmental expression and response to hypocretin (orexin) The Journal of comparative neurology. 2001;433:349–63. doi: 10.1002/cne.1144. [DOI] [PubMed] [Google Scholar]
- 63.van den Pol AN, Gao XB, Obrietan K, Kilduff TS, Belousov AB. Presynaptic and postsynaptic actions and modulation of neuroendocrine neurons by a new hypothalamic peptide, hypocretin/orexin. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1998;18:7962–71. doi: 10.1523/JNEUROSCI.18-19-07962.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Uramura K, Funahashi H, Muroya S, Shioda S, Takigawa M, Yada T. Orexin-a activates phospholipase C- and protein kinase C-mediated Ca2+ signaling in dopamine neurons of the ventral tegmental area. Neuroreport. 2001;12:1885–9. doi: 10.1097/00001756-200107030-00024. [DOI] [PubMed] [Google Scholar]
- 65.Bartfai T, Iverfeldt K, Fisone G, Serfozo P. Regulation of the release of coexisting neurotransmitters. Annu Rev Pharmacol Toxicol. 1988;28:285–310. doi: 10.1146/annurev.pa.28.040188.001441. [DOI] [PubMed] [Google Scholar]