Abstract
Purpose
To examine the relation of increased ocular asymmetry over time on vision-related quality of life in keratoconus.
Methods
Subjects were in the Collaborative Longitudinal Evaluation of Keratoconus (CLEK) Study and had complete data on a least one scale of the NEI VFQ and examination data at baseline and at least one follow-up visit. Three measures of disease asymmetry (visual acuity, corneal curvature, and refractive error) and better eye status were assessed. Multilevel models were fit to the data.
Results
Analyses were completed using 961 subjects. Six scales on the NEI VFQ had adequate variability to model (distance activity, driving, mental health, near activity, ocular pain, and role difficulties). Refractive error changes were not associated with statistically significant quality of life differences. Except for ocular pain, statistically significant, but not clinically meaningful, differences were found for visual acuity changes and corneal curvature changes. For a 0.1-unit logMAR visual acuity change, the quality of life scales decreased between 0.20 and 0.99 units. For a 1.00-D steepening of corneal curvature these decreases were on the order of 0.20 to 0.59 units. Changes related to asymmetry were small as well: decreases on the order of 0.20 to 0.38 units.
Conclusions
Increasing ocular asymmetry and decreases in visual acuity and corneal steepening in the better eye were associated with decreasing vision-related quality of life, though the magnitudes of the changes were not clinically meaningful. Of these two disease status indicators, the vision in the better eye had greater effect on vision-related quality of life.
Keywords: Keratoconus, quality of life, longitudinal
Keratoconus is a progressive, binocularly asymmetric thinning of the cornea.1-2 Corneal thinning leads to irregular astigmatism and distorted vision, which negatively affects keratoconus patients’ vision-related quality of life, as measured by the National Eye Institute Visual Function Questionnaire (NEI-VFQ).3-4 Despite relatively good visual acuity,5-6 keratoconus patients report vision-related quality of life scores similar to patients with moderate to severe age-related macular degeneration.3 With time, most aspects of keratoconus patients’ vision-related quality of life decline. The factors most closely associated with poor vision-related quality of life are poor visual acuity and steep corneal curvature.3 Over time, decreases in visual acuity and corneal steepening are associated with decreases in vision-related quality of life.7
Asymmetry increases with disease severity in keratoconus,8 so the vision-related quality of life may decline as asymmetry increases; however, it is assumed that binocular visual acuity is equal to or better than the vision of the eye that sees most clearly9 so vision-related quality of life would not necessarily decline with increasing asymmetry. No previous publications have examined the association between disease asymmetry and vision-related quality of life in keratoconus. This paper will explore the effect of increased ocular asymmetry as measured by visual acuity, corneal curvature, and refractive error (controlling for disease severity) on vision-related quality of life.
Methods
The Collaborative Longitudinal Evaluation of Keratoconus (CLEK) Study is a multi-center, observational study of the natural progression of keratoconus. Details of the CLEK Study methods have been previously presented,5 but a brief description follows. Between May, 1995 and June, 1996, 1,209 subjects were enrolled. The protocol was approved by the institutional review board of each clinic, and all subjects provided informed consent. The inclusion criteria for entrance into the study were that the potential subject was at least 12 years of age; an irregular cornea in either eye as determined by the distortion of keratometric mires or of the red or retinoscopic reflex; and at least one biomicroscopic sign, including Vogt’s striae, Fleischer’s ring of 2 mm or more of arc, or corneal scarring typical of keratoconus. Potential subjects did not qualify if they had bilateral corneal transplants or non-keratoconic eye disease in either eye.
Three indicators of disease status were considered in this study: visual acuity, corneal curvature and refractive error. Binocular, high contrast logMAR visual acuity (VA) was assessed according to a standardized protocol5, 10 while patients wore their habitual vision correction. Corneal curvature was specified by computing the average of two keratometric measurements of the steep corneal meridian of each eye. Refractive error was measured by manifest refraction using standard subjective techniques with additional methods such as large steps between choices, high powered Jackson crossed cylinder lenses, and subjective cylinder axis orientation. Spherical equivalent refractive error was calculated from the manifest sphere and cylinder.
All CLEK subjects completed the 25-item National Eye Institute Visual Function Questionnaire (NEI-VFQ) annually beginning with the second examination; the NEI-VFQ was not readily available before the second visit.11-13 Subjects used for the analyses presented here were required to have completed the NEI-VFQ at the second examination. All eligible data on or after this visit were used beginning with the second examination. In what follows, “baseline” refers to the second visit. Data from a visit were included if there were NEI-VFQ responses and if indicators of disease status were available for both eyes. If an eye had a corneal transplant, all data collected after the transplant were treated as missing.
Twelve scales are contained in the NEI-VFQ. The scale scores were computed according to the algorithm developed by Mangione and colleagues, and they range from 0 (worst) to 100 (best). The distribution of the responses presented an issue for some of the quality of life scales. Two scales, the dependency scale and the social functioning scale, showed signs of a ceiling effect where at least 70% of the responses were 100. Four other scales had limited variability (i.e., no more than five different responses): health perception, general vision, color vision, and peripheral vision. The analyses, therefore, used only these scales: distance activities, driving, mental health, near activities, ocular pain, and role difficulties.
The outcome of interest was the change in quality of life. The goal was to assess whether deterioration in the disease status of a subject’s better eye or increase in the difference in the status of a subject’s two eyes correlated with a change in quality of life. For each disease indicator, the better eye was determined by comparing the within-eye averages of the indicator over the subject’s visits. The better eye was: for visual acuity – the eye with the lower average logMAR visual acuity; for spherical equivalent refractive error – the eye with the spherical equivalent closest to plano; and for keratometry in the steep meridian – the eye with the flatter average keratometry value. Asymmetry was defined as the difference between the two eyes for each of these variables. Binocular visual acuity was also included in models as a predictor to determine whether there was a different effect than the one seen for the visual acuity in the better eye.
For each quality of life scale and each indicator of disease status, a multilevel model was fit to assess the relation between the change in quality of life and the change in disease status. The model assumed the change in a quality of life scale was continuous and normally distributed. It controlled for the passage of time, gender, baseline age, disease status, and scale score. For each scale, a visual inspection of the scale score change over time indicated that a linear model in time was an acceptable fit. Information from all visits were incorporated into the model to account for the variation in quality of life over the course of the study, rather than just assessing change as a simple difference between the last and first study visit.
The model included one interaction between baseline scale score and time to account for those with higher baseline scores who have less room to improve on a given scale score due to ceiling effects (100 points). The model also adjusted for the baseline value of the disease indicator in the better eye and the baseline value of disease asymmetry. The predictors of most interest to this research were the change in the disease severity in the better eye and the change in asymmetry, both relative to baseline.
For the purpose of modeling, visual acuity was converted to logMAR and multiplied by a factor of 10. This results in visual acuity effect estimates that represent a change in logMAR of 0.1 units (1 line).
In addition to considering change in quality of life as a continuous variable, we dichotomized the change. A decrease in quality of life relative to baseline of 10 or more units was viewed as indicating a meaningful change in quality of life. Generalized estimating equations were used to assess the relation between at least a 10-point decrease in a quality of life scale and measures of asymmetry, while accounting for the repeated measures.
Results
There were 961 subjects who had complete data for at least one scale and one disease status indicator at baseline and at least one subsequent visit. The number of subjects and visits used to fit a model depended on the scale and the disease status indicator. The most data were available for the visual acuity models, where four of the six scales used 5,692 observations from the 961 subjects. The least data were available for the corneal curvature models, where four of the six scale analyses used 5,636 observations from 953 of the subjects. One scale analysis, driving, used only 5,337 observations from 911 of the subjects.
Demographic data and the pertinent clinical features considered in the paper are presented in Table 1. The average age of the subjects included in these analyses was 40.2 ± 10.8 years (range 13 to 78 years old). Forty-five percent of the subjects were female, and 71% were white. The number of visits ranged from two to eight, with a mean follow-up time in years of 6.3 years ± 1.7.
Table 1.
Demographic and clinical variables of the subjects analyzed in this paper.
| Number | Percent or mean ± SD | |
|---|---|---|
| Age (years) | 961 | 40.2 ± 10.8 |
| Gender (% female) | 431 | 45.8 |
| Race | ||
| African-American | 168 | 17.5 |
| Asian/Pacific Islander | 17 | 1.8 |
| Hispanic | 73 | 7.6 |
| Native American | 7 | 0.7 |
| White | 686 | 71.4 |
| Other | 10 | 1.0 |
| Educational level | ||
| Less than high school | 35 | 3.6 |
| High school graduate | 147 | 15.3 |
| Some College | 269 | 28.0 |
| College graduate | 218 | 22.7 |
| Some graduate school | 292 | 30.4 |
| Contact lens wear | ||
| None | 141 | 14.7 |
| One eye only | 33 | 3.4 |
| Both eyes | 787 | 81.9 |
| Baseline logMAR visual acuity of the better eye | 961 | 0.08 ± .15 |
| Baseline logMAR visual acuity of the worse eye | 961 | 0.29 ± 0.33 |
| Baseline logMAR visual acuity asymmetry | 961 | 0.24 ± 0.28 |
| Baseline spherical equivalent of the better eye (D) | 953 | -5.28 ± 4.59 |
| Baseline spherical equivalent of the worse eye (D) | 953 | -8.01 ± 6.11 |
| Baseline spherical equivalent asymmetry (D) | 853 | 3.26 ± 4.02 |
| Baseline keratometry steep meridian of the better eye (D) | 953 | 48.42 ± 4.42 |
| Baseline keratometry steep meridian of the worse eye (D) | 953 | 52.86 ± 6.06 |
| Baseline keratometry steep meridian asymmetry (D) | 953 | 4.61 ± 4.87 |
Eighty-five percent of the subjects wore at least one contact lens. There was no asymmetry in contact lens wear (i.e., same wearing time between eyes) for almost 94% of the visits, and for a little less than half of the visits the reported change in the wearing time between visits was zero. Due to the negligible asymmetry, contact lens wear was not considered further in the analyses.
Also shown in Table 1 are the baseline values, by worse and better eye, for the three different visual status variables. The average baseline logMAR VA in the better eye was 0.08 (about 20/24), while the logMAR VA in the other eye was, on average, 0.29 (20/39). The average logMAR VA asymmetry at baseline was about 0.24 (± 0.28). The average spherical equivalent refractive error in the better eye was −5.28 D compared to an average of about −8.00 D in the weaker eye. This represents an average asymmetry of about 2.75 D. The average asymmetry at baseline in the keratometric reading of the steep meridian was about 4.60 D, with the better eye value being 48.40 D on average. Because identification of the better eye was determined looking across all visits within a subject, the baseline asymmetry was not simply the difference between the better and the worse eyes at baseline.
Table 2 displays the average observed change from baseline for each scale. Four of the scales have, on average, exhibited a small increase in the score relative to baseline (distance activity, driving, mental health, ocular pain), while the other two scales decreased slightly (near activity, role difficulty).
Table 2.
Average change by scale.
| Scale | Mean Change in Scale |
sd | Maximum decrease in scale |
Maximum improvement in scale |
|---|---|---|---|---|
| Distance Activity | 1.07 | 11.43 | −41.70 | 72.63 |
| Driving | 0.41 | 10.94 | −75.00 | 56.25 |
| Mental Health | 3.78 | 14.66 | −65.17 | 71.40 |
| Near Activity | −0.27 | 11.97 | −41.66 | 58.35 |
| Ocular Pain | 0.59 | 13.82 | −43.75 | 56.25 |
| Role Difficulty | −2.12 | 17.05 | −71.43 | 91.07 |
Table 3 displays, by disease indicators, the average observed change in asymmetry and status of the better eye relative to the baseline values. On average, all measures of disease status became more extreme. Asymmetry increased over all the indicators, visual acuity became worse in the better eye, corneal curvature steepened, and the refractive error became more myopic.
Table 3.
Observed average change in asymmetry and status of the better eye relative to baseline from univariate models. A positive change in asymmetry indicates increasing asymmetry. A positive change in the better eye indicates the eye declined for logMAR and corneal curvature. A negative change in spherical equivalent indicates increased myopia.
| Indicator | Type of change | Mean change | sd | Minimum change |
Maximum change |
|---|---|---|---|---|---|
| LogMAR*10 | Change in Asymmetry |
0.11 | 2.19 | −10.44 | 11.20 |
| LogMAR*10 | Change in Better Eye |
0.07 | 1.15 | −9.11 | 7.73 |
| LogMAR*10 OU acuity |
Change in Asymmetry |
0.11 | 2.19 | −10.44 | 11.20 |
| LogMAR*10 OU acuity |
Change in OU acuity |
0.03 | 0.94 | −7.02 | 6.50 |
| Steep Keratometric reading (D) |
Change in Asymmetry |
0.16 | 2.14 | −13.21 | 12.83 |
| Steep Keratometric reading (D) |
Change in Better Eye |
0.39 | 1.52 | −7.02 | 13.21 |
| Spherical equivalent (D) |
Change in Asymmetry |
0.30 | 2.98 | −31.00 | 15.80 |
| Spherical equivalent (D) |
Change in Better Eye |
−0.50 | 2.12 | −12.86 | 9.29 |
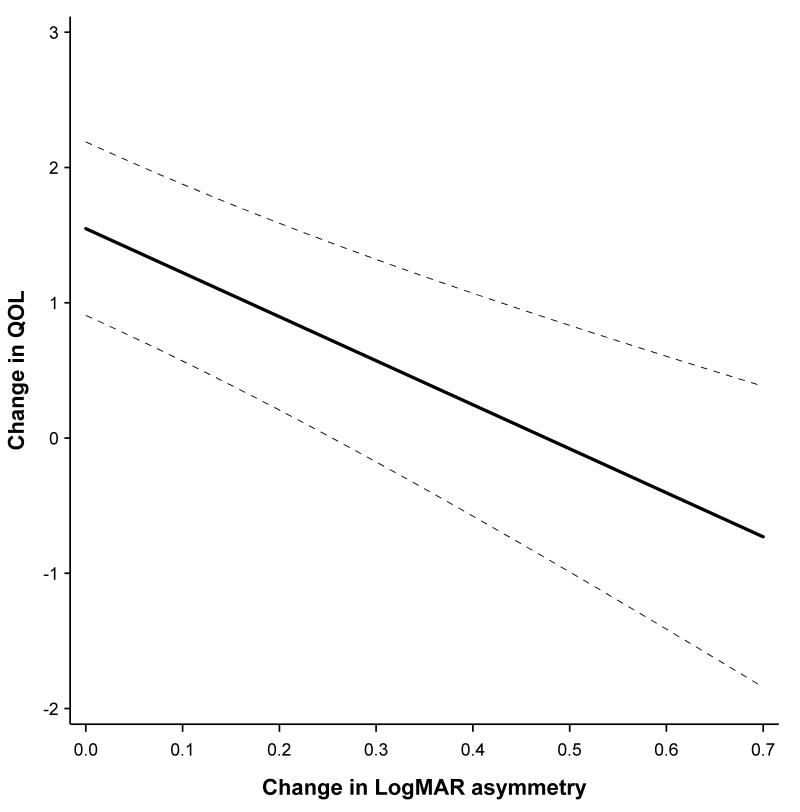
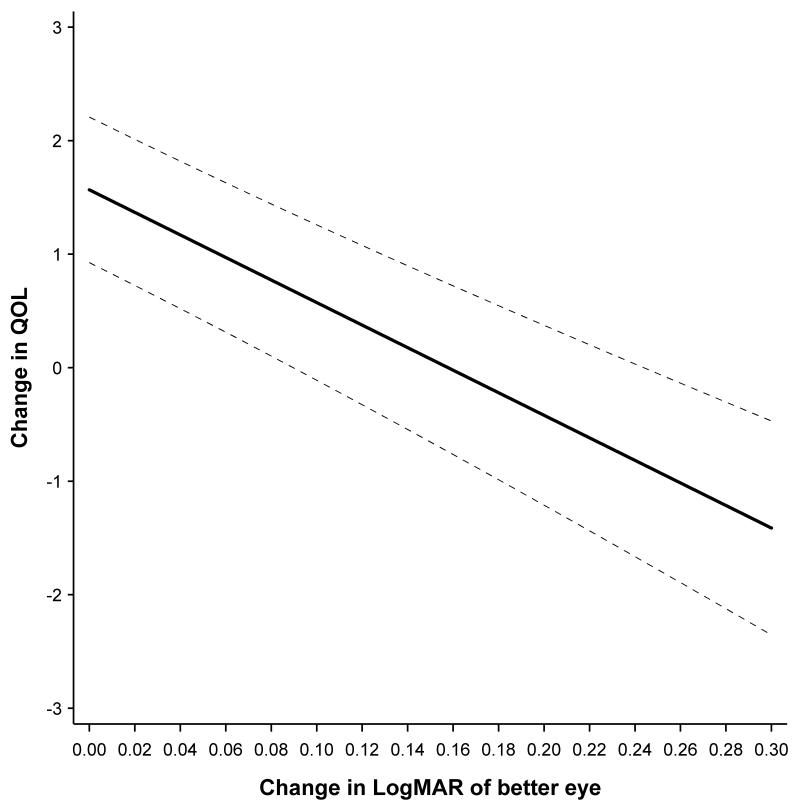
Tables 4 presents the results of interest to this study, namely – the effects of change in performance of the better eye and change in disease asymmetry on quality of life. Model parameter estimates are adjusted for the following variables: gender, baseline age, baseline quality of life scale score, an interaction between baseline score and time, the baseline status in the better eye, and baseline asymmetry. Across all of the scales except ocular pain, there was considerable evidence that there was a decrease in quality of life as asymmetry increased and the better eye worsened in visual acuity and its cornea steepened. So for example, for the distance activity scale, an increase in asymmetry of 0.1 units logMAR acuity resulted in a decrease of 0.33 units on this scale. A decrease in 0.1 units logMAR acuity in the better eye resulted in a drop of 0.99 units on the distance activity scale. To demonstrate the effect graphically the distance activity scale is chosen as an example, Figure 1 presents the predicted change in quality of life based on the change in the better eye. Figure 2 shows the predicted change quality of life based on the change in asymmetry for the distance activity scale.
Table 4.
Summary results for the parameters of change in asymmetry and change in the better eye. For binocular acuity, the ‘change in the better eye’ is simply the change in ‘binocular acuity.’
| Indicator | Type of change |
LogMAR*10 | LogMAR*10 OU acuity |
Corneal Curvature |
Spherical equivalent |
|---|---|---|---|---|---|
| Distance Activity |
Change in asymmetry |
−0.33 (−0.46,-0.19)** |
−0.26 (−0.39, −0.13) ** |
−0.23 (−0.36,−0.09) * |
−0.05 (−0.16, 0.05) |
| Distance Activity |
Change in better eye |
−0.99 (−1.22,−0.77) ** |
−1.57 (−1.85, −1.29) ** |
−0.43 (−0.64,−0.22) ** |
0.04 (−0.08, 0.17) |
| Driving | Change in asymmetry |
−0.37 (−0.51,−0.23) ** |
−0.30 (−0.44, −0.17) ** |
−0.34 (−0.49,−0.2) ** |
−0.09 (−0.19, 0.02) |
| Driving | Change in better eye |
−0.99 (−1.23,−0.75) ** |
−1.29 (−1.60, −0.98) ** |
−0.13 (−0.34, 0.09) |
0.06 (−0.07, 0.19) |
| Mental Health | Change in asymmetry |
−0.05 (−0.21, 0.10) |
−0.02 (−0.18, 0.13) |
−0.30 (−0.47,−0.14) * |
0.004 (−0.12, 0.12) |
| Mental Health | Change in better eye |
−0.59 (−0.86,−0.32) ** |
−1.06 (−1.40, −0.72) ** |
−0.56 (−0.81,−0.31) ** |
0.14 (−0.02, 0.29) |
| Near Activity | Change in asymmetry |
−0.38 (−0.53,−0.23) ** |
−0.32 (−0.47, −0.18) ** |
−0.26 (−0.41,−0.1) * |
0.01 (−0.10, 0.13) |
| Near Activity | Change in better eye |
−0.92 (−1.18,−0.67) ** |
−1.38 (−1.70, −1.06) ** |
−0.34 (−0.58,−0.11) * |
0.05 (−0.09, 0.19) |
| Ocular Pain | Change in asymmetry |
−0.13 (−0.31, 0.05) |
−0.13 (−0.30, 0.05) |
−0.21 (−0.4,−0.03) * |
−0.05 (−0.19, 0.08) |
| Ocular Pain | Change in better eye |
−0.14 (−0.45, 0.17) |
−0.19 (−0.58, 0.20) |
−0.27 (−0.54, 0.01) |
0.04 (−0.13, 0.21) |
| Role Difficulty | Change in asymmetry | −0.20 (−0.39,−0.01) * |
−0.14 (−0.33, 0.04) |
−0.17 (−0.37, 0.02) |
−0.03 (−0.18, 0.12) |
| Role Difficulty | Change in better eye |
−0.88 (−1.21,−0.55) ** |
−1.52 (−1.93, −1.11) ** |
−0.59 (−0.88,−0.29) ** |
0.21 (0.02,0.39) * |
- p< 0.05
- p< 0.0001
Figure 1.
Distance Activities - Predicted change in QOL as a function of change in LogMAR visual acuity of better eye.
Figure 2.
Distance Activities - Predicted change in QOL as a function of change in visual acuity asymmetry
For the steep meridian on keratometry, a 1.00-diopter (D) increase in asymmetry between eyes resulted in a decrease in the distance activity scale of 0.23 units, while a 1.00-D increase in the better eye resulted in a decrease of 0.43 units. There was little evidence that a refractive error change in asymmetry or in the better eye changed quality of life. On those scales with statistically significant effects for both change in asymmetry and change in the better eye, the change in the better eye represented the larger of the two effects. This indicates that the better eye had more influence on the quality of life outcome than asymmetry.
Table 5 presents the associated odds ratios for the effect of change in asymmetry and the change in the better eye on the chance of a 10-point decrease in the NEI VFQ scales. As in quality of life for the continuous measure, refractive error was not associated with a decrease in any scale of quality of life, either for change in asymmetry or for change in better eye. For the remaining indicators of visual status, a change in the better eye was associated with a statistically significant increase in the odds of a 10-unit decrease in quality of life with few exceptions (ocular pain and visual acuity measures, driving and corneal curvature). The magnitudes of the odds ratios were small, between 1.07 and 1.36. Increasing asymmetry was statistically significantly associated with a 10-point decrease in the driving and near activity quality of life scales for both visual acuity measures and corneal curvature, as well as for monocular visual acuity and corneal curvature for the distance activity quality of life scale. The magnitude of the effect was quite small though, on the order of odds ratios from 1.03 to 1.06.
Table 5.
Summary odds ratio results from multivariate models for a 10-unit change in quality of life by type of change - change in asymmetry and change in the better eye. For binocular acuity, the ‘change in the better eye’ is simply the change in ‘binocular acuity.’
| Indicator | Type of change |
LogMAR*10 OR (95% CI) |
LogMAR*10 OU acuity OR (95% CI) |
Corneal Curvature OR (95% CI) |
Spherical equivalent OR (95% CI) |
|---|---|---|---|---|---|
| Distance Activity |
Change in asymmetry |
1.04 (1.00, 1.08) * |
1.03 (0.99, 1.07) |
1.06 (1.02, 1.10) * |
1.01 (0.98, 1.04) |
| Distance Activity |
Change in better eye |
1.18 (1.11, 1.25) ** |
1.36 (1.26, 1.48) ** |
1.09 (1.04, 1.14) * |
0.97 (0.93, 1.01) |
| Driving | Change in asymmetry |
1.04 (1.01, 1.08) * |
1.03 (1.00, 1.06) * |
1.06 (1.03, 1.10) ** |
1.01 (0.99, 1.03) |
| Driving | Change in better eye |
1.16 (1.09, 1.23) ** |
1.19 (1.11, 1.28) ** |
1.01 (0.97, 1.06) |
1.00 (0.97, 1.03) |
| Mental Health | Change in asymmetry |
1.02 (0.99, 1.06) |
1.02 (0.98, 1.06) |
1.03 (0.99, 1.07) |
1.02 (0.99, 1.05) |
| Mental Health | Change in better eye |
1.09 (1.02, 1.16) * |
1.13 (1.04, 1.23) |
1.12 (1.06, 1.18) ** |
0.99 (0.95, 1.02) |
| Near Activity | Change in asymmetry |
1.06 (1.02, 1.10) * |
1.05 (1.02, 1.09) * |
1.03 (1.00, 1.07) * |
1.01 (0.99, 1.04) |
| Near Activity | Change in better eye |
1.13 (1.07, 1.20) ** |
1.23(1.15, 1.32) ** | 1.09 (1.04, 1.13) ** |
0.99 (0.97, 1.01) |
| Ocular Pain | Change in asymmetry | 1.02 (0.99, 1.05) |
1.02 (0.99, 1.04) |
1.03 (1.00, 1.06) * |
1.01 (0.99, 1.03) |
| Ocular Pain | Change in better eye |
1.03 (0.98, 1.08) |
1.06 (0.99, 1.13) |
1.08 (1.04, 1.12) ** |
0.98 (0.95, 1.00) |
| Role Difficulty |
Change in asymmetry |
1.02 (0.99, 1.05) |
1.01 (0.98, 1.04) |
1.01 (0.98, 1.03) |
1.01 (0.98, 1.02) |
| Role Difficulty |
Change in better eye |
1.13 (1.08, 1.19) ** |
1.20 (1.13, 1.27) ** |
1.07 (1.02, 1.11) * |
0.98 (0.95, 1.00) |
- p< 0.05
- p< 0.0001
Discussion
While the changes in quality of life in CLEK’s keratoconus subjects have been explored previously,4 we were interested in looking at the possible relation between the change in asymmetry and quality of life. Of particular interest was a comparison of the effect of the better eye compared to the effect of the asymmetry. Consistent with the original paper, average quality of life changes were small,7 and the various asymmetry parameters described were consistent with other publications.8, 14-15
Changes in the quality of life scales were associated with changes in the asymmetry of visual acuity and corneal curvature as well as the changes in the better eye, with few exceptions. Differences for refractive error, either measured by change in the better eye or change in asymmetry, were not statistically significant, for the most part, possibly because changes in refractive error are generally correctable by simple changes in refractive correction; however, none of the changes in the quality of life scales would be considered clinically relevant; the largest change approaches one unit on the quality of life scale that ranges from 0 to 100. Overall, if one were to pick the variable, asymmetry or better eye, that had a larger effect size, and therefore more influence, it would be change in the better eye.
Comparing the results of the changes in quality of life for the visual acuity in the better-seeing eye to the binocular visual acuity indicated that there was very little difference between these two measures of acuity. This was true whether the outcome was the continuous measure of quality of life or a large decrease in quality of life as measured by a 10-unit decrease.
Relatively few studies have presented longitudinal data related to the NEI-VFQ and disease state. Matza et al.16 examined data in a study of visual acuity in subjects with diabetic retinopathy. Over 18 months, changes on the same scales as we present ranged from small (0.1 units) to large (−22.9 units), depending on the scale and the change in visual acuity. For example, a decrease in visual acuity of 10 letters or more corresponded to an average decrease in the driving scale of 22.9 units. Those with little change in visual acuity experienced quality of life changes between −1.8 units and 2.2 units.16
The Age-Related Eye Disease Study Research Group looked at a 15-letter decrease in visual acuity over a one- to four-year time frame from questionnaire administration. There was relatively no change in ocular pain (+0.08 units), while the largest difference seen was for the driving scale, a decrease of 22.2 units.17
Subjects in the Los Angeles Latino Eye Study (LALES) reported differences between −0.3 units (driving) and +3.5 units (mental health), on average, from baseline to the four-year follow-up visit.18 For those experiencing no visual acuity change (within two lines of 20/20), the mean change in the quality of life ranged from −0.05 units (distance activities) to +3.1 units (ocular pain), while among those subjects who experienced at least two lines, the mean change was between 0.6 units (near activities) to −12.7 units (driving).
Visual changes in diabetic retinopathy and macular degeneration cannot be corrected by changes in refractive correction, whereas most changes in visual acuity, corneal curvature, and refractive error (as measured in this investigation and LALES) can be corrected with some form of visual correction. This is why the changes in quality of life are relatively small for this study, compared to other eye diseases.
Kymes et al.4evaluated the same large change in quality of life (10 points) in the CLEK Study for a large decrease in binocular acuity (10 letters) and a large increase (3.00 D) in corneal curvature. The odds ratios associated with a 10-point decline in these quality of life scales were of a much larger magnitude (ORs = 1.62 to 3.49 for corneal curvature and 1.20 to 2.19 for binocular visual acuity) than we saw for a 1-D change in corneal curvature asymmetry or a 0.1 unit (5 letters/1 line) change in visual acuity asymmetry. This indicates that using the same sample, the impact of asymmetry changing over time is minor compared to other characteristics of the disease process.
Limitations
Visual changes over time due to keratoconus are relatively slow,19primarily due to the ability of gas permeable contact lenses to correct the irregular astigmatism of many keratoconus patients, but perhaps also due to the subjects being slightly older at baseline (on average 40.2 years) so that they may be progressing less. Because the visual acuity does not change significantly over time for most keratoconus patients, the quality of life changes very little. The changes in quality of life in this study were generally not clinically relevant, thereby limiting the results to small changes. Perhaps larger changes in asymmetry versus larger changes in the better eye would have different effects on the quality of life, but it is difficult to determine from the relatively stable vision of this sample.
Conclusions
Most clinicians assume that the eye with the best visual acuity drives a patient’s quality of life, however prior to this investigation, there has never been a comparison of the effects of the best eye compared to differences between the eyes. Because keratoconus leads to relatively large asymmetries between the eyes, the question is particularly important in this disease. According to our findings, vision of the better eye and asymmetry are both important, but the vision of the better eye typically has a stronger effect on vision-related quality of life than the difference between the eyes.
ACKNOWLEDGMENTS
The CLEK Study Group (as of April 2004)
Clinical Centers
University of Alabama at Birmingham School of Optometry, Birmingham, AL: William J. “Joe” Benjamin, OD PhD (Principal Investigator), Carol Rosenstiel, OD (Co-Investigator); Maria S. Voce (Study Coordinator); Brian Marshall, OD (Co-Investigator, 1994-1995), C. Denise Pensyl, OD MS (Co-Investigator 1994-2000)
University of California, Berkeley School of Optometry, Berkeley, CA: Nina E. Friedman, OD MS (Principal Investigator), Dennis S. Burger, OD (Co-Investigator), Kelly A. McCann, MFA (Administrative Assistant, 2000-2001), Pamela Qualley, MA (Study Coordinator, 1994-2001), Karla Zadnik, OD PhD (Principal Investigator, 1994-1996)
University Hospitals of Cleveland and Case Western Reserve University, Department of Ophthalmology, Cleveland, OH: Loretta B. Szczotka, OD MS (Principal Investigator), Beth Ann Benetz, MA (Photographer), Ellen Burnside (Photographer), Stephanie Burke (Back-up Photographer), Janet Edgerton, COT (Technician), Mark Harrod (Photographer), Patricia Kane (Back-up Photographer), Jonathan H. Lass, MD (Co-Investigator), Jeffrey C. Lerner (Technician), Dawn McInture (Technician), Kristee Mines (Back-up Study Coordinator), Stephanie M. Shaffer, MA (Study Coordinator), Thomas Stokkermans, OD PhD (Co-Investigator), Pamela A. Smith (Technician, 1999-2002), Kimberly D. Supp (Technician, 1994-1999), Bonita Darby (Study Coordinator, 1994-1996), Ellen M. Stewart (Photographer, 1995-1997), Laura A. Teutsch (Technician, 1995-1999), Kimberly L. Schach (Study Coordinator, 2000-2002)
Gundersen Lutheran, La Crosse, WI: John L. Sterling, OD (Principal Investigator), Thomas M. Edwards, OD (Co-Investigator), Lisa J. Feuerhelm (Technician), Janet M. Hess (Study Coordinator/Technician), John D. Larson, OD (Co-Investigator), Jill A. Nelson (Study Coordinator/Technician), John M. Sake (Photographer), Lorna J. Plenge (Technician, 1995-2001), Eric M. Sheahan (Photographer, 1995-1999)
University of Illinois at Chicago Department of Ophthalmology, Chicago, IL: Timothy T. McMahon, OD (Principal Investigator), S. Barry Eiden, OD (Co-Investigator), Charlotte E. Joslin, OD (Co-Investigator), Tina M. Laureano (Study Coordinator), George A. Rosas (Technician), Brenda Smith (Technician), Tim Ehrecke (Photographer, 1994-1995), Mildred Santana (Technician, 1997), Jamie L. Brahmbatt (Study Coordinator, 1994-2000)
Indiana University School of Optometry, Bloomington, IN and Indianapolis Eye Care Center, Indianapolis, IN: Colleen Riley, OD MS (Principal Investigator), Gerald E. Lowther, OD PhD (Co-Investigator) Carolyn G. Begley, OD MS (Co-Investigator), Donna K. Carter (Study Coordinator/Technician), Nikole L. Himebaugh, OD (Co-Investigator), Pete S. Kollbaum, OD (Co-Investigator), Stephanie K. Sims (Back-up Study Coordinator), Lee M. Wagoner, MHA (Study Coordinator, 1996-2000)
Jules Stein Eye Institute UCLA, Los Angeles, CA: Barry A. Weissman, OD PhD (Principal Investigator), Lilian L. Andaya (Study Coordinator), Doris M. Boudaie, OD (Co-Investigator), Melissa W. Chun, OD (Co-Investigator), Ronit Englanoff, OD (Co-Investigator), Elisabeth T. Lim (Technician), Louis Rosenberg, OD (Co-Investigator), Arti S. Shah, OD (Co-Investigator), Lisa A. Barnhart, OD (Co-Investigator, 1995-2001), Karen K. Yeung, OD (Co-Investigator, 1999-2001)
University of Missouri-St. Louis School of Optometry, St. Louis, MO: Larry J. Davis, OD (Principal Investigator), Edward S. Bennett, OD MSEd (Co-Investigator), Beth A. Henderson, OD (Co-Investigator), Bruce W. Morgan, OD (Co-Investigator), Patricia Sanders, BS (Study Coordinator), Ivetta S. Siedlecki, OD (Co-Investigator), Zansheree L. Blue (Study Coordinator, 2000-2001), Monica J. Harris, OD (Co-Investigator, 2000-2001), Amber A. Reeves, MA (Study Coordinator, 1998-2000), Bruce W. Morgan, OD (Co-Investigator), Nancy M. Duquette (Study Coordinator, 1995-1998), Janene R. Sims, OD (Co-Investigator, 2000-2002)
State University of New York State College of Optometry, New York, NY: David P. Libassi, OD (Principal Investigator), Ralph E. Gundel, OD (Co-Investigator)
Northeastern Eye Institute, Scranton, PA: Joseph P. Shovlin, OD (Principal Investigator), John W. Boyle, OD (Co-Investigator), J. Bradley Flickinger, OD (Co-Investigator), M. Elizabeth Flickinger, OD (Co-Investigator), Stephen C. Gushue (Photographer), Patricia McMasters (Study Coordinator), Cheryl Haefele (Study Coordinator, 1994-2000), Stephen E. Pascucci, MD (Medical Monitor)
Nova Southeastern University College of Optometry, Ft. Lauderdale, FL: Heidi Wagner, OD (Principal Investigator), Andrea M. Janoff, OD (Co-Investigator), Chris Woodruff, OD (Photographer), Arnie Patrick, OD (Study Coordinator), Julie A. Tyler, OD (Study Coordinator), Karla E. Rumsey, OD (Co-Investigator 1995)
The Ohio State University College of Optometry, Columbus, OH: Barbara A. Fink, OD PhD (Principal Investigator), Lindsay Florkey (Study Coordinator), Gregory J. Nixon, OD (Co-Investigator), Jason J. Nichols, OD MS (Co-Investigator; Coordinator 1996-2001), Susan L. Sabers, OD (Study Coordinator, 1994-1996), Lisa Badowski, OD MS (Co-Investigator, 1995-1996)
Pennsylvania College of Optometry, Philadelphia, PA: Joel A. Silbert, OD (Principal Investigator), Kenneth M. Daniels, OD (Co-Investigator), Mary Jameson (Back-up Study Coordinator), Theresa E. Sanogo (Study Coordinator), David T. Gubman, OD, MS (Co-Investigator, 1998-2000)
Southern California College of Optometry, Fullerton, CA: Julie Yu, OD (Principal Investigator), Raymond H. Chu, OD (Co-Investigator), Timothy B. Edrington, OD MS (Co-Investigator; Principal Investigator, 1994-2002), Eunice Myung, OD (Co-Investigator), Julie A. Schornack, OD MEd (Co-Investigator), Terry Y. Tsang, OD (Co-Investigator, 1998-2000)
University of Utah, John Moran Eye Center, Department of Ophthalmology, Salt Lake City, UT: Harald E. Olafsson, OD (Principal Investigator), Doug M. Blanchard (Photographer), Deborah Y. Harrison, MS (Study Coordinator), Mark McKay, OD (Co-Investigator), Paula F. Morris (Photographer), Kimberley Wegner (Study Coordinator/Technician), Libbi A. Tracy, OD (Co-Investigator, 1995-1998), Kate M. Landro (Study Coordinator, 1995-1998), Lizbeth A. Malmquist (Technician, 1998), Marie Cason (Technician, 1995-1999), Craig M. Fehr (Technician, 1997-1999)
Former Clinical Centers
University of Texas at San Antonio Health Science Center Department of Ophthalmology, San Antonio, TX (1996): Julie A. Yu, OD (Principal Investigator), Beth Ann Benetz, MA (Photographer), E. Joseph Zayac, OD (Principal Investigator 1994-1996), Paul D. Comeau (Photographer 1994-1996), Ray V. Reil (Photographer 1994-1996), Sandra J. Hunt (Technician 1994-1996)
Resource Centers
Chairman’s Office, The Ohio State University College of Optometry, Columbus, OH: Karla Zadnik, OD PhD (Chairman), Lanna Blue (Secretary), Jodi M. Malone, RN (Study Coordinator), Jeffrey J. Walline, OD PhD (Optometrist), Dione Allen (Secretary, 1997-2000), Nora McFadden (Secretary, 2000-2002),
CLEK Photography Reading Center, The Ohio State University College of Optometry, Columbus, OH: Joseph T. Barr, OD MS (Director), Gilbert E. Pierce, OD PhD (Reader), Marjorie J. Rah, OD PhD (Reader, based at the New England College of Optometry), Mohinder Merchea, OD MS (Reader, based at Bausch & Lomb), Beth Oglevee (Study Coordinator), Gloria Scott-Tibbs (Study Coordinator), Robert Steffen, OD MS (Reader 1994-1995), Roanne Flom, OD (Reader 1998-2001)
Coordinating Center, Washington University Medical School, Department of Ophthalmology &Visual Sciences and the Division of Biostatistics, St. Louis, MO: Mae O. Gordon, PhD (Director), Joel Achtenberg, MSW (Senior Research Analyst), Patricia A. Nugent (Data Assistant), Teresa A. Roediger (Project Manager), Kenneth B. Schechtman, PhD (Statistician), Brad S. Wilson, MA (Statistical Data Analyst), Steven Kymes, PhD (Statistical Data Analyst), Karen Steger-May (Statistical Data Analyst), Michael Richman (Project Manager, 1994-1996)
CLEK Topography Reading Center, Department of Ophthalmology & Visual Sciences, University of Illinois at Chicago, Chicago, IL: Timothy T. McMahon, OD (Director), Robert J. Anderson, PhD (Biostatistician), Michi Goto (Research Assistant), Cynthia Roberts, PhD (Consultant), George A. Rosas (Study Coordinator), Loretta B. Szczotka, OD MS (Consultant), Mark Wright, MS (Programmer/Analyst), Stephanie K. Schoepfer-Grosskurth (Reader), Stephanie Walter Cooper (Reader, 1998), Thomas W. Raasch, OD PhD (Consultant 2000-2002), Dasia Corado (Reader, 2001)
Project Office, National Eye Institute, Rockville, MD: Donald F. Everett, MA
Committees
Executive Committee: Karla Zadnik, OD PhD (Chairman), Joseph T. Barr, OD MS, Mae O. Gordon, PhD, Timothy B. Edrington, OD MS, Donald F. Everett, MA, Timothy T. McMahon, OD
CLEK Topography Analysis Group: Loretta B. Szczotka, OD MS (Co-Chairman), Timothy T. McMahon, OD (Co-Chairman), Robert J. Anderson, PhD, Nina E. Friedman, OD MS, Larry J. Davis, OD, Thomas W. Raasch, OD PhD
Data Monitoring and Oversight Committee: Gary R. Cutter, PhD (Chairman), Robin L. Chalmers, OD, Bruce A. Barron, MD
Support: National Eye Institute/National Institutes of Health grants EY10419, EY10069, EY10077, EY12656, and EY02687, Conforma Contact Lenses, Paragon Vision Sciences, CIBA Vision Corporation, and the Ohio Lions Eye Research Foundation.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Krachmer JH, Feder RS, Belin MW. Keratoconus and related noninflammatory corneal thinning disorders. Surv Ophthalmol. 1984;28:293–322. doi: 10.1016/0039-6257(84)90094-8. [DOI] [PubMed] [Google Scholar]
- 2.Rabinowitz YS. Keratoconus. Surv Ophthalmol. 1998;42:297–319. doi: 10.1016/s0039-6257(97)00119-7. [DOI] [PubMed] [Google Scholar]
- 3.Kymes SM, Walline JJ, Zadnik K, Gordon MO. Quality of life in keratoconus. Am J Ophthalmol. 2004;138:527–35. doi: 10.1016/j.ajo.2004.04.031. [DOI] [PubMed] [Google Scholar]
- 4.Kymes SM, Walline JJ, Zadnik K, Sterling J, Gordon MO. Changes in the quality-of-life of people with keratoconus. Am J Ophthalmol. 2008;145:611–7. doi: 10.1016/j.ajo.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zadnik K, Barr JT, Edrington TB, Everett DF, Jameson M, McMahon TT, Shin JA, Sterling JL, Wagner H, Gordon MO. Baseline findings in the Collaborative Longitudinal Evaluation of Keratoconus (CLEK) Study. Invest Ophthalmol Vis Sci. 1998;39:2537–46. [PubMed] [Google Scholar]
- 6.Zadnik K, Barr JT, Edrington TB, Nichols JJ, Wilson BS, Siegmund K, Gordon MO. Corneal scarring and vision in keratoconus: a baseline report from the Collaborative Longitudinal Evaluation of Keratoconus (CLEK) Study. Cornea. 2000;19:804–12. doi: 10.1097/00003226-200011000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Kymes SM, Walline JJ, Zadnik K, Sterling J, Gordon MO. Changes in the Quality-of-Life of People with Keratoconus. Am J Ophthalmol. 2008;145:611–7. e1. doi: 10.1016/j.ajo.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nichols JJ, Steger-May K, Edrington TB, Zadnik K. The relation between disease asymmetry and severity in keratoconus. Br J Ophthalmol. 2004;88:788–91. doi: 10.1136/bjo.2003.034520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Comas M, Castells X, Acosta ER, Tuni J. Impact of differences between eyes on binocular measures of vision in patients with cataracts. Eye. 2007;21:702–7. doi: 10.1038/sj.eye.6702305. [DOI] [PubMed] [Google Scholar]
- 10.Gordon MO, Schechtman KB, Davis LJ, McMahon TT, Schornack JA, Zadnik K, Group CS. Repeatability of visual acuity in keratoconus. Impact on sample size. Optometry and Vision Science. 1998;75:249–57. doi: 10.1097/00006324-199804000-00021. [DOI] [PubMed] [Google Scholar]
- 11.Mangione CM, Lee PP, Pitts J, Gutierrez P, Berry S, Hays RD, NEI-VFQ Field Test Investigators Psychometric properties of the National Eye Institute Visual Function Questionnaire (NEI-VFQ) Arch Ophthalmol. 1998;116:1496–504. doi: 10.1001/archopht.116.11.1496. [DOI] [PubMed] [Google Scholar]
- 12.Mangione CM, Lee PP, Gutierrez PR, Spritzer K, Berry S, Hays RD. Development of the 25-item National Eye Institute Visual Function Questionnaire. Arch Ophthalmol. 2001;119:1050–8. doi: 10.1001/archopht.119.7.1050. [DOI] [PubMed] [Google Scholar]
- 13.Mangione CM, Berry S, Spritzer K, Janz NK, Klein R, Owsley C, Lee PP. Identifying the content area for the 51-item National Eye Institute Visual Function Questionnaire: results from focus groups with visually impaired persons. Arch Ophthalmol. 1998;116:227–33. doi: 10.1001/archopht.116.2.227. [DOI] [PubMed] [Google Scholar]
- 14.Chopra I, Jain AK. Between eye asymmetry in keratoconus in an Indian population. Clin Exp Optom. 2005;88:146–52. doi: 10.1111/j.1444-0938.2005.tb06687.x. [DOI] [PubMed] [Google Scholar]
- 15.Zadnik K, Steger-May K, Fink BA, Joslin CE, Nichols JJ, Rosenstiel CE, Tyler JA, Yu JA, Raasch TW, Schechtman KB. Between-eye asymmetry in keratoconus. Cornea. 2002;21:671–9. doi: 10.1097/00003226-200210000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Matza LS, Rousculp MD, Malley K, Boye KS, Oglesby A. The longitudinal link between visual acuity and health-related quality of life in patients with diabetic retinopathy. Health Qual Life Outcomes. 2008;6:95. doi: 10.1186/1477-7525-6-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lindblad AS, Clemons TE. Responsiveness of the National Eye Institute Visual Function Questionnaire to progression to advanced age-related macular degeneration, vision loss, and lens opacity: AREDS Report no. 14. Arch Ophthalmol. 2005;123:1207–14. doi: 10.1001/archopht.123.9.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McKean-Cowdin R, Varma R, Hays RD, Wu J, F C, Azen SP, Group LAES. Longitudinal Changes in Visual Acuity and Health-related Quality of Life. Ophthalmology. 2010 Publish ahead of print. [Google Scholar]
- 19.Davis LJ, Schechtman KB, Wilson BS, Rosenstiel CE, Riley CH, Libassi DP, Gundel RE, Rosenberg L, Gordon MO, Zadnik K. Longitudinal changes in visual acuity in keratoconus. Invest Ophthalmol Vis Sci. 2006;47:489–500. doi: 10.1167/iovs.05-0381. [DOI] [PubMed] [Google Scholar]