Abstract
Background
In left ventricular (LV) pressure-overload hypertrophy, lack of adaptive capillary growth contributes to progression to failure. Remodeling of the hypertrophied myocardium requires proteolysis of the extracellular matrix (ECM) carried out by matrix metalloproteinases (MMPs). MMPs, specifically MMP-9, are known to cleave ECM components to generate angiogenesis inhibitors (angiostatin, endostatin, tumstatin). We hypothesize that MMP-9 releases antiangiogenic factors during compensated and decompensated hypertrophy, which results in lack of adaptive capillary growth.
Methods
Newborn rabbits underwent aortic banding. Myocardial tissue from age-matched and banded animals at compensated (4 weeks) and decompensated hypertrophy (7 weeks), as identified by serial echocardiography, was analyzed by immunoblotting for angiostatin, endostatin, and tumstatin. MMP-9 activity was determined by zymography. A cell-permeable, potent, selective MMP-9 inhibitor was administered intrapericardially to animals with hypertrophied hearts and tissue was analyzed.
Results
MMP-9 is activated in hypertrophied myocardium versus in control hearts (22 ± 2 versus 16 ± 1; p = 0.04), which results in significantly increased levels of angiostatin (115 ± 10 versus 86 ± 7; p = 0.02), endostatin (33 ± 1 versus 28 ± 1; p = 0.006), and tumstatin (35 ± 6 versus 17 ± 4; p = 0.04). Zymography confirms inhibition of MMP-9 (hypertrophy + MMP-9 inhibitor, 14 ± 0.6 versus hypertrophy + vehicle, 17 ± 1; p = 0.01) and angiostatin, endostatin, and tumstatin are down-regulated, accompanied by up-regulation of capillary density (hypertrophy + MMP-9 inhibitor, 2.99 ± 0.07 versus hypertrophy + vehicle, 2.7 ± 0.05; p = 0.002).
Conclusions
Up-regulation of angiogenesis inhibitors prevents adaptive capillary growth in pressure-overload hypertrophied myocardium. Therapeutic interventions aimed at inhibition of angiogenesis inhibitors are useful in maintaining capillary density and thereby preventing heart failure.
The extracellular matrix (ECM) is a 3-dimensional structure that serves as a scaffold for cardiomyocytes. Cardiomyocyte attachment to the ECM is mediated through the basement membrane, which surrounds the cell and serves as an extracellular anchor between the cardiomyocyte and the connective tissue of the ECM [1]. Because of the basement membrane, cardiomyocytes maintain their shape, and enzymatic removal of the basement membrane results in cardiomyocytes becoming spherical [2]. As an integral process of hypertrophic remodeling, cardiomyocytes increase in size. During this process, the basement membrane is degraded and newly deposited to provide structural support for hypertrophying cardiomyocytes. Degradation of the basement membrane is carried out by specific proteases, the so-called matrix metalloproteinases (MMPs). MMPs underlie tight regulation by their endogenous inhibitors—the tissue inhibitors of matrix metalloproteinases (TIMPs). MMPs regulate several physiologic processes, such as embryogenesis or angiogenesis, but also contribute to pathologic processes such as tumor metastasis, inflammation, and arthritis [3–5]. In particular, MMP-2 and MMP-9 (gelatinase A and gelatinase B) are involved in cardiovascular diseases, including atherosclerosis, stroke, heart failure, ischemic heart disease, and aneurysm [6]. Our group has previously shown that adaptive capillary growth in hypertrophied myocardium is directly linked to MMP-2 and MT-MMP-1 activation, whereas TIMPs remain unchanged [4]. In contrast, MMP-9 has been associated with antiangiogenic disease states such as myocardial infarction and diabetes [7, 8]. In a model of myocardial infarction, MMP9 gene deletion yielded increased angiogenesis; it is also increased in chronic hypertension–induced left ventricular (LV) hypertrophy [7, 9].
MMP-9 has a broad range of targets and can cleave ECM components to create low-molecular-weight fragments that possess antiangiogenic properties. Matrix-bound plasminogen is cleaved by MMP-9 to generate an NH2-terminal fragment that inhibits endothelial cell proliferation and is known as angiostatin [10]. Endostatin is the C-terminal fragment of the α1-chain of type XVIII collagen (a component of the basement membrane), and MMP-9 overexpression has been shown to release endostatin, which results in tumor regression [11, 12]. Tumstatin is a cleavage product of type IV collagen, which is the main protein of all basement membranes. Mice deficient in MMP-9 display decreased circulating levels of tumstatin, which is accompanied by accelerated tumor growth [13]. In the myocardium, angiostatin and endostatin were identified as contributing to the rarefaction of coronary vessels in myocardial ischemia, but the role of tumstatin on myocardial vascularization has never been studied [13, 14].
Based on these observations, we hypothesize that pressure-overload–induced remodeling of the ECM activates MMP-9 and results in up-regulation of low-molecular-weight cleavage products of ECM components such as angiostatin, endostatin, and tumstatin. Up-regulation of angiogenesis inhibitors in hypertrophied myocardium prevents adaptive capillary growth and contributes to progression to failure.
Material and Methods
Ethics Statement
All animals received humane care from the Animal Resources of Children’s Hospital Boston, and the investigation conforms to the “Guide for the Care and Use of Laboratory Animals” prepared by the National Academy of Sciences and published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996). The protocol was reviewed and approved by the Institutional Animal Care and Use Committee at Children’s Hospital Boston.
Animal Model of LV Hypertrophy and Transthoracic Echocardiography
Pressure-overload hypertrophy was achieved by banding the descending aorta in 10-day-old New Zealand White rabbits (Millbrook Farms, Amherst, MA) as we have previously described in more detail [15]. All animals were followed by weekly transthoracic echocardiography to determine the stage of hypertrophy. Compensated hypertrophy with normal contractile function was detected until 4 weeks of age. At 7 weeks of age, LV dilatation had occurred and contractile function was significantly impaired (decompensated hypertrophy). At this time point we had already established that capillary density was significantly lower than that in age-matched, nonhypertrophied hearts [16]. Compensated hypertrophy (4 weeks) and decompensated hypertrophy (7 weeks) were selected for evaluation of MMP-9 activation and the release of angiogenesis inhibitors, angiostatin, endostatin, and tumstatin. Tissue was selected from hearts evaluated by echocardiography.
Immunoblotting
Protein levels of angiostatin, endostatin (Millipore, Temecula, MA), and tumstatin (Santa Cruz Biotechnology, Santa Cruz, CA) were determined by immunoblotting using standard protocols as we have previously reported [17]. The following were investigated: controls representing sham-operated littermates with no hypertrophy (n = 6 per group), untreated hypertrophy at the stage of compensated hypertrophy (n = 8 –10 per group) and at the stage of decompensated hypertrophy (n = 8 –10 per group), and hypertrophied hearts treated with an MMP inhibitor (n = 6 per group) or treated with vehicle only (n = 6 per group). Equal protein loading was confirmed by staining of gels with Coomassie brilliant blue (Bio-Rad Laboratories, Inc, Hercules, CA). For specific protein detection, endostatin and tumstatin tissue samples underwent immunoprecipitation before immunoblotting [17]. Horseradish peroxidase– conjugated antibodies were used as secondary antibodies (GE Healthcare, Piscataway, NJ). The bound antibody was detected by enhanced chemiluminescence. After exposure on films, quantitative protein analysis was conducted using laser densitometry. Immunoblotting data are expressed as arbitrary densitometry units.
Treatment with MMP-9 Inhibitor
Animals with hypertrophied myocardium were treated with a cell-permeable inhibitor of MMP-9 (EMD Chemicals, Gibbstown, NJ). At the concentration used in this study, this inhibitor selectively blocks MMP-9, according to the manufacturer. Following a previously described surgical method, 0.5 μg/kg inhibitor or vehicle at 0.25 mL total volume was administered intrapericardially at 4 weeks of age, and animals were euthanized 1 week later or followed by echocardiography until 7 weeks of age [4, 16]. MMP-9 activity was measured by zymography, and angiostatin, endostatin, and tumstatin protein levels were determined by immunoblotting.
Gelatinase Activity Measurement by Zymography
LV myocardial tissue extraction to determine gelatinase activity was performed following a previously described method [4]. LV myocardial tissue was homogenized on ice in extraction buffer (2 mol/L NaCl; 10 mmol/L tris(hydroxymethyl)aminomethane [Tris], 0.02%; NaN3, pH 7.0) and nutated at 4°C for 24 hours. Samples were then centrifuged for 15 minutes (4°C, 15,000 g). The supernatant was saved on ice and samples were dialyzed against assay buffer (50 mmol/L Tris, pH 7.6; 0.2 mol/L NaCl; 1 mmol/L CaCl2) at 4°C. Total protein content was determined and the myocardial extracts were stored at −80°C for further analysis. All chemicals were obtained from Sigma-Aldrich, Corp, St. Louis, MO.
The myocardial extracts were mixed with substrate sample buffer (10% sodium dodecyl sulfate; 4% glycerol; 0.25 mmol/L Tris hydrochloride, pH 6.8; 0.1% bromophenol blue) without boiling and loaded onto electrophoresis gels (sodium dodecyl sulfate polyacrylamide gel electrophoresis) containing 1 mg/mL gelatin (Invitrogen, Carlsbad, CA) under nonreducing conditions [4]. After electrophoresis, the gels were soaked in renaturing buffer containing 2.5% Triton X-100 with gentle shaking at 21°C for 30 minutes, rinsed with water, and incubated in substrate buffer (50 mmol/L Tris hydrochloride, pH 8.0; 5 mmol/L CaCl2; 0.02% NaN3) at 37°C overnight. Thereafter gels were rinsed with water, stained using 0.5% Coomassie Blue R-250 (Bio-Rad Laboratories, Inc) and destained in a mixture of acetic acid and isopropyl alcohol. A MMP-9 zymographic standard was included in the gels and served as a positive control (R&D Systems Inc, Minneapolis, MN).
Determination of Microvascular Density by Immunohistochemistry
Frozen cross-sections of the left ventricle were stained with CD-31 using a red Alexa-594 fluorescent secondary antibody, 4′,6-diamidino-2-phenylindole to identify nuclei, and Alexa Fluor 488 phalloidin for cardiomyocytes (Invitrogen, Carlsbad, CA). We have previously shown that CD-31 staining and lectin perfusion determined microvascular density equally well [18]. Coverslips were applied with fluorescent mounting medium (Dako Corp, Carpinteria, CA). Microvascularity was quantified by measuring CD-31–stained vessels and expressed as number of microvessels per individual cardiomyocyte on histologic sections obtained from vehicle-treated hypertrophied hearts and MMP-9 inhibitor–treated hypertrophied hearts. Three cardiomyocytes per field of view and 15 fields per slide were analyzed by a blinded observer using a Zeiss Axiovert 35 Microscope with a Nikon 20× objective (numerical aperture [NA] = 20×/0.75).
Statistical Analysis
Data were analyzed using SPSS software package (version 16.0, SPSS Inc, Chicago, IL) and are reported as mean ± standard error of the mean. After confirming normal distribution, a 2-tailed unpaired Student’s t test or analysis of variance with Bonferroni post hoc analysis when applicable were used for comparison between groups if normality was passed. A p value less than or equal to 0.05 was considered statistically significant.
Results
Animal Model: Compensated Versus Decompensated Hypertrophy
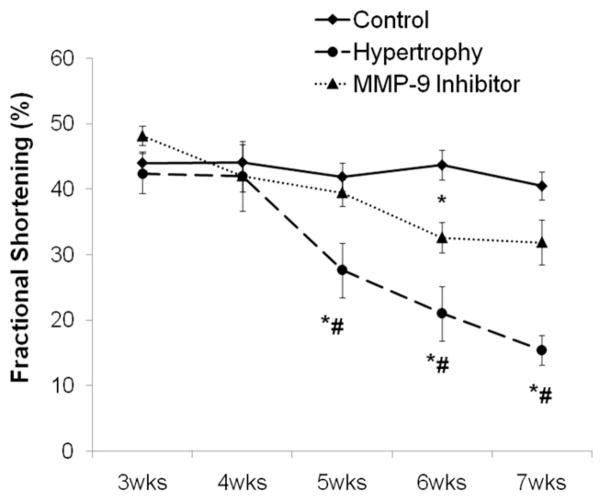
The initial stages of hypertrophy are considered to involve adaptive changes to maintain contractile function. We decided to determine ECM remodeling at early hypertrophy when increase of muscle mass is still compensating to normalize wall stress and contractile function is normal (Fig 6). At this time point, however, we have previously reported that irreversible changes on the cardiomyocyte level are already occurring [16]. This stage of compensated hypertrophy is then followed by ventricular dilatation and contractile dysfunction—the characteristics of decompensation that are accompanied by extracellular changes involving the microvasculature [4, 16].
Fig 6.
Echocardiography measurements of contractile function (% shortening fraction) are shown. Hypertrophied and control samples represent cumulative data obtained from all hearts used for analysis in this study. There was a significant decline in contractile function as hypertrophy progresses, which was prevented by administration of a MMP-9 inhibitor (*p < 0.05 versus control; #p < 0.05 versus MMP-9 Inhibitor).
Protein Levels of Angiostatin, Endostatin, and Tumstatin
Measurements of protein levels for angiostatin, endostatin, and tumstatin were obtained at 2 different time points during progressive hypertrophy, which was determined by echocardiography (Figs 1 and 2). All angiogenesis inhibitors (angiostatin, endostatin, and tumstatin) are already significantly up-regulated during the compensated stage of hypertrophy in association with MMP-9 activation and remain elevated during the decompensated stage, corresponding to increased MMP-9 activity.
Fig 1.
Angiogenesis inhibitors during compensated hypertrophy: all angiogenesis inhibitors. (A) Angiostatin (88 kDa), (B) endostatin (30 kDa), and (C) tumstatin (20 kDa) were significantly up-regulated in hypertrophied myocardium (angiostatin, **p = 0.001; endostatin, *p = 0.02; tumstatin, *p = 0.03). Equal protein loading was confirmed by staining of gels with Coomassie brilliant blue.
Fig 2.
Angiogenesis inhibitors during decompensated hypertrophy. (A) Angiostatin (88 kDa), (B) endostatin (30 kDa), and (C) tumstatin (20 kDa) remained significantly up-regulated in hypertrophied myocardium versus in age-matched control hearts (angiostatin, *p = 0.02; endostatin, *p = 0.006; tumstatin, *p = 0.04). Equal protein loading was confirmed by staining of gels with Coomassie brilliant blue.
MMP-9 Activity in Compensated and Decompensated Hypertrophy
MMP-9 activation was determined by zymography. At the stage of compensated hypertrophy, MMP-9 activity is already significantly up-regulated compared with that in age-matched control hearts (hypertrophy, 23 ± 1 versus control, 17 ± 1; p = 0.003). The same is found at the stage of decompensated hypertrophy, with significantly increased MMP-9 activation (hypertrophy, 22 ± 2 versus control, 16 ± 1; p = 0.04).
MMP-9 Inhibition and Angiogenesis Inhibitors
The cell-permeable and selective MMP-9 blocker, administered into the pericardium, results in inhibition of MMP-9 activity determined by zymography, lasting for 1 week (hypertrophy + vehicle, 17 ± 1 versus hypertrophy + MMP-9 inhibitor, 14 ± 0.7; p = 0.01) (Fig 3). Inhibition of MMP-9 results in significant down-regulation of all 3 angiogenesis inhibitors: angiostatin (hypertrophy + vehicle, 201 ± 7 versus hypertrophy + MMP-9 inhibitor, 182 ± 3; p = 0.04), endostatin (hypertrophy + vehicle, 189 ± 3 versus hypertrophy + MMP-9 inhibitor, 167 ± 3; p = 0.002) and tumstatin (hypertrophy + vehicle: 120 ± 9 versus hypertrophy + MMP-9 inhibitor, 93 ± 3; p = 0.05) (Fig 4).
Fig 3.
MMP-9 activity levels were determined by gelatin zymography. A MMP-9 –specific standard indicates the molecular weight of active MMP-9. (A) and (B) Representative zymograms are shown for compensated hypertrophy and decompensated hypertrophy and a summary of density data. Compared with controls, active MMP-9 was significantly higher in compensated (*p = 0.003) and decompensated hypertrophied hearts (*p = 0.04). (C) A representative zymogram and data summary show a significant decrease in active MMP-9 after inhibitor treatment of hypertrophied compared with vehicle-treated hypertrophied hearts (*p = 0.01).
Fig 4.
Representative immunoblots for (A) angiostatin, (B) endostatin, and (C) tumstatin after MMP-9 inhibition. As indicated by the bar graphs, there was a significant down-regulation of angiostatin (*p = 0.04), endostatin (*p = 0.002), and tumstatin (*p = 0.05) in hypertrophied hearts receiving an MMP-9 inhibitor. Equal protein loading was confirmed by staining of gels with Coomassie brilliant blue.
Microvascular Density
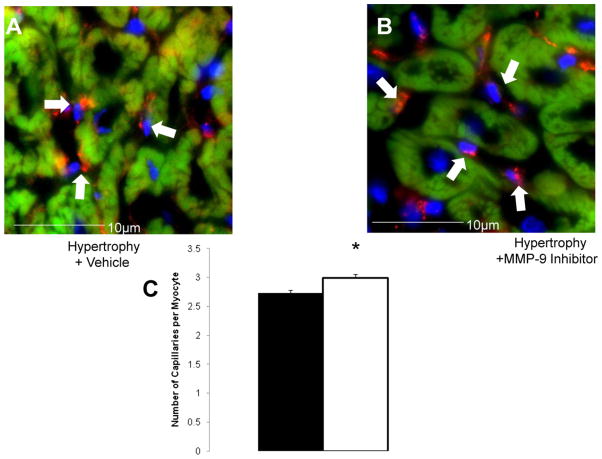
As we have previously shown, microvascular density is significantly decreased in hypertrophied myocardium [4, 18]. Blocking the release of all 3 ECM-derived angiogenesis inhibitors with an MMP-9 –specific inhibitor results in microvascular growth despite the presence of pressure overloading (Fig 5). The number of microvessels per cardiomyocyte is significantly up-regulated in hypertrophied hearts in the presence of MMP-9 inhibition (p = 0.002).
Fig 5.
(A) Representative immunohistochemical sections show the increase in capillary density following inhibition of MMP-9. Cardiomyocytes are stained in red, nuclei in blue, and vessels in green. (B) Microvessels are expressed per cardiomyocyte. (C) A summary of microvascular counts is shown. Blocking of MMP-9 with subsequent inhibition of angiogenesis inhibitors angiostatin, endostatin and tumstatin lead to an increase in capillary numbers (*p = 0.002).
Transthoracic Echocardiography
Shortening fraction is used as an indicator of contractile function. Figure 6 depicts a summary of all hearts examined in this study. As we have previously reported for this animal model [4, 18], contractile function deteriorates as hypertrophy progresses. Inhibition of angiogenesis inhibitors through administration of an MMP-9 inhibitor in hypertrophied hearts prevents the deterioration of contractile function from adaptive microvascular growth (p = 0.05 hypertrophy versus hypertrophy + MMP-9 inhibitor).
Comment
Our results are consistent with the hypothesis that pressure-overload–mediated remodeling of the ECM induces activation of MMP-9. As a consequence, matrix-derived/bound angiogenesis inhibitors are released early during hypertrophy and continue to be produced as hypertrophy progresses to failure. The release of matrix-derived angiogenesis inhibitors is tightly regulated by MMP-9 because blocking of MMP-9 inhibits the release of the angiogenesis inhibitors angiostatin, endostatin, and tumstatin. Inhibition of angiogenesis inhibitor release through blocking of MMP-9 induces a proangiogenic response, with an adaptive increase in microvascular growth that results in preservation of contractile function in progressive hypertrophy.
Hypertrophied myocardium displays an overall shift toward enhanced proteolytic activity. Concurrent development of progressive cardiomyocyte hypertrophy requires degradation of ECM components such as the basement membrane, which is carried out by MMPs. The activity of MMPs can be regulated at the levels of gene transcription and translation by posttranslational modifications and by TIMPs by inhibiting MMP activity through binding in a 1:1 stoichiometric ratio [19]. The 4 members of the TIMP family (TIMP-1 through TIMP-4) are expressed in the myocardium, as we and others have previously reported [4, 20], but TIMP protein levels are unchanged in this model of pressure-overload hypertrophy and thus are not likely contributing to the regulation of MMP-9 [4].
Both gelatinases MMP-2 and MMP-9 are ubiquitously expressed in the heart and are distinguished based on their molecular weights as determined with zymography. MMP-2 and MMP-9 share considerable functional similarity but their promoters are structurally different. Therefore they underlie different regulation, but the stimulus responsible for MMP activation is not clear. MMPs can be induced by mechanical stretch as a result of increased wall stress or by secretion of substances such as tumor necrosis factor-alpha (TNF-α) [21, 22]. Since elevated TNF-α levels have been implicated in heart failure, it is plausible that activation of MMPs may occur through a cytokine-mediated pathway, which is in accordance with our data because we have previously reported that TNF-α levels are directly associated with progression of hypertrophy to heart failure [23]. Conversely, increasing wall tension in pressure-overload hypertrophy can trigger MMP release. In our LV hypertrophy model, both stimuli for MMP activation, TNF-α and mechanical stretch, are present. MMPs in the heart have been implicated in early heart development in which mainly MMP-2 has been shown to be important for heart valve development and angiogenesis [24]. MMP-2– deficient mice are viable at birth but display significantly retarded growth compared with their wild-type controls [25]. As we have shown, MMP-2 is directly linked to proangiogenic events in the hypertrophied myocardium [4]. In contrast, up-regulation of MMP-9 has been demonstrated in failing human hearts [26, 27]. Also, MMP-9 activation has been directly linked to impaired myocardial contractile performance, and targeted deletion of MMP-9 attenuates left ventricle remodeling [28]. MMP-9 activation directly correlates with the release of angiogenesis inhibitors in certain cancers, and mice deficient in MMP-9 display decreased circulating levels of tumstatin and accelerated tumor growth [11, 14]. In this study we found that MMP-9 activation is directly associated with the progression of hypertrophy and regulates the release of matrix-derived/bound angiogenesis inhibitors. MMP-9 is activated early in the compensated stage of the disease and continues to be activated throughout the observation period until 7 weeks of age.
MMP-9 targets include plasminogen, collagen XVIII, or collagen IV. Angiostatin is an NH2-terminal cleavage product of plasminogen inhibiting endothelial cell proliferation [10]. Components of the basement membrane include collagen IV, laminin, fibronectin, and proteoglycans such as collagen XVIII [29]. Collagen XVIII is a protein of unknown function but is necessary for normal development of the vasculature in the retina [30]. The C-terminal fragment of the α1-chain of type XVIII collagen is called endostatin. Endostatin inhibits endothelial cell proliferation and migration [31] and binds integrins on the cell surface, which destabilizes cell-cell and cell-matrix interaction counteracting angiogenesis [32, 33]. It also has direct effects on vascular endothelial growth factor and its receptor, and furthermore inhibits MMP-2 activity [34, 35]. Angiostatin and endostatin up-regulation have both been implicated in rarefaction of coronary vessels in coronary artery disease [12, 36]. Collagen IV is the main component of all basement membranes and it is crucial for their stability and assembly [37]. Its cleavage product is tumstatin, which exerts its antiangiogenic effect by inducing apoptosis of proliferating endothelial cells [38]. Our data show that angiostatin, endostatin, and tumstatin are released early in hypertrophy and continue to be significantly up-regulated during hypertrophy progressing to heart failure. ECM turnover in pressure-overload hypertrophy is accompanied by continuous release of angiogenesis inhibitors, which directly coincides with MMP-9 activation. To determine whether MMP-9 regulates the release of these angiogenesis inhibitors, we blocked MMP-9 at the earliest time point of activation (4 weeks of age), the compensated stage of hypertrophy. Selective inhibition of MMP-9 can be accomplished by tetracyclines; however, we failed to demonstrate this in our model (data not shown) [39]. We therefore used a synthetic inhibitor that specifically targets the catalytic domain of MMP-9 and selectively inhibits MMP-9 at the concentration used. After inhibition of MMP-9 in hypertrophied myocardium, which we confirmed through zymography, release of matrix-derived/bound angiogenesis inhibitors is significantly reduced. Despite remodeling of the ECM, MMP-9 inhibition prevents the release of all 3 angiogenesis inhibitors, which results in the stimulation of angiogenesis. Preserving adaptive capillary growth maintains contractile function, as shown by our echocardiography data, which is in accordance with our previously published findings on the tight association of angiogenesis and contractile function [4, 16–18, 40].
In conclusion, pressure-overload–induced remodeling of the ECM activates MMP-9, which enhances the release of low-molecular-weight cleavage products of ECM components, the angiogenesis inhibitors angiostatin, endostatin, and tumstatin. Up-regulation of angiogenesis inhibitors prevents adaptive capillary growth in pressure-overload hypertrophied myocardium. An intervention aimed at blocking of MMP-9 results in the down-regulation of antiangiogenic ECM fragments and stimulates capillary growth. Tipping the balance of angiogenesis inhibitors and stimulators in a favorable direction proves to be useful in maintaining capillary growth and thereby preserves contractile function in pressure-overload hypertrophied hearts.
Acknowledgments
This work was supported by grants from National Heart, Lung, and Blood Institute HL-075430 (Dr Friehs), HL-74734 (Dr. McGowan) and HL-063095 (Dr Del Nido) and the Eleanor and Miles Shore Fellowship Program for Scholars in Medicine, Harvard Medical School, Children’s Hospital Boston (Dr Friehs). Dr Ablasser was sponsored by the Austrian Cardiological Society and Ms Nikolova by the Jack Kent Cooke Foundation and Harvard University.
References
- 1.Timpl R. Molecular aspects of the basement membrane structure. Prog Clin Biol Res. 1985;171:63–74. [PubMed] [Google Scholar]
- 2.Lundgren E, Gullberg D, Rubin K, Borg TK, Terracio MJ, Terracio L. In vitro studies on adult cardiac myocytes: attachment and biosynthesis of collagen type IV and laminin. J Cell Physiol. 1988;136:43–53. doi: 10.1002/jcp.1041360106. [DOI] [PubMed] [Google Scholar]
- 3.Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006;25:9–34. doi: 10.1007/s10555-006-7886-9. [DOI] [PubMed] [Google Scholar]
- 4.Friehs I, Margossian R, Moran AM, Cao-Danh H, Moses MA, del Nido PJ. Vascular endothelial growth factor delays onset of failure in pressure-overload hypertrophy through matrix metalloproteinase activation and angiogenesis. Basic Res Cardiol. 2005;100:1–10. doi: 10.1007/s00395-005-0581-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohammed FF, Smookler DS, Khokha R. Metalloproteinases, inflammation, and rheumatoid arthritis. Ann Rheum Dis. 2003;62(Suppl 2):ii43–7. doi: 10.1136/ard.62.suppl_2.ii43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dollery CM, McEwan JR, Henney AM. Matrix metalloproteinases and cardiovascular disease. Circ Res. 1995;77:863–8. doi: 10.1161/01.res.77.5.863. [DOI] [PubMed] [Google Scholar]
- 7.Lindsey ML, Escobar GP, Dobrucki LW, et al. Matrix metalloproteinase-9 gene deletion facilitates angiogenesis after myocardial infarction. Am J Physiol Heart Circ. 2006;290:H232–9. doi: 10.1152/ajpheart.00457.2005. [DOI] [PubMed] [Google Scholar]
- 8.Vu TH, Werb Z. Matrix metalloproteinases: effectors of development and normal physiology. Genes Dev. 2000;14:2123–33. doi: 10.1101/gad.815400. [DOI] [PubMed] [Google Scholar]
- 9.Franz M, Berndt A, Altendorf-Hofmann A, et al. Serum levels of large tenascin-C variants, matrix metalloproteinase-9, and tissue inhibitors of matrix metalloproteinases in concentric versus eccentric left ventricular hypertrophy. Eur J Heart Fail. 2009;11:1057–62. doi: 10.1093/eurjhf/hfp128. [DOI] [PubMed] [Google Scholar]
- 10.Cornelius LA, Nehring LC, Harding E, et al. Matrix metalloproteinases generate angiostatin: effects on neovascularization. J Immunol. 1998;161:6845–52. [PubMed] [Google Scholar]
- 11.Bendrik C, Robertson J, Gauldie J, Dabrosin C. Gene transfer of matrix metalloproteinase-9 induces tumor regression of breast cancer in vivo. Cancer Res. 2008;68:3405–12. doi: 10.1158/0008-5472.CAN-08-0295. [DOI] [PubMed] [Google Scholar]
- 12.Panchal VR, Rehman J, Nguyen AT, et al. Reduced pericardial levels of endostatin correlate with collateral development in patients with ischemic heart disease. J Am Coll Cardiol. 2004;43:1383–7. doi: 10.1016/j.jacc.2003.10.063. [DOI] [PubMed] [Google Scholar]
- 13.Hamano Y, Zeisberg M, Sugimoto H, et al. Physiological levels of tumstatin, a fragment of collagen IV alpha3 chain, are generated by MMP-9 proteolysis and suppress angiogenesis via alphaV beta3 integrin. Cancer Cell. 2003;3:589–601. doi: 10.1016/s1535-6108(03)00133-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tyagi SC, Kumar SG, Haas SJ, et al. Post-transcriptional regulation of extracellular matrix metalloproteinase in human heart end-stage failure secondary to ischemic cardiomyopathy. J Mol Cell Cardiol. 1996;28:1415–28. doi: 10.1006/jmcc.1996.0132. [DOI] [PubMed] [Google Scholar]
- 15.Moran AM, Friehs I, Takeuchi K, et al. Noninvasive serial evaluation of myocardial mechanics in pressure overload hypertrophy of rabbit myocardium. Herz. 2003;28:52–62. doi: 10.1007/s00059-003-2392-0. [DOI] [PubMed] [Google Scholar]
- 16.Friehs I, Barillas R, Vasilyev NV, Roy N, McGowan FX, del Nido PJ. Vascular endothelial growth factor (VEGF) prevents apoptosis and preserves contractile function in hypertrophied infant heart. Circulation. 2006;114(Suppl I):I290–5. doi: 10.1161/CIRCULATIONAHA.105.001289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friehs I, Cao-Danh H, Nathan M, McGowan FX, del Nido PJ. Impaired insulin-signaling in hypertrophied hearts contributes to increased susceptibility to ischemic injury. Biochem Biophys Res Commun. 2005;331:15–22. doi: 10.1016/j.bbrc.2005.03.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friehs I, Moran AM, Stamm C, et al. Promoting angiogenesis protects severely hypertrophied hearts from ischemic injury. Ann Thorac Surg. 2004;77:2004–11. doi: 10.1016/j.athoracsur.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 19.Brew K, Dinakarpandian D, Nagase H. Tissue inhibitors of metalloproteinases: evolution, structure and function. Biochim Biophys Acta. 2000;1477:267–83. doi: 10.1016/s0167-4838(99)00279-4. [DOI] [PubMed] [Google Scholar]
- 20.Li YY, McTiernan CF, Feldman AM. Proinflammatory cytokines regulate tissue inhibitors of metalloproteinases and disintegrin metalloproteinase in cardiac cells. Cardiovasc Res. 1999;42:162–72. doi: 10.1016/s0008-6363(98)00297-1. [DOI] [PubMed] [Google Scholar]
- 21.Bradham WS, Bozkurt B, Gunasinghe H, Mann D, Spinale FG. Tumor necrosis factor-alpha and myocardial remodeling in progression of heart failure: a current perspective. Cardiovasc Res. 2002;53:822–30. doi: 10.1016/s0008-6363(01)00503-x. [DOI] [PubMed] [Google Scholar]
- 22.Li YY, Feng Y, McTiernan CF, et al. Downregulation of matrix metalloproteinases and reduction in collagen damage in the failing human heart after support with left ventricular assist devices. Circulation. 2001;104:1147–52. doi: 10.1161/hc3501.095215. [DOI] [PubMed] [Google Scholar]
- 23.Stamm C, Friehs I, Cowan DB, et al. Inhibition of tumor necrosis factor-alpha improves postischemic recovery of hypertrophied hearts. Circulation. 2001;104(12 Suppl 1):I350–5. doi: 10.1161/hc37t1.094851. [DOI] [PubMed] [Google Scholar]
- 24.Linask KK, Han M, Cai DH, Brauer PR, Maisastry SM. Cardiac morphogenesis: matrix metalloproteinase coordination of cellular mechanisms underlying heart tube formation and directionality of looping. Dev Dyn. 2005;233:739–53. doi: 10.1002/dvdy.20377. [DOI] [PubMed] [Google Scholar]
- 25.Itoh T, Ikeda T, Gomi H, Nakao S, Suzuki T, Itohara S. Unaltered secretion of beta-amyloid precursor protein in gelatinase A (matrix metalloproteinase 2)-deficient mice. J Biol Chem. 1997;272:22389–92. doi: 10.1074/jbc.272.36.22389. [DOI] [PubMed] [Google Scholar]
- 26.Li YY, Feldman AM, Sun Y, McTiernan CF. Differential expression of tissue inhibitors of metalloproteinases in the failing human heart. Circulation. 1998;98:1728–34. doi: 10.1161/01.cir.98.17.1728. [DOI] [PubMed] [Google Scholar]
- 27.Uemura S, Matsushita H, Li W, et al. Diabetes mellitus enhances vascular matrix metalloproteinase activity: role of oxidative stress. Circ Res. 2001;88:1291–8. doi: 10.1161/hh1201.092042. [DOI] [PubMed] [Google Scholar]
- 28.Ducharme A, Frantz S, Aikawa M, et al. Targeted deletion of matrix metalloproteinase-9 attenuates left ventricular enlargement and collagen accumulation after experimental myocardial infarction. J Clin Invest. 2000;106:55–62. doi: 10.1172/JCI8768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martin GR, Timpl R. Laminin and other basement membrane components. Annu Rev Cell Biol. 1987;3:57–85. doi: 10.1146/annurev.cb.03.110187.000421. [DOI] [PubMed] [Google Scholar]
- 30.Menzel O, Bekkeheien RC, Reymond A, et al. Knobloch syndrome: novel mutations in COL18A1, evidence for genetic heterogeneity, and a functionally impaired polymorphism in endostatin. Hum Mutat. 2004;23:77–84. doi: 10.1002/humu.10284. [DOI] [PubMed] [Google Scholar]
- 31.Dhanabal M, Ramchandran R, Waterman MJ, et al. Endostatin induces endothelial cell apoptosis. J Biol Chem. 1999;274:11721–6. doi: 10.1074/jbc.274.17.11721. [DOI] [PubMed] [Google Scholar]
- 32.Dixelius J, Cross M, Matsumoto T, Sasaki T, Timpl R, Claesson-Welsh L. Endostatin regulates endothelial cell adhesion and cytoskeletal organization. Cancer Res. 2002;62:1944–7. [PubMed] [Google Scholar]
- 33.Romanic AM, Harrison SM, Bao W, et al. Myocardial protection from ischemia/reperfusion injury by targeted deletion of matrix metalloproteinase-9. Cardiovasc Res. 2002;54:549–58. doi: 10.1016/s0008-6363(02)00254-7. [DOI] [PubMed] [Google Scholar]
- 34.Kim YM, Jang JW, Lee OH, et al. Endostatin inhibits endothelial and tumor cellular invasion by blocking the activation and catalytic activity of matrix metalloproteinase. Cancer Res. 2000;60:5410–3. [PubMed] [Google Scholar]
- 35.Kim YM, Hwang S, Pyun BJ, et al. Endostatin blocks vascular endothelial growth factor-mediated signaling via direct interaction with KDR/Flk-1. J Biol Chem. 2002;277:27872–9. doi: 10.1074/jbc.M202771200. [DOI] [PubMed] [Google Scholar]
- 36.Sodha NR, Clements RT, Boodhwani M, et al. Endostatin and angiostatin are increased in diabetic patients with coronary artery disease and associated with impaired coronary collateral formation. Am J Physiol Heart Circ Physiol. 2009;296:H428–34. doi: 10.1152/ajpheart.00283.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Toyota E, Matsunaga T, Chilian WM. Myocardial angiogenesis. Mol Cell Biochem. 2004;264:35–44. doi: 10.1023/b:mcbi.0000044372.65864.18. [DOI] [PubMed] [Google Scholar]
- 38.Maeshima Y, Colorado PC, Torre A, et al. Distinct antitumor properties of a type IV collagen domain derived from basement membrane. J Biol Chem. 2000;275:21340–8. doi: 10.1074/jbc.M001956200. [DOI] [PubMed] [Google Scholar]
- 39.Golub LM, Lee HM, Ryan ME, Giannobile WV, Payne J, Sorsa T. Tetracyclines inhibit connective tissue breakdown by multiple non-antimicrobial mechanisms. Adv Dent Res. 1998;12:12–26. doi: 10.1177/08959374980120010501. [DOI] [PubMed] [Google Scholar]
- 40.Kaza E, Ablasser K, Poutias D, et al. Up-regulation of soluble vascular endothelial growth factor receptor-1 prevents angiogenesis in hypertrophied myocardium. Cardiovasc Res. 2011;89:410–8. doi: 10.1093/cvr/cvq321. [DOI] [PMC free article] [PubMed] [Google Scholar]