Abstract
Methamphetamine (METH) dependence is frequently comorbid with HIV infection. Both factors are independently characterized by inhibitory deficits, which may manifest as increased motor activity, inappropriate perseverative behavior, and elevated exploratory responses to novel stimuli, but the effect of combined METH exposure and HIV is not well understood. In this study, we administered a chronic escalation/binge regimen of METH or vehicle treatment to wildtype (WT) or transgenic (tg) mice expressing the HIV-1 gp120 envelope protein and quantified disinhibition during the 7 days following drug withdrawal. We hypothesized that gp120tg mice administered chronic METH would exhibit more pronounced inhibitory deficits compared to vehicle-treated WT or gp120tg animals. Our results showed that METH treatment alone increased novel object interaction while female METH-treated gp120tg mice exhibited the highest level of exploration (holepoking) compared to other female mice. Transgenic mice exhibited fewer rears relative to WT, slightly less locomotion, and also demonstrated a trend towards more perseverative motor patterns. In summary, both METH treatment and gp120 expression may modify inhibition, but such effects are selective and dependent upon variations in age and sex that could impact dopamine and frontostriatal function. These findings illustrate the need to improve our knowledge about the combined effects of HIV and substance use and facilitate improved treatment methods for comorbid disease and drug dependence.
Keywords: HIV, methamphetamine, gp120, inhibition, behavioral pattern monitor
1. Introduction
The use of illicit drugs such as methamphetamine (METH) is a significant risk factor for HIV infection and concurrent METH use is associated with more rapid progression of the disease, treatment non-compliance, and greater mortality [1–3]. Recent studies indicate that comorbid METH dependence increases cognitive deficits observed in HIV infection, including deficits in inhibition linked to neuronal loss and dysfunction in frontal cortex [4, 5]. Inhibitory deficits, or the inability to withhold an action or thought, have a critical impact on drug-seeking behavior and high-risk activities that transmit disease. For example, METH use is associated with an increased number of sex partners, risky sexual behaviors, and greater likelihood of sex with an HIV-infected partner [6, 7]. Impaired inhibition in both METH dependence and HIV has also been demonstrated in standard neuropsychological tests, including abnormalities in response inhibition and perseverative behavior on the Stroop color-word task, stop-signal task, and Wisconsin Card Sorting Task (WCST) [8–11]. Further, recent work suggests that METH may exacerbate HIV-induced neuropathology, including contributing to the disruption of the blood brain barrier, upregulation of HIV co-receptors such as CCR5, increasing viral load in the brain, and augmentation of gp120-induced neurotoxicity [12–15].
In contrast to self-report forms and neuropsychological tests administered to human subjects, the effects of stimulants such as METH are typically quantified in rodents using open-field paradigms [16]. While some studies assess relatively simple measures of total motor activity, this approach provides limited information about more subtle phenomena generated by the differential activation of distinct neurobiological systems [17, 18]. Application of multivariate methods that simultaneously quantify exploration, locomotion, and motor patterns thus enable much more sophisticated evaluation of multifaceted and competing behaviors [19]. To address this issue, we have developed the Behavioral Pattern Monitor (BPM), an automated open-field paradigm used in both rats [16, 20] and mice [21], that provides multiple and independent measures of locomotion and exploration [20]. This tool is particularly useful when considering the concept of inhibitory deficits, which may manifest in several domains, including: 1) motor hyperactivity; 2) perseveration, an inability to inhibit ongoing responses, resulting in repeated and persistent behaviors; 3) increased novelty-seeking, or elevated interaction with novel stimuli [22].
While rodents cannot be infected effectively with HIV-1 virus, several murine models of HIV have been developed, including transgenic mice that express the gp120 protein (gp120tg) (Toggas et al., 1994), a viral envelope protein believed to play a critical role in HIV neuropathogenesis [23]. This glycoprotein is part of the outer layer of the virus, forming “spike” complexes comprised of three gp120 proteins that bind to the CD4 receptor and facilitate viral entry into host cells, including macrophages, microglia, and T-cells. Infected macrophages cross the blood brain barrier as early as a few weeks after the initial HIV infection, enabling the virus to penetrate the brain and lead to subsequent development of NeuroAIDS [24]. In addition to initiating infection, there is considerable evidence that gp120 exerts both direct and indirect neurotoxic effects in the brain, including inducing apoptosis in lymphocytes and neurons, engendering excitotoxicity, and stimulating the release of pro-inflammatory agents, including cytokines and chemokines [25]. In turn, the clinical symptoms of HIV-associated neurocognitive disorders (HAND) are correlated with the extent of excitatory neurotoxins in cerebrospinal fluid, neuronal loss, and the level of inflammatory markers present in microglia and astrocytes [26].
The gp120 transgenic animals exhibit a number of neuropathological features similar to NeuroAIDS in the human brain, including decreased synaptic and dendritic density, astrocytosis and activated microglia in the cortex and hippocampus, and impaired hippocampal neurogenesis [27, 28]. Such phenomena emerge as early as 2–3 months of age and persist throughout the first year of life [26, 29, 30]. While behavioral characterization of the mice is relatively sparse, previous studies report impairment in spatial memory and contextual fear conditioning at 9–13 months of age [26, 31]. One paper has examined the effect of acute METH exposure in gp120tg mice, reporting an increase in observer-rated stereotypic behavior (head-up sniffing) and vertical movement in transgenic mice compared to wildtype [32], but the consequence of extended METH treatment has not been assessed.
The objective of the current study was to examine the effect of a chronic METH regimen on disinhibition in gp120tg and WT mice at various ages (4 and 9 month-old mice) using the BPM, a novel object approach task, and the light-dark box test. Since the combined effect of METH dependence and HIV infection is demonstrated to impair cognition even in drug-abstinent individuals [5], we proposed to model human function in mice by assessing behavior after several days of METH withdrawal. We hypothesized that gp120 transgenic mice treated with METH would exhibit a pattern of behavior consistent with altered inhibition, including increased motor activity, greater perseveration, and an elevated response to novelty compared to wildtype and vehicle-treated mice.
2. Methods
2.1 Animals
Male and female transgenic mice expressing the HIV-1 envelope glycoprotein gp120 were obtained from the lab of Dr. Eliezer Masliah at the University of California San Diego. The gp120 expression occurs in astrocytes under the control of a modified murine glial fibrillary acidic protein (GFAP) in mice generated from a mixed C57BL/6 x Sv129 (SJL/BL6/129) background [28]. For the current study, transgenic mice on the BL6/129 background were crossed with wildtype BDF1 mice (both male and female) obtained from Charles River. Non-transgenic littermates (wildtype, WT) were used as controls. Two separate cohorts of mice were tested, including one cohort of 8–9 month-old gp120tg and WT animals (n = 12–13 per condition, from the F6 BL6/BDF1 generation) and a second cohort of 4 month-old mice (n = 9–11 per condition, from the F7–F8 BL6/BDF1 generation). Genotype was confirmed by PCR analysis of tail DNA.
All mice were housed in groups of 2–4 per cage, separated by sex, in a climate-controlled animal colony on a reversed day/night cycle (lights on at 8:00 PM, off at 8:00 AM). All behavioral testing was conducted between 9:00 AM and 6:00 PM. The animals were given free access to food (Haran Teklad, Madison, WI) and water through the experiments. Mice were brought to the testing room for an acclimation period of 60 minutes prior to behavioral assessment. All procedures were approved by the UCSD Institutional Animal Care and Use Committee and conformed to NIH guidelines.
2.2 Drug Regimen
Methamphetamine (Sigma, St. Louis, MO, USA) was dissolved in saline and administered subcutaneously with a 5 ml/kg injection volume (freebase weight). Fresh syringes were used for every injection given to each mouse and saline (0.9%) utilized as the vehicle treatment. Stock solutions of METH were prepared every 3–4 days and diluted as needed during the drug regimen.
We administered an escalating dose-multiple binge METH regimen first tested in rat [33] and subsequently piloted in mice (Dr. Masliah, personal communication). While the neurotoxic effects of METH have been typically induced by an acute “binge” procedure (4 injections of high doses in drug-naïve rodents) [34], it has been postulated that the inclusion of an escalating dose pretreatment regimen represents a more accurate simulation of the gradual dose progression in typical human abusers [35]. Prior work indicates that inclusion of this escalation paradigm attenuates the hyperthermic effects of higher METH doses in rats, while still inducing neuropathological effects in both rats and mice [33] (Dr. Masliah, personal communication). In this study, gp120tg and WT mice were injected three times per day (10:00 AM; 1:15 PM; 5:30 PM) for 14 days with vehicle or escalating doses of METH starting with 0.1 mg/kg and increasing to 4.0 mg/kg, with a step-wise increase of 0.1 mg/kg per injection (as illustrated in Figure 1). After this 14-day period, animals were exposed to an 11-day “binge” period and administered four daily injections of 6.0 mg/kg METH or vehicle at 2 hour intervals (10:00 AM, 12:00 PM, 2:00 PM, 4:00 PM). Behavioral tests were subsequently administered as described below.
Fig. 1.
Schematic representation of experiment timeline. Mice were administered a chronic 25 day regimen of methamphetamine (METH), including an escalation period of 14 days, where the dose was increased from 0.1 to 4.0 mg/kg (freebase), and an 11 day “binge” interval of 6 mg/kg injections. In experiment 1, mice were tested in the Behavioral Pattern Monitor after 7 days of withdrawal from METH and subsequently challenged 7 days later with a low dose of the drug (0.45 mg/kg). In experiment 2, mice were assessed in the Novel Object Approach Task (NOA) after three days of withdrawal and the Light-Dark box (LD) during days 4–5 after METH treatment
2.3 Behavioral Assessment
2.3.1 Behavioral Pattern Monitor (BPM)
Locomotor and exploratory activity were quantified as previous described [36] using the mouse BPM (San Diego Instruments, San Diego, CA), a system that includes ten chambers enclosed in individual cabinets. Each chamber consists of a 30.5 × 61 × 38-cm Plexiglas area equipped with 11 holes (3 in the floor, 3 along each long wall, and 1 on each short wall). The location of the mouse is tracked every 0.1 seconds by a grid of 12 × 24 infrared photobeams placed 1 cm off the floor, quantifying the position of the animal across 9 unequal regions (4 corners, 4 wall regions, and the center area). Rearing behavior is detected by a second set of 16 photobeams placed 2.5 cm above the floor and aligned with the long axis of the chamber. Each of the 11 holes is also equipped with a photobeam to record mouse holepokes. Photobeam data are stored as ASCII computer files that comprise the X-Y axis location of the animal (1.25 cm resolution), time (e.g., length of time spent in a region), and number and type of events (e.g., holepokes or rears).
Dependent measures of inhibition fall into 3 categories: 1) the quantity of motor activity, measured by number of transitions between the 9 regions and total distance traveled; 2) exploratory behavior, quantified as the number of times a mouse investigates a hole (holepokes) and rears; 3) the structure of locomotor activity assessed by spatial d and the spatial coefficient of variation (CV). Spatial d, measured between values of 1 and 2, indicates the extent to which a subject travels in a straight line (close to 1) or adopts a more convoluted, meandering path, such as very localized, circumscribed movements (close to 2). This measure is calculated by plotting the successive x-y coordinates of the path traveled against varying lengths of measuring resolutions (e.g., measuring the distance traveled with a small versus large ruler) [37]. Values at either end of this range may indicate repetitive, perseverative behavior; for example, acute treatment with amphetamine induces an abnormal sequential pattern characterized by repeated running around the perimeter of the chamber, resulting in lower d values [36]. Such activity may be interpreted to represent a failure of motor inhibition, insofar as it reflects the inability of a rodent to normally pause when encountering a corner or another object. In similar fashion, CV quantifies variation in the pattern of transitions among the 9 regions in the BPM. Repeated transitions between the same regions also reflects a more consistent and perseverative pattern of locomotion [16], resulting in higher values of CV.
2.3.2 Novel Object Approach Task (NOA)
The novel object test was conducted using the EthoVision (Tracksys, Nottingham, UK) system as previously described [38]. Mice are placed into four adjacent white Plexiglas enclosures (41 cm × 41 cm × 34 cm), with one animal in each field. Motor activity and mouse location was quantified using EthoVision 3.0 to generate the x-y coordinate location of the animal and the time spent at each location. Each enclosure is divided into a center region (20 × 20 cm), four corners (10 × 10 cm) and four rectangular areas adjacent to the walls (20 × 10 cm). The mouse is initially allowed to explore the area for 5 minutes without any object, followed by a 5-minute period when a novel object is placed into the center of the field. The novel object was a 50 ml conical tube filled with clean corn cob bedding (standard mouse bedding) attached to the bottom of the enclosure. Prior to testing, each tube was cleaned in a 5% bleach solution, rinsed with water, and dried with Kimwipes. Each object was used only once for one mouse. This tube was selected because it is sufficiently novel to provoke exploration and has an appropriate size, shape, and color to enable the video system to clearly track adjacent mouse movements. Primary dependent variables include the time spent in the center, latency to enter the center region, and number of center entries. While center duration does not directly measure object interaction, previous observations indicate that it is highly correlated with object exploration [39].
2.3.3 Light-Dark Box (LD)
This apparatus consists of two adjoining Plexiglas boxes (each 20 cm × 18.4 cm × 19 cm) that allow the mouse to transit between them. One is composed of black Plexiglas and is kept dark and covered during the test; the other is white and illuminated (800 lux). For this test, the mouse is placed initially in the light side and allowed free access to both the dark and light fields of the enclosure for a 5 minute period. Mouse location is tracked by EthoVision and the primary dependent variable is time spent in the light side. The LD test is commonly considered to be an indicator of anxiety-like behavior; however, increased time spent in the brightly lit (aversive) chamber has been interpreted as disinhibition of exploration mediated by drug treatment (e.g., benzodiazepines) [40, 41]. Therefore, we chose to include this test, in conjunction with the BPM and a traditional object interaction task, in order to provide a more comprehensive assessment of behaviors related to inhibition.
2.4 Experimental Design
2.4.1 Experiment 1: BPM assessment in 8–9 month-old gp120tg mice
Administration of the escalation/binge METH regimen or vehicle was initiated in 4 groups of 8–9 month-old mice (male WT, male gp120tg, female WT, female gp120tg) with 12–13 mice in each treatment condition. We decided to test animals in this age range based on prior work indicating the presence of marked neuropathology and behavioral deficits in 9 – 12 month-old gp120tg mice [26, 31]. After 7 days of withdrawal from the chronic METH regimen, motor and exploratory behavior was quantified in the mouse BPM for a period of 30 minutes (Figure 1). This 7-day withdrawal period was selected based on evidence of impaired performance in the Morris Water maze at this time point in METH-treated gp120tg mice (Dr. Eliezer Masliah, personal communication). After another 7 days with no drug exposure, mice in each group were randomly assigned to receive a low challenge dose of METH (0.45 mg/kg, s.c.) or vehicle (6–7 mice per group) to assess for evidence of behavioral sensitization.
2.4.2 Experiment 2: BPM, NOA, LD assessment in 4 month-old gp120tg mice
The second experiment examined METH withdrawal in a younger cohort to determine if the drug may exert age-dependent effects in gp120tg mice tested in the BPM. Similar to the first study, the METH regimen or vehicle treatment was administered to male and female WT and gp120tg mice and BPM activity was measured after 7 days of withdrawal (n = 9–11 per group). Given that stimulant exposure may impact behavior after shorter periods of drug withdrawal [42], we also administered the NOA test to this cohort after 3 days of METH withdrawal, followed by testing with the LD box during days 4–5 of withdrawal. We chose to administer this battery rather than simply repeat the BPM test on multiple days in order to avoid habituation to the same open-field enclosure. To investigate possible hyperthermic effects of drug treatment, we quantified mouse temperature at baseline (a week before the session) and during the first day of the METH binge (Day 15 of treatment). Temperature was assessed using a non-invasive infrared monitor (153-IRB, EB Instruments) placed against the base of the mouse tail. On treatment day 15, mouse temperature was measured two hours after the third drug injection (mice received a total of 18 mg/kg METH in the preceding 6 hours), following a previous protocol adopted for the escalation/binge regimen [43].
2.5 Statistics
Statistical analyses were performed using SPSS. Activity during the 30 minute BPM session and 5 minute LD test was analyzed with three-way analysis of variance (drug × genotype × sex). A mixed ANOVA was used to quantify measures across the three 10 minute epochs in the BPM, including time as a within-subject variable. The novel object approach task was also analyzed with a mixed ANOVA (drug × genotype × sex × epoch), including two 5 minute epochs (with and without the novel object in the enclosure). Motor activity (transitions) during the sensitization test in experiment 1 was assessed with a four-way ANOVA (chronic drug treatment × acute drug × genotype × sex). Mouse weight was evaluated separately in males and females with a three-way mixed ANOVA (drug × genotype × treatment day), with day as a repeated-measure. Post hoc differences were assessed with Tukey’s HSD using an alpha level of 0.05.
3. Results
3.1 Experiment 1
3.1.1 BPM test after 7 days of METH withdrawal
Quantity of Motor Activity
For the 30-minute session in Experiment 1, females exhibited trends towards decreased transitions [F(1,95) = 3.3, p = 0.07] and reduced distance traveled [F(1,95) = 3.3, p = 0.07] relative to male mice, but there were no main effects or interactions with drug or genotype (Figure 2). When the data were analyzed in 10 minute epochs, we observed a significant epoch by sex by genotype interaction for both transitions [F(2,94) = 8.0, p < 0.001] and distance traveled [F(2,94) = 4.0, p < 0.05]. Subsequent 2 × 2 (sex by genotype) ANOVAs conducted separately for each epoch revealed that gp120tg mice exhibited trends towards reduced transitions [F(1,103) = 3.0, p = 0.08] and decreased distance traveled [F(1,103) = 3.3, p = 0.07] compared to WT mice in epoch 3.
Fig. 2.
Effect of METH withdrawal on activity in the mouse Behavioral Pattern Monitor for 8–9 month-old wildtype (WT) and gp120tg mice (gp120) administered methamphetamine (METH) or vehicle (VEH), n = 12–13 per group. Total motor activity (transitions, distance traveled), exploration (holepokes, rears), and locomotor structure (spatial d, spatial CV) were quantified during a 30-minute session (a–f) comprised of three 10-minute epochs. Genotype and drug treatment did not affect the quantity or structure of motor activity. Holepokes were increased in METH-treated female mice compared to vehicle over the total 30 minutes (c), during the middle 10 minutes of the session (Epoch 2) (g), and in gp120tg mice in Epoch 3 (h). Rears were decreased in gp120tg mice compared to WT over 30 minutes (d). * p <0.05, ** p < 0.01, # indicates a trend towards decreased holepokes in female WT groups (p < 0.1) relative to female METH-treated gp120tg mice
Exploration
There was a significant sex by drug interaction for holepokes over the 30 minute period [F(1,95) = 5.3, p < 0.05]. Post hoc analyses indicated that METH-treated female mice showed more holepokes relative to vehicle-treated females (p < 0.05) (Figure 2c). Analysis of the 10 minute epoch data revealed a 4-way epoch by sex by genotype by drug interaction for holepokes [F(2,94) = 4.9, p < 0.01]. Neither drug nor genotype affected holepokes in male mice. However, METH-treated females exhibited more holepokes compared to vehicle-treated females in epoch 2 [F(1,48) = 7.5, p < 0.01] (Figure 2g) and there was a marginal interaction between genotype and drug for female mice in epoch 3 [F(1,48) = 4.0, p = 0.05] (Figure 2h). Subsequent pairwise comparisons in female mice revealed that gp120tg mice treated with METH exhibited a trend towards elevated epoch 3 holepokes relative to WT (p = 0.06) and more holepokes compared to gp120tg treated with vehicle (p < 0.05). In contrast to the holepoke data, female mice exhibited fewer rears compared to males [F(1,95) = 5.3, p < 0.05] and gp120tg mice exhibited fewer rears compared to WT [F(1,95) = 6.8, p < 0.05] (Figure 2d).
Structure of Motor Activity
No group differences were detected over 30 minutes for spatial d or spatial CV (Figure 2e and 2f), but we did observe a sex by epoch interaction [F(2,94) = 3.9, p < 0.05] for spatial CV. Post hoc analyses showed that spatial CV trended higher in female mice in epoch 1 relative to males (p < 0.10), but no sex differences were observed in epochs 2 or 3.
3.1.2 METH challenge
When mice were challenged with a single METH dose (0.45 mg/kg) two weeks after withdrawal from the chronic METH regimen, acute drug exposure significantly increased transitions [F(1,87) = 30.5, p < 0.001] compared to the acute vehicle treatment. We also observed a significant interaction between sex, genotype, and acute drug [F(1,87) = 4.2, p < 0.05], and a trend towards a three-way interaction between genotype, chronic drug treatment, and acute drug treatment F(1,87) =3.2, p = 0.08]. Subsequent analyses indicated a significant interaction between chronic and acute METH treatment in WT [F(1,47) = 5.0, p < 0.05], but not gp120tg mice [F(1,47) = 0.21, NS]. Post hoc comparisons showed that WT mice exposed to chronic METH exhibited greater transitions in response to the acute drug relative to mice pre-treated with saline (p < 0.05), indicating behavioral sensitization (Figure 3a). This phenomenon was not observed in gp120tg, as acute METH induced a similar response regardless of prior drug exposure (Figure 3b). A trend towards a significant interaction between genotype and acute METH was also observed in males [F(1,47) = 3.8, p = 0.06], where vehicle-treated gp120tg males tended to be less active than vehicle-treated WT (p < 0.10), but both groups showed equivalent transitions following the METH injection (Figure 3c).
Fig. 3.
Chronic METH administration induces sensitization in wildtype (WT) but not gp120tg (gp120) mice during a 30 minute test in the Behavioral Pattern Monitor. Two weeks after chronic METH treatment, mice received one injection of METH (0.45 mg/kg) or vehicle. Acute METH administration increased transitions in both wildtype (WT) (a) and gp120tg (gp120) mice (b) compared to acute vehicle, n = 12–14 per group. WT mice treated with chronic METH exhibited increased locomotion in response to the acute drug compared to the chronic vehicle condition (p < 0.05) (a), but this effect was not observed in gp120tg mice. Male gp120tg mice also exhibited a trend towards fewer transitions relative to WT in the acute vehicle condition (c), but similar locomotion after acute METH injection * p < 0.05; # p < 0.10
3.2 Experiment 2
3.2.1 BPM test after 7 days of METH withdrawal
Quantity of Motor Activity
Similar to the older cohort of mice and independent of genotype, females exhibited less locomotion relative to males during the full 30 minute BPM session, showing a decrease in transitions [F(1,70) = 7.4, p < 0.01] and distance traveled [F(1,70) = 7.5, p < 0.01] (Figure 4a and 4b). There were no significant effects of drug or genotype on total motor activity.
Fig. 4.
Effect of METH withdrawal on activity in the mouse Behavioral Pattern Monitor for 4 month-old wildtype (WT) and gp120tg mice, n = 9–11 per group. Total motor activity (transitions, distance traveled), exploration (holepokes, rears), and locomotor structure (spatial d, spatial CV) were quantified during a 30-minute session (a–f) comprised of three 10-minute epochs. Similar to experiment 1, female mice were less active compared to males, with fewer transitions (a), less distance traveled (b), and decreased rears (d), but did exhibit more holepokes in the first 10 minutes (Epoch 1) (G). METH treatment increased spatial d (e) with a trend towards increased spatial CV in Epoch 1 (h) compared to vehicle. The gp120tg mice also exhibited a trend towards increased spatial CV in Epoch 1 relative to WT mice (f) (p < 0.10)
Exploration
Female mice exhibited fewer rears over 30 minutes relative to males [F(1,70) = 5.5, p < 0.05] (Figure 4d), with a trend towards a sex by drug interaction [F(1,70) = 3.6, p = 0.06], where METH-treated but not vehicle-treated females tended to show fewer rears. Holepokes did not differ across groups over the 30 minute test. However, there was a sex by epoch interaction [F(2,69) = 4.1, p < 0.05], where female mice engaged in more holepokes than males in epoch 1 (p < 0.05) (Figure 4g), but not epochs 2 or 3.
Structure of Motor Activity
There was a trend towards increased spatial CV in gp120tg mice relative to WT [F(1,70) = 3.2, p = 0.08] (Figure 4f). METH treatment significantly increased spatial d compared to vehicle [F(1,70) = 5.0, p < 0.05] (Figure 4e), although the average values remained in the middle of the range for this variable (1.39 for vehicle, 1.43 for METH). A drug by epoch interaction was noted for spatial CV [F(2,69) = 3.6, p < 0.05], where METH-treated mice exhibited a trend towards higher spatial CV compared to vehicle in epoch 1 (p < 0.10), but drug treatment groups did not differ in the remainder of the session.
3.2.2 NOA (Day 3 of METH withdrawal) and LD (Days 4–5 of METH withdrawal)
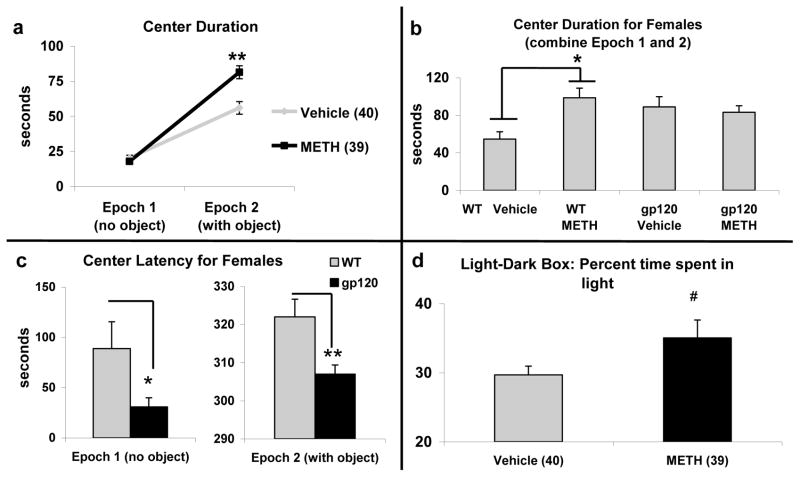
Analyses of the novel object approach task indicated a main effect of drug [F(1,71) = 8.6, p < 0.01] and a significant interaction between drug and epoch [F(1,71) = 27.0, p < 0.001] on time spent in the center region (Figure 5a). Post hoc analyses revealed that METH-treated mice spent significantly more time in the center with the object compared to vehicle-treated mice (p < 0.01), but there was no effect of drug on center duration without an object in the enclosure. We did not observe any interaction between genotype and epoch; however, significant 3-way sex by genotype by drug interactions were noted for time spent in the center [F(1,71) = 4.7, p < 0.05] and latency to enter the center region [F(1,71) = 4.9, p < 0.001]. Follow-up analyses indicated that, regardless of the presence or absence of the novel object, vehicle-treated female WT mice spent significantly less time in the center over the total 10 minute task compared to the METH-treated WT females (p < 0.05), with a trend towards less center time relative to the gp120tg female groups (Figure 5b); no difference was observed for males. Similarly, the latency to enter the center region was also reduced in vehicle gp120tg females compared to vehicle WT female mice for both epoch 1 and 2 (Figure 5c). In contrast to the NOA findings, no effect or interaction with genotype was observed for the LD test, but the data indicated a trend towards increased time spent in the light in METH-treated mice relative to vehicle [F(1,71) = 3.3, p = 0.07] (Figure 5d).
Fig. 5.
Novel Object Approach Task (NOA, a–c) and Light-Dark box (LD, d) during METH withdrawal in 4 month-old wildtype (WT) and gp120tg mice (gp120). NOA was administered after 3 days of METH withdrawal and LD task was tested after 4–5 days of withdrawal. METH-treated mice exhibited increased response to novelty in the NOA, spending more time in the center region with the novel object compared to vehicle (p < 0.01) (a). Center duration was increased in METH-treated female mice compared to vehicle-treated females over the whole test (with and without the object) (b), while gp120tg female mice also exhibited decreased latency to enter the center relative to female WT under both conditions (c). METH-treated mice exhibited a trend towards increased time in the light side of the LD box relative to vehicle (p = 0.07) (d). * p < 0.05; ** p < 0.01; # p < 0.10
3.3 Temperature and body weight results
The METH regimen administered to both cohorts of mice was tolerated well by the animals; of 182 total mice tested, only 1 animal died during METH treatment. In Experiment 1, there was no main effect of drug or drug by genotype interaction for weight during METH exposure, but analyses indicated a test day by drug condition interaction for both male [F(5,43) = 12.7, p < 0.001] and female mice [F(5,44) = 8.1, p < 0.001]. Female METH-treated mice exhibited significantly lower body weight compared to vehicle-treated female mice on Day 11 of the drug escalation period (p < 0.05), but did not differ thereafter. Male METH-treated mice exhibited lower weight relative to vehicle only near the end of the binge regimen (Day 22, p < 0.05) (Figure 6a). In Experiment 2, there was neither a main effect of drug nor any drug by genotype interaction on mouse weight; METH-treated mice did not differ in weight from vehicle during any of the test days (Figure 6b). In both cohorts of mice, however, gp120tg mice did exhibit lower weight overall compared to WT (experiment 1 [F(1,47) = 4.4, p < 0.05]; experiment 2 [F(1,35) = 16.6, p < 0.001]).
Fig. 6.
Weight and temperature data during the 25 day chronic METH regimen. Mouse weight was quantified during METH treatment (escalation days 1–14, binge days 15 – 25) in both experiment 1 (a) and experiment 2 (b). There was no main effect of drug, genotype, or drug-genotype interaction for either cohort, but METH-treated mice did show modest but significant decreases in weight relative to vehicle only on Day 11 (females) and Day 22 (males) in experiment 1 (p < 0.05). In experiment 2, temperature was quantified via a non-invasive infrared sensor before drug treatment and two hours after the third METH injection on the 15th day of chronic drug treatment (c). Mouse temperature was slightly elevated on the treatment day in all groups compared to baseline, but not affected by METH exposure or genotype
Temperature data obtained from the infrared monitor in Experiment 2 indicated that mouse temperature was slightly but significantly elevated on the first day of the METH binge (treatment day 15) compared to the baseline reading before the drug regimen [F(1,69) = 106.7, p < 0.001], but there was no effect or interaction with drug treatment or genotype (Figure 6c).
3.4 Combined cohort data for BPM
Finally, we assessed age effects by comparing BPM activity after 7 days of METH withdrawal in both the younger (4 month-old) and older (8–9 month-old) groups of mice. When the data for both cohorts were combined, we continued to observe a main effect of sex on motor activity, as female mice exhibited decreased transitions [F(1,165) = 10.4, p < 0.01] and distance traveled [F(1,165) = 11.8, p < 0.01] relative to males. There was no significant effect of age on transitions, but a trend towards increased distance traveled in the younger animals [F(1,165) = 3.4, p = 0.07]. Rears were decreased in females compare to males [F(1,165) = 12.4, p < 0.01], in gp120tg mice relative to WT [F(1,165) = 8.7, p < 0.01], and in older mice contrasted with the younger group [F(1,165) = 8.0, p < 0.01]. Holepokes were also reduced in the older mice versus the younger cohort [F(1,165) = 17.9, p < 0.001], but we also observed a main effect of drug such that all METH-treated mice exhibited increased holepokes compared to vehicle [F(1,165) = 4.4, p < 0.05]. Spatial d values were slightly but significantly elevated with chronic METH treatment compared to vehicle [F(1,165) = 6.4, p < 0.05] and in older mice relative to younger mice [F(1,165) = 7.6, p < 0.01].
4. Discussion
The purpose of this study was to examine the effect of chronic METH exposure on disinhibition in mice expressing the HIV-1 gp120 viral envelope protein. Our objective was to model the combined influence of comorbid human METH dependence and HIV infection on behaviors altered by inhibitory deficits, such as the response to novel stimuli. Overall, the findings indicated that both chronic METH administration and gp120 expression impact these measures, but effects are selective and dependent on age and sex. METH treatment increased BPM holepokes in 9 month-old female, but not male mice. During the last ten minutes of the task, METH-treated female gp120tg mice exhibited greater holepokes relative to the other female groups, in support of our hypothesis that disinhibited exploration would be elevated in animals exposed to the dual effects of stimulant treatment and HIV protein expression. While the METH-treated female gp120tg mice in the younger cohort continued to demonstrate the highest number of holepokes, drug and genotype effects were no longer significant. Comparison of age data revealed that the 4 month-old mice exhibited a greater number of holepokes compared to their older counterparts, so it is possible that a higher baseline level of activity may have obscured this finding in the younger mice. However, there were also apparent effects of METH treatment in this age group, including greater time spent in the center region with a novel object during the NOA task, a trend towards increased time in the light side of the LD enclosure, and a trend towards elevated spatial CV in the BPM relative to vehicle mice, indicative of repeated perseverative movements. These findings support a substantial body of work in human subjects and rodents that document inhibitory deficits associated with chronic METH exposure [42, 44]. METH administration for 2–3 weeks in rat is reported to increase impulsive responding in the 5-choice serial reaction time task and delay discounting paradigms for up to two weeks after withdrawal [42, 45]. Similarly, abstinent METH-dependent human participants are characterized by inhibitory deficits and perseveration on neuropsychological tests that include stop-signal, Stroop, delay discounting, and the Wisconsin Card Sorting Task [10, 46–48]. A recent study also reported that METH-dependent participants exhibited increased object interactions compared to drug-free comparison subjects in a novel human open-field apparatus [49], supporting the cross-species translational utility of this paradigm.
In contrast to the increased holepokes observed in METH-treated mice, gp120tg animals exhibited significantly fewer rears compared to WT in the older cohort, while the younger female METH and gp120tg groups tended to show fewer rears and slightly less total motor activity relative to the vehicle-treated WT females. Although group differences did not reach significance, gp120tg mice in Experiment 1 also demonstrated a trend towards fewer transitions and less distance traveled in Epoch 3 compared to WT. While rears are a measure of exploration, these results suggest that this measure, unlike holepokes, may have been reduced by an overall decrease in total locomotion. Previous work indicates that general open-field locomotion may be reduced in older (12 month-old) gp120tg mice [31]; thus decreased rearing may reflect subtle motor abnormalities that present with the gp120 protein expression. The 4 month-old female gp120tg mice did exhibit a trend towards increased spatial CV in the BPM relative to WT, similar to METH-treated mice, and also showed a decreased latency to enter the center region during the entire NOA task, but did not demonstrate specific responding to the novel object. Our findings diverge from a previous report [32] indicating that gp120tg mice demonstrated increased vertical activity when exposed to single acute high doses of METH (10 and 30 mg/kg), but may reflect the differences between acute and chronic METH exposure. Roberts et al. also reported increased stereotypy in the acute METH-treated gp120tg mice, a finding that corresponds with our observation of a trend towards higher spatial CV in the transgenic animals, reflecting more repetitive patterns of locomotor behavior[32].
Sex effects and interactions were prominent in this dataset and genotype differences were observed primarily in the female mice. Several factors may contribute to differential effects of METH treatment in male and female rodents (and humans), including drug metabolism, endocrine function, and variations in the dopamine (DA) system [50, 51]. In both species, there is evidence that amphetamines induce a greater release of striatal DA in males relative to females, accompanied by greater neurotoxicity (and in humans, more emergency fatalities in men) [50, 52]. These effects may be mediated by higher DA transporter (DAT) density in female basal ganglia in both human and rodent, more active DAT function (enabling more efficient removal of stimulant-released DA in females), and the protective effects of estrogen [53, 54]. In contrast, female rodents appear to metabolize METH more slowly than males [55], an effect that may explain reports of elevated locomotor and stereotypical activation in female rats [51] and more severe cognitive impairments in female mice [56]. The current findings indicate that female mice exposed to our chronic escalation/binge METH regimen may be more sensitive at least to the behavioral effects of the drug, while potential sex differences on markers of neurotoxicity have yet to be assessed.
The effect of sex on immune function and the inflammatory response may also be an important factor. Females of many species exhibit enhanced immune responses that may ultimately lead to detrimental consequences, as illustrated by the higher incidence of autoimmune disorders in older women [57]. Preclinical studies observe that female rodents may also demonstrate a more robust CNS response to infection or injury, including greater astrocytosis relative to males [58, 59]. Some work indicates a possible sex by age interaction, as male rodents appear more sensitive to immune challenge early in life, while adolescent and adult female mice exhibit higher quantities of microglia and astrocytes [57]. Previous studies with the gp120tg mice have not examined potential sex effects on neuropathology or behavior [28, 31], but the present data support the relevance and importance of this variable.
As with all animal representations of human HIV infection, the gp120 transgenic model includes a number of caveats and limitations. HIV induces CNS injury via several structural, regulatory, and accessory viral proteins predominantly mediated by a monocyte/macrophage brain infection [60], in contrast to the gp120 astrocyte expression of the current mouse model. However, key characteristics of NeuroAIDS are successfully recapitulated in the gp120tg mice, including activated microglia, decreased synaptic/dendritic density, and astrocytosis, phenomena that are associated with neurocognitive dysfunction in HIV dementia [26–28]. Inhibitory behaviors relevant to the current study are regulated by frontostriatal circuitry directly impacted by the gp120 protein, as both in vitro and in vivo gp120 administration is reported to induce apoptosis and neuronal loss in the caudate putamen and cortex [61–63]. These effects appear to be mediated by factors that include caspase-3 activation, inhibition of brain-derived neurotrophic factor (BDNF), glutamate-induced excitotoxicity, and pro-inflammatory mechanisms that involve the CXCR4 chemokine receptor [61, 64, 65]. While gp120 expression as a function of age has not yet been characterized (surprisingly) in gp120tg mice, striatal expression of the protein is reported to increase in older (10 month) vs. younger (2–3 month) HIV-1 transgenic rats [66], an effect that could potentially account for the age differences observed in the present study. Despite the distinction between the origin of gp120 expression (macrophages in human HIV infection, astrocytes for the gp120tg mouse), the similar cross-species pattern of neurodegeneration suggests that critical processes remain the same, including the release of neurotoxic factors that impair neuronal survival/function and the inflammatory response by the host organism.
The neurobiological mechanisms underlying the combined effect of METH and HIV have not been elucidated fully, but may involve a number of common mechanisms and pathways [67]. METH use induces excitotoxicity and neuronal death via increased glutamate and DA release [34, 68], similar to the effects of HIV gp120 and Tat proteins [69, 70]. Drug administration also exacerbates inflammatory function, increasing microglial activation and GFAP in mice [71], and may thus contribute to HIV inflammation-mediated neurodegeneration in frontostriatal circuitry regulating inhibitory function and cognitive performance. Effects of both drug and virus on the DA system itself may also be a key aspect of their combined influence. Exposure to gp120 induces dendritic shrinkage in midbrain DA neurons, degeneration of DA nigrostriatal neurons, and impairment of DAT function, with reduced striatal DAT also reported in HIV-positive individuals [72–75]. Similarly, chronic METH treatment decreases DA receptors and reduces DAT in both rodent and human, an effect proposed to contribute the development of behavioral sensitization [76, 77]. In conjunction with previous studies, we observed increased sensitization to a challenge dose of METH in WT mice previously treated with the chronic METH regimen, but this effect was not observed in the gp120tg mice, as higher transitions were observed in both the chronic METH and chronic vehicle conditions. One interpretation of this finding would suggest that the gp120tg mice show increased reactivity to an acute dose of METH even in drug-naive mice, but it is also appropriate to note that the genotype difference in METH-induced transitions did not reach significance. While relatively few studies have examined chronic METH exposure in models of HIV, studies with HIV-1 transgenic rats indicate that these animals exhibit increased sensitization to a subchronic METH regimen (6 days) compared to WT, an effect related to elevated DA receptor 1 expression in prefrontal cortex [78, 79]. Potential alterations in the DA system, including DAT expression, have not been characterized in gp120tg mice, but will be the focus of future investigations.
Limitations of the current study include aspects of experimental design and interpretation. METH-induced impairment of human cognitive function may vary over time, since some recovery appears to occur during protracted abstinence [80], while the time point of measurement in rodent is just as critical. We decided to perform behavioral testing within a range of 3 to 7 days of drug withdrawal, based on pilot data with gp120tg mice and prior reports of METH effects on inhibitory functioning in rodent [42]. However, it is possible that the behavioral effects of METH may vary during earlier and later points, changes that may also depend on the genetic background of the animals being tested [81]. We also quantified temperature effects of drug and genotype to address the issue of hyperthermia-induced neurotoxicity using a non-invasive infrared monitor. Similar to previous work in rat [33], inclusion of the METH escalation treatment appears to mitigate the temperature effects of the drug by the time the mice begin receiving the high dose binge regimen; however, temperature effects may also vary at different days during the treatment regimen and core body temperature was not recorded. Temperature was slightly elevated across all groups during the treatment regimen relative to baseline, an effect possibly mediated by the stress of receiving multiple injections. Finally, activity in the behavioral tasks we assessed may be interpreted to reflect different cognitive constructs. While measures in the BPM, NOA, and LD tests are related to aspects of inhibition, time spent in the light side of the light-dark box may also indicate levels of anxiety. We did observe, however, a consistent pattern of increased exploration with METH use across all three tests (e.g., holepokes, time in center with a novel object), indicative of a reliable phenotype of reduced inhibition. Future studies may augment our existing battery and clarify construct interpretation by assessing other measures of inhibitory function in rodents such as the go/no-go test, 5-choice continuous performance task, and set-shifting paradigms [82, 83].
In conclusion, administration of a chronic escalation/binge METH regimen induced a discrete pattern of disinhibition characterized by age-dependent increases in holepokes, object exploration, and trends towards perseverative locomotion in transgenic mice expressing the HIV gp120 protein. Future studies will examine the biological mechanisms that may mediate these effects, including the abundance and distribution of gp120 expression, potential alterations in DA signaling, and markers of excitotoxity and inflammation.
HIV infection and comorbid methamphetamine (METH) dependence impair inhibition.
We studied open-field activity in chronic METH-treated HIV/gp120 transgenic mice.
METH-treated gp120 female mice exhibited inhibitory deficits relative to control.
METH and gp120 expression selectively impact inhibition based on age and sex.
Additional studies are needed to elucidate the combined effects of HIV and METH.
Acknowledgments
We hereby declare we have no conflicts of interest involving this research and did not receive funds from a commercial sponsor. M.A. Geyer holds an equity interest in San Diego Instruments. The manuscript was supported by the Translational Methamphetamine AIDS Research Center (TMARC) (P50DA026306) funded by the National Institute On Drug Abuse (NIDA) and a grant from the National Institute of Mental Health (NIMH) (R01-MH071916).
The Translational Methamphetamine AIDS Research Center (TMARC) is affiliated with the University of California, San Diego (UCSD) and the Sanford-Burnham Medical Research Institute (SBMRI). The TMARC is comprised of: Director – Igor Grant, M.D.; Co-Directors – Ronald J. Ellis, M.D., Ph.D., Cristian L. Achim, M.D., Ph.D., and Scott L. Letendre, M.D.; Center Manager – Steven Paul Woods, Psy.D.; Assistant Center Manager – Aaron M. Carr, B.A.; Clinical Assessment and Laboratory (CAL) Core: Scott L. Letendre, M.D. (Core Director), Ronald J. Ellis, M.D., Ph.D., Rachel Schrier, Ph.D.; Neuropsychiatric (NP) Core: Robert K. Heaton, Ph.D. (Core Director), J. Hampton Atkinson, M.D., Mariana Cherner, Ph.D., Thomas D. Marcotte, Ph.D.; Neuroimaging (NI) Core: Gregory Brown, Ph.D. (Core Director), Terry Jernigan, Ph.D., Anders Dale, Ph.D., Thomas Liu, Ph.D., Miriam Scadeng, Ph.D., Christine Fennema-Notestine, Ph.D., Sarah L. Archibald, M.A.; Neurosciences and Animal Models (NAM) Core: Cristian L. Achim, M.D., Ph.D. (Core Director), Eliezer Masliah, M.D., Stuart Lipton, M.D., Ph.D.; Administrative Coordinating Core (ACC) – Data Management and Information Systems (DMIS) Unit: Anthony C. Gamst, Ph.D. (Unit Leader), Clint Cushman (Unit Manager); ACC – Statistics Unit: Ian Abramson, Ph.D. (Unit Leader), Florin Vaida, Ph.D., Reena Deutsch, Ph.D., Anya Umlauf, M.S.; ACC – Participant Unit: J. Hampton Atkinson, M.D. (Unit Leader), Jennifer Marquie-Beck, M.P.H. (Unit Manager); Project 1: Arpi Minassian, Ph.D. (Project Director), William Perry, Ph.D., Mark Geyer, Ph.D., Brook Henry, Ph.D.; Project 2: Amanda B. Grethe, Ph.D. (Project Director), Martin Paulus, M.D., Ronald J. Ellis, M.D., Ph.D.; Project 3: Sheldon Morris, M.D., M.P.H. (Project Director), David M. Smith, M.D., M.A.S., Igor Grant, M.D.; Project 4: Svetlana Semenova, Ph.D. (Project Director), Athina Markou, Ph.D., James Kesby, Ph.D.; Project 5: Marcus Kaul, Ph.D. (Project Director). The views expressed in this article are those of the authors and do not reflect the official policy or position of the United States Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lucas GM, Cheever LW, Chaisson RE, Moore RD. Detrimental effects of continued illicit drug use on the treatment of HIV-1 infection. J Acquir Immune Defic Syndr. 2001;27:251–9. doi: 10.1097/00126334-200107010-00006. [DOI] [PubMed] [Google Scholar]
- 2.Lucas GM, Griswold M, Gebo KA, Keruly J, Chaisson RE, Moore RD. Illicit drug use and HIV-1 disease progression: a longitudinal study in the era of highly active antiretroviral therapy. Am J Epidemiol. 2006;163:412–20. doi: 10.1093/aje/kwj059. [DOI] [PubMed] [Google Scholar]
- 3.Nelson KE, Galai N, Safaeian M, Strathdee SA, Celentano DD, Vlahov D. Temporal trends in the incidence of human immunodeficiency virus infection and risk behavior among injection drug users in Baltimore, Maryland, 1988–1998. Am J Epidemiol. 2002;156:641–53. doi: 10.1093/aje/kwf086. [DOI] [PubMed] [Google Scholar]
- 4.Chang L, Ernst T, Speck O, Grob CS. Additive effects of HIV and chronic methamphetamine use on brain metabolite abnormalities. Am J Psychiatry. 2005;162:361–9. doi: 10.1176/appi.ajp.162.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rippeth JD, Heaton RK, Carey CL, Marcotte TD, Moore DJ, Gonzalez R, et al. Methamphetamine dependence increases risk of neuropsychological impairment in HIV infected persons. J Int Neuropsychol Soc. 2004;10:1–14. doi: 10.1017/S1355617704101021. [DOI] [PubMed] [Google Scholar]
- 6.Freeman P, Walker BC, Harris DR, Garofalo R, Willard N, Ellen JM. Methamphetamine use and risk for HIV among young men who have sex with men in 8 US cities. Arch Pediatr Adolesc Med. 2011;165:736–40. doi: 10.1001/archpediatrics.2011.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mausbach BT, Semple SJ, Strathdee SA, Zians J, Patterson TL. Efficacy of a behavioral intervention for increasing safer sex behaviors in HIV-negative, heterosexual methamphetamine users: results from the Fast-Lane Study. Ann Behav Med. 2007;34:263–74. doi: 10.1007/BF02874551. [DOI] [PubMed] [Google Scholar]
- 8.Hinkin CH, Castellon SA, Hardy DJ, Granholm E, Siegle G. Computerized and traditional stroop task dysfunction in HIV-1 infection. Neuropsychology. 1999;13:306–16. doi: 10.1037//0894-4105.13.2.306. [DOI] [PubMed] [Google Scholar]
- 9.Martin HP. Mild cognitive impairment in HIV disease. Nurse Pract. 1995;20:94–7. [PubMed] [Google Scholar]
- 10.Monterosso JR, Aron AR, Cordova X, Xu J, London ED. Deficits in response inhibition associated with chronic methamphetamine abuse. Drug Alcohol Depend. 2005;79:273–7. doi: 10.1016/j.drugalcdep.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 11.Salo R, Nordahl TE, Possin K, Leamon M, Gibson DR, Galloway GP, et al. Preliminary evidence of reduced cognitive inhibition in methamphetamine-dependent individuals. Psychiatry Res. 2002;111:65–74. doi: 10.1016/s0165-1781(02)00111-7. [DOI] [PubMed] [Google Scholar]
- 12.Mahajan SD, Aalinkeel R, Sykes DE, Reynolds JL, Bindukumar B, Adal A, et al. Methamphetamine alters blood brain barrier permeability via the modulation of tight junction expression: Implication for HIV-1 neuropathogenesis in the context of drug abuse. Brain Res. 2008;1203:133–48. doi: 10.1016/j.brainres.2008.01.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marcondes MC, Flynn C, Watry DD, Zandonatti M, Fox HS. Methamphetamine increases brain viral load and activates natural killer cells in simian immunodeficiency virus-infected monkeys. Am J Pathol. 2010;177:355–61. doi: 10.2353/ajpath.2010.090953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nair MP, Saiyed ZM. Effect of methamphetamine on expression of HIV coreceptors and CC-chemokines by dendritic cells. Life Sci. 2011;88:987–94. doi: 10.1016/j.lfs.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silverstein PS, Shah A, Gupte R, Liu X, Piepho RW, Kumar S, et al. Methamphetamine toxicity and its implications during HIV-1 infection. J Neurovirol. 2011;17:401–15. doi: 10.1007/s13365-011-0043-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geyer MA, Russo PV, Masten VL. Multivariate assessment of locomotor behavior: pharmacological and behavioral analyses. Pharmacol Biochem Behav. 1986;25:277–88. doi: 10.1016/0091-3057(86)90266-2. [DOI] [PubMed] [Google Scholar]
- 17.Geyer M, Paulus M. Multivariate analyses of locomotor and investigatory behavior in rodents. In: Ossenkopp K, Kavaliers M, Sanberg P, editors. Measuring Movement and Locomotion: From Invertebrates to Humans. Austin, TX.: R.G. Landes Co; 1996. pp. 253–71. [Google Scholar]
- 18.Geyer MA. Approaches to the Characterization of Drug Effects on Locomotor Activity in Rodents. In: Adler M, Cowan A, editors. Testing and Evaluation of Drugs of Abuse. New York: Wiley-Liss; 1990. pp. 81–99. [Google Scholar]
- 19.Robbins T. A Critique of the Methods Available for Measurement of Spontaneous Motor Activity. In: Iversen L, Iversen S, Snyder S, editors. Handbook of Psychopharmacology. New York: Plenum Press; 1979. pp. 37–82. [Google Scholar]
- 20.Paulus MP, Geyer MA. Three independent factors characterize spontaneous rat motor activity. Behav Brain Res. 1993;53:11–20. doi: 10.1016/s0166-4328(05)80262-1. [DOI] [PubMed] [Google Scholar]
- 21.Risbrough VB, Masten VL, Caldwell S, Paulus MP, Low MJ, Geyer MA. Differential contributions of dopamine D1, D2, and D3 receptors to MDMA-induced effects on locomotor behavior patterns in mice. Neuropsychopharmacology. 2006;31:2349–58. doi: 10.1038/sj.npp.1301161. [DOI] [PubMed] [Google Scholar]
- 22.Goodwin FK, Jamison KR. Manic-depressive illness. New York: Oxford UP; 1990. [Google Scholar]
- 23.Teixeira C, Gomes JR, Gomes P, Maurel F, Barbault F. Viral surface glycoproteins, gp120 and gp41, as potential drug targets against HIV-1: brief overview one quarter of a century past the approval of zidovudine, the first anti-retroviral drug. Eur J Med Chem. 2011;46:979–92. doi: 10.1016/j.ejmech.2011.01.046. [DOI] [PubMed] [Google Scholar]
- 24.Purohit V, Rapaka R, Shurtleff D. Drugs of abuse, dopamine, and HIV-associated neurocognitive disorders/HIV-associated dementia. Mol Neurobiol. 2011;44:102–10. doi: 10.1007/s12035-011-8195-z. [DOI] [PubMed] [Google Scholar]
- 25.Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410:988–94. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- 26.Maung R, Medders KE, Sejbuk NE, Desai MK, Russo R, Kaul M. Genetic Knockouts Suggest a Critical Role for HIV Co-Receptors in Models of HIV gp120-Induced Brain Injury. J Neuroimmune Pharmacol. 2012;7:306–18. doi: 10.1007/s11481-011-9328-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okamoto S, Kang YJ, Brechtel CW, Siviglia E, Russo R, Clemente A, et al. HIV/gp120 decreases adult neural progenitor cell proliferation via checkpoint kinase-mediated cell-cycle withdrawal and G1 arrest. Cell Stem Cell. 2007;1:230–6. doi: 10.1016/j.stem.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 28.Toggas SM, Masliah E, Rockenstein EM, Rall GF, Abraham CR, Mucke L. Central nervous system damage produced by expression of the HIV-1 coat protein gp120 in transgenic mice. Nature. 1994;367:188–93. doi: 10.1038/367188a0. [DOI] [PubMed] [Google Scholar]
- 29.Garden GA, Budd SL, Tsai E, Hanson L, Kaul M, D’Emilia DM, et al. Caspase cascades in human immunodeficiency virus-associated neurodegeneration. J Neurosci. 2002;22:4015–24. doi: 10.1523/JNEUROSCI.22-10-04015.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kang YJ, Digicaylioglu M, Russo R, Kaul M, Achim CL, Fletcher L, et al. Erythropoietin plus insulin-like growth factor-I protects against neuronal damage in a murine model of human immunodeficiency virus-associated neurocognitive disorders. Ann Neurol. 2010;68:342–52. doi: 10.1002/ana.22070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.D’Hooge R, Franck F, Mucke L, De Deyn PP. Age-related behavioural deficits in transgenic mice expressing the HIV-1 coat protein gp120. Eur J Neurosci. 1999;11:4398–402. doi: 10.1046/j.1460-9568.1999.00857.x. [DOI] [PubMed] [Google Scholar]
- 32.Roberts AJ, Maung R, Sejbuk NE, Ake C, Kaul M. Alteration of Methamphetamine-induced stereotypic behaviour in transgenic mice expressing HIV-1 envelope protein gp120. J Neurosci Methods. 2010;186:222–5. doi: 10.1016/j.jneumeth.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuczenski R, Everall IP, Crews L, Adame A, Grant I, Masliah E. Escalating dose-multiple binge methamphetamine exposure results in degeneration of the neocortex and limbic system in the rat. Exp Neurol. 2007;207:42–51. doi: 10.1016/j.expneurol.2007.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davidson C, Gow AJ, Lee TH, Ellinwood EH. Methamphetamine neurotoxicity: necrotic and apoptotic mechanisms and relevance to human abuse and treatment. Brain Res Brain Res Rev. 2001;36:1–22. doi: 10.1016/s0165-0173(01)00054-6. [DOI] [PubMed] [Google Scholar]
- 35.Segal DS, Kuczenski R. An escalating dose “binge” model of amphetamine psychosis: behavioral and neurochemical characteristics. J Neurosci. 1997;17:2551–66. doi: 10.1523/JNEUROSCI.17-07-02551.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Young JW, Goey AK, Minassian A, Perry W, Paulus MP, Geyer MA. GBR 12909 administration as a mouse model of bipolar disorder mania: mimicking quantitative assessment of manic behavior. Psychopharmacology (Berl) 2010;208:443–54. doi: 10.1007/s00213-009-1744-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paulus MP, Geyer MA. A scaling approach to find order parameters quantifying the effects of dopaminergic agents on unconditioned motor activity in rats. Prog Neuropsychopharmacol Biol Psychiatry. 1991;15:903–19. doi: 10.1016/0278-5846(91)90018-v. [DOI] [PubMed] [Google Scholar]
- 38.Ali SS, Young JW, Wallace CK, Gresack J, Jeste DV, Geyer MA, et al. Initial evidence linking synaptic superoxide production with poor short-term memory in aged mice. Brain Res. 2011;1368:65–70. doi: 10.1016/j.brainres.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Powell SB, Geyer MA, Gallagher D, Paulus MP. The balance between approach and avoidance behaviors in a novel object exploration paradigm in mice. Behav Brain Res. 2004;152:341–9. doi: 10.1016/j.bbr.2003.10.020. [DOI] [PubMed] [Google Scholar]
- 40.Costall B, Naylor RJ. The influence of 5-HT2 and 5-HT4 receptor antagonists to modify drug induced disinhibitory effects in the mouse light/dark test. Br J Pharmacol. 1997;122:1105–18. doi: 10.1038/sj.bjp.0701513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Crawley JN. Exploratory behavior models of anxiety in mice. Neurosci Biobehav Rev. 1985;9:37–44. doi: 10.1016/0149-7634(85)90030-2. [DOI] [PubMed] [Google Scholar]
- 42.Dalley JW, Laane K, Theobald DE, Pena Y, Bruce CC, Huszar AC, et al. Enduring deficits in sustained visual attention during withdrawal of intravenous methylenedioxymethamphetamine self-administration in rats: results from a comparative study with d-amphetamine and methamphetamine. Neuropsychopharmacology. 2007;32:1195–206. doi: 10.1038/sj.npp.1301220. [DOI] [PubMed] [Google Scholar]
- 43.O’Neil ML, Kuczenski R, Segal DS, Cho AK, Lacan G, Melega WP. Escalating dose pretreatment induces pharmacodynamic and not pharmacokinetic tolerance to a subsequent high-dose methamphetamine binge. Synapse. 2006;60:465–73. doi: 10.1002/syn.20320. [DOI] [PubMed] [Google Scholar]
- 44.Scott JC, Woods SP, Matt GE, Meyer RA, Heaton RK, Atkinson JH, et al. Neurocognitive effects of methamphetamine: a critical review and meta-analysis. Neuropsychol Rev. 2007;17:275–97. doi: 10.1007/s11065-007-9031-0. [DOI] [PubMed] [Google Scholar]
- 45.Richards JB, Sabol KE, de Wit H. Effects of methamphetamine on the adjusting amount procedure, a model of impulsive behavior in rats. Psychopharmacology (Berl) 1999;146:432–9. doi: 10.1007/pl00005488. [DOI] [PubMed] [Google Scholar]
- 46.Hoffman WF, Moore M, Templin R, McFarland B, Hitzemann RJ, Mitchell SH. Neuropsychological function and delay discounting in methamphetamine-dependent individuals. Psychopharmacology (Berl) 2006;188:162–70. doi: 10.1007/s00213-006-0494-0. [DOI] [PubMed] [Google Scholar]
- 47.Salo R, Ursu S, Buonocore MH, Leamon MH, Carter C. Impaired prefrontal cortical function and disrupted adaptive cognitive control in methamphetamine abusers: a functional magnetic resonance imaging study. Biol Psychiatry. 2009;65:706–9. doi: 10.1016/j.biopsych.2008.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Woods SP, Rippeth JD, Conover E, Gongvatana A, Gonzalez R, Carey CL, et al. Deficient strategic control of verbal encoding and retrieval in individuals with methamphetamine dependence. Neuropsychology. 2005;19:35–43. doi: 10.1037/0894-4105.19.1.35. [DOI] [PubMed] [Google Scholar]
- 49.Henry BL, Minassian A, van Rhenen M, Young JW, Geyer MA, Perry W. Effect of methamphetamine dependence on inhibitory deficits in a novel human open-field paradigm. Psychopharmacology (Berl) 2011;215:697–707. doi: 10.1007/s00213-011-2170-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dluzen DE, Liu B. Gender differences in methamphetamine use and responses: a review. Gend Med. 2008;5:24–35. doi: 10.1016/s1550-8579(08)80005-8. [DOI] [PubMed] [Google Scholar]
- 51.Milesi-Halle A, McMillan DE, Laurenzana EM, Byrnes-Blake KA, Owens SM. Sex differences in (+)-amphetamine- and (+)-methamphetamine-induced behavioral response in male and female Sprague-Dawley rats. Pharmacol Biochem Behav. 2007;86:140–9. doi: 10.1016/j.pbb.2006.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bourque M, Liu B, Dluzen DE, Di Paolo T. Sex differences in methamphetamine toxicity in mice: effect on brain dopamine signaling pathways. Psychoneuroendocrinology. 2011;36:955–69. doi: 10.1016/j.psyneuen.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 53.Bhatt SD, Dluzen DE. Dopamine transporter function differences between male and female CD-1 mice. Brain Res. 2005;1035:188–95. doi: 10.1016/j.brainres.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 54.Rivest R, Falardeau P, Di Paolo T. Brain dopamine transporter: gender differences and effect of chronic haloperidol. Brain Res. 1995;692:269–72. doi: 10.1016/0006-8993(95)00611-s. [DOI] [PubMed] [Google Scholar]
- 55.Milesi-Halle A, Hendrickson HP, Laurenzana EM, Gentry WB, Owens SM. Sex- and dose-dependency in the pharmacokinetics and pharmacodynamics of (+)-methamphetamine and its metabolite (+)-amphetamine in rats. Toxicol Appl Pharmacol. 2005;209:203–13. doi: 10.1016/j.taap.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 56.Acevedo SF, Pfankuch T, van Meer P, Raber J. Role of histamine in short- and long-term effects of methamphetamine on the developing mouse brain. J Neurochem. 2008;107:976–86. doi: 10.1111/j.1471-4159.2008.05673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schwarz JM, Bilbo SD. Sex, glia, and development: Interactions in health and disease. Horm Behav. 2012 doi: 10.1016/j.yhbeh.2012.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cordeau P, Jr, Lalancette-Hebert M, Weng YC, Kriz J. Live imaging of neuroinflammation reveals sex and estrogen effects on astrocyte response to ischemic injury. Stroke. 2008;39:935–42. doi: 10.1161/STROKEAHA.107.501460. [DOI] [PubMed] [Google Scholar]
- 59.Zhang XM, Zhu SW, Duan RS, Mohammed AH, Winblad B, Zhu J. Gender differences in susceptibility to kainic acid-induced neurodegeneration in aged C57BL/6 mice. Neurotoxicology. 2008;29:406–12. doi: 10.1016/j.neuro.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 60.Ellis R, Langford D, Masliah E. HIV and antiretroviral therapy in the brain: neuronal injury and repair. Nat Rev Neurosci. 2007;8:33–44. doi: 10.1038/nrn2040. [DOI] [PubMed] [Google Scholar]
- 61.Chen L, Liu J, Xu C, Keblesh J, Zang W, Xiong H. HIV-1gp120 induces neuronal apoptosis through enhancement of 4-aminopyridine-senstive outward K+ currents. PLoS One. 2011;6:e25994. doi: 10.1371/journal.pone.0025994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Louboutin JP, Reyes BA, Agrawal L, Van Bockstaele EJ, Strayer DS. HIV-1 gp120-induced neuroinflammation: relationship to neuron loss and protection by rSV40-delivered antioxidant enzymes. Exp Neurol. 2010;221:231–45. doi: 10.1016/j.expneurol.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 63.Singh IN, Goody RJ, Dean C, Ahmad NM, Lutz SE, Knapp PE, et al. Apoptotic death of striatal neurons induced by human immunodeficiency virus-1 Tat and gp120: Differential involvement of caspase-3 and endonuclease G. J Neurovirol. 2004;10:141–51. doi: 10.1080/13550280490441103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li W, Galey D, Mattson MP, Nath A. Molecular and cellular mechanisms of neuronal cell death in HIV dementia. Neurotox Res. 2005;8:119–34. doi: 10.1007/BF03033824. [DOI] [PubMed] [Google Scholar]
- 65.Nosheny RL, Bachis A, Acquas E, Mocchetti I. Human immunodeficiency virus type 1 glycoprotein gp120 reduces the levels of brain-derived neurotrophic factor in vivo: potential implication for neuronal cell death. Eur J Neurosci. 2004;20:2857–64. doi: 10.1111/j.1460-9568.2004.03764.x. [DOI] [PubMed] [Google Scholar]
- 66.Peng J, Vigorito M, Liu X, Zhou D, Wu X, Chang SL. The HIV-1 transgenic rat as a model for HIV-1 infected individuals on HAART. J Neuroimmunol. 2010;218:94–101. doi: 10.1016/j.jneuroim.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 67.Cadet JL, Krasnova IN. Interactions of HIV and methamphetamine: cellular and molecular mechanisms of toxicity potentiation. Neurotox Res. 2007;12:181–204. doi: 10.1007/BF03033915. [DOI] [PubMed] [Google Scholar]
- 68.Marshall JF, O’Dell SJ, Weihmuller FB. Dopamine-glutamate interactions in methamphetamine-induced neurotoxicity. J Neural Transm Gen Sect. 1993;91:241–54. doi: 10.1007/BF01245234. [DOI] [PubMed] [Google Scholar]
- 69.Haughey NJ, Nath A, Mattson MP, Slevin JT, Geiger JD. HIV-1 Tat through phosphorylation of NMDA receptors potentiates glutamate excitotoxicity. J Neurochem. 2001;78:457–67. doi: 10.1046/j.1471-4159.2001.00396.x. [DOI] [PubMed] [Google Scholar]
- 70.Langford D, Hurford R, Hashimoto M, Digicaylioglu M, Masliah E. Signalling crosstalk in FGF2-mediated protection of endothelial cells from HIV-gp120. BMC Neurosci. 2005;6:8. doi: 10.1186/1471-2202-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thomas DM, Dowgiert J, Geddes TJ, Francescutti-Verbeem D, Liu X, Kuhn DM. Microglial activation is a pharmacologically specific marker for the neurotoxic amphetamines. Neurosci Lett. 2004;367:349–54. doi: 10.1016/j.neulet.2004.06.065. [DOI] [PubMed] [Google Scholar]
- 72.Bennett BA, Rusyniak DE, Hollingsworth CK. HIV-1 gp120-induced neurotoxicity to midbrain dopamine cultures. Brain Res. 1995;705:168–76. doi: 10.1016/0006-8993(95)01166-8. [DOI] [PubMed] [Google Scholar]
- 73.Chang L, Wang GJ, Volkow ND, Ernst T, Telang F, Logan J, et al. Decreased brain dopamine transporters are related to cognitive deficits in HIV patients with or without cocaine abuse. Neuroimage. 2008;42:869–78. doi: 10.1016/j.neuroimage.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nosheny RL, Ahmed F, Yakovlev A, Meyer EM, Ren K, Tessarollo L, et al. Brain-derived neurotrophic factor prevents the nigrostriatal degeneration induced by human immunodeficiency virus-1 glycoprotein 120 in vivo. Eur J Neurosci. 2007;25:2275–84. doi: 10.1111/j.1460-9568.2007.05506.x. [DOI] [PubMed] [Google Scholar]
- 75.Wallace DR, Dodson S, Nath A, Booze RM. Estrogen attenuates gp120- and tat1-72-induced oxidative stress and prevents loss of dopamine transporter function. Synapse. 2006;59:51–60. doi: 10.1002/syn.20214. [DOI] [PubMed] [Google Scholar]
- 76.McCann UD, Wong DF, Yokoi F, Villemagne V, Dannals RF, Ricaurte GA. Reduced striatal dopamine transporter density in abstinent methamphetamine and methcathinone users: evidence from positron emission tomography studies with [11C]WIN-35,428. J Neurosci. 1998;18:8417–22. doi: 10.1523/JNEUROSCI.18-20-08417.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pierce RC, Kalivas PW. A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res Brain Res Rev. 1997;25:192–216. doi: 10.1016/s0165-0173(97)00021-0. [DOI] [PubMed] [Google Scholar]
- 78.Kass MD, Liu X, Vigorito M, Chang L, Chang SL. Methamphetamine-induced behavioral and physiological effects in adolescent and adult HIV-1 transgenic rats. J Neuroimmune Pharmacol. 2010;5:566–73. doi: 10.1007/s11481-010-9221-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu X, Chang L, Vigorito M, Kass M, Li H, Chang SL. Methamphetamine-induced behavioral sensitization is enhanced in the HIV-1 transgenic rat. J Neuroimmune Pharmacol. 2009;4:309–16. doi: 10.1007/s11481-009-9160-8. [DOI] [PubMed] [Google Scholar]
- 80.Iudicello JE, Woods SP, Vigil O, Scott JC, Cherner M, Heaton RK, et al. Longer term improvement in neurocognitive functioning and affective distress among methamphetamine users who achieve stable abstinence. J Clin Exp Neuropsychol. 2010;32:704–18. doi: 10.1080/13803390903512637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Grace CE, Schaefer TL, Herring NR, Graham DL, Skelton MR, Gudelsky GA, et al. Effect of a neurotoxic dose regimen of (+)-methamphetamine on behavior, plasma corticosterone, and brain monoamines in adult C57BL/6 mice. Neurotoxicol Teratol. 2010;32:346–55. doi: 10.1016/j.ntt.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tanaka S, Young JW, Gresack JE, Geyer MA, Risbrough VB. Factor analysis of attentional set-shifting performance in young and aged mice. Behav Brain Funct. 2011;7:33. doi: 10.1186/1744-9081-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Young JW, Light GA, Marston HM, Sharp R, Geyer MA. The 5-choice continuous performance test: evidence for a translational test of vigilance for mice. PLoS One. 2009;4:e4227. doi: 10.1371/journal.pone.0004227. [DOI] [PMC free article] [PubMed] [Google Scholar]