Abstract
Using fMRI, this study examined the relationship between repetition-related changes in the medial temporal lobe (MTL) activation during encoding and subsequent memory for similarity of repetitions. During scanning, subjects classified pictures of objects as natural or man-made. Each object-type was judged twice with presentations of either identical pictures or pictures of different exemplars of the same object. After scanning, a surprise recognition test required subjects to decide whether a probe word corresponded to pictures judged previously. When a subject judged the word as “old”, a second judgment was made concerning the physical similarity of the two pictures. Repetition related changes in the MTL activation varied depending on whether or not subjects could correctly state that pictures were different. Moreover, psychophysiological interactions analyses showed that accuracy in recalling whether the two pictures were different was predicted by repetition-related changes in the functional connectivity of MTL with frontal regions. Specifically, correct recollection was predicted by increased connectivity between the left posterior hippocampus and the right inferior frontal gyrus, and also by decreased connectivity between the left posterior hippocampus and the left precentral gyrus on the second stimulus presentation. The opposite pattern was found for trials that were incorrectly judged on the nature of the repetition. These results suggest that successful encoding is predicted by a combination of increases and decreases in both the MTL activation and functional connectivity, and not merely by increases in activation and connectivity as suggested previously.
Keywords: fMRI, psychophysiological interaction, hippocampus, priming, subsequent memory
1. Introduction
Recently, a number of studies have explored how repetition-related changes in brain activation during encoding are related to subsequent memory (Wagner et al., 2000; Turk-Browne et al., 2006; Manelis et al., 2011; Xue et al., 2010, 2011). It would seem that subsequent memory should be predicted by the repetition-related changes in the MTL, given that successful (compared to unsuccessful) encoding is associated with increased activation in the medial temporal lobe (MTL) including hippocampus (HPC), perirhinal (PRc) and parahippocampal (PHc) cortices (e.g., Brewer et al., 1998; Wagner et al., 1998; Kirchhoff et al., 2000; Otten et al., 2001; Jackson and Schacter, 2004; Staresina and Davachi, 2006; Wimber et al., 2010; for reviews, see Henson, 2005; Spaniol et al., 2009; Kim, 2010). Surprisingly, however, only the study of Turk-Browne et al. (2006) reported that subsequent memory effects correlate with the magnitude of changes in activation across repetitions in the MTL. The Turk-Browne et al. study found that the PHc exhibited greater repetition-related decreases for hits than for misses. A different study looked at the effect of repetition in the MTL subregions for the pictures that were continuously recognized in the experiment (Yassa and Stark, 2008). They found repetition-related decreases in activation of bilateral PHc and anterior PRc and repetition-related increases in activation of bilateral posterior PRc. Neither study, however, reported any hippocampal effects.
One potential explanation for the failure to observe HPC effects in these studies involves the idea that the HPC is engaged in pattern separation to avoid interference from the similar representations (e.g., Yassa and Stark, 2008). Both studies used identical repetitions of the stimuli so that each repetition strengthened the existing representation, obviating any need for pattern separation. Another possible explanation for the failure to find HPC effects might be the nature of the recognition requirements at test. Both Turk-Browne et al. (2006) and Yassa and Stark (2008) used a recognition task that did not allow them to discriminate between judgments that were based on familiarity versus recollection. HPC plays a critical role in encoding the details of the study episode (e.g., Davachi et al., 2003; Ranganath et al., 2004; Uncapher & Rugg, 2009); therefore, it may be necessary to test subjects’ recollection in order to detect changes in HPC activation (e.g., Wheeler and Buckner, 2004; Montaldi et al., 2006; Diana et al., 2007; Eichenbaum et al., 2007).
The latter explanation derives support from recent studies of Gagnepain et al. (2011) and Poppenk et al. (2010). These studies examined the effect of recollection vs. familiarity judgments for stimuli that had been exposed prior to scanning compared to stimuli that had not been so primed. They found that HPC activation is modulated by both stimulus repetition and whether the subjects give a recollection response. Thus, there were stronger source memory effects (i.e., source memory vs. no source memory) for repeated (primed) compared with novel (unprimed) stimuli in the bilateral posterior HPC (Poppenk et al., 2010). In contrast, the right anterior HPC showed stronger source memory effects (Poppenk et al., 2010) and stronger recollection effects (i.e., “Remember” > “Know”) for novel compared to repeated stimuli (Gagnepain et al., 2011). Given that the subjects in these studies were not scanned during the priming phase of the experiment, the relationship between the repetition-related dynamics in the HPC and other MTL subregions during encoding and whether the stimuli are subsequently recollected is yet to be specified.
The present study addresses this matter by examining the relationship between repetition-related changes in the MTL during encoding and subsequent memory for pictures of objects. Subjects viewed either two identical pictures of an object or two different exemplars from the same basic category (e.g., a red apple and a green apple). We refer to the first case as a same-exemplar repetition and the second as a different-exemplar repetition. Some previous studies have also examined subsequent memory effects as a function of whether a probe stimulus was an identical or different exemplar from the same category of objects (e.g., Garoff et al., 2005), but they did not involve stimulus repetition during encoding.
A surprise memory test was then given outside the scanner that required subjects to make old/new judgments about the images when probed with words that could correspond to previously viewed pictures. For each “old” judgment, subjects had to indicate whether they remembered two different or two identical pictures that correspond to the probe word. Both memory judgments, old and same/different, also required a confidence decision (“sure” or “unsure”) about the judgment. Given that increased activity in the HPC and PHc often predicts subsequent memory strength (e.g., Kirwan et al., 2008; Wais, 2008; Song et al., 2011) rather then memory accuracy, this latter procedure allowed us to compare different types of subsequent memory while controlling for memory strength. Trials in which a subject correctly and confidently recognized a probe word as “old” and then correctly and confidently recalled whether the two presentations were the same or different were classified as correct recollection. Conversely, trials for which a subject correctly and confidently recognized a probe word as “old” but then confidently misremembered whether the two presentations were the same or different were classified as gist recollection.
The current paper examines neural priming in the MTL for correct recollection vs. gist recollection and reports a different set of analyses on different subsets of data than those previously published in Manelis et al. (2011). That previous report differs from the present one in that it compared neural priming for the items that were subsequently confidently recollected with the items that were subsequently forgotten (i.e., trials we define as gist recollection were not included in the previous report and the current paper does not analyze misses). The main finding reported in Manelis et al. (2011) was that the degree of neural attenuation in the temporo-occipital and frontal regions depended on the interaction between perceptual similarity across repeated presentations and the quality of their encodings.
Based on findings from some previous studies (e.g., Davachi et al., 2003; Ranganath et al., 2004; Weis et al., 2004), we predict that there should be a main effect of subsequent memory in the MTL but with greater activation in HPC and PHc for correct compared to gist recollection. While there is no consensus on the role of anterior and posterior HPC in memory (e.g., Henson, 2005), some studies suggest that the posterior aspect of the HPC is associated with recollection, while its anterior aspect is associated with novelty (e.g., Daselaar et al., 2006). Based on these findings, it is possible that in our study, correct recollection compared to gist recollection will elicit greater activation in the posterior portion of the HPC. Conversely, anterior HPC may show a larger effect for different-exemplar compared to same-exemplar repetition because of the novelty introduced by the presentation of a different exemplar in the different-exemplar condition.
A growing body of work on pattern completion and pattern separation in HPC suggests that HPC serves as a pattern separation device (e.g., Bakker et al., 2008; Yassa and Stark, 2008, 2011). From that view it follows that one should find the greatest HPC involvement in our study for different-exemplar repetitions when subjects actually remember that there were two different exemplars of the same object category. Different exemplars taken from the same basic category of objects share multiple perceptual features (e.g., Rosch et al., 1976). Therefore, to tease apart the two presentations and recollect that they were actually two different exemplars requires that a subject overcome the interference that comes from the similarity of perceptual features in the two exemplars by separating the exemplars’ patterns.
The second goal of this paper is to explore the repetition-related changes in the functional connectivity associated with the different levels of memory. Few studies have examined the changes in the functional connectivity as a function of repetition. One of these studies reported that repetition-related attenuation in regions responsible for processing of object and spatial information also showed increases in effective connectivity between these same regions (Büchel et al., 1999). Another study using MEG (Ghuman et al., 2008) found results consistent with those of Büchel et al. such that stimulus repetition strengthens the interactions among brain regions. No study, however, has examined repetition-related changes in connectivity of MTL and neocortex, especially as a function of subsequent memory. The results of several previous studies that involve subsequent memory (but not the interaction of subsequent memory and priming) suggest that functional and effective connectivity between the MTL subregions (especially, HPC) and neocortex are important factors predicting the subjects’ performance on memory tasks (e.g., Ranganath et al., 2005; Hannula and Ranganath, 2009; Gagnepain et al., 2011; Westerberg et al., 2011). For example, the analysis of effective connectivity in the Gagnepain et al. study revealed that successful stimulus encoding is related to the increased connectivity from the anterior aspect of the superior temporal gyrus (involved in processing of auditory stimuli) to the HPC. Hannula and Ranganath reported that functional connectivity between HPC and lateral prefrontal cortex (PFC) predicts accuracy for associative memory. Taken together, these findings suggest that successful encoding may be related to the increased HPC-neocortex connectivity over repetitions.
2. Materials and methods
2.1 Subjects
Fourteen volunteers (20 – 35 years old, all right-handed, eight female) with normal or corrected to normal vision participated in this fMRI study. One subject was excluded from the study due to biased responses in the subsequent recognition test (always answered “old, confident”). All subjects were fluent in English and were treated in accordance with the CMU and Pittsburgh University IRB guidelines.
2.2 Design and Procedure
Before conducting the experiment, we created a database of pictures that consisted of two different images for each of 508 different concepts pertaining to a basic level category (e.g. squirrel, hammer, etc.) The stimuli have gone through multiple revisions to ensure correspondence between the pictures and the concept label. All lab members generated labels for each picture in random order. When one of the photographs was not correctly labeled, we discarded that category and both pictures. The photographs comprising a pair for a given category differed from each other along several dimensions. They were not, for example, pictures of the same apple taken from different perspective, but they were pictures of two different types of apples, chairs, etc.
While inside the scanner, each subject viewed 336 pictures that corresponded to 168 concepts randomly selected from the entire pool with the constraint that half be natural (e.g., apple) and half man-made (e.g., chair). Furthermore, for half the natural and half the man-made concepts the two pictures that were shown were identical, while for the other half the two pictures were different. The subject’s task was to indicate whether the picture was natural or man-made by making a button press with either the left or right response glove (Figure 1A). The presentation order of the pictures was randomly determined with the constraint that the second picture for any concept was not shown until all the concepts had had one of their two pictures shown. The mean lag between stimulus presentations was 415.8 sec (SE=10.6). The experiment was self-paced such that a stimulus stayed on until the subject responded. We chose a self-paced stimulus presentation for two reasons. First this allows fast-responders to spend less time lying in the scanner off task. Second, slow-responders will miss fewer trials. After the response, a fixation cross against a gray background was shown for 1.5 seconds before the next stimulus appeared.
Figure 1.
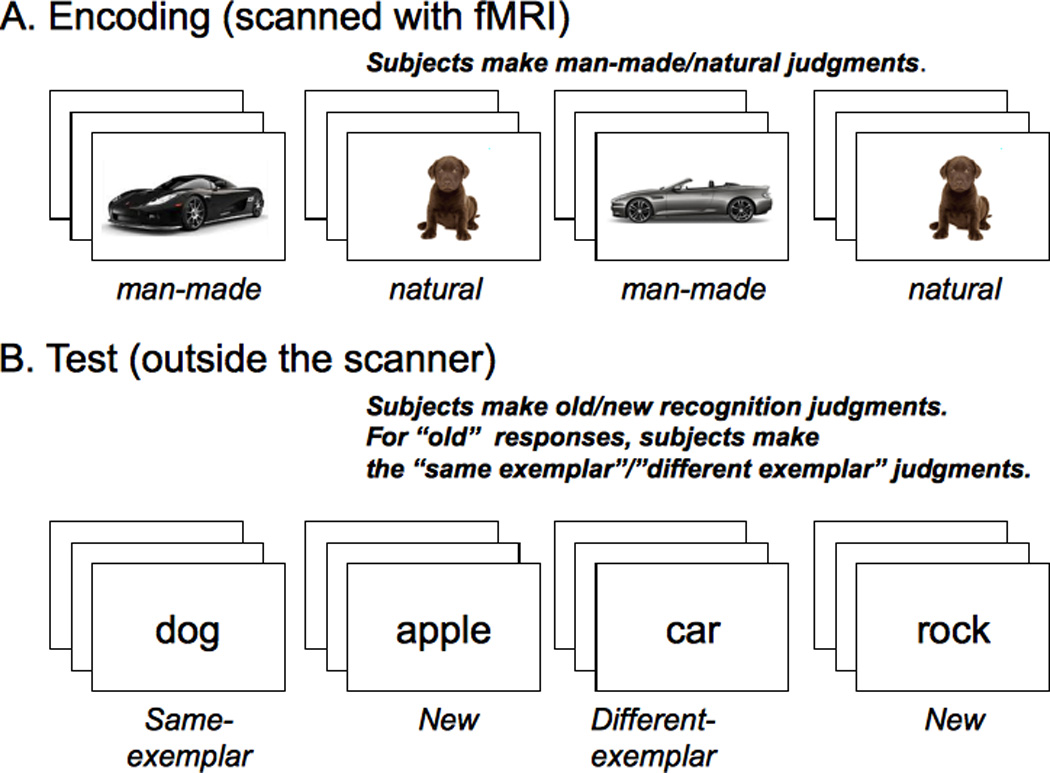
The experimental design.
After finishing the encoding phase, subjects took a break for ten to fifteen minutes outside the scanner. Subjects were then informed that they had seen two pictures for each concept/word and given a surprise recognition test in which they saw the words (the names of the concepts) that corresponded to the pictures shown in the scanner. Two-thirds of the concepts were old and one-third of the concepts were new (after Turk-Browne et al., 2006) for a total of 252 test words. For each test word, subjects made a “first-order” judgment as to whether they had seen a picture during the encoding phase that corresponds to the probe word. They made this judgment by pressing one of three buttons on a keyboard corresponding to “old confident,” “old maybe,” or “new.” When subjects responded either “old confident” or “old maybe”, they then made a “second-order” judgment as to whether the two pictures seen at encoding were identical or different. Subjects were instructed to press the key labeled “same, sure” if they remembered two identical pictures appearing for the concept and “different, sure” if they remembered two different pictures appearing for the concept. They were instructed to press either “same, unsure” or “different, unsure” if they were less confident in their judgment.
2.3 Image acquisition
Functional and anatomical images were acquired on a Siemens 3 T Allegra MR system. A high-resolution structural image (TR = 1540 ms, TE = 3.04 ms, slice thickness = 1mm, FOV = 205, FA = 8°, number of slices = 192, resolution 1 × 1 × 1mm) was acquired using an MPRAGE (a magnetization-prepared rapid acquisition in gradient echo) sequence in the beginning of the experiment. Functional data (BOLD signal) were collected using a gradient echo, echo-planar sequence (TR = 2000 ms, TE = 30 ms, slice thickness = 3.2 mm, FOV = 205, FA = 79°, number of slices = 35, resolution = 3.2 × 3.2 × 3.2). Stimuli were presented in a self-paced manner. This resulted in a variable number of volumes in subjects’ fMRI data (ranged from 377 to 547 volumes).
2.4 fMRI Data analysis
The images were processed and analyzed with FSL 4.1.7 (FMRIB’s Software Library, www.fmrib.ox.ac.uk/fsl) software. On each raw BOLD dataset, nonlinear noise reduction was done with SUSAN (Smallest Univalue Segment Assimilating Nucleus), motion correction with MCFLIRT (Jenkinson et al., 2002), slice-timing correction with Fourier-space time-series phase-shifting non-brain removal with BET (Smith, 2002), and spatial smoothing with a Gaussian kernel of FWHM 6mm. Multiplicative mean intensity normalization of the volume at each time point along with high-pass temporal filtering was done with a Gaussian-weighted least-squares straight line fitting using sigma=25.0s.
The preprocessed data served as an input to GLM using FEAT (http://www.fmrib.ox.ac.uk/fsl/feat5/index.html). A hemodynamic response function (HRF) was modeled using a Gamma function. Co-registration was carried out using FLIRT (Jenkinson & Smith, 2001; Jenkinson et al., 2002). BOLD images were registered to the high-resolution structural (MPRAGE) images, the high-resolution images were registered to the MNI152_T1_2mm template, and the two resulting transformations were concatenated and applied to the original BOLD image (http://www.fmrib.ox.ac.uk/fsl/flirt/gui.html) to transform it to the MNI space. Functional localization of the MTL regions (i.e., right and left HPC, PHc and PRc) was determined using the Harvard-Oxford cortical and subcortical structural probability atlases (http://www.fmrib.ox.ac.uk/fsl/fslview/atlas.html) with the probability of a voxel being in the region of interest (ROI) at or above 30%. Given our interest in the role of anterior and posterior HPC for accurate memory encoding, we divided HPC on anterior and posterior aspects along y=−20 (following Henson, 2005).
The FEAT (FMRI Expert Analysis Tool, v5.98) was used for the first- and higher-level analysis. The length of each event was modeled in the GLM by specifying the duration of the response in the second column of a three-column event file. We back-sorted trials based on subsequent recognition (e.g., Brewer et al., 1998; Wagner et al., 1998). Only trials on which the probes were confidently recognized as “old” and then, confidently judged as same-exemplar or different-exemplar repetitions were selected for the analyses. Table 1 describes the trials that are the focus of the present paper in more detail. One first-level analysis computed a main effect of memory for whether the two exemplars were identical or different by contrasting correct recollection with gist recollection for different-exemplar repetitions and a main effect of repetition type by contrasting same-exemplar correct recollection with different-exemplar correct recollection in the MTL (collapsed across Presentation 1 and Presentation 2). Another first-level analysis calculated the magnitude of neural priming by contrasting the first and second stimulus presentations for correct and gist recollection as a function of same- vs. different-exemplar repetition. The summary of all fMRI data analyses described in the paper is presented in the Supplement (Table S1).
Table 1.
Trials of interest selected for behavioral and neuroimaging data analyses
| Repeated exemplars were | ||
|---|---|---|
| Same | Different | |
| Subjects said that the two exemplars were “same” |
Same-exemplar correct recollection |
Different-exemplar gist recollection |
| Subjects said that the two exemplars were “different” |
Same-exemplar gist recollection |
Different-exemplar correct recollection |
Note. Only trials on which the probes were confidently recognized as “old” and then, confidently judged as same-exemplar or different-exemplar repetitions were selected for the analyses.
All higher-level analyses were carried out using OLS (ordinary least squares) mixed effects that models the subject variability (e.g., Mumford and Poldrack, 2007). Group means were computed for each of the first-level comparisons. In addition, we contrasted the magnitudes of neural priming for different-exemplar correct recollection vs. different-exemplar gist recollection, for same-exemplar correct recollection vs. different-exemplar correct recollection, and for same-exemplar correct recollection vs. different-exemplar gist recollection. This last category of trials was of interest because in both cases the subjects were confident that they saw the two identical images, but the judgment was correct only for the same-exemplar repetition type. Given that previous research suggests that it is often difficult to image the HPC due to an inherent low signal-to-noise ratio (Greicius et al. 2003; Zeineh et al. 2003), the resulting images of the MTL ROIs were thresholded at p<0.005 (uncorrected) and cluster extent threshold of n = 5 voxels. If the images passed this threshold, we tested whether the voxels in the cluster (or at least the voxel at the maximum z-score) pass the threshold of p<0.05 corrected for multiple comparisons. The correction was performed at the voxel level using Gaussian Random Field (GRF) theory (Worsley, 1992) within the pre-defined MTL ROIs (anterior and posterior HPC, PHc and PRc).
2.5. Correlation analysis
The number of trials with correctly remembered exemplars and those with incorrectly remembered exemplars varied from subject to subject. Moreover, a small number of the gist recollection responses for same-exemplar repetitions did not allow us to compare this category of responses with other response categories. Therefore, we conducted a correlation analysis in order to examine whether the subjects with a greater magnitude of neural priming (Presentation 1 – Presentation 2) for collapsed correct and gist recollection judgments also had a higher percentage of correct recollection judgments relative to the total number of correct and gist recollection judgments. The images were thresholded as explained in the section 2.4.
2.6. Psychophysiological interactions (PPI)
We explored whether and how functional connectivity between each of the MTL regions (revealed by the analyses described in sections 2.4 and 2.5) and the rest of the brain changed as a function of subsequent recollection and similarity of repeated stimuli using the PPI method (Friston et al., 1997). In the PPI analysis models, there are a) three psychological regressors (same-exemplar correct recollection presentation 1 vs. same-exemplar correct recollection presentation 2, different-exemplar correct recollection presentation 1 vs. different-exemplar correct recollection presentation 2, and different-exemplar gist recollection presentation 1 vs. different-exemplar gist recollection presentation 2); b) one physiological regressor – a mean time course for one of the MTL regions revealed by the 2.4 and the 2.5 analyses; and c) three interaction terms between the physiological and one of the psychological variables (PPI regressor). The contrasts between the first and second presentations were modeled by assigning “−1” and “1” to presentations 1 and 2 respectively. In each PPI model, trial types that were not part of the psychological regressor were included as covariates of no interest. All PPI analyses were conducted on the whole brain. Cluster size limits for corrected threshold (pcorrected < 0.05) were generated by Monte Carlo simulations (1000 iterations) using the AlphaSim program (http://afni.nimh.nih.gov/pub/dist/doc/manual/275AlphaSim.pdf) with FWHM = 6 mm, uncorrected voxel-wise p-value=0.001 and the image of whole brain as a mask. According to AlphaSim, the cluster size for this analysis had to be at least 38 voxels.
3. Results
In this paper we chose to focus on trials for which subjects were confident that they had seen pictures corresponding to the word and for which they were also confident in their second decision concerning the similarity of the two pictures, regardless of whether the second judgment was correct or not. Therefore, we present here only the analyses pertaining to confident correct recollection and confident gist recollection, while omitting the analysis of misses and all unconfident responses from either the first or second recognition judgment. Some of the omitted analyses can be found elsewhere (Manelis et al., 2011). If a picture was miscategorized during the encoding phase (e.g. a “hammer” was categorized as natural) or if a subject took longer than 6 seconds to make a response, then this concept was excluded from both behavioral and neuroimaging analyses. However, this happened infrequently (less than 7% of trials were excluded on this basis).
3.1. Behavioral results
Subjects confidently recognized the word corresponding with the encoded pictures 71% of time both when the two pictures were identical (same-exemplar condition) and when the two pictures were different (different-exemplar condition). Of the confident hits for different pairs, subjects were sure and correct that the two pictures were different 32% of time (correct recollection) and confident but incorrect that the two pictures were identical 25% of time (gist recollection). Of the confident hits for identical pairs, slightly more than half (63%) were confidently and correctly reported as two identical pictures (correct recollection) and confidently but incorrectly reported as two different pictures 7% of the time (gist recollection). This bias in the direction of errors (to say “same” more often than “different”) was also reflected in the fact that only 9 of the 13 subjects made gist recollection responses for identical pairs.
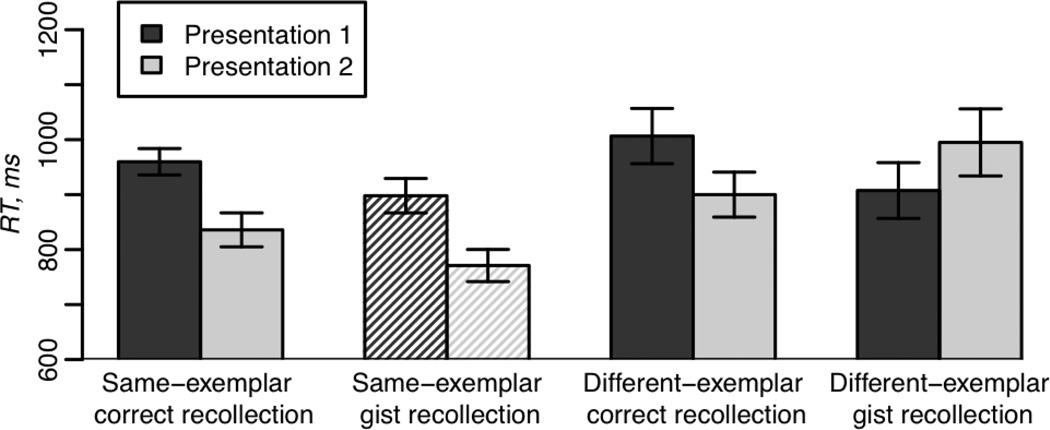
Figure 2 illustrates subjects’ response times (RT) to make artificial/natural judgments during encoding. A repetition type (same- vs. different-exemplar) × Presentation (1 vs. 2) × subsequent-memory (correct vs. gist recollection) ANOVA for mean correct RTs was performed on the 9 subjects that had observations in all conditions. This analysis revealed a main effect of repetition type (F(1,8)=17.3, p<0.005) with faster responses for the same-exemplar than for the different-exemplar repetition condition, driven by the greater speed up on the second presentation for identical repetitions. That is, there was a reliable repetition type × presentation interaction (F(1,8)=5.8, p<0.05).
Figure 2.
Response times to make man-made/natural judgments about the pictures on the first and second presentations for same- and different-exemplar repetitions for correct recollection and gist recollection. The same-exemplar gist recollection category of responses is differently shaded to indicate that this condition represents data from only 9 of 13 subjects. Standard error bars represent “within-subject” estimated variability of the differences in means across conditions within subjects.
Given that the ANOVA described above was limited to only 9 subjects, we conducted three 2-way ANOVAs that excluded the same-exemplar gist recollection category in order to use all 13 subjects. A 2 × 2 ANOVA (presentation number × subsequent-memory) conducted on the different-exemplar correct recollection vs. different-exemplar gist recollection responses revealed a marginally significant presentation × memory interaction effect on RT (F(1,12)=3.5, p=0.09) suggesting greater repetition-related decreases for correct than gist recollection. Another ANOVA that also examined presentation number × subsequent-memory effects but for same-exemplar correct recollection vs. different-exemplar gist recollection, revealed an interaction effect (F(1,12)=5.3, p<0.05) with greater decreases for same-exemplar correct recollection than for different-exemplar gist recollection. A 2 × 2 ANOVA (presentation number × repetition type) conducted on the same-exemplar correct recollection vs. different-exemplar correct recollection judgments revealed a main effect of presentation, (F(1,12)=8.9, p<0.05), with slower RT on the first stimulus presentation than on the second one.
Further analyses of behavioral priming were carried out using four paired t-tests comparing RT on the presentations 1 and 2 on the four conditions described in Table 1. Bonferroni adjusted alpha levels for these tests were 0.0125 per test (0.05/4). These analyses revealed significant decreases in RT on the second presentation for the same-exemplar trials (t(12)=4.3, p<0.01 for correct recollection; t(8)=3.5, p<0.01 for gist recollection). There was no significant facilitation, however, for the different-exemplar trials for either correct or gist recollection (all p-values > 0.1).
3.2. Neuroimaging results
The tables 2 and 3 report the results thresholded at p<0.005 uncorrected, cluster size > 5 voxels. In addition, unless specified otherwise, the results pertaining to the MTL ROIs analyses passed the p<0.05 threshold corrected for multiple comparisons at least in the voxel with the maximum z-score.
Table 2.
Comparison of the magnitude of neural priming (defined as the difference in activation on Presentation 1 vs. Presentation 2) in hippocampus, parahippocampal and perirhinal cortices
| Hemisphere | Region | N voxels |
Z- Max |
MNI coordinates | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
|
Main effect of subsequent memory accuracy correct vs. gist recollection Different-exemplar correct recollection > Different-exemplar gist recollection | ||||||
| R | Hippocampus, anterior | 9 | 3.09 | 22 | −8 | −22 |
| R | Hippocampus, posterior | 7 | 3.01 | 26 | −36 | −6 |
| L | Hippocampus, anterior | 5 | 3.04 | −22 | −6 | −22 |
| R | Parahippocampal cortex | 12 | 3.59 | 18 | −32 | −18 |
| L | Parahippocampal cortex | 12 | 3.42 | −26 | −40 | −12 |
|
Main effect of repetition type Same-exemplar correct recollection > Different-exemplar correct recollection | ||||||
| R | Hippocampus, posterior | 6 | 3.2 | 34 | −32 | −8 |
|
Presentation (1 vs 2) × Memory (correct vs. gist) interaction Different-exemplar correct recollection < Different-exemplar gist recollection | ||||||
| R | Hippocampus, anterior | 11 | 3.3 | 24 | −4 | −28 |
| L | Hippocampus, posterior | 8 | 3.0 | −32 | −30 | −10 |
|
Presentation × Repetition type interaction Same-exemplar correct recollection > Different-exemplar correct recollection | ||||||
| L | Perirhinal cortex | 16 | 3.5 | −16 | −10 | −24 |
| R | Hippocampus/Parahippocampal cortex | 12 | 3.2 | 22 | −26 | −12 |
| L | Parahippocampal cortex | 11 | 3.5 | −28 | −28 | −24 |
| R | Hippocampus, anterior | 10 | 3.4 | 26 | −4 | −28 |
|
Presentation × Repetition type interaction for the “same” judgments Same-exemplar correct recollection < Different-exemplar gist recollection | ||||||
| L | Hippocampus, posterior | 14 | 3.37 | −34 | −34 | −6 |
Table 3.
The results of the correlation analysis between the magnitude of neural priming (Presentation 1 – Presentation 2) and subsequent recollection of the exemplar similarity
| Hemisphere | Region | N voxels |
Z- max |
MNI coordinates | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
|
Negative correlation between neural priming (Presentation1 – Presentation 2) and proportion of correct-exemplar responses Different-Exemplar repetitions | ||||||
| R | Hippocampus | 83 | 3.6 | 30 | −38 | −4 |
| L | Hippocampus | 6 | 2.9 | −28 | −38 | 0 |
| R | PHc | 32 | 3.55 | 30 | −30 | −16 |
|
Negative correlation between neural priming (Presentation1 – Presentation 2) and proportion of correct-exemplar responses Same-Exemplar repetitions | ||||||
| R | Hippocampus | 60 | 4.3 | 36 | −26 | −8 |
| L | Hippocampus | 11* | 3.02 | −34 | −16 | −20 |
Note.
This region did not survive correction for multiple comparisons.
Subjects rarely responded “different, sure” when the two presentations were actually the same. Therefore, to examine the differences between correct recollection vs. gist recollection in MTL, we contrasted these two types of judgments for different-exemplar repetition. We found a main effect of recollection in bilateral anterior HPC, right posterior HPC and bilateral PHc with greater activation for correct compared to gist recollection (see Table 2). For different-exemplar repetitions there was also an interaction between presentation (1 vs. 2) and memory accuracy (correct vs. gist recollection) in the right anterior HPC and left posterior HPC. Both areas revealed greater neural priming for gist than for correct recollection (Table 2, Figure 3 C,E). A follow-up analysis showed that, in the left posterior HPC, this effect was due to significant repetition-related decreases for different-exemplar gist recollection, t(12)=3.2, p<0.01, and a lack of significant changes for different-exemplar correct recollection (p>0.1). In the right anterior HPC, the interaction effect was explained by the finding that activity on the second presentation for trials that produced correct recollection was significantly greater than for ones that resulted in gist recollection, t(12)=3.1, p<0.05.
Figure 3.
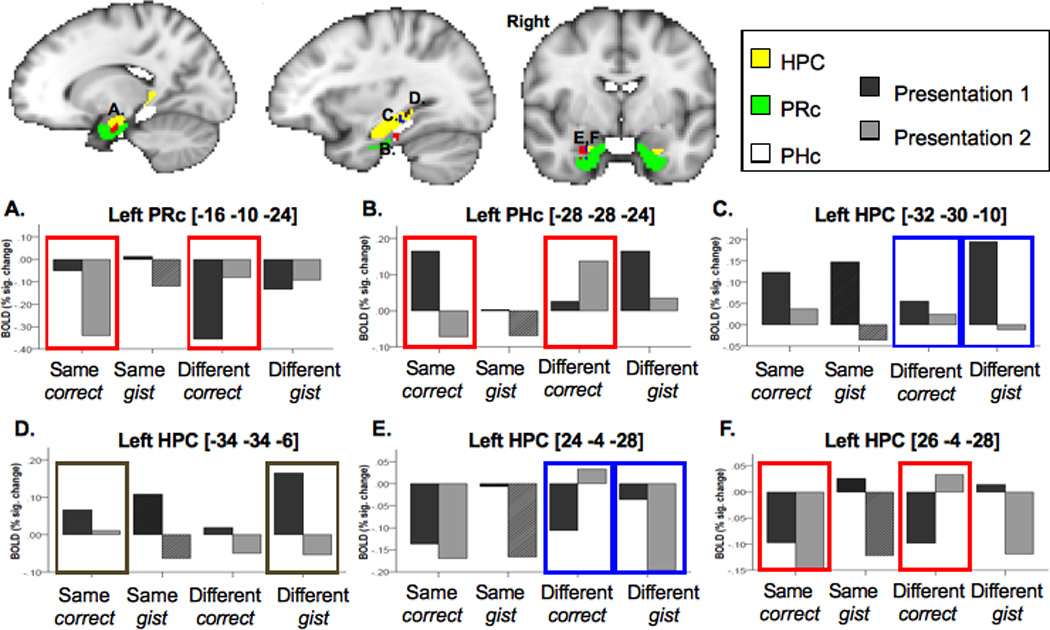
Differences in repetition-related changes in hippocampus (HPC), parahippocampal (PHc) and perirhinal (PRc) cortices. Boxes around different conditions refer to the contrast. Red color: same-exemplar correct-detail hits > different-exemplar correct-detail hits contrast. Blue color: different-exemplar correct-detail hits < different-exemplar incorrect-detail hits contrast. Brown color: same-exemplar correct-detail hits < different-exemplar incorrect-detail hits contrast. Same-exemplar incorrect-detail hits that were available only for 9 of 13 subjects are illustrated by the slanted line pattern.
The main effect of repetition type (same-exemplar vs. different-exemplar) on the MTL activation for correctly recollected exemplars was found in the right posterior HPC. The same-exemplar correct recollection judgments elicited stronger HPC activation than the different-exemplar correct recollection judgments. A presentation (1 vs. 2) × repetition type (same vs. different exemplar) interaction effect was found in left PRc, left PHc and right anterior and posterior HPC (Table 2, Figure 3). The anterior HPC area overlapped with the right anterior HPC area found in the different-exemplar correct recollection vs. different-exemplar gist recollection contrast. A follow-up analysis showed that the interaction effects in the above MTL regions were explained by the significant repetition-related decreases for the same-exemplar trials (left PRc: t(12)=2.5, p<0.05; left PHc: t(12)=3.4, p<0.01; right HPC/PHc region: t(12)=2.6, p<0.05), but marginally significant increases (left PRc: t(12)=−2.1, p=0.06; right anterior HPC, t(12)=−1.8, p=0.09) or sustained activation (left PHc and right HPC/PHc) across presentations for the different-exemplar trials.
The effect of veridical memory (subjects say that the two presentations were the same on the same-exemplar trials) vs. imprecise memory (subjects say that the two presentations were the same on the different-exemplar trials) was investigated by contrasting repetition-related changes for same-exemplar correct recollection vs. different-exemplar gist recollection. In both conditions, subjects were confident that the two presentations were identical pictures; however, in the latter case, this judgment was false. The only region that showed the effect of veridical vs. imprecise memory was left posterior HPC (Table 2, Figure 3 D) that was located slightly posterior to the left posterior HPC area identified in the different-exemplar correct recollection vs. different-exemplar gist recollection contrast. A follow-up analysis showed that this effect was primarily due to a significant attenuation of the fMRI signal from Presentation 1 to Presentation 2 for the different-exemplar gist recollection judgments, t(12)=3.6, p<0.005, but not for the same-exemplar correct recollection judgments.
3.3. Correlation analysis
The goal of this analysis was to provide converging evidence that less neural priming in MTL is associated with better exemplar recall. Table 3 illustrates the results of the correlational analysis, across subjects, between the magnitude of neural priming (Presentation 1 – Presentation 2) and proportion of trials for which the nature of the repetition (identical vs. different exemplar) was correctly recollected. We found a significant negative correlation between the magnitude of neural priming and exemplar recall in bilateral HPC for same-exemplar repetition and in the bilateral HPC and right PRc for different-exemplar repetition (Table 3). Consistent with the results described in section 3.2, subjects with better memory for whether the two exemplars were identical or different showed, respectively, less repetition-related neural attenuation for same-exemplar repetitions but more of an increase for different-exemplar repetitions in the MTL regions shown in Figure 4.
Figure 4.
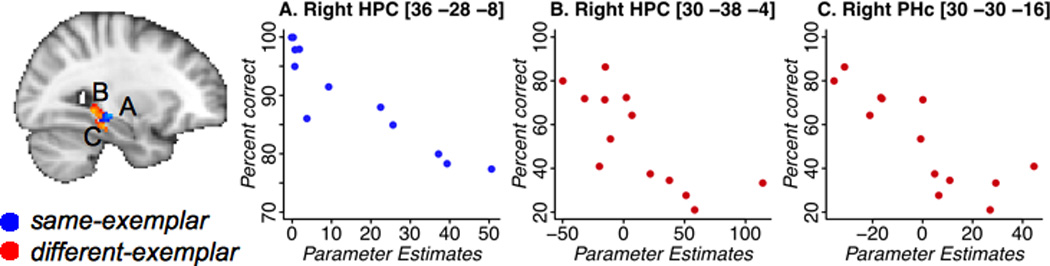
Correlation between repetition-related neural attenuation and percent of correctly recalled exemplars for same-exemplar (blue) and different-exemplar (red) repetitions. Negative values of parameter estimates mean increased activation on Presentation 2 relative to Presentation 1.
3.4 PPI analysis
The goal of this analysis was to examine whether subsequent exemplar recollection (i.e., subjects’ ability to remember that there were two different exemplars in the different-exemplar condition) can be predicted by the repetition-related changes in the functional connectivity between HPC and other brain regions. For this purpose, we compared the repetition-related changes in the functional connectivity for the different-exemplar correct recollection and for the different-exemplar gist recollection responses using the right anterior HPC and the left posterior HPC as the seed regions, identified in Table 2 and Figure 3 C and 3 E. We found no significant presentation number (1 vs. 2) × memory accuracy (correct vs. gist recollection) interaction effect on the functional connectivity between the right anterior HPC and other brain regions (at least at the pcorrected < 0.05).
There was, however, a significant presentation number (1 vs. 2) × memory accuracy (correct vs. gist recollection) on the functional connectivity between the left posterior HPC and two regions in the frontal cortex. The functional connectivity between the left posterior HPC and the right inferior frontal cortex (IFG; z-max=4.16 at [58 20 18], number of voxels = 48) increased on the second presentation for the correct recollection responses, but decreased on the second presentation for the gist recollection responses (Figure 5). A planned comparison analysis revealed that both the increases for correct recollection and the decreases for gist recollection were statistically significant (t(12)=2.9, p<0.05 for correct recollection; t(12)=−4.5, p<0.005 for gist recollection). In addition, the connectivity was significantly stronger for gist than correct recollection on the first presentation (t(12)=3.4, p<0.01). On the second presentation, the connectivity was marginally stronger for correct than gist recollection (t(12)=1.9, p<0.1).
Figure 5.
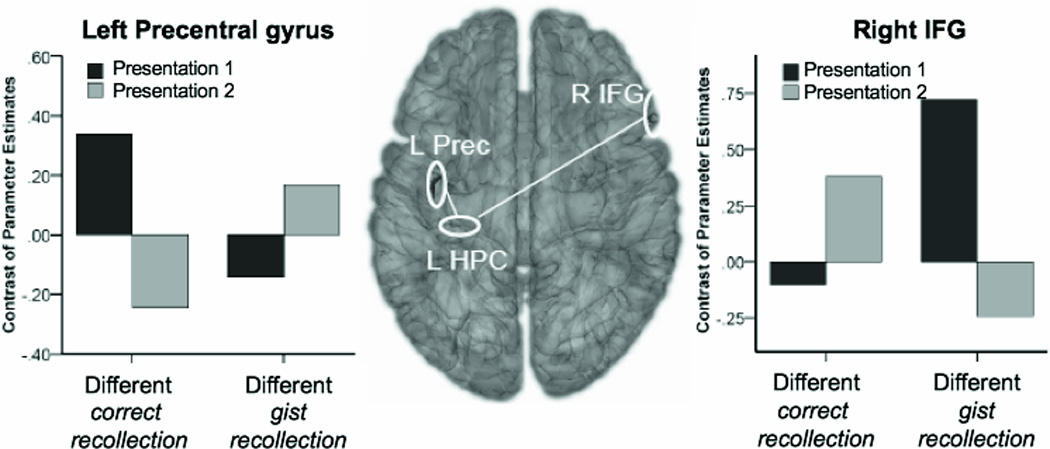
Functional connectivity between the left posterior HPC and frontal regions for different-exemplar pairs subsequently remembered as old, as a function of presentation (1 vs. 2) and accuracy for type of repetition (correct vs. gist recollection).
A pattern of functional connectivity between the left posterior HPC and the left precentral gyrus (z-max=4.4 at [−38 −14 54], number of voxels = 40) was opposite to that described above. The functional connectivity decreased on the second presentation for correct recollection but increased for gist recollection (Figure 5). A planned comparison analysis showed that both the decreases for correct recollection and the increases for gist recollection were statistically significant (t(12)=−3.5, p<0.01 for correct recollection; t(12)=2.6, p<0.05 for gist recollection). The functional connectivity was significantly stronger for correct than gist recollection (t(12)=2.6, p<0.05) on the first presentation; however, on the second presentation the connectivity was significantly stronger for gist than correct recollection (t(12)=2.4, p<0.05).
4. Discussion
4.1 Neural priming in the MTL predicts subsequent memory accuracy in judging whether encoded exemplars were identical or different
Recent studies have shown that the magnitude of repetition-related neural attenuation can be predictive for whether the stimuli are subsequently remembered of forgotten (Turk-Browne et al., 2006; Manelis et al., 2011; Xue et al., 2011). It was unclear, however, whether this effect exists only in cortical regions or also in the HPC and whether the differences in neural priming can predict subsequent recollection for two identical or different study episodes. The results of our study provide novel evidence that the patterns of neural priming in the MTL, including HPC, predict subsequent recollection.
The repetition-related changes in activation of the right anterior HPC and the left posterior HPC were predictive for whether subjects would correctly recollect seeing two different exemplars or incorrectly recollect seeing two identical exemplars on the different-exemplar repetition trials. In both HPC areas, correct recollection was characterized by sustained activation over the two encoding presentations. In contrast, gist recollection (i.e., when subjects were confident that they saw two identical pictures that were in fact different exemplars) was characterized by the significant decreases in activation on the second presentation. The finding of sustained activation for correct recollection is, in general, consistent with the recent report that activation patterns across repetitions are more similar for subsequently recognized and recalled stimuli than for the stimuli that are later forgotten (Xue et al., 2010).
Thus far, there is no consensus on the role of anterior and posterior HPC on memory performance (e.g., Henson, 2005). Some studies suggest that the posterior aspect of the HPC is associated with recollection, while its anterior aspect is associated with novelty (e.g., Daselaar et al., 2006). Another view is that anterior HPC is more specialized for successful associative encoding compared to the posterior HPC (Sperling et al., 2003; Jackson and Schacter, 2004; Chua et al., 2007). Still another view proposes that the anterior HPC is involved in flexible retrieval of relational information, while the posterior HPC is involved in retrieval of repeated representations (Giovanello et al., 2009). In our study, anterior and posterior regions showed similar sensitivity to memory for exemplars suggesting that co-activation of these two regions may be especially important for recollection (and reintegration over repetitions, and categorization) of two encoding episodes that appear at two different time points of the experiment. This idea is consistent with the recent finding that right anterior and left posterior HPC were co-activated when subjects had to remember the order of the stimuli presented in the experiment (Tubridy and Davachi, 2011).
In addition to a joint role of anterior and posterior HPC in recollection, it is likely that each of the regions makes a unique contribution during successful encoding. For example, sustained activation in the posterior HPC may be important for binding each representation to a specific time frame and to a specific category of objects in the experiment, which later helps determine whether the two instances of an object were identical or different. One piece of evidence for this view comes from the finding that sustained activation in the posterior HPC was found not only for the contrast between different-exemplar correct recollection and different-exemplar gist recollection, but also in the contrast that compares same-exemplar correct recollection vs. different-exemplar gist recollection. The left posterior HPC region that showed sustained activation in the latter contrast was in close proximity to the posterior HPC region found in the different-exemplar correct recollection vs. different-exemplar gist recollection contrast. Notably, both of these regions in the left posterior HPC correspond to the HPC area [−32 −30 −12 in Kim, 2011] that, according to a recent meta-analysis (Kim, 2011), is more active for associative encoding than for item encoding of pictures.
Another piece of evidence for the role of posterior HPC in correct recollection of two encoding episodes came from the correlation analysis between the magnitude of neural priming (for correct and gist recollection collapsed) and the proportion of correctly recollected exemplars. The findings support the idea that formation of strong representations occurs when activation of the posterior HPC on the second presentation is equivalent or stronger than on the first. We found a negative correlation between the magnitude of neural priming and the proportion of correct responses in the right posterior HPC for same-exemplar repetitions and the bilateral posterior HPC and the right PHc for different-exemplar repetitions. This indicates that subjects with better recollection of whether the two presentations were identical or different showed less neural priming in HPC and PHc. Moreover, in the different-exemplar condition, subjects with better exemplar recollection showed repetition-related increases in HPC and PHc activation, while subjects with poor recollection showed repetition-related decreases in these regions.
Functional specificity of the right anterior HPC was revealed through the analysis involving the same- vs. different-exemplar correct recollection judgments. Although both conditions represent confident, correct responses, the area in the right anterior HPC (that overlapped with the right anterior HPC area observed in the different-exemplar correct recollection vs. different-exemplar gist recollection contrast) showed differential activation patterns for these two conditions. The slight repetition-related decreases in activation of the right anterior HPC for same-exemplar repetitions were paralleled by the repetition-related increases for different-exemplar repetitions. This finding may be related to the fact that presentation of a different exemplar from the same category of objects elicited a novelty response in HPC, a view consistent with earlier work (e.g., Tulving et al., 1996; Kirchhoff et al., 2000; Daselaar et al., 2006; Kumaran and Maguire, 2006; Poppenk et al., 2010).
Some previous studies suggest that novelty detection in HPC occurs automatically (e.g., Yamaguchi et al., 2004). Automaticity in novelty detection predicts no difference in repetition-related changes in HPC activation for correct and gist recollection in the different-exemplar condition because the second stimulus presentation is different from the first presentation in both cases. Despite this prediction, we found that the HPC response depended on whether the subjects actually remembered having seen two different exemplars. This finding suggests that the novelty detection is not an automatic property of HPC but depends on the quality of encoding.
4.2. Pattern separation and pattern completion in HPC
Our findings contribute to the growing body of literature on pattern separation and completion in the HPC (e.g., Bakker et al., 2008; Yassa and Stark, 2011) as these two phenomena seem to account for subjects’ successes and failures to recollect whether the two instances are of the same object. It is important to emphasize that the analyses reported in this paper excluded unsure responses (as well as misses) from the first-order judgments and unsure responses from the second-order judgments concerning with whether the two presentations were identical. Therefore, both correct recollection and gist recollection responses were confident judgments. To confidently decide that a stimulus is “old" requires only that there be a strong memory trace for one of the two presentations. Whether the subsequent judgment is correct recollection or gist recollection depends on whether both presentations have strong traces in memory.
If both traces are strong, subjects can correctly and confidently recollect whether the two exemplars were identical or different objects. Such recollection requires “pattern separation” to overcome the interference that comes from the similarity in perceptual features between the two exemplars from the same category. The “pattern separation” is thought to be expressed in brain regions as sustained activation on the second presentation of the stimulus (“the activity should resemble that of an initial presentation” ~ Yassa and Stark, 2011, p.518). Consistent with this view, our study demonstrated that correct recollection of different exemplars relies on sustained activation in HPC, while gist recollection is associated with significant neural attenuation on the second presentation. On the other hand, the results of our correlational analysis indicate that even identical repetitions can produce sustained activation because subjects with better recollection did not show repetition-related decreases. That is, “pattern separation” seems to be required not only for recollection of two different exemplars but also for recollection of two identical exemplars.
An alternative explanation of the encoding processes, that better fits with the correlation analyses, is that correct recollection involves having recollected the first presentation when the second one is presented and storing that the two presentations differed. Support for this view comes from a similar paradigm using ERP in which we found evidence that the amplitude of recollection (LPC) signal during the second presentation predicts the level memory accuracy at test (DeWolfe et al., in preparation).
Gist recollection can occur whenever only one of the two presentations has a strong trace in memory. There was a bias to respond “same, sure” and presumably subjects used this whenever they were confident that the saw a picture of the object probed but could not remember two different pictures. Consider the case where the first presentation of two identical pictures is well encoded, but the second presentation is not. All features encoded on the second presentation will reestablish the features already encoded on the first presentation. This will create a feeling that the two presentations match (supported by “pattern completion”), which will result in the subsequent correct response that the two presentations are identical. Given that in this scenario, the correct responses are not based on two strong memory representations, this correct response should be considered more of a “lucky guess” than a correct recollection. Given that separating “lucky guesses” from pure recollection was not possible, the correct recollection responses for same-exemplar repetitions were the mixture of true correct recollection and “lucky guesses”.
The proportion of “lucky guesses” would be of no concern if it were the same in all conditions. However, the probability of “lucky guesses” in the different-exemplar condition was much lower than in the same-exemplar condition. Whether the subjects formed a strong memory trace for the first presentation and a weak one for the second or vice versa, they will tend to respond that they saw two identical exemplars because they remember only one exemplar but know that there were two presentations of each object (because we told them so). This explains why there was a bias to respond “same” and why there were almost twice as many correct recollection responses for same-exemplar than for different-exemplar repetitions even though the number of “confident hits” was equivalent for both conditions. This also explains why, according to the correlation analysis, the subjects that were able to correctly recollect that there were two different exemplars in the different-exemplar condition increased HPC activation on the second presentation, while the subjects that were able to correctly recollect that there were two identical exemplars in the same-exemplar condition showed no changes between Presentation 1 and Presentation 2.
4.3 Differences in functional connectivity related to type of stimulus repetition and quality of subsequent memory
The HPC is anatomically connected to a wide range of areas in the brain, thereby enabling information encoded by different regions to be bound together (e.g., Suzuki and Eichenbaum, 2000). It is useful to then ask whether the subsequent memory effect in HPC is related to the changes in the functional connectivity of HPC with other regions in the brain. The increases in connectivity between HPC and prefrontal regions were observed in several previous studies (e.g., Hannula and Ranganath, 2009; Westerberg et al., 2011). Our study adds to these findings by showing that successful encoding is not necessarily predicted by greater HPC activation or stronger functional connectivity but rather by the changes in neural dynamics from one presentation to the next (that includes local activation and functional coupling of HPC with other brain regions). Specifically, we found that for correct recollection judgments, there was strengthening of connectivity on the second stimulus presentation between the left posterior HPC and the right IFG, but weakening of functional connectivity between the left posterior HPC and the left precentral gyrus. The opposite was true for gist recollection that showed repetition-related decreases in the functional connectivity between the left posterior HPC and the right IFG but repetition-related increases in the functional connectivity between the left posterior HPC and the left precentral gyrus.
One way to think about successful encoding is that memory for distinct encoding episodes requires a chain of events to occur. Both frontal regions, right IFG and left precentral gyrus, that were functionally connected to HPC in our study, are involved in subsequent memory effects (see Kim, 2011 for review). Successful encoding on the first presentation establishes the coupling between the posterior HPC and precentral gyrus. One previous study (Zysset et al., 2002) reported that the brain region located in close proximity to the left precentral region we report here (Talairach coordinates [−39 −14 47] in the Zysset et al. study and MNI coordinates [−38 −14 54] in our study) showed a stronger response when subjects had to make semantic judgments (e.g., “Leipzig is the capital of Germany”) compared to evaluative judgments (“I like Leipzig”). This suggests that the coupling between the posterior HPC and left precentral gyrus may be related to semantic processing of a picture to make an artificial/natural judgment. The second presentation strengthens the connectivity with IFG. Given that some studies found greater involvement of prefrontal cortex in associative memory than for item memory (Hales and Brewer, 2010), the increased functional connectivity between the HPC and IFG for successful encoding may determine the formation of an associative link between the two presentations based on their semantic similarity.
Not much is known about how decreases in functional connectivity can support memory encoding. One recent study, however, sheds light on this phenomenon by examining changes in the functional connectivity related to consolidation of face-location associations (Takashima et al., 2009). According to this study, decreases in the functional connectivity of posterior HPC with fusiform face area and posterior parietal cortex characterized the consolidation of information in memory. Given these recent findings, we speculate that decreases in the functional connectivity between the left posterior HPC and left precentral gyrus (that characterized successful encoding of details) reflect a consolidation process. A problem with this explanation is that consolidation occurs gradually, often during sleep (like in the Takashima et al. study). On the other hand, it has been demonstrated that under certain circumstances the consolidation process can occur quite rapidly, maybe even on-line (Tse et al., 2007).
An alternative explanation for decreased connectivity between HPC and left precentral gyrus on the second presentation for correctly recollected different exemplars is that during the first stimulus presentation subjects had to make a semantic judgment about the stimulus to classify it as natural or man-made. On the second presentation, subjects were already primed with that specific category of objects, so making artificial/natural judgment was easier with less semantic involvement required.
4.4 Summary
In summary, our study provides novel findings that whenever encoding involves two stimulus presentations, subsequent memory for these presentations is determined by the changes in brain activation between the two stimulus presentations in the HPC as well as by the changes in functional connectivity between the HPC and frontal cortex. Importantly, successful encoding is predicted by the combination of increases and decreases in HPC activation and functional connectivity, not by mere increases as suggested in previous studies. This paper also adds to the understanding of the functional dissociation between anterior and posterior aspects of HPC. Both of these regions jointly contribute to recollection of two distinct encoding episodes. However, the role of posterior HPC may be to bind memory representations to specific time points in the experiment, while the role of anterior HPC may be to detect whether the exemplars from the same category of objects are identical or different.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health grants 5R01MH052808, T32MH019983 and T32GM081760. It was also supported by the National Science Foundation grant DGE0549352. We thank Lisa Storey for help conducting the study.
References
- Bakker A, Kirwan CB, Miller M, Stark CEL. Pattern separation in the human hippocampal CA3 and dentate gyrus. Science. 2008;319:1640–1642. doi: 10.1126/science.1152882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer JB, Zhao Z, Desmond JE, Glover GH, Gabrieli JD. Making memories: Brain activity that predicts how well visual experience will be remembered. Science. 1998;281:1185–1187. doi: 10.1126/science.281.5380.1185. [DOI] [PubMed] [Google Scholar]
- Büchel C, Coull JT, Friston KJ. The predictive value of changes in effective connectivity for human learning. Science. 1999;283:1538–1541. doi: 10.1126/science.283.5407.1538. [DOI] [PubMed] [Google Scholar]
- Chua EF, Schacter DL, Rand-Giovannetti E, Sperling RA. Evidence for a specific role of the anterior hippocampal region in successful associative encoding. Hippocampus. 2007;17:1071–1080. doi: 10.1002/hipo.20340. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Fleck MS, Cabeza R. Triple dissociation in the medial temporal lobes: Recollection, familiarity, and novelty. J Neurophysiol. 2006;96:1902–1911. doi: 10.1152/jn.01029.2005. [DOI] [PubMed] [Google Scholar]
- Davachi L, Mitchell JP, Wagner AD. Multiple routes to memory: Distinct medial temporal lobe processes build item and source memories. Proc Natl Acad Sci USA. 2003;100:2157–2162. doi: 10.1073/pnas.0337195100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C. Imaging recollection and familiarity in the medial temporal lobe: A three-component model. Trends Cogn Sci. 2007;11:379–386. doi: 10.1016/j.tics.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annu Rev Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. NeuroImage. 1997;6(3):218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Gagnepain P, Henson R, Chételat G, Desgranges B, Lebreton K, Eustache F. Is neocortical-hippocampal connectivity a better predictor of subsequent recollection than local increases in hippocampal activity? New insights on the role of priming. J Cogn Neurosci. 2011;23:391–403. doi: 10.1162/jocn.2010.21454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garoff RJ, Slotnick SD, Schacter DL. The neural origins of specific and general memory: the role of the fusiform cortex. Neuropsychologia. 2005;43:847–859. doi: 10.1016/j.neuropsychologia.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Giovanello KS, Schnyer D, Verfaellie M. Distinct hippocampal regions make unique contributions to relational memory. Hippocampus. 2009;19:111–117. doi: 10.1002/hipo.20491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghuman AS, Bar M, Dobbins IG, Schnyer DM. The effects of priming on frontal-temporal communication. Proc Natl Acad Sci USA. 2008;105:8405–8409. doi: 10.1073/pnas.0710674105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Boyett-Anderson JM, Eliez S, Schatzberg AF, Reiss AL, Menon V. Regional analysis of hippocampal activation during memory encoding and retrieval: fMRI study. Hippocampus. 2003;13:164–174. doi: 10.1002/hipo.10064. [DOI] [PubMed] [Google Scholar]
- Hales JB, Brewer JB. Activity in the hippocampus and neocortical working memory regions predicts successful associative memory for temporally discontiguous events. Neuropsychologia. 2010;48:3351–3359. doi: 10.1016/j.neuropsychologia.2010.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannula DE, Ranganath C. The eyes have it: Hippocampal activity predicts expression of memory in eye movements. Neuron. 2009;63:592–599. doi: 10.1016/j.neuron.2009.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson RNA. A mini-review of fMRI studies of human medial temporal lobe activity associated with recognition memory. Q J Exp Psychol B. 2005;58:340–360. doi: 10.1080/02724990444000113. [DOI] [PubMed] [Google Scholar]
- Jackson O, Schacter DL. Encoding activity in anterior medial temporal lobe supports subsequent associative recognition. Neuroimage. 2004;21:456–462. doi: 10.1016/j.neuroimage.2003.09.050. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimisation for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Kim H. Neural activity that predicts subsequent memory and forgetting: A meta-analysis of 74 fMRI studies. Neuroimage. 2011;54:2446–2461. doi: 10.1016/j.neuroimage.2010.09.045. [DOI] [PubMed] [Google Scholar]
- Kirchhoff BA, Wagner AD, Maril A, Stern CE. Prefrontal-temporal circuitry for episodic encoding and subsequent memory. J Neurosci. 2000;20:6173–6180. doi: 10.1523/JNEUROSCI.20-16-06173.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirwan CB, Wixted JT, Squire LR. Activity in the medial temporal lobe predicts memory strength, whereas activity in the prefrontal cortex predicts recollection. J Neurosci. 2008;28:10541–10548. doi: 10.1523/JNEUROSCI.3456-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaran D, Maguire EA. An unexpected sequence of events: Mismatch detection in the human hippocampus. PLoS Biol. 2006;4:e424. doi: 10.1371/journal.pbio.0040424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manelis A, Wheeler ME, Paynter CA, Storey L, Reder LM. Opposing patterns of neural priming in same-exemplar vs. different-exemplar repetition predict subsequent memory. Neuroimage. 2011;55:763–772. doi: 10.1016/j.neuroimage.2010.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montaldi D, Spencer TJ, Roberts N, Mayes AR. The neural system that mediates familiarity memory. Hippocampus. 2006;16:504–520. doi: 10.1002/hipo.20178. [DOI] [PubMed] [Google Scholar]
- Mumford JA, Poldrack RA. Modeling group fMRI data. Soc Cogn Affect Neurosci. 2007;2:251–257. doi: 10.1093/scan/nsm019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otten LJ, Henson RN, Rugg MD. Depth of processing effects on neural correlates of memory encoding: Relationship between findings from across- and within-task comparisons. Brain. 2001;124:399–412. doi: 10.1093/brain/124.2.399. [DOI] [PubMed] [Google Scholar]
- Poppenk J, McIntosh AR, Craik FIM, Moscovitch M. Past experience modulates the neural mechanisms of episodic memory formation. J Neurosci. 2010;30:4707–4716. doi: 10.1523/JNEUROSCI.5466-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C, Heller A, Cohen MX, Brozinsky CJ, Rissman J. Functional connectivity with the hippocampus during successful memory formation. Hippocampus. 2005;15:997–1005. doi: 10.1002/hipo.20141. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Yonelinas AP, Cohen MX, Dy CJ, Tom SM, D’Esposito M. Dissociable correlates of recollection and familiarity within the medial temporal lobes. Neuropsychologia. 2004;42:2–13. doi: 10.1016/j.neuropsychologia.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Rosch E, Mervis CB, Gray WD, Johnson DM, Boyes-Braem P. Basic objects in natural categories. Cognitive Psychology. 1976;8(3):382–439. [Google Scholar]
- Smith S. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z, Wixted JT, Smith CN, Squire LR. Different nonlinear functions in hippocampus and perirhinal cortex relating functional MRI activity to memory strength. Proc Natl Acad Sci USA. 2011;108:5783–5788. doi: 10.1073/pnas.1103225108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaniol J, Davidson PSR, Kim ASN, Han H, Moscovitch M, Grady CL. Event-related fMRI studies of episodic encoding and retrieval: Meta-analyses using activation likelihood estimation. Neuropsychologia. 2009;47:1765–1779. doi: 10.1016/j.neuropsychologia.2009.02.028. [DOI] [PubMed] [Google Scholar]
- Sperling R, Chua E, Cocchiarella A, Rand-Giovannetti E, Poldrack R, Schacter DL, Albert M. Putting names to faces: Successful encoding of associative memories activates the anterior hippocampal formation. Neuroimage. 2003;20:1400–1410. doi: 10.1016/S1053-8119(03)00391-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staresina BP, Davachi L. Differential encoding mechanisms for subsequent associative recognition and free recall. J Neurosci. 2006;26:9162–9172. doi: 10.1523/JNEUROSCI.2877-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki WA, Eichenbaum H. The neurophysiology of memory. Ann N Y Acad Sci. 2000;911:175–191. doi: 10.1111/j.1749-6632.2000.tb06726.x. [DOI] [PubMed] [Google Scholar]
- Takashima A, Nieuwenhuis ILC, Jensen O, Talamini LM, Rijpkema M, Fernández G. Shift from hippocampal to neocortical centered retrieval network with consolidation. J Neurosci. 2009;29:10087–10093. doi: 10.1523/JNEUROSCI.0799-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse D, Langston RF, Kakeyama M, Bethus I, Spooner PA, Wood ER, Witter MP, Morris RGM. Schemas and memory consolidation. Science. 2007;316:76–82. doi: 10.1126/science.1135935. [DOI] [PubMed] [Google Scholar]
- Tubridy S, Davachi L. Medial temporal lobe contributions to episodic sequence encoding. Cereb Cortex. 2011;21:272–280. doi: 10.1093/cercor/bhq092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E, Markowitsch HJ, Craik FE, Habib R, Houle S. Novelty and familiarity activations in PET studies of memory encoding and retrieval. Cereb Cortex. 1996;6:71–79. doi: 10.1093/cercor/6.1.71. [DOI] [PubMed] [Google Scholar]
- Turk-Browne NB, Yi DJ, Chun MM. Linking implicit and explicit memory: Common encoding factors and shared representations. Neuron. 2006;49:917–927. doi: 10.1016/j.neuron.2006.01.030. [DOI] [PubMed] [Google Scholar]
- Uncapher M, Rugg M. Selecting for memory? The influence of selective attention on the mnemonic binding of contextual information. J Neurosci. 2009;29:8270–8279. doi: 10.1523/JNEUROSCI.1043-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AD, Maril A, Schacter DL. Interactions between forms of memory: When priming hinders new episodic learning. J Cogn Neurosci. 2000;12:52–60. doi: 10.1162/089892900564064. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Schacter DL, Rotte M, Koutstaal W, Maril A, Dale AM, Rosen BR, Buckner RL. Building memories: Remembering and forgetting of verbal experiences as predicted by brain activity. Science. 1998;281:1188–1191. doi: 10.1126/science.281.5380.1188. [DOI] [PubMed] [Google Scholar]
- Wais PE. fMRI signals associated with memory strength in the medial temporal lobes: A meta-analysis. Neuropsychologia. 2008;46:3185–3196. doi: 10.1016/j.neuropsychologia.2008.08.025. [DOI] [PubMed] [Google Scholar]
- Weis S, Specht K, Klaver P, Tendolkar I, Willmes K, Ruhlmann J, Elger CE, Fernández G. Process dissociation between contextual retrieval and item recognition. Neuroreport. 2004;15:2729–2733. [PubMed] [Google Scholar]
- Westerberg CE, Voss JL, Reber PJ, Paller KA. Medial temporal contributions to successful face-name learning. Hum Brain Mapp. 2011 doi: 10.1002/hbm.21316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler ME, Buckner RL. Functional-anatomic correlates of remembering and knowing. Neuroimage. 2004;21:1337–1349. doi: 10.1016/j.neuroimage.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Wimber M, Heinze HJ, Richardson-Klavehn A. Distinct frontoparietal networks set the stage for later perceptual identification priming and episodic recognition memory. J Neurosci. 2010;30:13272-1328. doi: 10.1523/JNEUROSCI.0588-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsley KJ, Evans AC, Marrett S, Neelin P. A three-dimensional statistical analysis for CBF activation studies in human brain. J Cereb Blood Flow Metab. 1992;12:900–918. doi: 10.1038/jcbfm.1992.127. [DOI] [PubMed] [Google Scholar]
- Xue G, Dong Q, Chen C, Lu Z, Mumford JA, Poldrack RA. Greater neural pattern similarity across repetitions is associated with better memory. Science. 2010;330:97–101. doi: 10.1126/science.1193125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue G, Mei L, Chen C, Lu Z-L, Poldrack R, Dong Q. Spaced learning enhances subsequent recognition memory by reducing neural repetition suppression. J Cogn Neurosci. 2011;23:1624–1633. doi: 10.1162/jocn.2010.21532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S, Hale LA, D’Esposito M, Knight RT. Rapid prefrontal-hippocampal habituation to novel events. J Neurosci. 2004;24:5356–5363. doi: 10.1523/JNEUROSCI.4587-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Stark CEL. Multiple signals of recognition memory in the medial temporal lobe. Hippocampus. 2008;18:945–954. doi: 10.1002/hipo.20452. [DOI] [PubMed] [Google Scholar]
- Yassa MA, Stark CEL. Pattern separation in the hippocampus. Trends Neurosci. 2011;34:515–525. doi: 10.1016/j.tins.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeineh MM, Engel SA, Thompson PM, Bookheimer SY. Dynamics of the hippocampus during encoding and retrieval of face-name pairs. Science. 2003;299:577–580. doi: 10.1126/science.1077775. [DOI] [PubMed] [Google Scholar]
- Zysset S, Huber O, Ferstl E, von Cramon DY. The anterior frontomedian cortex and evaluative judgment: an fMRI study. Neuroimage. 2002;15:983–991. doi: 10.1006/nimg.2001.1008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.