Abstract
Maintenance of normal myocardial function depends intimately on synchronous tissue contraction driven by electrical activation and on adequate nutrient perfusion in support thereof. Bioreactors have been used to mimic aspects of these factors in vitro to engineer cardiac tissue, but due to design limitations, previous bioreactor systems have yet to simultaneously support nutrient perfusion, electrical stimulation, and unconstrained (i.e., not isometric) tissue contraction. To the best of our knowledge, the bioreactor system described herein is the first to integrate in concert these three key factors. We present the design of our bioreactor and characterize its capability in integrated experimental and mathematical modeling studies. We then culture cardiac cells obtained from neonatal rats in porous, channeled elastomer scaffolds with the simultaneous application of perfusion and electrical stimulation, with controls excluding either one or both of these two conditions. After eight days of culture, constructs grown with the simultaneous perfusion and electrical stimulation exhibited substantially improved functional properties, as evidenced by a significant increase in contraction amplitude (0.23±0.10% vs. 0.14±0.05, 0.13±0.08, or 0.09±0.02% in control constructs grown without stimulation, without perfusion, or either stimulation or perfusion, respectively). Consistently, these constructs had significantly improved DNA contents, cell distribution throughout the scaffold thickness, cardiac protein expression, cell morphology and overall tissue organization than either control group. Thus, the simultaneous application of medium perfusion and electrical conditioning enabled by the use of the novel bioreactor system may accelerate the generation of fully functional, clinically sized cardiac tissue constructs.
Keywords: Bioreactor, cardiac tissue engineering, perfusion, electrical stimulation, imaging
1. Introduction
Heart disease and stroke, the principal components of cardiovascular disease, are the first and third leading cause of death in the US, accounting for nearly 40% of all deaths, more than all cancer combined (Lloyd-Jones et al., 2009). Upon myocardial infarction, a patient may lose up to 50 grams of muscle mass, as a result of hypoxia that leads to a release of apoptotoic factors and cell death, and limited ability of the damaged heart to regenerate following injury (Bergmann et al., 2009). Furthermore, congenital heart defects, which occur in nine out of every 1000 births, are the leading cause of death from birth defects (Lloyd-Jones et al., 2009). In recent years tissue engineering has emerged as a field of great interest, with the promise of creating in vitro biological substitutes that are capable of replacement, repair, and regeneration of damaged tissue in vivo. Several groups, including our own, have pioneered techniques to recapitulate cardiac tissue in vitro, by using different combinations of cells (Bursac, 2009, Klug et al., 1996, Serena et al., 2009, Tandon et al., 2009, Ugurlucan et al., 2009), biomaterials (Chen et al., 2008, Grayson et al., 2009, Ott et al., 2008, Shin et al., 2004, Wang et al., 2002, Engelmayr et al., 2008), and culture techniques (Radisic et al., 2008, Radisic et al., 2004, Zimmermann et al., 2004, Cheng et al., 2009), with the expectation that a cardiac “patch”, if clinically sized and electromechanically functional, could be used to repair damage following myocardial infarction (Zimmermann et al., 2006), or as a test platform for novel therapeutic techniques (Song et al., 2009).
Several key features of native myocardium that should be taken into account when trying to grow cardiac tissue in vitro include: (i) a high density of myocytes and supporting fibroblasts and vascular cells, (ii) efficient oxygen transport to these highly metabolically active cells, and (iii) synchronous contractions orchestrated by electrical signal propagation through interconnected cells (Vunjak-Novakovic et al., 2010). In order to achieve such highly specified tissue, it was proposed to provide cells with an in vivo-like (“biomimetic”) environment, so that they can differentiate and assemble into functional cardiac tissue. To achieve effective control over the cellular microenvironment, several groups have developed bioreactors which are advanced culture systems that can be used to provide environmental control, mechanical (Zimmermann et al., 2002) or electrical stimulation (Radisic et al., 2004, Tandon et al., 2009), or enhanced nutrient transport (Radisic et al., 2004) to developing cardiac tissue constructs.
In our previous studies, bioreactors designed to deliver electrical signals mimicking those in the native heart have been used to generate electrically functional and contractile cardiac tissue patches. Specifically, the application of electrical stimulation resulted in the progressive development of conductive and contractile properties characteristic of cardiac tissue, including cell coupling, increased amplitude of synchronous contractions, and ultrastructural organization (Radisic et al., 2006, Radisic et al., 2004). However, the thickness of viable cardiac tissue cultured in these early bioreactors (Petri dishes fitted with carbon rod electrodes) is limited to about 100 μm, corresponding to the penetration depth of oxygen by diffusion (Radisic et al., 2006). Cultivation of cardiac tissues with a clinically relevant size requires enhanced transport of nutrients – and most critically oxygen.
Bioreactors that perfuse culture medium through a porous scaffold (e.g., collagen sponge) seeded with cells bulk have been used to achieve uniform tissue formation in millimeter-scale thickness cardiac constructs (Carrier et al., 2002, Radisic et al., 2008, Radisic et al., 2004). By perfusing culture medium through a construct, critical nutrients such as oxygen are delivered by convective transport, whereas in traditional static cultures (e.g. Petri dishes) diffusion is the primary transport mechanism. Although perfusion enhanced the uniformity of engineered cardiac tissue, it also subjected the cardiac myocytes to hydrodynamic shear, a non-physiologic stimulus that alters cell morphology. In the heart, the blood supply flows through a dense capillary network that minimizes transport distances but also protects myocytes from shear. To recapitulate this situation in vitro, we have pioneered the use of scaffolds with a parallel array of small diameter (250 μm) channels (Radisic et al., 2005, Radisic et al., 2006) and developed techniques to uniformly seed these constructs homogeneously with a cardiac cell population without the use of a gel carrier that would block the channels (Maidhof et al., 2010). Perfusion of culture medium through scaffold channels decreased the distances for diffusional transport while protecting the cells from hydrodynamic shear, by mechanisms similar to those utilized by the capillary flow in the heart.
Recently, a cardiac tissue engineering system has been proposed that enables simultaneous application of perfusion and electrical stimulation, but at the expense of external fixation (Barash et al., 2010). In this system, constructs are held in a cartridge sandwiched between two screens, and not allowed to contract freely in response to electrical stimuli. Since it has previously been shown that induction of myocyte contraction, either by electrical pacing or mechanical stretch, improves tissue organization and assembly (Radisic et al., 2004, Zimmermann et al., 2002), fixing the constructs in place for perfusion culture is a severe limitation, motivating the bioreactor design developed in the present work.
In this study we propose the design of a bioreactor to deliver simultaneous culture medium perfusion and electrical conditioning during culture of engineered cardiac constructs while allowing the cultured tissues to freely contract. We hypothesized that a combination of two key biophysical stimuli – perfusion and electrical stimulation - will lead to a more viable and functional tissue than could be created using either stimulus. These principles of biophysical conditioning should prove useful for the development of cardiac patches starting from clinically relevant cardiac cells such as embryonic (Yang et al., 2008) or induced plouripotent stem cells (Moretti et al, 2010).
2. Methods
Ethics statement Cardiac cells were obtained from 1- to 2-day-old neonatal Sprague-Dawley rats (Harlan) according to procedures approved by the Columbia University Animal Care and Use Committee (IACUC protocol AC-AAAB2757).
2.1 Scaffolds
Porous poly(glycerol sebacate) (PGS) scaffolds were fabricated by means of a salt leaching technique as previously described (Radisic et al., 2008). The PGS scaffolds used for this study had an elastic modulus in tension of 34.55±1.26 kPa, a porosity of 76%, and pore sizes in the range of 75–150 μm, as measured previously (Gao et al., 2006, Wang et al., 2002). An array of cubically packed parallel channels (250 μm diameter, 500 μm wall-to-wall spacing) were created in the 1 mm thick sheet of the porous scaffold material using a computer controlled 30 Watt CO2 laser cutter (VersaLaser, VLS 2.30). The scaffold sheet was then cored into disks (8 mm diameter, 1 mm thickness) via biopsy punch, that were sterilized by autoclave and ethanol gradient (75, 50, 25, and 0% EtOH), incubated for 2 hours in a 10 μg/ml laminin solution (to enhance cell attachment to PGS) (Lee et al., 2009), and soaked overnight in culture medium containing 10% FBS to enhance cell retention on the scaffolds.
2.2 Cardiac cells
Cardiac cells were obtained from 1- to 2-day-old neonatal Sprague-Dawley rats (Harlan) according to procedures approved by the Columbia University Animal Care and Use Committee (IACUC protocol AC-AAAB2757), as in our previous studies (Radisic et al., 2008). Briefly, ventricles were quartered, incubated at 4°C in a 0.06% (w/v) solution of trypsin in Hank’s balanced salt solution (HBSS, Gibco), and subjected to a series of digestions (3–4 min, 37°C, 150 rpm) in a 0.1% (w/v) solution of collagenase type II in HBSS. The cell suspensions from the 4–5 digestions were collected and labeled unseparated. The cells were preplated in T75 flasks for one 75-min period to enrich for cardiomyocytes. The non-adherent cells were then collected and counted using a hemocytometer prior to seeding.
2.3 Cell seeding and culture
For cell seeding, perfusion loops consisting of cartridges and tubing were assembled as in our previous studies (Maidhof et al., 2010). Briefly, two channeled scaffolds were stacked on top of each other in perfusion cartridges and held in place on the edge by gaskets made from silicone tubing. In this configuration, perfusion of culture medium was forced through the inner 5 mm core of the scaffolds and the flow was mainly through the PGS pores since the channels were unaligned. Freshly isolated cardiac cells were injected into the tubing loop (3.3 M cells per scaffold, seeding density of 150 M cells/cm3 tissue) that was then transferred to a cell culture incubator and connected to a peristaltic pump (Ismatec). Forward-reverse flow was applied (0.1 mm/sec flow velocity, reversal of flow direction every 3 minutes) over a 2 hr seeding period. As previously demonstrated, this procedure yields constructs that are homogeneously seeded with viable cells, and channels that remain unblocked and can be perfused with culture medium (Maidhof et al., 2010).
For culture, constructs were removed from perfusion cartridges and placed in one of four experimental groups: (i) static (non-perfused) culture without electrical stimulation, (ii) static culture with electrical stimulation, (iii) perfusion culture without electrical stimulation, or (iv) perfusion culture with electrical stimulation. For static culture groups, constructs were placed in Petri dishes fitted with carbon rod electrodes (1 cm spacing) that were set up as in our previous studies (Tandon et al., 2009). For perfusion culture groups, custom designed bioreactors were assembled under sterile conditions as shown (Figure 1). These bioreactors were machined from alternating layers of Teflon, silicone, and plastics (polyetherimide), forming a “Y” shaped channel that routes culture medium flow simultaneously through two constructs to a single perfusion outlet.
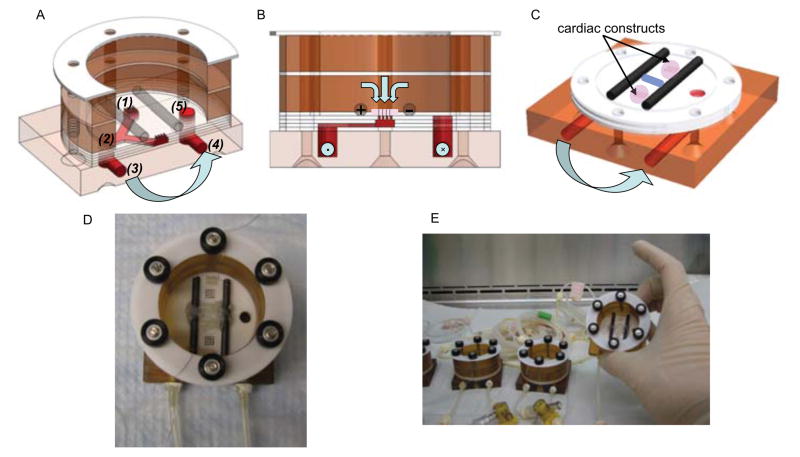
Figure 1. Perfusion-stimulation bioreactor setup.
(A) Alternating layers of custom machined Teflon, silicone, and plastic are stacked to a “perfused dish” culture chamber where medium flows from the medium bath (1), into the “Y” shaped channel (2), out of the bioreactor (3) and through a peristaltic pump, where it is pumped back to the bioreactor inlet (4), and back into the medium bath (5). Light blue arrows denote medium flow direction. (B) Cross-sectional view of the bioreactor showing flow path through the construct and carbon rod electrodes that provide electrical stimulation. (C) Isometric view showing placement of scaffolds over perfusion holes, between carbon rods. (D) Final assembly of the bioreactor with spacers to keep construct loosely in place and platinum wires to connect electrodes to a cardiac stimulator. (E) After autoclaving, bioreactors and tubing are assembled under sterile conditions in a tissue culture hood.
Constructs were placed into the space between silicone spacers and carbon rods (which also served as electrodes for stimulation). Notably, the constructs were not fixed in any way and instead they were perfused with culture medium that was flowing downwards through the constructs placed on a circular array of perforated holes. By sizing the construct to be larger in size than the array of holes (8 mm vs 5 mm in diameter, respectively), the fluid was routed through the construct without the need for a seal or fixation.
Culture medium above the constructs was constantly equilibrated with the incubator gas, and at the same time served as a trap for any bubbles generated during perfusion. A silicone tubing loop connected the bioreactor inlet and outlet and a peristaltic pump was used to flow culture medium through the system at a flow rate of 18 μL / minute.
For electrical stimulation, pulses (3 V/cm, 3 Hz, monophasic square wave) were applied by connecting platinum wires attached to the carbon rod electrodes to cardiac stimulators (Grass). The culture (with electrical stimulation alone, medium perfusion, alone, both the electrical stimulation and perfusion, or without any conditioning) was initiated on the third day following seeding to allow time for cell attachment to the scaffold, a factor previously shown to be important for the functional development of engineered cardiac tissue (Radisic et al., 2004). Constructs were cultured for an additional 5 days, and ¼ of culture medium was changed every 3 days (corresponding to 10 ml medium per construct). Culture medium consisted of high glucose DMEM supplemented with 10% fetal bovine serum, as in our previous studies (Radisic et al., 2008).
2.4 Modeling of the electrical field
To characterize the novel perfusion-stimulation bioreactor we modeled the electrical field generated by the carbon rod electrodes using commercially available software (Multiphysics, Comsol, electrostatics module). The electroquasistatic approximation is generally considered appropriate for simple cases such as homogeneous, isotropic media, where the system under consideration is much smaller than the wavelengths of interest (Voldman, 2006). In our case, with the application of monophasic rectangular pulses 2 ms in duration, greater than 99% of the power of the applied signal lies in the frequency band below 10 kHz (Stem et al., 2004). Furthermore, we assumed an isotropic medium with conductance of 1.5 S/m (a value reported previously for cell culture medium) (Cannizzaro et al., 2007) and could thus calculate the electric fields by solving Maxwell’s equations with the quasistatic approximation for the electrical field (Durand and Bronzino 1995). We also assumed that the bioreactor surface provides an electrically insulating boundary condition, and solved the equations using triangular mesh elements with an average area of 2.04 × 10−3 mm2 per element.
2.5 Flow visualization
To visualize flow through the bioreactor and channeled scaffold, bioreactors were assembled and filled with PBS and channeled scaffolds were placed appropriately. Above each scaffold, 5 μl of trypan blue dye was injected and flow was initiated via the peristaltic pump. After 3 minutes images of the construct were taken at 4x magnification using a stereoscope to visualize the presence of the dye within the scaffold. As a control the procedure was repeated without perfusion to visualize the effects of diffusion alone.
2.6 Functional analysis
Contractile activity of engineered cardiac constructs in response to electrical field stimulation was assessed after 8 days of culture by measuring the excitation threshold (the minimum stimulation voltage required for synchronous contraction), maximum capture rate (the maximum beating frequency that could be induced by electrical pacing), and the amplitude of contraction (fractional area change) using methods previously established in our lab (Tandon et al., 2009) (n=10–14 per group, video frame rate = 36 / second). The excitation threshold and maximum capture rate were also measured for freshly isolated neonatal rat heart ventricles (n=5).
2.7 Analysis of motion and deformation during contraction
Microscopic images of the patches were acquired in the RGB format, which stores three color values, red, green, and blue, for each pixel. The RGB images were converted to grayscale images in MATLAB® software (The MathWorks, Natick, MA). 2D motion between each image frame in the video (i.e., comparison frame) and a fixed frame before contraction (i.e., reference frame) was estimated using a highly efficient normalized cross-correlation method as previously described (Luo and Konofagou, 2010). An observation reference sub-image (i.e., window) was defined in the reference frame and subsequently compared with different candidate sub-images from the comparison frame, within a pre-defined search range. The normalized cross-correlation function was calculated to quantify the similarity, or ‘matching’, between the reference and comparison windows. The 2D motion of the reference window was estimated from the spatial shift between the reference window and the best-match comparison window, which gave the maximum normalized cross-correlation value. The reference window was then moved pixel by pixel across the image in order to obtain the 2D motion image (Luo and Konofagou, 2010).
Each recorded image had a size of 240 × 320 pixels. In motion analysis, the window size was set to 8 × 8 pixels. The search range was ±3 pixels, sufficient to cover the maximum displacement experienced by the constructs. The estimated 2D motion vectors were superimposed onto the construct images, and color coded for the motion amplitude (in pixels). The global strain was calculated as the difference between the highest motion amplitudes estimated on opposite edges of the construct normalized by the distance between the two regions.
2.8 DNA quantification
Constructs were cut in half (n=5–7 per group), and each half was weighed, and digested overnight at 56°C in 0.5 ml of 1 mg/ml proteinase K solution (pH 7.5) prepared in 10 mM Tris with 1 mM EDTA (Sigma). All samples were then analyzed by Quant-iT PicoGreen dsDNA Assay Kit (Invitrogen) according to the manufacturer’s protocol. DNA concentrations were compared to reference lambda DNA standards supplied in the kit. The total DNA content of each construct was determined by adjusting by the weight percent of the digested construct.
2.9 Cell distribution
For histological analysis, one half of each scaffold was fixed overnight in 4% neutral buffered formalin, embedded in paraffin, sectioned perpendicular to the scaffold diameter in 5 μm thick cross-sections, and stained with hematoxylin and eosin (H&E). Color images were acquired by a Q-imaging camera mounted to a light microscope (Olympus). Each scaffold was divided into four equal regions starting from the top edge (e.g. facing the medium bath) and the number of cells in each region was hand counted using image analysis software (ImageJ). The number of cells in each region was then divided by the total number of cells in the cross section, to obtain the percentage of cells as a function of depth in the tissue.
2.10 Immunofluoresence imaging
To visualize cell morphology we stained cross sections using antibodies for troponin-I using previously described techniques (Radisic et al., 2008). Images were acquired using a LSM 510 Meta confocal fluorescence microscope with 1 μm Z-stack stepping, a magnification of 400x, and a resolution of 512 × 512 pixels. Images for presentation were captured as screen snapshots of extended focus using Volocity LE (Improvision) and prepared using Adobe Photoshop. Due to micropores in the PGS scaffolds, the sections contained PGS that appeared to fluoresce in the blue channel, which was the same as our TO-PRO-3 nuclear stain. To correct this in each image we selected the scaffold portions based upon characteristic size and irregular shape and lowered the intensity of the signal in these selections to effectively increase the contrast between the nuclei and scaffold.
2.11 Western blot
Construct homogenates were diluted (1:4) in Laemmili buffer (Bio-Rad) containing 5% mercaptoethanol and 2% sodium dodecyl sulfate (SDS) and were boiled for 10 minutes to denature proteins. Protein lysates containing 30 μg of protein were separated on 4–15% SDS-PAGE gel (Biorad) and transferred to polyvinylidene difluoride membranes (Millipore, Bedford, MA). The membranes were incubated at 4°C overnight in blocking buffer (5% nonfat dry milk in TBST buffer). For immunoblotting, the primary antibodies used were monoclonal mouse anti-cardiac troponin T (1:1000, Abcam), polyclonal rabbit anti-connexin 43 (1:1000, Abcam), and polyclonal goat anti-creatine kinase-M (1:100, Santa Cruz). After multiple washes, blots were incubated with secondary goat anti-rabbit IgG HRP, goat anti-mouse IgG HRP antibodies, or donkey anti-goat IgG HRP (1:2000, Santa Cruz) for 30 min at room temperature, and developed using chemiluminescence (Supersignal, Pierce). Protein lysate was also obtained from neonatal rat hearts as positive control and the blots were probed for β-actin (1:2000 dilution, Sigma), which was used as equal loading control. Quantification of Western blots was performed using Image J (National Institutes of Health, Bethesda, MD). The results were presented as relative density after correction with β-actin.
2.12 Statistical analysis
Results are presented as mean ± standard deviation. Differences between the groups were analyzed by one-way ANOVA followed by Tukey’s test for pairwise comparisons and p < 0.05 was considered significant.
3. Results
The novel design of our bioreactor is based on the concept of a “perfused dish” with holes that route the medium flow through the interstitium of a tissue construct and not around the construct, without any external fixation. The lack of external fixation is essential for the mechanical contractions of the constructs in response to electrical stimulation.
Each bioreactor houses two tissue constructs. The bioreactor chamber is composed of several machined layers that, when assembled, form a perforated bottom plate underneath the constructs, enabling medium flow downwards from the bath and through the tissue (Figure 1). Fluid is carried through the tubing via peristaltic pump and returned to the bath via a separate channel in the bioreactor. The bioreactors are easy to sterilize, assemble, prime with culture medium, and fit with the tissue constructs under a tissue culture hood. A glass Petri dish cover serves as a cover for the medium bath, providing sterility throughout the culture duration.
Electrical stimulation was provided via two parallel carbon rods serving as the positive and negative electrode. Constructs were placed between the rods. Platinum wires attached to the electrodes were then connected to a cardiac stimulator providing repeating electrical pulses. In this configuration, application of supra-threshold electrical stimuli caused the constructs to contract freely in response to the pacing. As previously established (Radisic et al., 2004), electrical pacing in this manner is an effective way to engineer synchronously contractile constructs.
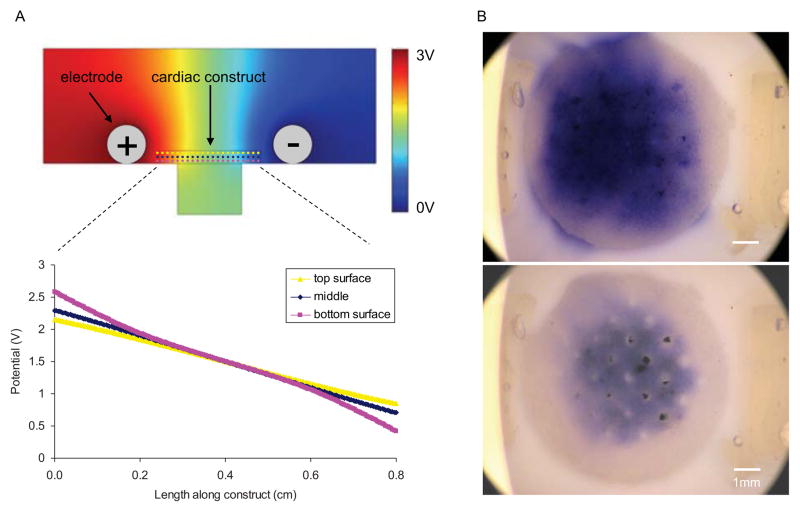
To verify the perfusion capability of our system, we performed a flow study using trypan blue dye (Figure 2A). Specifically, we confirmed that the culture medium was flowing through the construct channels, and not around the construct. Also, when fluid flow was disconnected the tracer dye diffused away, while with flow the dye was perfused through the scaffold channels.
Figure 2. Bioreactor characterization.
(A) Modeling of electrical field in the bioreactor at different slices through the construct depth (dotted lines) and corresponding potential along these lines. (B) Flow study showing progress of trypan blue dye three minutes after injection with either no flow (top) or flow via peristaltic pump (bottom).
To characterize the electrical properties of our bioreactor we modeled the electrical potential generated by the carbon rod electrodes and examined the field in the area where cardiac constructs were placed (Figure 2B). Modeling showed that the drop in electric potential is nearly linear along the length of the construct, and does not vary significantly between the top and bottom planes of the construct. The modeling results indicate that the cells throughout the construct volume are exposed to the same linear electrical field.
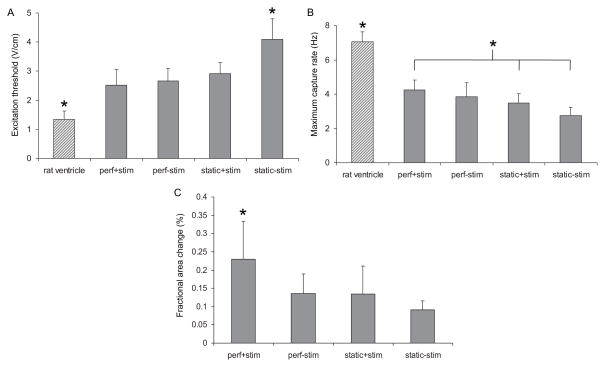
After eight days of culture, we observed several trends with respect to measured functional parameters (Figure 3). Although not statistically significantly different, excitation threshold (ET), the voltage gradient necessary to induce macroscopic contractions, was the lowest in the perf+stim group (2.5±0.5 V/cm), and showed an increasing trend to 2.7±0.4, 2.9±0.4 and 4.1±0.7 V/cm for the perf-stim, static+stim and static-stim groups, respectively. All ET values were higher than those measured for freshly isolated neonatal rat ventricle (1.3±0.3 V/cm).
Figure 3. Cardiac construct functionality after 8 days of culture.
Measured excitiation threshold (A), maximum capture rate (B), and fractional area change (C) of constructs cultured in either simultaneous perfusion and electrical stimulation (perf+stim), perfusion without electrical stimulation (perf-stim), no perfusion with electrical stimulation (static+stim), or no perfusion and no electrical stimulation (static-stim). Error bars represent standard deviation, * denotes statistical difference from all other groups (A) and (C) or difference between perf+stim group and static controls (B).
Maximum capture rate (MCR) was the highest in the perf+stim group (4.3±0.6 Hz) and decreased to 3.8±0.8, 3.5±0.5, and 2.8±0.5 Hz for the perf-stim, static+stim and static-stim groups, respectively (and the perf+stim group having a significantly higher MCR than static+stim and static-stim groups). All MCR values were lower than those measured for freshly isolated neonatal rat ventricles (7.1±0.6 Hz).
Although, due to their differing shapes, it is difficult to compare the fractional area change of engineered tissue to native tissue, among the experimental groups, fractional area change was significantly higher for the perf+stim group (0.23±0.10%) than for the perf-stim, static+stim, and static-stim groups (0.14±0.05, 0.13±0.08, 0.09±0.02%, respectively, perf+stim group significantly greater than all other experimental groups).
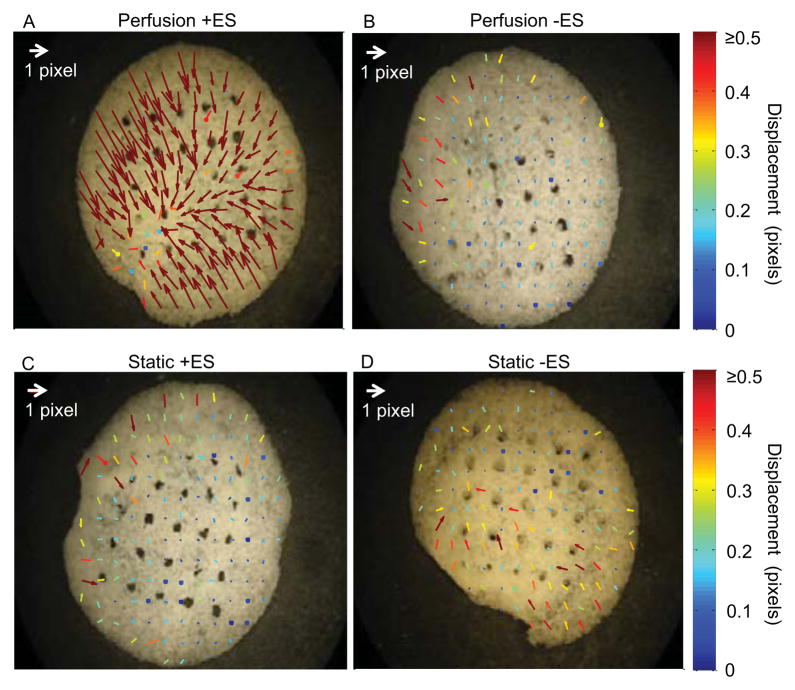
As an additional functional assay we used a custom image analysis program (Luo and Konofagou, 2010) to track the local displacements of the contracting constructs (Figure 4). The overall motion and strain during contraction in the perf+stim group was both greater in magnitude and more uniformly oriented towards the construct center than in the other groups, indicating the development of a more homogeneous and differentiated tissue. The global strain (i.e., deformation during contraction towards the center) of the entire construct in the perf+stim group was approximately 2%, substantially larger than that in the static-stim group, as determined by a T test.
Figure 4. Motion analysis during a contraction.
Vector map showing maximum motion after a contraction for a construct cultured in either the simultaneous perfusion and electrical stimulation group (A), the perfusion and no electrical stimulation group (B), the no perfusion with electrical stimulation group (C), or the no perfusion and no electrical stimulation group (D). Arrows represent relative motion of each pixel from relaxed to contracted state.
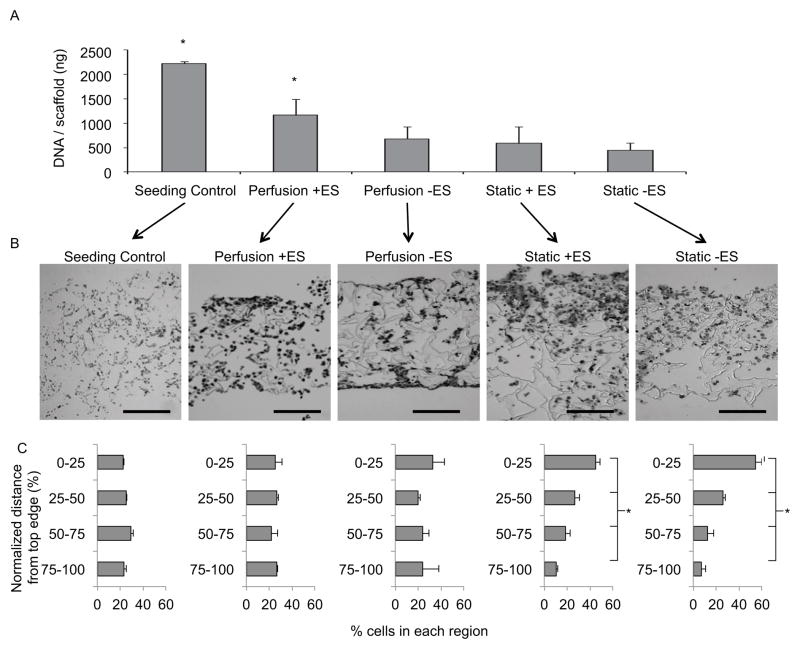
We conducted a DNA assay to measure construct cellularity immediately after cardiac cell seeding, after three days of static preculture, and after five additional days of culture (Figure 5A). Constructs analyzed at the end of the three-day preculture period had a DNA content of 1,231±419 ng/scaffold. After an additional 5 days of culture, constructs from the perf+stim group had a significantly higher DNA content (1,167±330 ng/scaffold) than constructs in the perf-stim, static+stim, and static-stim groups that had comparable and markedly lower DNA contents (680±257, 591±337, 451±147 ng/scaffold, respectively). Specifically, only constructs from the perf+stim group maintained the DNA content established at the end of preculture.
Figure 5. DNA content and cell distribution after 8 days of culture.
(A) DNA content for each group was assessed for each culture condition via a fluorescence assay and compared to control scaffolds analyzed immediately after the seeding procedure. (B) H&E stained cross sections approximately 1 mm thick were divided into four sections of equal length and the total number of cells in each section was counted (scale bars = 500 μm). (C) Histograms show the percentage of cells in each quadrant of the scaffold thickness. * denotes statistical difference from all other groups (A) or difference between all regions for a particular culture condition (C).
To determine the homogeneity of cell distribution in the constructs, images of cross-sections taken throughout the construct thickness (~1 mm) were analyzed (Figure 5B). Each section was divided from top to the bottom edge into four equal regions, and the number of cells in each region was counted (Figure 5C). Resulting histograms showed that after seeding the cells were relatively uniformly distributed throughout the construct volume, and that this uniform distribution was maintained in both perfused groups. However, significant variation in cell numbers between the top and bottom edges was found in the constructs cultured statically.
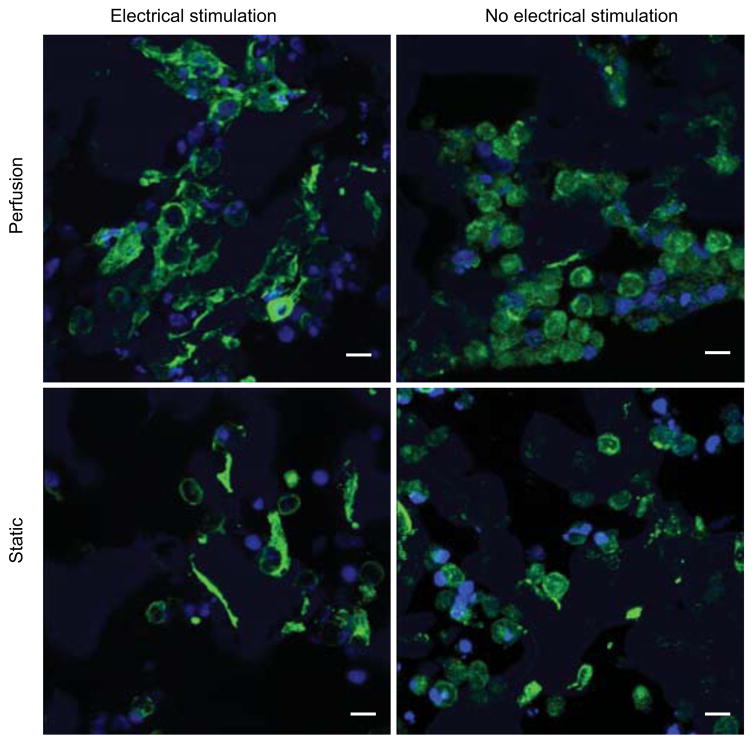
To visualize cell morphology construct cross sections were immunostained for troponin-I and confocal imaging was performed (Figure 6). In all groups, a majority of cells stained positive for troponin-I, indicating that cardiomyocytes remained the predominant cell type present after culture. Cells were most clustered and most abundant in the perf+stim group. Additionally, both the perfusion and electrical stimulation induced cell elongation and the organization of internal cell structures, whereas in non-stimulated groups the cells were mostly rounded and had less organized internal structure.
Figure 6. Immunostaining for troponin I.
After 8 days of culture constructs were fixed, embedded in paraffin and sectioned through the 1 mm thick cross section, stained for troponin I (green) and cell nuclei (blue), and imaged via confocal microscopy. Note: PGS scaffold is autofluorescent (blue). Scale bars = 10 μm.
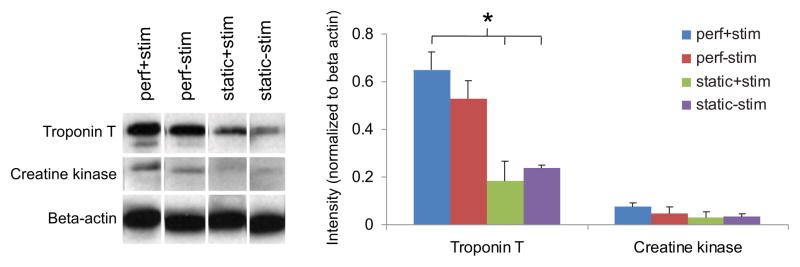
Immunoblotting further characterized the impact of culture conditions on cell phenotype. Western blot revealed that the expression of troponin T (a component of the thin filament involved in cardiomyocyte contraction) and creatine kinase (an enzyme involved in generating phosphocreatine, an energy reservoir in cardiomyocytes) was the greatest for constructs from the perf+stim group, and decreased for non-perfused groups.
4. Discussion
We designed a bioreactor system for cardiac tissue engineering with a unique capability to provide simultaneous perfusion (interstitial flow) and electrical field stimulation of tissue constructs that are not fixed in place and can be freely contracted by the cells. The constructs were formed from neonatal rat heart cells and channeled microporous elastomer scaffolds. By systematically comparing the compositions, structural and contractile properties of constructs cultured with perfusion and electrical stimulation (perf+stim), perfusion only (perf-stim), electrical stimulation only (static+stim) or neither stimulus (static-stim), we determined the individual and combined effects of each stimulus. When evaluating the performance of engineered cardiac tissue, several factors, including electrical excitability, cellular interconnectivity, and contractility must all be considered together. From this perspective, an optimal tissue culture regime is the one resulting in not only low excitation threshold (indicative of electrical excitability), but also high maximum capture rate (indicative of cellular interconnectivity) and high amplitude of contraction (indicative of contractile behavior). When taken together, the low excitation threshold and high maximum capture rate observed for the perf+stim group, when compared with other experimental groups (although not significantly different from all other groups), combined with the significantly higher amplitude of contraction, show significant advantages of the simultaneous perfusion and electrical stimulation in terms of contractible capability of the resulting constructs, a design that provides some of the mass transport and signaling conditions that are present in vivo.
Over the past decade, advances in the field of cardiac tissue engineering have been achieved through the application of various types of biophysical stimuli: electrical (Radisic et al., 2004), mechanical (Zimmermann et al., 2002), and hydrodynamic (Carrier et al., 2002), via specialized bioreactors. Generally, the bioreactor designs have been motivated by the understanding of the native cardiac environment, and the notion that the factors present in the developing cardiac tissue in vivo need to be recapitulated to engineer functional cardiac tissues in vitro.
Bioreactors that permit the study of a single stimulus – mechanical, electrical, or hydrodynamic – are certainly useful and advantageous when compared to simple Petri dishes. However, the need to choose which one of these three stimuli to provide, at the expense of the other two, remains a major limitation of the existing bioreactor designs for cardiac tissue engineering. For example, electrical stimulation bioreactors consisting of a Petri dish fitted with electrodes are limited in their ability to grow constructs with a thickness of the compact and viable tissue beyond ~100 μm (Radisic et al., 2004, Tandon et al., 2009). On the other hand, perfusion bioreactors that enhance nutrient transport by shortening the diffusional distances do not allow the simultaneous application of electrical stimulation (Radisic et al., 2008, Radisic et al., 2006).
Recently, a cardiac tissue engineering system has been proposed that enables simultaneous application of perfusion and electrical stimulation, but at the expense of external fixation (Barash et al., 2010). In this system, constructs are held in a cartridge sandwiched between two screens, and not allowed to contract freely in response to electrical stimuli. In our previous studies involving culture medium perfusion, cardiac constructs were also placed in cartridges and held in place in between gaskets and screens to route flow through the tissue (Carrier et al., 2002, Radisic et al., 2004). Although the engineered tissue developed some measures of electrical functionality, its contractile properties were limited. Since it has previously been shown that induction of myocyte contraction, either by electrical pacing or mechanical stretch, improves tissue organization and assembly (Radisic et al., 2004, Zimmermann et al., 2002), fixing the constructs in place for perfusion culture is a severe limitation of both systems, motivating the novel bioreactor design developed in the present work.
A major advantage of our bioreactor, which combines the ability to simultaneously electrically stimulate and perfuse culture medium through the bulk of a tissue construct, is that the tissue is not fixed in place and can be freely contracted by the cells. The constructs are prevented from floating away because of the combined forces of gravity and downward medium flow, and the sizing of the constructs relative to the perforated bottom provides a seal at the construct edge. For our experiments we chose a relatively low flow rate, that resulting in low hydrodynamic shear (< 1 dyn/cm2) that does not hinder contractions due to the hydrodynamic forces. This way, the constructs are free to contract while being perfused by culture medium.
Another advantage of our bioreactor is that it can support a wide range of perfusion-stimulation conditions of interest. For example, a recent study showed that pulsatile perfusion flow improved the contraction amplitude of engineered cardiac tissue over that achieved with a constant flow rate (Brown et al., 2008). Pulsatile flow mimics the in vivo situation wherein blood is perfused through the blood vessels in a cyclic profile with each heartbeat. The mechanical compression of cells with each pulse may provide a source of mechanical stimulation that enhances cardiac tissue development. The proposed perfusion-stimulation bioreactor can provide pulsatile flow, and the electrical pacing could be coupled to the flow profile so that the cells are excited in phase with the flow. We could also tailor the applied stimulus waveform via a computer-controlled stimulator to study electrical signals that could further improve cardiac tissue organization.
The carbon electrodes provide a relatively linear electric field that is not affected by medium flow (Figure 2B). This is consistent with previous characterization of dishes fitted with carbon rods (Tandon et al., 2009, Tandon et al., 2008), therefore we can assume that any field applied will be relatively uniform across the construct. Although field stimulation is a non-physiologic phenomenon, it has previously been shown as an important factor that significantly enhances contractile development in engineered cardiac tissues (Radisic et al., 2004). Flow studies showed that, with the initiation of perfusion, fluid is drawn through the scaffold channels, (Figure 2A). Within our experimental conditions, we have not observed fluid flow around the scaffold, and have not varied the flow rate. A more detailed investigation on the effect of flow rates on tissue development and the occurrence of the secondary flow around the scaffold should be carried out if the experimental conditions are to be extended into a broader range of flow parameters.
For this study, we chose to initiate our culture conditions after three days of culture without electrical stimulation or medium perfusion. Previous results showed that this preculture period was needed to allow cardiac cells to recover from isolation and begin to regenerate gap junction and contractile proteins (Radisic et al., 2004). During the preculture period perfusion is probably not necessary since the pore structure remains mostly open until cells begin to organize into tissue. After only 8 days of culture with simultaneous medium perfusion and electrical stimulation, the cells developed a remarkable level of functionality compared to any control group (Figure 3). The effect was most evident with respect to contractile behavior, as measured by fractional area change. Combined perfusion and stimulation resulted in tissue that contracted with an amplitude that was almost twice as high as that achieved by application of perfusion or stimulation separately. In addition to amplitude, the uniformity and directionality of contractions were clearly better established in the perf+stim group than in any of the controls (Figure 4). It appears that perfusion and stimulation indeed have a synergistic effect and, when combined, lead to a more functional engineered cardiac tissue.
We attempted to seed the cells at a physiologic density (~108 cells/cm3), since neonatal cardiomyocytes do not proliferate in culture (Radisic et al., 2003). Even so, cells were lost during culture, likely because unattached cells cannot survive and are washed away. Thus the DNA content of scaffolds immediately after seeding decreased over the first 3 days of preculture (Figure 5A). However, over the time of subsequent construct cultivation, DNA content was found to be significantly higher in the simultaneous perfusion and stimulation group than in the controls. After the three-day preculture period, simultaneous perfusion and stimulation maintained the cell content for the duration of the eight day culture. Therefore we suspect that initiation of perfusion at an earlier time point could lead to higher cell content after culture. As expected from previous studies, a nonuniform cell distribution was inherent to non-perfused cultures (Figure 5B, 5C), with most of the cells clustered near the top surface of the construct that was in contact with the culture medium. The perfused groups were able to maintain even cell distribution established during the initial seeding, presumably due to enhanced mass transport throughout the constructs.
A limitation of the current study is that although the cardiac cells form dense clusters throughout the tissue, they do not form uniformly interconnected tissue. Since the cells do not readily adhere to the PGS scaffold and are not encapsulated in a gel phase, they must secrete extracellular matrix within the scaffold pores in order to elongate and form intracellular connections. This process requires time in culture, and by the end of 8 days some of the cultured cells spread and form compact tissue. A longer culture period might lead to enhanced tissue uniformity in future studies. In addition, a gel used to deliver cells and lock them in place within the scaffold would likely allow them to elongate and develop compact tissue within a shorter time, by providing a substrate for attachment and trapping secreted cell products. In this study we chose not to use a gel carrier because gelation would block the small-diameter channels, and new techniques to seed the porous channeled scaffolds may be required.
Troponin I and troponin T are both subunits of troponin (which regulates the generation of force in cardiomyocytes), and either troponin can be used as a cardiomyocyte marker (Saggin et al., 1988, Westfall et al., 1996). Immunostaining for troponin revealed that the majority of cells after culture were cardiomyocytes (Figure 6), which is important because the initial cell population contains about 65% cardiomyocytes and 35% fibroblasts (Radisic et al., 2008). In the present study we focused on this simplified starting cell population. However, the heart actually contains a mixture of cardiomyocytes, fibroblasts and endothelial cells that interact dynamically via secretion of paracrine signals and extracellular matrix components to regulate tissue development and organization. Previous work has shown that seeding PGS scaffolds with fibroblasts several days prior to cardiomyocyte seeding leads to enhanced contraction amplitude, superior DNA content, and higher glucose consumption, presumably due to the interactions between cardiomyocytes and extracellular matrix secreted by the pre-seeded fibroblasts (Radisic et al., 2008). Other studies have shown that coculture of cardiac fibroblasts and cardiomyocytes induces cellular alignment (Nichol et al., 2008) and coculture of endothelial cells with cardiomyocytes can improve cardiac function in ischemic hearts (Sekine et al., 2008). Therefore, coculture of the various cardiac cell types could further enhance tissue organization and functional development, and this is an ongoing area of research by our group.
Immunostaining also showed that the cell structure and organization were affected by culture conditions (Figure 6). In electrically stimulated groups, myocytes appeared to elongate and form defined intracellular structures, whereas without stimulation they remained round and without organized structure. Cells appeared most dense and interconnected in the simultaneous perfusion and stimulation group, which correlates with the measured higher DNA content and greater functional properties observed for this condition. In addition, this group exhibited greater troponin content as compared to controls, and expressed other important cardiac markers including creatine kinase (Figure 7).
Figure 7. Immunoblot analysis of cardiac proteins.
After 8 days of culture, constructs were analyzed via Western blot for tropnin T, connexin 43 (Cx-43), and creatine kinase. Band intensity was quantified by image analysis software and normalized by beta-actin expression to compare relative amounts of protein expressed in each group. * denotes statistically significant difference between perf+stim group and controls.
Taken together, these results indicate that simultaneous perfusion and stimulation enhance the development of engineered cardiac tissue, especially in terms of contraction amplitude. There appears to be a synergistic effect between these two conditioning stimuli, leading to enhanced organization and functionality of engineered cardiac tissue. The bioreactor we created could thus be an important tool in the development of engineered constructs for cardiac repair or in vitro disease study.
Acknowledgments
We gratefully acknowledge Yadong Wang and Jin Gao for providing PGS scaffolds and George Eng for harvesting the rat hearts.
We gratefully acknowledge the support of NIH (HL076485, EB002520, HL088913 and RR026244 to GVN; training grant T 32 HL087745 support to RTM) and Columbia University (Presidential Fellowship to NT).
Footnotes
Ethics statement
All studies have been conducted in accordance to the approved protocols at the Columbia University, and the ethical and responsible conduct of research.
Contributor Information
Robert Maidhof, Email: rtm2001@columbia.edu, Columbia University, Department of Biomedical Engineering, 622 West 168thStreet, MC 104B, New York, NY 10032, 212-305-9239
Nina Tandon, Email: nmt2004@columbia.edu, Columbia University, Department of Biomedical Engineering, 622 West 168thStreet, MC 104B, New York, NY 10027, 212-305-9239
Eun Jung Lee, Email: el368@email.med.yale.edu, Yale University, Department of Anesthesiology, 10 Amistad Street 314, P.O.Box. 208089, New Haven, CT 06520, 203-737-1428 203-737-1484 (fax)
Jianwen Luo, Email: jl2767@columbia.edu, Columbia University, Department of Biomedical Engineering, 500 West 120thStreet, 351 Engineering Terrace, MC 8904, New York, NY 10027, 212-342-1611
Yi Duan, Email: yd2210@columbia.edu, Columbia University, Department of Biomedical Engineering, 622 West 168thStreet, MC 104B, New York, NY 10032, 212-305-9239
Keith Yeager, Email: ky2161@columbia.edu, Columbia University, Department of Biomedical Engineering, 500 West 120thStreet, 351 Engineering Terrace, New York, NY 10027, 212-854-0290
Elisa Konofagou, Email: ek2191@columbia.edu, Columbia University, Department of Biomedical Engineering, 622 West 168thStreet, New York, NY 10032, 212-342-0863/212-342-1648 (fax)
Gordana Vunjak-Novakovic, Email: gv2131@columbia.edu, Columbia University, Department of Biomedical Engineering, 622 West 168thStreet, MC 104B, New York, NY 10032, 212-305-2304 / 212-305-4692 (fax)
References
- Barash Y, Dvir T, Tandeitnik P, et al. Electric field stimulation integrated into perfusion bioreactor for cardiac tissue engineering. Tissue Eng Part C Methods. 2010 doi: 10.1089/ten.TEC.2010.0068. [DOI] [PubMed] [Google Scholar]
- Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabé-Heider F, et al. Evidence for Cardiomyocyte Renewal in Humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MA, Iyer RK, Radisic M. Pulsatile perfusion bioreactor for cardiac tissue engineering. Biotechnol Prog. 2008;24(4):907–20. doi: 10.1002/btpr.11. [DOI] [PubMed] [Google Scholar]
- Bursac N. Cardiac tissue engineering using stem cells. IEEE Eng Med Biol Mag. 2009;28(2):80, 2, 4, 6, 8–9. doi: 10.1109/MEMB.2009.931792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrier RL, Rupnick M, Langer R, et al. Perfusion improves tissue architecture of engineered cardiac muscle. Tissue Eng. 2002;8(2):175–88. doi: 10.1089/107632702753724950. [DOI] [PubMed] [Google Scholar]
- Chen QZ, Bismarck A, Hansen U, et al. Characterisation of a soft elastomer poly(glycerol sebacate) designed to match the mechanical properties of myocardial tissue. Biomaterials. 2008;29(1):47–57. doi: 10.1016/j.biomaterials.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Cheng M, Moretti M, Engelmayr GC, et al. Insulin-like growth factor-I and slow, bidirectional perfusion enhance the formation of tissue-engineered cardiac grafts. Tissue Eng Part A. 2009;15(3):645–53. doi: 10.1089/ten.tea.2008.0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand D, Bronzino JD. The Biomedical Engineering Handbook. CRC Press; Boca Raton: 1995. [Google Scholar]
- Engelmayr GC, Jr, Cheng M, Bettinger CJ, et al. Accordion-like honeycombs for tissue engineering of cardiac anisotropy. Nat Mater. 2008;7(12):1003–10. doi: 10.1038/nmat2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Crapo PM, Wang Y. Macroporous elastomeric scaffolds with extensive micropores for soft tissue engineering. Tissue Eng. 2006;12(4):917–25. doi: 10.1089/ten.2006.12.917. [DOI] [PubMed] [Google Scholar]
- Grayson WL, Martens TP, Eng GM, et al. Biomimetic approach to tissue engineering. Semin Cell Dev Biol. 2009;20(6):665–73. doi: 10.1016/j.semcdb.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klug MG, Soonpaa MH, Koh GY, et al. Genetically selected cardiomyocytes from differentiating embronic stem cells form stable intracardiac grafts. J Clin Invest. 1996;98(1):216–24. doi: 10.1172/JCI118769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EJ, Vunjak-Novakovic G, Wang Y, et al. A biocompatible endothelial cell delivery system for in vitro tissue engineering. Cell Transplant. 2009;18(7):731–43. doi: 10.3727/096368909X470919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Jones D, Adams R, Carnethon M, et al. Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480–486. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- Luo J, Konofagou EE. A fast normalized cross-correlation calculation method for motion estimation. IEEE Trans Ultrason Ferroelectr Freq Control. 2010;57(6):1347–57. doi: 10.1109/TUFFC.2010.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maidhof R, Marsano A, Lee EJ, et al. Perfusion seeding of channeled elastomeric scaffolds with myocytes and endothelial cells for cardiac tissue engineering. Biotechnol Prog. 2010;26(2):565–72. doi: 10.1002/btpr.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretti A, Bellin M, Jung CB, et al. Mouse and human induced pluripotent stem cells as a source for multipotent Isl1+ cardiovascular progenitors. FASEB J. 2010;24(3):700–11. doi: 10.1096/fj.09-139477. [DOI] [PubMed] [Google Scholar]
- Nichol JW, Engelmayr GC, Jr, Cheng M, et al. Co-culture induces alighment in engineered cardiac constructs via MMP-2 expression. Biochem Biophys Res Commun. 2008;373(3):360–5. doi: 10.1016/j.bbrc.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott HC, Matthiesen TS, Goh SK, et al. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat Med. 2008;14(2):213–21. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- Radisic M, Deen W, Langer R, et al. Mathematical model of oxygen distribution in engineered cardiac tissue with parallel channel array perfused with culture medium containing oxygen carriers. Am J Physiol Heart Circ Physiol. 2005;288(3):H1278–89. doi: 10.1152/ajpheart.00787.2004. [DOI] [PubMed] [Google Scholar]
- Radisic M, Euloth M, Yang L, et al. High-density seeding of myocyte cells for cardiac tissue engineering. Biotechnol Bioeng. 2003;82(4):403–14. doi: 10.1002/bit.10594. [DOI] [PubMed] [Google Scholar]
- Radisic M, Malda J, Epping E, et al. Oxygen gradients correlate with cell density and cell viability in engineered cardiac tissue. Biotechnol Bioeng. 2006;93(2):332–43. doi: 10.1002/bit.20722. [DOI] [PubMed] [Google Scholar]
- Radisic M, Marsano A, Maidhof R, et al. Cardiac tissue engineering using perfusion bioreactor systems. Nat Protoc. 2008;3(4):719–38. doi: 10.1038/nprot.2008.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radisic M, Park H, Chen F, et al. Biomimetic approach to cardiac tissue engineering: oxygen carriers and channeled scaffolds. Tissue Eng. 2006;12(8):2077–91. doi: 10.1089/ten.2006.12.2077. [DOI] [PubMed] [Google Scholar]
- Radisic M, Park H, Martens TP, et al. Pre-treatment of synthetic elastomeric scaffolds by cardiac fibroblasts improves engineered heart tissue. J Biomed Mater Res A. 2008;86(3):713–24. doi: 10.1002/jbm.a.31578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radisic M, Park H, Shing H, et al. Functional assembly of engineered myocardium by electrical stimulation of cardiac myocytes cultured on scaffolds. Proc Natl Acad Sci U S A. 2004;101(52):18129–34. doi: 10.1073/pnas.0407817101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radisic M, Yang L, Boublik J, et al. Medium perfusion enables engineering of compact and contractile cardiac tissue. Am J Physiol Heart Circ Physiol. 2004;286(2):H507–16. doi: 10.1152/ajpheart.00171.2003. [DOI] [PubMed] [Google Scholar]
- Saggin L, Ausoni S, Gorza L, et al. Troponin T switching in the developing rat heart. J Biol Chem. 1988;263(34):18488–92. [PubMed] [Google Scholar]
- Sekine H, Shimizu T, Hobo K, et al. Endothelial cell coculture within tissue-engineered cardiomyocyte sheets enhances neovascularization and improves cardiac function of ischemic hearts. Circulation. 2008;118(14 Suppl):S145–52. doi: 10.1161/CIRCULATIONAHA.107.757286. [DOI] [PubMed] [Google Scholar]
- Serena E, Figallo E, Tandon N, et al. Electrical stimulation of human embryonic stem cells: cardiac differentiation and the generation of reactive oxygen species. Exp Cell Res. 2009;315(20):3611–9. doi: 10.1016/j.yexcr.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin M, Ishii O, Sueda T, et al. Contractile cardiac grafts using a novel nanofibrous mesh. Biomaterials. 2004;25(17):3717–23. doi: 10.1016/j.biomaterials.2003.10.055. [DOI] [PubMed] [Google Scholar]
- Song H, Yoon C, Kattman SJ, et al. Regenerative Medicine Special Feature: Interrogating functional integration between injected pluripotent stem cell-derived cells and surrogate cardiac tissue. Proc Natl Acad Sci U S A. 2009;107(8):3329–34. doi: 10.1073/pnas.0905729106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stem HP, Mahmoud SA, Stem LE. Communication Systems: Analysis and Design. Prentice Hall; Upper Saddle River, NJ, USA: 2004. [Google Scholar]
- Tandon N, Cannizzaro C, Chao PH, et al. Electrical stimulation systems for cardiac tissue engineering. Nat Protoc. 2009;4(2):155–73. doi: 10.1038/nprot.2008.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandon N, Goh B, Marsano A, et al. Alignment and elongation of human adipose-derived stem cells in response to direct-current electrical stimulation. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:6517–21. doi: 10.1109/IEMBS.2009.5333142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandon N, Marsano A, Cannizzaro C, et al. Design of electrical stimulation bioreactors for cardiac tissue engineering. Conf Proc IEEE Eng Med Biol Soc. 2008;2008:3594–7. doi: 10.1109/IEMBS.2008.4649983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugurlucan M, Yerebakan C, Furlani D, et al. Cell sources for cardiovascular tissue regeneration and engineering. Thorac Cardiovasc Surg. 2009;57(2):63–73. doi: 10.1055/s-2008-1039235. [DOI] [PubMed] [Google Scholar]
- Voldman J. Electrical forces for microscale cell manipulation. Annu Rev Biomed Eng. 2006;8:425–54. doi: 10.1146/annurev.bioeng.8.061505.095739. [DOI] [PubMed] [Google Scholar]
- Vunjak-Novakovic G, Tandon N, Godier A, et al. Challenges in Cardiac Tissue Engineering. Tissue Eng Part B Rev. 2010;16(2):169–87. doi: 10.1089/ten.teb.2009.0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Ameer GA, Sheppard BJ, et al. A tough biodegradable elastomer. Nat Biotechnol. 2002;20(6):602–6. doi: 10.1038/nbt0602-602. [DOI] [PubMed] [Google Scholar]
- Westfall MV, Samuelson LC, Metzger JM. Troponin I isoform expression is developmentally regulated in differentiating embryonic stem cell-derived cardiac myocytes. Dev Dyn. 1996;206(1):24–38. doi: 10.1002/(SICI)1097-0177(199605)206:1<24::AID-AJA3>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Yang L, Soonpaa MH, Adler ED, et al. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature. 2008;453(7194):524–8. doi: 10.1038/nature06894. [DOI] [PubMed] [Google Scholar]
- Zimmermann WH, Melnychenko I, Eschenhagen T. Engineered heart tissue for regeneration of diseased hearts. Biomaterials. 2004;25(9):1639–47. doi: 10.1016/s0142-9612(03)00521-0. [DOI] [PubMed] [Google Scholar]
- Zimmermann WH, Melnychenko I, Wasmeier G, et al. Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts. Nat Med. 2006;12(4):452–8. doi: 10.1038/nm1394. [DOI] [PubMed] [Google Scholar]
- Zimmermann WH, Schneiderbanger K, Schubert P, et al. Tissue engineering of a differentiated cardiac muscle construct. Circ Res. 2002;90(2):223–30. doi: 10.1161/hh0202.103644. [DOI] [PubMed] [Google Scholar]