Summary
Compulsive over-consumption of rewards characterizes disorders ranging from binge eating to drug addiction. Here, we provide evidence that enkephalin surges in an anteromedial quadrant of dorsal neostriatum contribute to generating intense consumption of palatable food. In ventral striatum, mu opioid circuitry contributes an important component of motivation to consume rewards [1–4]. In dorsal neostriatum, mu opioid receptors are concentrated within striosomes that receive inputs from limbic regions of prefrontal cortex [5–13]. We employed advanced opioid microdialysis techniques that allow detection of extracellular enkephalin levels. Endogenous >150% enkephalin surges in anterior dorsomedial neostriatum were triggered as rats began to consume palatable chocolates. By contrast, dynorphin levels remained unchanged. Further, a causal role for mu opioid stimulation in over-consumption was demonstrated by observations that microinjection in the same anterior dorsomedial quadrant of a mu receptor agonist (DAMGO) generated intense >250% increases in intake of palatable sweet food (without altering hedonic impact of sweet tastes). Mapping by “Fos plume” methods confirmed the hyperphagic effect to be anatomically localized to the anterior medial quadrant of the dorsal neostriatum, whereas other quadrants were relatively ineffective. These findings reveal that opioid signals in anteromedial dorsal neostriatum are able to code and cause motivation to consume sensory rewards.
Results and Discussion
Dorsal neostriatum has been traditionally viewed to mediate movement and habits [9, 14–16], and to respond to learned cues [17], whereas ventral striatum is well known to generate reward and motivation to consume incentives (in large part mediated by opioid circuitry) [2, 18]. Dorsal striatum recently has also become implicated in reward-related functions [19–24] and here we report that opioid signaling in an extremely dorsal region of neostriatum contributes to generating intense motivation to over-consume palatable food rewards. In dorsal neostriatum, mu opioid receptors are localized mainly in “patch” or “striosome” compartments [5, 7]. Patches or striosomes [23] in neostriatum receive converging inputs from limbic regions of prefrontal cortex, including from orbitofrontal, prelimbic, and anterior cingulate regions [5, 6, 9–13]. We focused here on the medial region of dorsal neostriatum, which has been implicated by previous studies in processing value of rewards [25].
In short, our microinjection results revealed that exogenous mu opioid stimulation by DAMGO microinjection into the anteromedial dorsal neostriatum potently enhanced eating of palatable M&M™ chocolates, more than doubling the total M&M™ intake. This hyperphagic effect was specifically localized within the anterior medial quadrant of the dorsal neostriatum (DAMGO = 251% average increase over vehicle levels). Accordingly, our microdialysis study of that same anteromedial quadrant of dorsal neostriatum found that endogenous enkephalin levels rose to 150% of baseline when rats were suddenly allowed to eat chocolates. These endogenous and exogenous results are described in detail below.
Endogenous enkephalin release
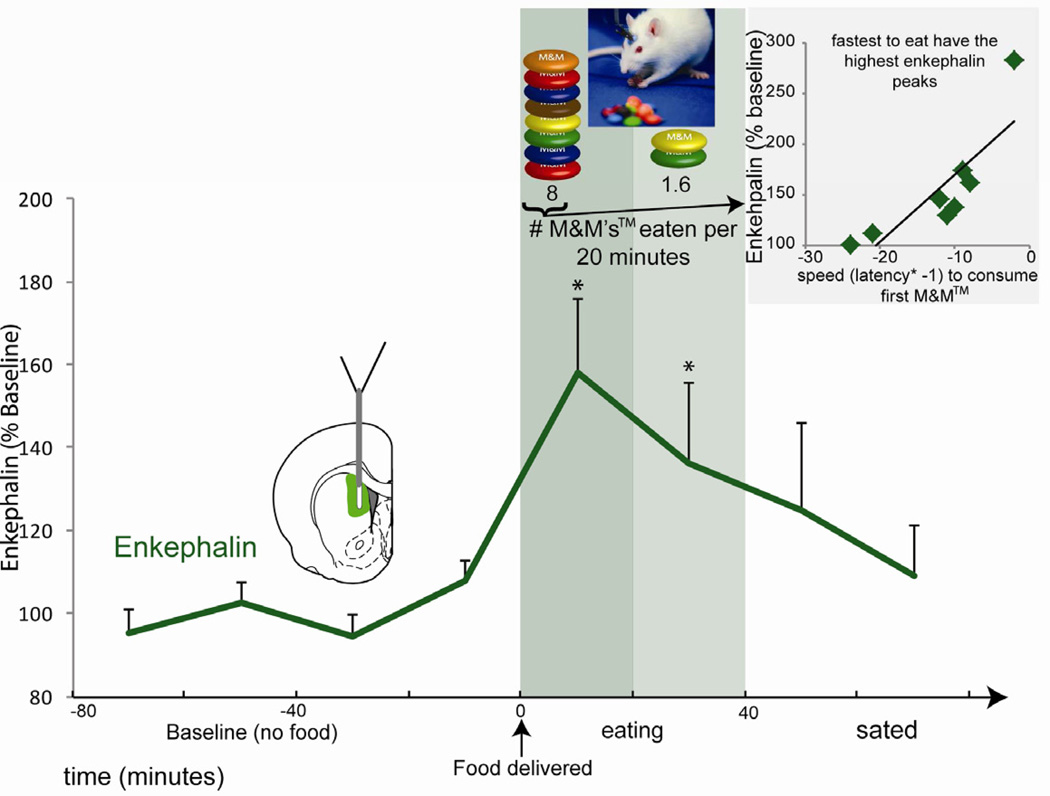
Microdialysis probes implanted in anteromedial dorsal neostriatum measured extracellular levels of endogenous striatal opioid peptides: enkephalin (likely released from ‘indirect path’ neurons that also express dopamine D2 receptors), and dynorphin (likely released from ‘direct path’ neurons that express dopamine D1 receptors). Enkephalin and dynorphin were measured first during a normal quiet behavioral state in mildly hungry rats before any meal to establish a baseline, and next when a large quantity of palatable chocolate candies (M&Ms™) was suddenly presented. Opportunity to eat chocolate M&Ms™ evoked avid consumption, averaging 10 M&Ms™ in 20 min (≈10 g), and elicited an immediate rise in endogenous levels of met-enkephalin and leu-enkephalin, reaching an elevation of >150% over pre-meal baseline (Baseline: met-enkephalin = 2.61 ± SEM 0.56 pM, leuenkephalin = 2.32 ± SEM 0.30 pM; Friedman’s test, p<0.01; baseline = 100%; Fig. 1 and Fig. 2A). Enkephalin levels remained elevated throughout the roughly 20–40 min period that each rat continued to eat, and then began to decline as rats slowed and gradually ceased eating, typically returning fully back to baseline within the next 40 minutes (1st baseline vs. last sample Wilcoxon’s test, n.s.).
Fig. 1. Endogenous extracellular opioid peptides in response to palatable food consumption.
Extracellular enkephalin levels surged when rats began to eat milk chocolate M&Ms™. Onset of eating coincided with a robust increase in extracellular enkephalin (met and leu), which remained sustained during eating, and gradually tapered off as eating declined. The magnitude of the enkephalin rise in individuals correlated with their latency to eat their first M&M™: higher enkephalin rise for the fastest eaters. The correlation between faster speed to start eating and higher enkephalin also remains significant if the highest outlier individual (upper right of inset) is removed (spearman’s rho=−0.85, p=0.013, 95% CI [−1,−0.4]). * indicates p<0.05. Error bars represent standard error of the mean.
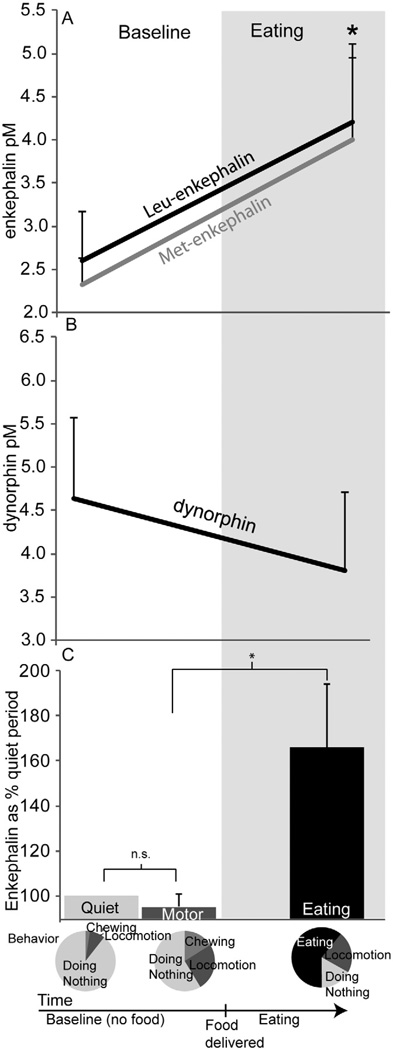
Fig. 2. Details of endogenous enkephalin surge.
Absolute concentrations of met- enkephalin and leu- enkephalin are displayed in panel A. In contrast, dynorphin remained relatively stable throughout resting, eating and other behaviors (panel B). Enkephalin levels did not rise during non-ingestive activities involving forelimbs, body, or orofacial movements (walking, rearing, gnawing, grooming), here the “motor” period. Enkephalin levels as percent “quiet” are represented. Average percent of time the rats engage in each behavior is represented in the pie charts (panel C). * indicates p<0.05. Error bars represent standard error of the mean.
In contrast to enkephalin, dynorphin levels failed to rise during eating, and instead remained unchanged throughout the meal (Friedman’s test, p>0.1; Fig. 2B). Therefore, only enkephalin in the anteromedial quadrant of dorsal neostriatum became dynamically elevated during consumption of palatable food.
Enkephalin surges did not seem to be a consequence of mere motor activity. Enkephalin changes were measured during “motor” periods when the rat performed non-ingestive active movements, such as oromotor gnawing of plastic or wood objects, spontaneous body grooming, or locomotion (walking and/or rearing) in the absence of food (Fig. 2C). During these non-ingestive activities, enkephalin levels never rose (Friedman’s test, p=1, n.s.), suggesting that the rise described above in the same rats to eating chocolates was not due simply to the motoric production of active movements involved in eating. By contrast, enkephalin levels did rise when M&Ms™ were presented and eaten, compared to the previous periods when rats engaged in locomotor and other movements (Friedman’s test, p=0.023), again suggesting that enkephalin reflected more than simply the occurrence of ongoing motor activities (Fig. 2C). Therefore it appears that enkephalin rose specifically with onset of the reward experience of eating palatable chocolates, remained elevated during eating, and declined soon after.
Finally, we found that the magnitude of enkephalin surge in each rat correlated with that individual’s speed or latency to begin consuming its first M&M (spearman’s rho=−0.90, p=0.002, CI [−1,−.392]; Fig 1 inset): the faster a rat started eating, the higher its relative increase in enkephalin levels. This correlation raises the possibility that anteromedial dorsal neostriatum opioid levels might contribute a motivational “eat now” command. That causal hypothesis was tested further in the microinjection study below.
Exogenous mu stimulation of intense eating
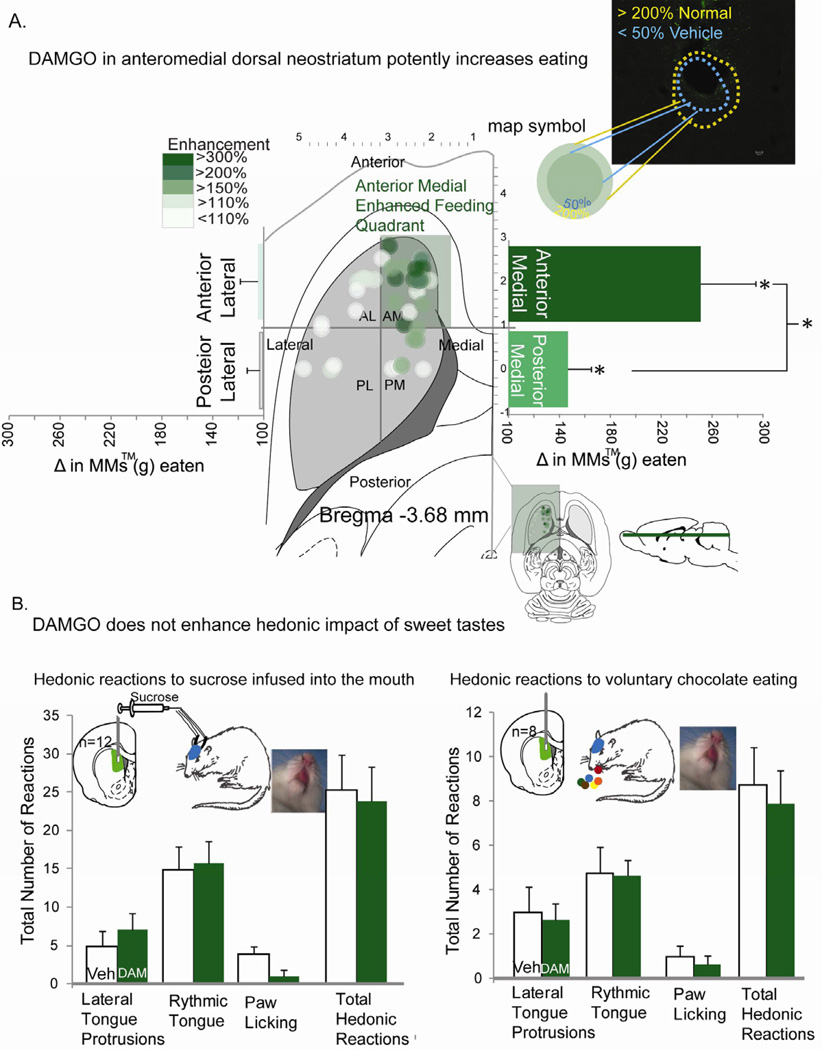
We found that DAMGO microinjection in dorsal neostriatum stimulated more intense eating of chocolates in non-deprived rats, but depending on precise site or quadrant. Sites within the anteromedial quadrant of dorsal neostriatum produced by far the most intense increases of >250% in intake of M&Ms™ (t(18)=5.1, p<.001, 95% CI [9.96, 4.15], Cohen’s d= 1.217; compared to vehicle control intake levels by the same rats; Fig. 3A). Anteromedial quadrant sites produced higher elevations of eating than all other quadrants of dorsal neostriatum (Anteromedial vs. other quadrants F(1,36)=8.44, p=.006, 95%CI [136,235], Cohen’s d=1.09). Localization of function was further determined by mapping the causal efficacy of neostriatal microinjection sites to stimulate eating, using symbols sized to the measured radius of Fos plumes surrounding DAMGO microinjections in dorsal neostriatum (Fos radius reflects the anatomical spread of drug impact, Fig 3). For sites that elevated eating >250%, at least 90% of the volume of DAMGO-induced local Fos plumes would have been contained entirely within the anteromedial quadrant of dorsal neostriatum. That is, DAMGO Fos plumes were measured to have a 0.18 mm total radius (0.02 mm3 volume), containing an inhibited small center (0.15mm radius, volume=0.016mm3 zone of halved Fos expression compared to vehicle baselines) surrounded by a larger excitatory Fos sphere (0.18mm radius, 0.02mm3 volume; zone of doubled Fos expression over normal baseline; center/surround Fos opposition possibly reflects reciprocal local inhibitory connections between the two zones; Fig. 2A inset). These plume measurements allow confidence that the intense over-consumption was generated by DAMGO stimulation of anteromedial dorsal neostriatum, rather than by diffusion to other regions of neostriatum, ventral striatum or nucleus accumbens.
Fig. 3. Map of microinjection causation of intense eating.
DAMGO microinjection into anterior dorsomedial neostriatum potently enhanced intake of M&M™ chocolate candies (high-fat & high-sugar). All DAMGO-evoked eating behavior and intake changes are expressed as percent increases over vehicle-evoked control levels measured in the same rat (panel A). A two layer “Fos plume” shows the maximal spread of Fos locally surrounding DAMGO microinjections. This measured radius was used to assign the size of symbol to the microinjection site for each behaviorally-tested rat. The color of each symbol depicts the magnitude of eating behavior stimulated by DAMGO microinjection at that site (relative to vehicle control level of the same rat). The largest increases in eating were localized to the anteromedial quadrant of dorsal neostriatum. Taste reactivity results show that DAMGO injected into the same dorsomedial area did not increase hedonic impact of sucrose (during oral infusions) or M&M™ chocolates (during voluntary eating) (panel B). * indicates p<0.05. Error bars represent standard error of the mean.
For sites in the highly effective anteromedial quadrant of dorsal neostriatum, most rats ate over 17g of M&Ms™, equal to about 5% of their 300g body weight (Fig. 3A). That level of elevated consumption (5% of body weight) is roughly proportional to a 150lb human consuming about 8 lbs. of M&Ms™ in a single hour, thus clearly overriding normal satiety signals [1, 26].
DAMGO microinjection in this anteromedial quadrant also made rats faster to begin to eat (in addition to making them eat more): decreasing the latency to begin eating their first M&M of the day (Vehicle=55.4 ± SEM 10.4. DAMGO = 28.7 ± SEM 4.2; t(25)=2.49, p=.019, 95% CI [4.69, 48.85], Cohen’s d= 2.781). Faster speed to eat supports the hypothesis that mu opioid receptor stimulation in this neostriatum region provides a command to “eat now” as well as to “eat more.”
In contrast to the anteromedial quadrant, as microinjection sites moved posteriorly in medial dorsal neostriatum the level of stimulated eating gradually declined. Strong elevation of eating was still produced at intermediate medial sites where the diameter of Fos plumes straddled the border between anteromedial and posteromedial quadrants of dorsal neostriatum (190% increase). No significant elevation was produced by more posterior sites fully contained within the posteromedial quadrant (i.e., where no part of a posterior site’s Fos plume would contact the anteromedial border; average 118%, n.s.).Thus, overall for the entire posteromedial quadrant, intermediate 150% elevations of eating were found, due mostly to the medial sites that straddled the anterior/posterior border (t(8)=2.52, p=.036, 95% CI [7.28, 0.33, Cohen’s d= 0.939). Comparing anterior versus posterior directly as entire quadrants, DAMGO in the anteromedial quadrant produced a greater increase in intense eating than DAMGO in the posteromedial quadrant (t(23)=2.21, p=.037, 95% CI [201.6, 6.96], Cohen’s d= 0.85).
Eating elevations fell off to zero abruptly as microinjection sites moved laterally from the anteromedial quadrant. Anterolateral quadrant sites produced no increase at all over vehicle levels (i.e., outside and lateral to effective sites in dorsomedial neostriatum; only 103%; t(9)=0.1, p=0.917; Fig 3A).
By contrast to mu stimulation of eating, delta opioid receptor stimulation by DPDPE microinjections failed to increase eating behavior or intake over vehicle control levels at all sites in dorsomedial neostriatum, even in the anteromedial quadrant (F(1,12)=.4, p>0.1; Fig. S1). Accordingly, M&M™ intake was much higher after mu agonist DAMGO microinjection than after delta agonist DPDPE microinjection at the same anteromedial dorsal neostriatum sites (DAMGO= 6.31 ± SEM 1.13, DPDPE= −0.46 ± SEM 0.68; t(36) = 5.10, p < 0.001, 95% CI [9.47, 4.09], Cohen’s d = 1.65). That difference suggests that enkephalin may act primarily at mu receptors in anterior dorsomedial neostriatum to stimulate increases in consumption, rather than at delta receptors.
Further, DAMGO microinjections in all areas of neostriatum, including the anterior dorsomedial quadrant, failed to produce any general increases in locomotor or oromotor activity, measured by cage crosses, rearing, grooming or treading behaviors, chow consumption, or wooden block gnawing (behaviors F(5,28)=0.151, n.s.; chow F(15,90)=0.68, n.s.; gnawing t(4)=2.1, n.s.).
Exogenous mu stimulation fails to alter hedonic ‘liking’ for sweetness
Finally, we used the affective taste reactivity test of orofacial ‘liking’ reactions to ask whether mu opioid enhancement of motivation to eat sweet food reflected purely generation of motivation to eat (similar to opioid effects in most of ventral striatum outside a cubic-millimeter hotspot and in central amygdala) or additionally involved any enhancement of hedonic impact or ‘liking’ for the taste of sweet reward (typical only of restricted hotspots in ventral striatum, ventral pallidum, etc.)[2, 4, 27]. This measure draws on rodent affective orofacial reactions (e.g. positive tongue protrusion and lip licking to sweetness versus aversive gapes to bitterness) that are homologous to human infant affective facial expressions elicited by tastes [2, 4, 28].[22] We tested for hedonic enhancement in a standard taste reactivity test using a sucrose solution infused directly in the mouth to control stimulus quality and duration, and separately for the taste of sweet/fatty chocolate as rats voluntarily ate 0.2 g fragments of M&Ms™, replicating the chocolate stimulus and conditions of eating enhancement [29]. Taste reactivity results of both tests showed that DAMGO microinjections in anterior dorsomedial neostriatum failed to enhance positive hedonic taste reactions at all, either to oral infusions of 1% sucrose solution (F(3,9)=1.875, p=.204; Fig. 3B) or to the chocolate taste of solid M&Ms™ fragments (F(3,5)=0.175, p=0.91; Fig 3B). Although no increase in hedonic impact was observed, the DAMGO microinjections again increased motivation to eat in the voluntary intake test so that the rats doubled their consumption of chocolate over vehicle control levels (t(5)=15.58, 95% CI [9.90, 7.09], Cohen’s d= 2.3). Thus DAMGO in dorsomedial neostriatum appeared to make rats selectively ‘want’ to eat M&Ms™ more intensely, in the sense of enhanced eating behavior and intake, without making them ‘like’ sweetness any more, in the sense of hedonic reactions to sucrose or chocolate tastes. We note that similar dissociations involving selective increases in ‘wanting’ without ‘liking’ are typical of opioid stimulation at many other brain sites, including ventral striatum (except in the hotspot of nucleus accumbens shell) and striatal-related structures [2, 4, 27]. This dissociation between ‘wanting’ versus ‘liking’ suggests that mu opioid stimulation in anterior dorsomedial neostriatum may generate increases of motivation as a specific psychological mechanism to drive intense eating and consumption of food rewards[6].
We acknowledge that our finding of intense over-consumption produced by mu opioid stimulation in the anteromedial dorsal neostriatum contrasts at first sight with previous reports of relative failure to observe any increase in eating after DAMGO microinjection in dorsal neostriatum [3, 30, 31]. However, earlier studies never distinguished anatomically among the four quadrants of dorsal neostriatum as defined here, and typically used placements that as a whole were more centrally located in the dorsal half of neostriatum (i.e., more ventral and posterior to our eating hotspot). Our finding of eating localization in the anteromedial quadrant of the dorsal level may explain why studies that mixed together different subregions failed to find eating stimulation in dorsal neostriatum.
Other hints of dorsal neostriatum involvement in motivation to consume rewards have emerged in the past decade [19–24]. In humans, dorsal striatum activation has been reported to be elicited by palatable food and its cues in obese binge eaters, and by cocaine and its cues in drug addicts [20–23]. Such dorsal striatal activations have remained slightly ambiguous, as they could be viewed either as reward motivation or as hedonic impact, incipient movements, habits, cognitive processing, or learned predictions. Similarly, dorsal neostriatal neuronal activations elicited by rewards or cues are reported in monkeys and rodents, and often have been interpreted as reflecting learned predictions or teaching signals (e.g., prediction error model) [16, 17, 32, 33]. Our results more specifically indicate that dorsal striatal activation can participate in generating intense motivation to over-consume a reward. Thus the generative role shown here might link some functions of dorsal neostriatum more closely to the reward motivation functions of ventral striatum (nucleus accumbens) [2, 34–36]. This also seems consistent with reports that restoring synaptic function to a region of dorsal neostriatum can rescue eating in an aphagic mutant mouse model, and supports the interpretation that such neostriatum mediated rescues may involve a motivational component [24].
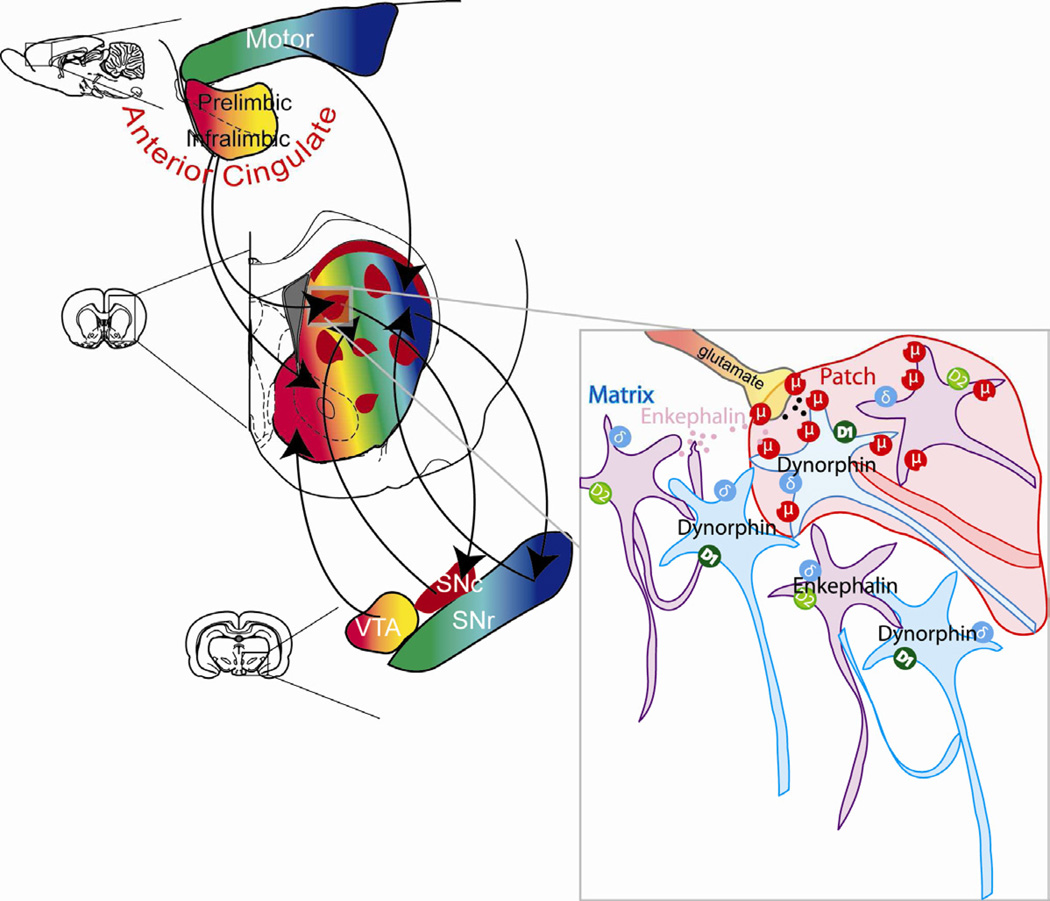
The hypothesis that opioid circuitry in dorsomedial neostriatum participates in generating motivation to over-consume a palatable food reward also seems concordant with its anatomical wiring from limbic prefrontal cortical inputs [6, 8, 10–13] (Fig. 4). For example, corticostriatal projections to the anteromedial region of dorsal neostriatum (“rostromedial sector of caudate-putamen”), similar to that studied here, originate from the prelimbic region of medial prefrontal cortex in rats [10]. Corticostriatal projections from “posterior [lateral] orbitofrontal/anterior insular cortex and the mediofrontal prelimbic/anterior cingulate cortex” similarly terminate in striosomes in the medial caudate in macaque primates, which probably overlaps with our eating site [6]. It is also noteworthy that direct mu opioid stimulation of prefrontal cortex regions, via DAMGO microinjections in orbitofrontal and prelimbic/infralimbic (ventral anterior cingulate) cortex, can stimulate eating in rats, raising the possibility of a larger opioid-related corticostriatal circuit involved in eating and motivation [37].
Fig. 4. Anatomical circuit for anteromedial dorsal neostriatum.
Neurons in patches/striosomes of the anteromedial dorsal neostriatum are rich in mu opioid receptors and receive cortical inputs from limbic areas of prefrontal cortex. Some patch/striosome neurons that express D1 receptors make direct projections to dopamine neurons within substantia nigra. D2 expressing matrix neurons release enkephalin that could act on mu opioid receptors, especially of patch neurons to generate motivation to eat.
We speculate that opioid surges particularly within anteromedial patches of dorsal striatum might modulate presynaptic corticostriatal glutamate release or modulate postsynaptic activity of eating-related neurons in striosomes that contain mu opioid receptors [38, 39]. In addition, some D1 receptor-expressing neurons in striosomes may uniquely project directly to dopamine neurons in substantia nigra [40], which might facilitate modulation of dopamine systems to [30] additionally help generate intense motivation. Beyond the neostriatum, the anterior dorsomedial neostriatal region described here likely interacts with other parts of the distributed mesocorticolimbic network involved in eating and intake, including hypothalamus, ventral striatum, limbic cortex, amygdala, and mesolimbic dopamine nuclei [1, 31, 37, 41, 42].
In conclusion, our results provide novel evidence that enkephalin surges and mu opioid stimulation in the same anteromedial dorsal neostriatum region contribute to signaling the opportunity to eat a sensory reward and to causally generating increased consumption of that reward. The neostriatum-generated increase in motivation can be powerful enough to more than double the amount of food a rat “wants” to eat, yet be functionally specific enough to the motivational component of reward, rather than the hedonic component, to not enhance “liking” for the same sweet chocolate treat. Opioid circuitry in anterior dorsomedial neostriatum could in this way participate in normal motivations, and perhaps even in generating intense pathological levels of motivation to over-consume rewards in binge eating disorders, drug addiction, and related compulsive pursuits. [4]
Experimental Procedures
Summary
All experiments were conducted in accordance with protocols approved by the University of Michigan Committee on Use and Care of Animals (UCUCA). Rats were anesthetized and implanted with bilateral (microinjection), or unilateral (microdialysis) chronic cannula. Rats used for sucrose taste reactivity received oral cannula in addition to bilateral cannula. After at least 5 days of recovery rats were habituated to the testing apparatus. For microinjection experiments 1 “sham” injection of vehicle was given during habituation prior to testing. After drug microinjection rats were placed in free eating apparatus and videotaped for 1 hour.
Hedonic impact was measured using the taste reactivity paradigm for the tastes of sucrose solution or M&M™ chocolates. Orofacial reactions elicited by sucrose taste (1% sucrose solution) were video recorded in a standard taste reactivity test [2, 4]. Orofacial reactions elicited by the combined sweet/fatty sensations of milk chocolate were video recorded in a voluntary ingestion modification of the taste reactivity paradigm, in which the rat was given fragments of chocolate M&Ms™ (0.2 g each) and allowed to eat voluntarily and emit postprandial hedonic reactions for 6s after each fragment (scoring was controlled for facial visibility)[29]. All behaviors were scored off line in slow motion by an observer blind to DAMGO/vehicle condition (frame-by-frame to 1/10th speed). For more information on experimental procedures please refer to supplementary materials.
For microdialysis experiments, on test days the probe was lowered and samples were collected every 20 minutes with a 0.6 µl/minute flow rate. A 60 minute baseline where rats engaged in various motor and oromotor behaviors was collected before food was introduced to the chamber. Samples were analyzed using liquid chromatography coupled with MS3 linear ion trap. Specificity of molecular structural information gained is high through the use of LC-MSn. After separation by LC, molecules are transferred to gas phase and charged by electrospray ionization for MS analysis. During MS3, 3 rounds of MS are imposed for each target peptide. Thus, within the ion trap mass spectrometer, charged molecules are first selected for their characteristic mass to charge ratio (m/z) and all other ions excluded from the ion trap. The trapped ions are then dissociated into characteristic fragments by introduction of He as a collision gas. Ionic products of a specific m/z (characteristic of the target peptide) from this reaction are then fragmented in a third stage and detected to generate a signal. Thus, peptides are detected based on retention time, m/z, and multiple fragmentation pathways. This method yields sequence specific detection.
Supplementary Material
Highlights.
First report of enkephalin surge triggered by opportunity to eat food reward
Localization of opioid eating generation to anteromedial dorsal neostriatum
Generates pure motivation to eat without impacting “liking” for sweet tastes
Acknowledgements
We thank Aaron Garcia for help with immunohistochemical analysis and Matthew Howe and Jocelyn Richard for comments on an earlier draft. We also thank Brian Vickers, Michael Shvartsman, and Dr. Cyril Pernet for assistance with the skipped correlation. This work was supported by NIH grants DA015188 and MH63649 to KCB, DA007267 to AGD, and R37 EB003320 to RTK and DA07268 to OSM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Information contains an additional figure (Fig S1) and detailed information on experimental procedures.
References
- 1.Kelley AE, Baldo BA, Pratt WE. A proposed hypothalamic-thalamic-striatal axis for the integration of energy balance, arousal, and food reward. J Comp Neurol. 2005;493:72–85. doi: 10.1002/cne.20769. [DOI] [PubMed] [Google Scholar]
- 2.Pecina S, Berridge KC. Hedonic hot spot in nucleus accumbens shell: where do mu-opioids cause increased hedonic impact of sweetness? J Neurosci. 2005;25:11777–11786. doi: 10.1523/JNEUROSCI.2329-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang M, Kelley AE. Enhanced intake of high-fat food following striatal mu-opioid stimulation: microinjection mapping and fos expression. Neuroscience. 2000;99:267–277. doi: 10.1016/s0306-4522(00)00198-6. [DOI] [PubMed] [Google Scholar]
- 4.Smith KS, Berridge KC. The ventral pallidum and hedonic reward: neurochemical maps of sucrose "liking" and food intake. J Neurosci. 2005;25:8637–8649. doi: 10.1523/JNEUROSCI.1902-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crittenden JR, Graybiel AM. Basal Ganglia disorders associated with imbalances in the striatal striosome and matrix compartments. Front Neuroanat. 2011;5:59. doi: 10.3389/fnana.2011.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eblen F, Graybiel AM. Highly restricted origin of prefrontal cortical inputs to striosomes in the macaque monkey. J Neurosci. 1995;15:5999–6013. doi: 10.1523/JNEUROSCI.15-09-05999.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerfen CR. The neostriatal mosaic: compartmentalization of corticostriatal input and striatonigral output systems. Nature. 1984;311:461–464. doi: 10.1038/311461a0. [DOI] [PubMed] [Google Scholar]
- 8.Gerfen CR. The neostriatal mosaic: striatal patch-matrix organization is related to cortical lamination. Science. 1989;246:385–388. doi: 10.1126/science.2799392. [DOI] [PubMed] [Google Scholar]
- 9.Graybiel AM. Habits, rituals, and the evaluative brain. Annu Rev Neurosci. 2008;31:359–387. doi: 10.1146/annurev.neuro.29.051605.112851. [DOI] [PubMed] [Google Scholar]
- 10.Levesque M, Parent A. Axonal arborization of corticostriatal and corticothalamic fibers arising from prelimbic cortex in the rat. Cereb Cortex. 1998;8:602–613. doi: 10.1093/cercor/8.7.602. [DOI] [PubMed] [Google Scholar]
- 11.Ragsdale CW, Jr, Graybiel AM. Fibers from the basolateral nucleus of the amygdala selectively innervate striosomes in the caudate nucleus of the cat. J Comp Neurol. 1988;269:506–522. doi: 10.1002/cne.902690404. [DOI] [PubMed] [Google Scholar]
- 12.Ragsdale CW, Jr., Graybiel AM. A simple ordering of neocortical areas established by the compartmental organization of their striatal projections. Proc Natl Acad Sci U S A. 1990;87:6196–6199. doi: 10.1073/pnas.87.16.6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kincaid AE, Wilson CJ. Corticostriatal innervation of the patch and matrix in the rat neostriatum. J Comp Neurol. 1996;374:578–592. doi: 10.1002/(SICI)1096-9861(19961028)374:4<578::AID-CNE7>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 14.Parkinson JA, Cardinal RN, Everitt BJ. Limbic cortical-ventral striatal systems underlying appetitive conditioning. Prog Brain Res. 2000;126:263–285. doi: 10.1016/S0079-6123(00)26019-6. [DOI] [PubMed] [Google Scholar]
- 15.Balleine BW, Delgado MR, Hikosaka O. The role of the dorsal striatum in reward and decision-making. J Neurosci. 2007;27:8161–8165. doi: 10.1523/JNEUROSCI.1554-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suto N, Wise RA, Vezina P. Dorsal as well as ventral striatal lesions affect levels of intravenous cocaine and morphine self-administration in rats. Neurosci Lett. 2011;493:29–32. doi: 10.1016/j.neulet.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schultz W, Dickinson A. Neuronal coding of prediction errors. Annu Rev Neurosci. 2000;23:473–500. doi: 10.1146/annurev.neuro.23.1.473. [DOI] [PubMed] [Google Scholar]
- 18.Baldo BA, Kelley AE. Discrete neurochemical coding of distinguishable motivational processes: insights from nucleus accumbens control of feeding. Psychopharmacology (Berl) 2007;191:439–459. doi: 10.1007/s00213-007-0741-z. [DOI] [PubMed] [Google Scholar]
- 19.Wise RA. Roles for nigrostriatal--not just mesocorticolimbic--dopamine in reward and addiction. Trends Neurosci. 2009;32:517–524. doi: 10.1016/j.tins.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nummenmaa L, Hirvonen J, Hannukainen JC, Immonen H, Lindroos MM, Salminen P, Nuutila P. Dorsal striatum and its limbic connectivity mediate abnormal anticipatory reward processing in obesity. PLoS One. 2012;7:e31089. doi: 10.1371/journal.pone.0031089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stice E, Spoor S, Bohon C, Veldhuizen MG, Small DM. Relation of reward from food intake and anticipated food intake to obesity: a functional magnetic resonance imaging study. J Abnorm Psychol. 2008;117:924–935. doi: 10.1037/a0013600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Volkow ND, Wang GJ, Fowler JS, Logan J, Jayne M, Franceschi D, Wong C, Gatley SJ, Gifford AN, Ding YS, et al. "Nonhedonic" food motivation in humans involves dopamine in the dorsal striatum and methylphenidate amplifies this effect. Synapse. 2002;44:175–180. doi: 10.1002/syn.10075. [DOI] [PubMed] [Google Scholar]
- 23.Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci. 2006;26:6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palmiter RD. Dopamine signaling in the dorsal striatum is essential for motivated behaviors: lessons from dopamine-deficient mice. Ann N Y Acad Sci. 2008;1129:35–46. doi: 10.1196/annals.1417.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yin HH, Ostlund SB, Knowlton BJ, Balleine BW. The role of the dorsomedial striatum in instrumental conditioning. Eur J Neurosci. 2005;22:513–523. doi: 10.1111/j.1460-9568.2005.04218.x. [DOI] [PubMed] [Google Scholar]
- 26.Woolley JD, Lee BS, Taha SA, Fields HL. Nucleus accumbens opioid signaling conditions short-term flavor preferences. Neuroscience. 2007;146:19–30. doi: 10.1016/j.neuroscience.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 27.Mahler SV, Berridge KC. What and when to "want"? Amygdala-based focusing of incentive salience upon sugar and sex. Psychopharmacology (Berl) 2011 doi: 10.1007/s00213-011-2588-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grill HJ, Norgren R. The taste reactivity test. I. Mimetic responses to gustatory stimuli in neurologically normal rats. Brain Res. 1978;143:263–279. doi: 10.1016/0006-8993(78)90568-1. [DOI] [PubMed] [Google Scholar]
- 29.Feurte S, Nicolaidis S, Berridge KC. Conditioned taste aversion in rats for a threonine-deficient diet: demonstration by the taste reactivity test. Physiol Behav. 2000;68:423–429. doi: 10.1016/s0031-9384(99)00202-4. [DOI] [PubMed] [Google Scholar]
- 30.Bakshi VP, Kelley AE. Striatal regulation of morphine-induced hyperphagia: an anatomical mapping study. Psychopharmacology (Berl) 1993;111:207–214. doi: 10.1007/BF02245525. [DOI] [PubMed] [Google Scholar]
- 31.Bakshi VP, Kelley AE. Feeding induced by opioid stimulation of the ventral striatum: role of opiate receptor subtypes. J Pharmacol Exp Ther. 1993;265:1253–1260. [PubMed] [Google Scholar]
- 32.Apicella P, Ljungberg T, Scarnati E, Schultz W. Responses to reward in monkey dorsal and ventral striatum. Exp Brain Res. 1991;85:491–500. doi: 10.1007/BF00231732. [DOI] [PubMed] [Google Scholar]
- 33.Haracz JL, Tschanz JT, Wang Z, White IM, Rebec GV. Striatal single-unit responses to amphetamine and neuroleptics in freely moving rats. Neurosci Biobehav Rev. 1993;17:1–12. doi: 10.1016/s0149-7634(05)80226-x. [DOI] [PubMed] [Google Scholar]
- 34.Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- 35.Carelli RM, Ijames SG. Selective activation of accumbens neurons by cocaine-associated stimuli during a water/cocaine multiple schedule. Brain Res. 2001;907:156–161. doi: 10.1016/s0006-8993(01)02604-x. [DOI] [PubMed] [Google Scholar]
- 36.Will MJ, Vanderheyden WM, Kelley AE. Striatal opioid peptide gene expression differentially tracks short-term satiety but does not vary with negative energy balance in a manner opposite to hypothalamic NPY. Am J Physiol Regul Integr Comp Physiol. 2007;292:R217–R226. doi: 10.1152/ajpregu.00852.2005. [DOI] [PubMed] [Google Scholar]
- 37.Mena JD, Sadeghian K, Baldo BA. Induction of hyperphagia and carbohydrate intake by mu-opioid receptor stimulation in circumscribed regions of frontal cortex. J Neurosci. 2011;31:3249–3260. doi: 10.1523/JNEUROSCI.2050-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiang ZG, North RA. Pre- and postsynaptic inhibition by opioids in rat striatum. J Neurosci. 1992;12:356–361. doi: 10.1523/JNEUROSCI.12-01-00356.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang H, Pickel VM. Dendritic spines containing mu-opioid receptors in rat striatal patches receive asymmetric synapses from prefrontal corticostriatal afferents. J Comp Neurol. 1998;396:223–237. [PubMed] [Google Scholar]
- 40.Fujiyama F, Sohn J, Nakano T, Furuta T, Nakamura KC, Matsuda W, Kaneko T. Exclusive and common targets of neostriatofugal projections of rat striosome neurons: a single neuron-tracing study using a viral vector. Eur J Neurosci. 2011;33:668–677. doi: 10.1111/j.1460-9568.2010.07564.x. [DOI] [PubMed] [Google Scholar]
- 41.Will MJ, Franzblau EB, Kelley AE. The amygdala is critical for opioid-mediated binge eating of fat. Neuroreport. 2004;15:1857–1860. doi: 10.1097/00001756-200408260-00004. [DOI] [PubMed] [Google Scholar]
- 42.Gosnell BA. Involvement of mu opioid receptors in the amygdala in the control of feeding. Neuropharmacology. 1988;27:319–326. doi: 10.1016/0028-3908(88)90050-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.