Abstract
The putative Ca2+-channel blocker LaCl3 prevented the gravitropic bending of cut snapdragon (Antirrhinum majus L.) spikes (S. Philosoph-Hadas, S. Meir, I. Rosenberger, A.H. Halevy [1996] Plant Physiol 110: 301–310) and inhibited stem curvature to a greater extent than vertical and horizontal stem elongation at the bending zone. This might indicate that LaCl3, which modulates cytosolic Ca2+, does not influence general stem-growth processes but may specifically affect other gravity-associated processes occurring at the stem-bending zone. Two such specific gravity-dependent events were found to occur in the bending zone of snapdragon spikes: sedimentation of starch-containing chloroplasts at the bottom of stem cortex cells, as seen in cross-sections, and establishment of an ethylene gradient across the stem. Our results show that the lateral sedimentation of chloroplasts associated with gravity sensing was prevented in cross-sections taken from the bending zone of LaCl3-treated and subsequently gravistimulated spikes and that LaCl3 completely prevented the gravity-induced, asymmetric ethylene production established across the stem-bending zone. These data indicate that LaCl3 inhibits stem curvature of snapdragon spikes by preventing several gravity-dependent processes. Therefore, we propose that the gravitropic response of shoots could be mediated through a Ca2+-dependent pathway involving modulation of cytosolic Ca2+ at various stages.
Various aboveground plant parts, such as coleoptiles, hypocotyls, seedlings, grass-shoot pulvini, and flowering stems, respond to a change in a gravity vector by differential growth that leads to upward bending. Most of the work on negative gravitropism has been performed with seedlings and specific graviresponsive organs such as coleoptiles, epycotyls and grass-shoot pulvini (Kaufman et al., 1985; Migliaccio and Galston, 1987; Gehring et al., 1990; Brock et al., 1992; Kim and Kaufman, 1995). However, there have been several reports of gravitropism of stem-like organs (Rorabaugh and Salisbury, 1989; Kiss et al., 1997; Strudwick et al., 1997), vegetative stems (Meicenheimer and Nackid, 1994), and flowering shoots (Kohji et al., 1979; Halevy and Mayak, 1981; Woltering, 1991; Philosoph-Hadas et al., 1995, 1996; Fukaki et al., 1996). In this respect, the graviresponding spikes of snapdragon (Antirrhinum majus L.; Philosoph-Hadas et al., 1996) provide an excellent model system for investigating the gravitropic phenomenon in mature inflorescence stems.
The common underlying mechanisms of negative gravitropism involve perception of the stimulus and its transduction into physiological processes that lead to a differential growth response (Salisbury, 1993). Gravity perception is considered to be accomplished by a mass pressure on the lower cell membrane following changes in organ orientation. This mass is believed to be either that of specific plastids, usually amyloplasts or chloroplasts, which sediment only in cells of a specific developmental stage and location (the starch-statolith model; Sack, 1997; Vitha et al., 1998), or that of the entire cell (the gravitational pressure model; Staves, 1997). According to the prevailing Cholodny-Went theory, this gravity perception leads to redistribution of auxin toward the lower side of the gravireacting organ, thereby increasing its growth rate and consequently the organ reorientation (Li et al., 1991; Trewavas, 1992; Salisbury, 1993). However, it is clear now that the gravitropic response is a complex, multistep event also influenced by factors such as ethylene (Wheeler et al., 1986; Salisbury, 1993; Philosoph-Hadas et al., 1996), responsiveness and sensitivity to auxin (Rorabaugh and Salisbury, 1989; Kim and Kaufman, 1995), and Ca2+ (Philosoph-Hadas et al., 1995, 1996; Belyavskaya, 1996; Sinclair and Trewavas, 1997), which might act in succession or in parallel.
In several shoot systems the gravitropic response has been reported to be accompanied by significantly higher amounts of ethylene produced by the lower half of a horizontally positioned stem (Clifford et al., 1983; Kaufman et al., 1985; Wheeler et al., 1986; Woltering, 1991; Philosoph-Hadas et al., 1996). However, in only a few cases could inhibitors of ethylene synthesis or action block the gravitropic response (Wheeler and Salisbury, 1981; Wheeler et al., 1986; Philosoph-Hadas et al., 1996). Therefore, the role of ethylene in gravitropism is still controversial.
Ca2+ ions have long been proposed to be essential for gravitropic competence in plants (Pickard, 1985; Poovaiah et al., 1987; Roux and Serlin, 1987; Trewavas, 1992; Bush, 1995; Belyavskaya, 1996; Sinclair and Trewavas, 1997). These ions were suggested to be involved as a second messenger in all steps of the signal transduction pathway leading to the gravitropic bending of higher plants: stimulus perception (Pickard, 1985; Belyavskaya, 1996), auxin redistribution (Migliaccio and Galston, 1987) or action (Saunders, 1990; Bush, 1995), and differential cell growth (Brock et al., 1992; Jackson and Hall, 1993). However, because of the difficulties in measuring [Ca2+]cyt in intact plant tissues, and because of the complexity of the gravitropic system (Sinclair and Trewavas, 1997), evidence for the role of [Ca2+]cyt in gravitropism remains circumstantial. Only one example of what is apparently a direct gravity-induced but only slightly sustained elevation of [Ca2+]cyt in maize coleoptiles has been reported so far (Gehring et al., 1990). This finding was challenged recently by Legué et al. (1997), who clearly demonstrated by direct measurements of [Ca2+]cyt that the gravitropic response of Arabidopsis roots is not associated with detectable changes in [Ca2+]cyt.
Alternatively, the use of Ca2+ agonists and antagonists has been extensively reported as providing indirect evidence for the involvement of changes in [Ca2+]cyt in the process of gravistimulation (Poovaiah et al., 1987; Roux and Serlin, 1987; Belyavskaya, 1996; Sinclair and Trewavas, 1997). Recently, we have shown that several Ca2+ chelators (e.g. EGTA, CDTA, and 1,2-bis[2-aminophenoxy]ethane-N,N,N′,N′-tetraacetic acid) and a Ca2+-channel blocker (LaCl3) significantly inhibited the gravitropic response of snapdragon spikes (Philosoph-Hadas et al., 1996). These agents had been similarly effective in inhibiting the gravitropic response of several other flowering stems capable of linear growth after harvest (Philosoph-Hadas et al., 1995).
The role of [Ca2+]cyt as a second messenger requires the activity of Ca2+ channels between Ca2+ stores and the cytoplasm that open upon signaling, thereby allowing the movement of Ca2+ down its electrochemical gradient. In plant and animal systems, La3+ ions inhibit various Ca2+-dependent processes by interacting with binding sites inside Ca2+ channels or by stimulating Ca2+-ATPases, thereby preventing elevation of [Ca2+]cyt (Tester, 1990; Bush, 1995; Belyavskaya, 1996). Therefore, LaCl3 has been widely used to show Ca2+ involvement in various physiological responses (Knight et al., 1992; Jackson and Hall, 1993; Bush, 1995; Polisensky and Braam, 1996; Rock and Quatrano, 1996), including graviperception (Belyavskaya, 1996; Staves, 1997).
Since LaCl3 showed the most pronounced inhibitory effect on the gravitropic response of snapdragon spikes (Philosoph-Hadas et al., 1996), it was of interest to further characterize its mode of action. For this purpose we examined whether LaCl3 inhibits curvature through inhibition of general stem-elongation processes and whether it affects other gravity-induced processes such as amyloplast sedimentation, which is associated with gravity perception, and differential ethylene production, which is associated with the physiological response. Our results indicate that LaCl3 significantly and specifically affects these gravitropism-associated events and therefore may provide additional evidence for the possible second-messenger role of [Ca2+]cyt in the gravitropic bending process of shoots.
MATERIALS AND METHODS
Plant Material and Treatments
Experiments were performed with several cultivars (Maryland White, Maryland Appleblossom, and Potomac Royal) of snapdragon (Antirrhinum majus L., F1 hybrid), purchased from the Pan-American Seed Company (West Chicago, IL). Freshly cut spikes bearing four to six open florets were obtained from local commercial growers. All treatments were performed as previously described (Philosoph-Hadas et al., 1996) in a standard, controlled-environment room maintained at 20°C with 60% to 70% RH and 24 h of light at an intensity of 14 μmol m−2 s−1 provided by cool-white fluorescent tubes. To enable their straightening after harvest and transport, spikes were held vertically overnight with the cut ends of the stems in distilled water. Spikes were subsequently trimmed to a length of 60 to 70 cm, and 10 to 30 flower stalks were placed in a 2-L plastic cylinder containing 400 mL of distilled water or a solution of LaCl3 (Sigma) at concentrations between 10 and 30 mm (pH 6.0–7.0) for 20 to 24 h. This duration of pulsing treatment was determined in preliminary experiments.
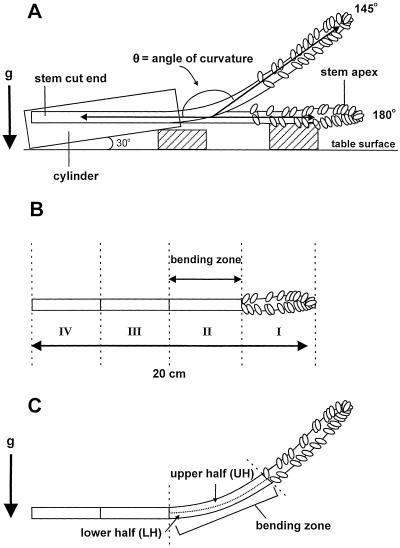
After the spikes were pulsed, they were divided into bunches of five stalks each and transferred to 1-L plastic cylinders containing 400 mL of preservative solution (TOG-6, Assia Reizel Ltd., Ramat Gan, Israel) containing 5 mg mL−1 chlorine complexed with sodium dichloro-isocyanureate to prevent microorganism contamination. Gravitropic stimulation was provided by tilting the cylinders horizontally at an angle of 30° to the table surface to avoid drifting of the liquid inside the cylinder. In this position stems could be kept with their cut ends inside the solution and still be oriented initially at an angle of 180°, which represents noncurved stems (Fig. 1A). The curvature angle (Fig. 1A) of 10 to 15 spikes was measured at hourly intervals with a protractor to monitor the kinetics of stem bending. The stem thickness at the bending zone of all of the snapdragon cultivars assayed ranged between 4 and 10 mm. The bending rate of all cultivars was similar and was not dependent on stem diameter (H. Friedman, S. Meir, I. Rosenberger, A.H. Halevy, and S. Philosoph-Hadas, unpublished data). Experiments were repeated three to five times with similar results, and data from individual representative experiments are presented.
Figure 1.
Schematic drawing illustrating the various experimental settings used for snapdragon spikes. A, Gravitropic stimulation of a snapdragon spike from a noncurved (180°) to a curved (145°) position and the angle (○̶) measured as the curvature of the stem. B, The numbers I through IV were given to each 5-cm segment starting from the stem apex to 20 cm below for determination of the elongation rate in vertical or horizontal spikes. C, Longitudinally halved stem sections, excised from the stem-bending zone during gravistimulation and used for ethylene measurements.
Determination of Stem Elongation
Elongation measurements were performed with spikes of the cvs Maryland White and Maryland Appleblossom. For stem-elongation measurements, leaves and florets were removed from the whole spike except for the top 5-cm apical section, which contains only buds. These bare spikes responded to gravistimulation in a manner similar to that of floret-bearing stalks (data not shown). Four sections at 5-cm intervals, designated I to IV counting from the apical part of the inflorescence stem, were marked on the bare stalk with a thin marker (Fig. 1B). The marker did not cause any obvious damage to stem cells, as judged from microscopic analysis and curvature measurements. The marked stems were then pulsed for 22 h with various LaCl3 concentrations (10–30 mm) and placed either vertically or horizontally in the preservative solution for an additional 24 h, after which time the elongation of each stem section was determined with a ruler. The elongation rate was calculated separately for each 5-cm stem section.
Staining of Starch-Containing Chloroplasts (Amyloplasts)
Snapdragon spikes were pulsed with the lowest concentration of LaCl3 that caused maximal inhibition of curvature (10–20 mm LaCl3 depending on spike diameter). Following pulsing, spikes were either kept vertical or gravistimulated for an additional 6 or 10 h (to obtain curvatures of 150° or 90°, respectively). Hand-cut cross-sections were prepared from the middle part of the stem-bending zone (section II) taken from LaCl3-treated or nontreated spikes at the indicated intervals during gravistimulation. The sections were stained with iodine solution (10% KI/5% I2 in distilled water), as described previously (Song et al., 1988) and immediately examined with a light microscope (Vanox, Olympus), and photographs were taken with Kodak 100-ASA film.
Ethylene Measurements
For measurements of ethylene-production rates during the gravitropic bending of snapdragon spikes, 5-cm stem segments were excised from the bending zone of treated and untreated spikes (Fig. 1C) at various intervals following gravistimulation, as described previously (Philosoph-Hadas et al., 1996). After leaves and florets were removed, the longitudinally halved stem sections (Fig. 1C) were weighed and individually placed in 25-mL Erlenmeyer flasks sealed with rubber serum caps for 1 h at 20°C. The upper and lower halves of horizontally placed stems were maintained in their original positions during ethylene measurement. The ethylene concentration in each flask was analyzed by withdrawing a 2-mL gas sample with a hypodermic syringe and injecting it into a gas chromatograph (Varian, Palo Alto, CA) equipped with an activated-alumina column and a flame-ionization detector.
RESULTS
Effect of LaCl3 on the Gravitropic Response of Snapdragon Spikes
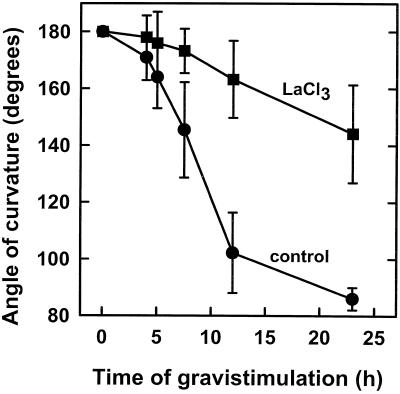
cv Maryland White spikes were pulsed with 20 mm LaCl3 and the effects on the bending kinetics are illustrated in Figure 2. The results show that LaCl3 delayed the onset of upward bending of this cultivar by 3 h and reduced the bending rate from 7° to 2° h−1. A similar curvature inhibition pattern was found for the other snapdragon cultivars after pulsing with LaCl3 concentrations between 10 and 40 mm, without any detectable damage (H. Friedman, S. Meir, I. Rosenberger, A.H. Halevy, and S. Philosoph-Hadas, unpublished data). It should be noted that spikes (7–10 mm thick) pulsed with 20 mm LaCl3 for 24 h absorbed an average amount of 0.9 mg LaCl3 g−1 fresh weight, which was effective in blocking their gravitropic response (Fig. 2). Therefore, for effective curvature inhibition of thinner spikes (4–6 mm thick), this amount of LaCl3 was obtained by shorter pulsing time or lower LaCl3 concentrations.
Figure 2.
Effect on the kinetics of the gravitropic response after pulsing cv Maryland White spikes with LaCl3. Flowering stems were pulsed with 20 mm LaCl3 for 22 h and placed horizontally. Each point and bar represent an average angle of curvature ± se of 15 spikes.
Effect of LaCl3 on Stem-Elongation Rate
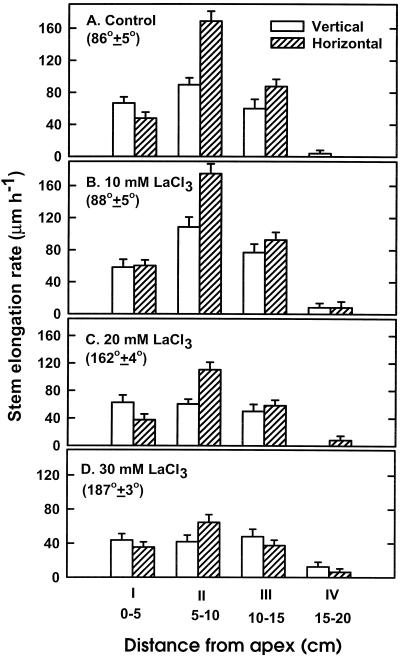
To examine the possibility that LaCl3 inhibits the gravitropic response by inhibiting stem elongation, we studied the effect of LaCl3 on the elongation rate of vertically and horizontally positioned spikes of two snapdragon cultivars; detailed results of cv Maryland White are presented in Figure 3. The elongation rates of four different sequential stem sections (I–IV; Fig. 1B) were determined for vertical and horizontal spikes after pulsing with various LaCl3 concentrations. The elongation rates of the three top sections (I–III) of vertical spikes were in the same range (60–90 μm h−1), whereas that of section IV was much lower (Fig. 3A). Unlike the elongation profile of vertical stems, section II of horizontally positioned spikes, i.e. the stem-bending zone, showed the highest elongation rate (almost twice as high as that of horizontal sections I and III or of vertical sections I–III; Fig. 3A). This indicates that gravistimulation induces stem elongation, particularly in the bending zone.
Figure 3.
Elongation profiles of vertical and horizontal stem sections of cv Maryland White spikes in the absence (A) or presence of 10 mm (B), 20 mm (C), or 30 mm (D) LaCl3. Spikes were pulsed for 22 h with the various LaCl3 concentrations, and elongation rates of each of the four designated stem sections (I–IV) were determined 24 h later. Each column and bar represent an average elongation rate ± se of 10 stem replicates. Numbers in parentheses represent the average angle of curvature ± se of 10 horizontally positioned spikes.
Pulsing spikes with 10 mm LaCl3 either promoted or did not significantly affect the elongation rates of the various sections of vertical and horizontal spikes (Fig. 3B), nor did this LaCl3 concentration change the curvature of horizontal spikes (Fig. 3B). On the other hand, higher LaCl3 concentrations, which markedly delayed stem curvature, significantly inhibited the elongation rate of all stem sections in both horizontal and vertical spikes (Fig. 3, C and D). The highest degree of inhibition was obtained in section II of vertical stems, whereas sections II and III of horizontal stems were equally inhibited by LaCl3 (Table I), indicating that LaCl3 does not interfere with growth of all stem sections but, rather, affects a specific region in both vertical and horizontal stems. It is noteworthy that, in general, the elongation rate of section II of horizontal spikes was higher than that of the corresponding section of vertical spikes at all LaCl3 concentrations examined, even at 30 mm, a concentration that completely eliminated the bending response.
Table I.
Effect of two LaCl3 concentrations on the percent inhibition of the elongation rates of the three top stem sections in vertical and horizontal cv Maryland White spikes
| Stem Section | Inhibition
|
|||
|---|---|---|---|---|
| 20 mm
LaCl3
|
30 mm
LaCl3
|
|||
| Vertical | Horizontal | Vertical | Horizontal | |
| % | ||||
| I | 6 | 22 | 35 | 26 |
| II | 33 | 35 | 54 | 62 |
| III | 17 | 34 | 20 | 57 |
Data were obtained from Figure 3 and are presented as percentages of control.
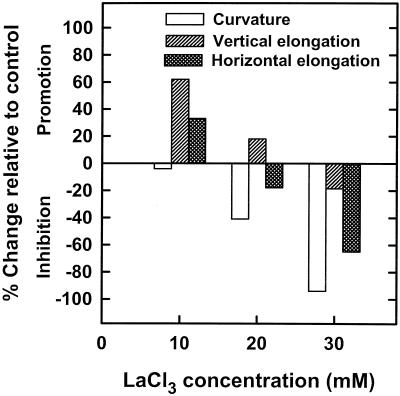
When the relative extents of inhibition by LaCl3 of stem curvature and elongation rate of section II of cv Maryland White spikes are analyzed, it can be seen that these two processes are not similarly inhibited. Thus, 20 and 30 mm LaCl3 inhibited the angle of spike curvature by 80% and 100%, respectively, although these concentrations inhibited the elongation rates of vertical and horizontal shoots similarly, but to a lesser extent, by only 33% to 35% and 54% to 60%, respectively (Fig. 3, C and D; Table I). The differential inhibition pattern of these two processes was even more pronounced in cv Maryland Appleblossom. Figure 4 shows the relative changes in the angle of curvature and stem-elongation rates of section II of vertical and horizontal spikes of this snapdragon cultivar after pulsing with various LaCl3 concentrations.
Figure 4.
Effect of three LaCl3 concentrations on the relative changes in curvature angles of gravistimulated spikes and in elongation rates of the stem-bending zone section II of cv Maryland Appleblossom spikes incubated for 24 h in either the vertical or the horizontal orientation. Results are expressed as percent inhibition or promotion, based on control values and calculated from the means of 15 spikes.
The results show that 10 mm LaCl3 enhanced the elongation rates of vertical and horizontal stems in this cultivar but did not affect the angle of curvature (Fig. 4), as it did for cv Maryland White (Fig. 3B). However, 20 and 30 mm LaCl3 reduced the angle of curvature of cv Maryland Appleblossom spikes by 40% and 90%, respectively. This significant inhibition was obtained even though 20 mm LaCl3 promoted vertical growth and inhibited horizontal growth by only 20%, and 30 mm LaCl3 inhibited elongation of vertical and horizontal shoots by only 20% and 60%, respectively (Fig. 4). These results indicate that (a) in all cases LaCl3 inhibited horizontal stem elongation more than it inhibited vertical elongation and (b) the extent of reduction of the angle of curvature by LaCl3 was in all cases almost double the extent of inhibition for the elongation of both vertical and horizontal spikes. It seems, therefore, that LaCl3 affects bending much more than it affects vertical or horizontal stem elongation.
Effect of LaCl3 on Sedimentation of Chloroplasts in Stems during Gravistimulation
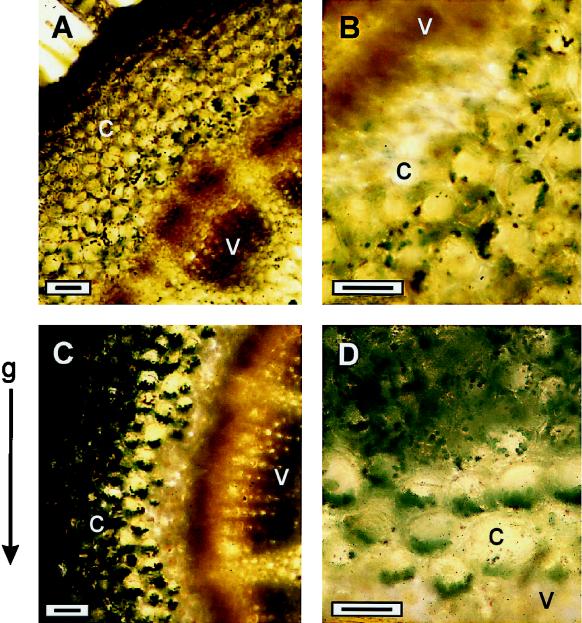
It has been suggested that amyloplast/chloroplast sedimentation is responsible for gravity stimulus perception (Sack, 1997). Therefore, the effect of LaCl3 on this gravity-dependent event was studied in cells of gravistimulated snapdragon spikes. Figure 5 illustrates the chloroplast distribution as seen in stem cross-sections prepared from the bending zones of vertically and horizontally positioned cv Potomac Royal spikes. In vertical spikes the chloroplasts usually sediment in the direction of the gravitational field in the lower part of the cortex stem cells (Brock et al., 1989). This typical pattern of chloroplast position appeared as a random distribution in stem cells seen in cross-sections of vertical spikes (Fig. 5, A and B). On the other hand, in gravistimulated spikes that had fully curved to 90°, the cross-sections were characterized by a polar position of chloroplasts at the bottoms of the cells (Fig. 5, C and D). It is noteworthy that this gravity-induced orientation of chloroplasts occurred only in the cortex cells close to the vascular system (Fig. 5D).
Figure 5.
Light micrographs of chloroplast distribution in cortical cells of the stem-bending zone as seen in cross-sections prepared from vertical (A and B) or gravistimulated (90°; C and D) cv Potomac Royal spikes. Cross-sections of the stem-bending zone taken from vertical or gravistimulation spikes were stained with KI/I2 solution and examined under the microscope. c, Cortex; v, vascular cylinder. Bars = 25 μm.
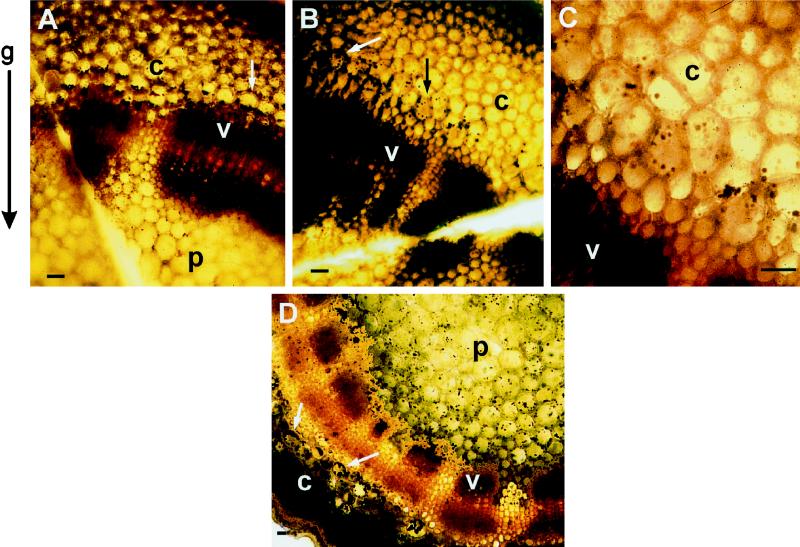
A similar sedimentation pattern was obtained in spikes curved only to 150° (Fig. 6A). Pulsing spikes with 10 mm LaCl3, which completely inhibited the gravitropic curvature of cv Potomac Royal, prevented the typical chloroplast sedimentation obtained in untreated spikes (Fig. 6, B and C). However, LaCl3 did not affect the chloroplast distribution in either cortex or pith cells of vertical shoots, as seen in stem cross-sections (Fig. 6D). Therefore, their chloroplast distribution was very similar to that of untreated vertical shoots (Fig. 5, A and B). A similar pattern of chloroplast sedimentation in the absence or presence of LaCl3 was also obtained in cross-sections of the other snapdragon cultivars assayed in this work (data not shown). Therefore, it seems that LaCl3 prevents the gravity-induced sedimentation of chloroplasts in snapdragon stem cells.
Figure 6.
Effect of LaCl3 on chloroplast distribution in cortical (c) and pith (p) cells of the stem-bending zone as seen in cross-sections prepared from gravistimulated (A–C) or vertical (D) cv Potomac Royal spikes. Spikes were pulsed with either distilled water or 10 mm LaCl3 for 20 h and gravistimulated or held vertically for an additional 6 h. Cross-sections of the stem-bending zones were stained and examined as detailed in Figure 5. The arrows point to individual cells in which the chloroplast distribution is clearly evident. A, Control gravistimulated spikes (150°); B and C, LaCl3-treated and gravistimulated spikes (180°); D, LaCl3-treated, vertical spikes. v, Vascular cylinder; c, cortex; p, pith. Bars = 25 μm.
Effect of LaCl3 on Ethylene-Production Rates during the Gravitropic Response
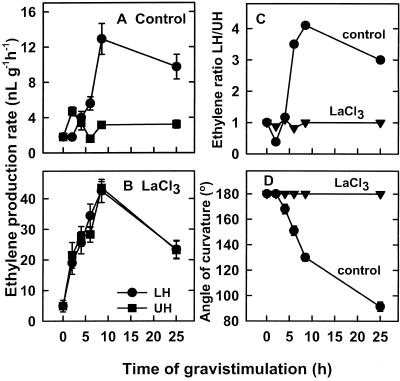
To further elucidate the mode of action of LaCl3 on physiological processes occurring during the gravitropic response, we examined its effect on ethylene-production rates of longitudinally halved stem sections taken from the spike-bending zone during gravistimulation (Fig. 1C). Figure 7A shows that the ethylene-production rates of lower stem halves were relatively low (2 nL g−1 h−1) for the first 2 h of gravistimulation, but it then started to increase, reaching a maximum of 13 nL g−1 h−1 after 8 h and declining slightly thereafter. However, except for an initial increase to 5 nL g−1 h−1 during the first 2 h, ethylene-production rates of upper stem halves remained low, in the range of 2 to 3 nL g−1 h−1, during 25 h of gravistimulation (Fig. 7A). Consequently, the ratio between the ethylene-production rates of lower and upper stem halves, which was approximately unity during the initial 4 h of gravistimulation, eventually reached values of 3 to 4 (Fig. 7C). This ethylene gradient coincided with the initiation of stem curvature and persisted during the progress of the bending response (Fig. 7D). Thus, the initial increase in ethylene-production rates in the lower stem halves (Fig. 7A) appeared together with bending initiation (Fig. 7D), and the maximal ethylene gradient (Fig. 7C) appeared when a curvature angle of 130° was attained (Fig. 7D).
Figure 7.
Effect of pulsing cv Maryland White spikes with LaCl3. Shown are the effects on the kinetics of ethylene evolution (A and B) from longitudinally halved stem sections, cut from their bending zone, on the ethylene-production ratio (C) and on the kinetics of spike curvature (D) during 25 h of gravistimulation. Spikes were pulsed with 20 mm LaCl3 for 24 h and placed horizontally for an additional 25 h. Stem sections (5 cm long) were excised from the bending zone at the indicated intervals, cut longitudinally into halves, and incubated for 1 h in sealed vials for ethylene accumulation. Points and bars are the averages ± se of seven replicates. LH, Lower halves; UH, upper halves.
Pulsing spikes with 20 mm LaCl3 prior to gravistimulation modified this pattern of ethylene-production rate (Fig. 7B); it markedly increased ethylene-production rates of both upper and lower stem halves (Fig. 7B) and prevented the development of the ethylene gradient between these two stem sections (Fig. 7C). In parallel, the bending response of LaCl3-treated spikes was completely inhibited and their angle of curvature remained at 180° during 25 h of gravistimulation (Fig. 7D).
DISCUSSION
In our previous study (Philosoph-Hadas et al., 1996) we showed that the putative plasma membrane Ca2+-channel blocker LaCl3 significantly inhibited the gravitropic response of snapdragon. The present study demonstrates that the inhibitory effect of LaCl3 on gravitropic bending may result from its inhibitory effect on various gravity-associated processes. This effect was found in several different snapdragon cultivars, with slight variations, indicating that the phenomenon may be widespread. Until now, LaCl3 has rarely been used to block the gravitropic response in plants: apart from inhibiting gravity-induced cytoplasmic streaming in characean algae (Staves, 1997), La3+ has been shown to suppress gravisensitivity only in maize coleoptiles and roots (for review, see Belyavskaya, 1996) and in pea roots (Belyavskaya, 1992).
Analysis of the elongation profiles of various stem sections of snapdragon revealed that, despite the elongation potential of all three top sections (sections I–III) of vertical spikes, only section II exhibited a significantly increased elongation rate following gravistimulation (Fig. 3A). This higher elongation rate of section II seems to contribute to the upward bending of gravistimulated spikes and could be attributed to the ability of cells in this particular region to perceive and/or respond to the gravity stimulus. Therefore, like the root cap (Sack, 1997), stems seem to have specific sites for gravity perception and response.
The elongation rate of section II of both vertical and horizontal spikes was also the most responsive to LaCl3 inhibition (Fig. 3, C and D; Table I). Therefore, LaCl3 interferes mainly with the elongation process, occurring specifically in this section of both vertical and horizontal spikes. Moreover, the following observations suggest that LaCl3 also interferes with several other distinct processes that lead to the asymmetric stem elongation induced by gravity, rather than inhibiting bending merely by blocking general stem elongation. LaCl3 inhibited the gravitropic response by reducing the bending rate and by extending the lag period until the visible response was apparent (Fig. 2), indicating that LaCl3 might interfere with initial processes occurring during this lag period. Also, LaCl3 inhibited the elongation rate of horizontal spikes to a greater extent than it inhibited the elongation rate of vertical spikes (Figs. 3 and 4; Table I), indicating that LaCl3 interferes with gravity-induced elongation processes. And finally, LaCl3 was more efficient at inhibiting the bending response than at inhibiting the elongation rate (Figs. 3 and 4). This was particularly pronounced in the cv Maryland Appleblossom (Fig. 4), in which LaCl3 could specifically inhibit both bending and elongation rates of horizontal stems in spite of its promotive effect on vertical spike elongation.
Additional support for the view that LaCl3 affects specific processes induced by gravistimulation is provided by studies of amyloplast/chloroplast sedimentation, which is believed to be one of the susceptors that trigger gravitropic sensing in roots and shoots of higher plants (Song et al., 1988; Sack, 1997; Vitha et al., 1998). As was previously found for columella cells of roots or for endodermis of stem seedlings (Sack, 1997), the bending zone of the snapdragon stem was found to be very rich in starch-containing chloroplasts (Figs. 5 and 6D). This might contribute to the high responsiveness of this spike to gravity. Although the chloroplasts were present in the cortex and pith, their gravity-induced sedimentation was restricted to specified stem zones in the inner cortex and around the vascular system in the stele (Figs. 5, C and D, and 6A).
The chloroplast-distribution pattern in cross-sections of vertical (Fig. 5, A and B) and horizontal (Fig. 5, C and D) snapdragon spikes was similar to that found in cross-sections of barley (Brock et al., 1989) and oat (Brock and Kaufman, 1990) pulvini taken from the corresponding shoot orientations. This provides additional correlative evidence for their important role in the gravitropic sensing of shoots. To our knowledge, our observations show for the first time that LaCl3 treatment prevented the chloroplast sedimentation in gravistimulated spikes (Fig. 6, B and C), which indicates that LaCl3 could inhibit bending by preventing the gravity-induced sedimentation of the chloroplast gravisensors. Similarly, LaCl3 has previously been demonstrated to disturb the polar distribution of amyloplasts in statocytes of pea roots (Belyavskaya, 1992). Since LaCl3 is known to inhibit Ca2+ fluxes across the plasma membrane, these results imply that the gravity-induced sedimentation of chloroplasts could be mediated by changes in [Ca2+]cyt. Alternatively or additionally, since LaCl3 can penetrate the cell, the possibility that LaCl3 may directly disturb the sedimentation process cannot be excluded.
Another gravity-related process that was influenced by LaCl3 was the development of an ethylene gradient across the stem (Fig. 7, A–C). It is possible that this gradient is necessary for curvature development, since the timing of its formation was correlated with the initiation of curvature development (Fig. 7, C and D), as reported previously (Philosoph-Hadas et al., 1996). Similar correlative evidence for the formation of curvature and the development of gravity-induced ethylene gradients across the stem, with more ethylene in the lower half, has been reported previously in various other systems (Clifford et al., 1983; Wheeler et al., 1986; Woltering, 1991). The relevance of this ethylene gradient to differential cell elongation and, hence, to the gravitropic bending of flowering stems was previously hypothesized as possibly reflecting an asymmetric auxin distribution across the stem (Philosoph-Hadas et al., 1996). Additionally, the direct contribution of such an asymmetric ethylene production to differential stem elongation cannot be excluded. This possibility is indicated by the close correlation found in the present study between the remarkably increased rates following gravistimulation of elongation in the stem-bending zone (Fig. 3A) and of the ethylene production in the lower half of the stem-bending zone (Fig. 7A). In this respect, the recent findings of Smalle et al. (1997) showing that ethylene can induce marked hypocotyl elongation in light-grown Arabidopsis may provide further support for this idea. However, such an ethylene-induced elongation seems to be dependent on ethylene levels in the tissue, because when endogenous ethylene-production rates reached very high values (40–45 nL g−1 h−1) in the presence of 20 mm LaCl3 (Fig. 7B), horizontal stem elongation was suppressed (Fig. 3C).
LaCl3 prevented ethylene gradient development by increasing the ethylene-production rates of both the upper and lower halves of horizontal spike stems (Fig. 7, A and B). This may be because LaCl3 induces expression of genes involved in ethylene production, as shown recently for mRNA levels of several other genes (Polisensky and Braam, 1996; Rock and Quatrano, 1996). On the other hand, CDTA, a Ca2+ chelator that also inhibited gravitropic bending, prevented the development of such an ethylene gradient in snapdragon shoots by reducing the ethylene-production rates in the lower stem section (Philosoph-Hadas et al., 1996). This indicates that, although these two Ca2+ antagonists affected ethylene production in an opposite manner, they both resulted in elimination of the ethylene gradient across the stem and in a parallel blocking of spike curvature. It seems, therefore, that the development of such an ethylene gradient could be an important prerequisite for development of stem curvature. In addition, the opposite effect of these two Ca2+ antagonists on ethylene production indicates that ethylene is induced directly by LaCl3 application (Fig. 7, A and B) rather than by modulation of [Ca2+]cyt.
LaCl3 can exert its inhibitory effect on various gravity-induced and possibly Ca2+-dependent processes by several modes of action. At the cellular level, La3+ ions can prevent increases in [Ca2+]cyt through a direct inhibitory effect on Ca2+ channels and a stimulatory effect on several Ca2+-ATPases, thereby interfering with free Ca2+ availability (Bush, 1995; Belyavskaya, 1996). It was demonstrated by using aequorin-containing transgenic plants that LaCl3 directly prevented the [Ca2+]cyt increase induced by cold treatment (Knight et al., 1992; Polisensky and Braam, 1996). Thus, it seems that the putative elevation of [Ca2+]cyt following gravistimulation may require extracellular Ca2+ and may derive from an influx through Ca2+ channels. This possibility is reinforced by our findings showing that, in addition to LaCl3, snapdragon bending was also inhibited by Ca2+ chelators such as CDTA or 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (Philosoph-Hadas et al., 1995, 1996), which are known to reduce Ca2+ fluxes across the plasma membrane. Nevertheless, it is still possible that, unlike the Ca2+ chelators, La3+ ions have more complex effects in addition to antagonizing Ca2+ (Polisensky and Braam, 1996).
It has been reported that LaCl3 can inhibit other intracellular ion channels (Tester, 1990), can directly induce mRNA levels of several genes (Polisensky and Braam, 1996; Rock and Quatrano, 1996), can bind proteins and various ligands in biological molecules, and can often mimic Ca2+ action inside the cell (Belyavskaya, 1996). These direct effects of LaCl3 may be attributed to the fact that it can be taken up by plant cells, whereas the Ca2+ chelator CDTA remains in the extracellular space (Bush, 1995; Polisensky and Braam, 1996). In addition, unlike the findings of Gehring et al. (1990) in oat coleoptiles, Legué et al. (1997) recently directly demonstrated that the gravitropic response of Arabidopsis roots is not associated with detectable changes in [Ca2+]cyt. Therefore, although these contradictory results could be ascribed to the different plant organs used in these studies and in this study (coleoptiles and stems versus roots), regarding the recent observations in roots and the direct effects of LaCl3, it is still possible that the inhibitory effect of LaCl3 might be unrelated to [Ca2+]cyt changes.
In summary, the present study shows that the putative Ca2+-channel blocker LaCl3 inhibits the negative-gravitropic curvature of snapdragon spikes by only partially inhibiting the stem-elongation rate. Its more pronounced inhibitory effect on the bending response seems to be exerted through prevention of several gravity-induced processes, including starch-containing chloroplast sedimentation and formation of an ethylene gradient across the stem.
The exact sequence of these gravity-induced events is not yet clear. One possibility is that the change in stem orientation leads to changes in [Ca2+]cyt, which may induce sedimentation of chloroplasts in the stem-bending zone. This sedimentation process may elicit, through interaction with cell membranes, additional transient changes in [Ca2+]cyt, which may affect membranous IAA receptors, altering IAA transport (Gross and Sauter, 1988) and leading to the asymmetric production of ethylene across the stem, as well as to differential stem growth. Accordingly, LaCl3 might prevent this cascade of events by blocking Ca2+ channels, preventing [Ca2+]cyt elevation, and thereby disturbing chloroplast sedimentation and subsequent gravity-associated processes. In addition, LaCl3 might prevent bending through direct disturbance of chloroplast sedimentation, direct induction of ethylene biosynthesis genes, and/or inhibition of IAA transport. A careful analysis is now being performed to examine the effects of LaCl3 on the differential stem-growth process and on endogenous IAA distribution in the bending zone of snapdragon spikes and may shed light on this hypothesis.
Abbreviations:
- [Ca2+]cyt
[Ca2+] of the cytosol
- CDTA
trans-1,2- cyclohexane dinitro-N,N,N′,N′-tetraacetic acid
Footnotes
This work was supported by grant no. IS-2434-94 from the United States-Israel Binational Agricultural Research and Development Fund, by grant no. 95/26 from the Joint Dutch-Israeli Agricultural Research Program, and by the Pearlstein Family Fund for Research in Ornamental Horticulture at the Hebrew University (to A.H.H.). This study is no. 407 in the Agricultural Research Organization (Volcani Center, Bet Dagan, Israel) series.
LITERATURE CITED
- Belyavskaya NA. The function of calcium in plant graviperception. Adv Space Res. 1992;12:83–91. doi: 10.1016/0273-1177(92)90267-2. [DOI] [PubMed] [Google Scholar]
- Belyavskaya NA. Calcium and graviperception in plants: inhibitor analysis. Int Rev Cytol. 1996;168:123–185. [Google Scholar]
- Brock TG, Burg J, Ghosheh NS, Kaufman PB. The role of calcium in growth induced by indole-3-acetic acid and gravity in the leaf sheath pulvinus of oat (Avena sativa) J Plant Growth Regul. 1992;11:99–103. doi: 10.1007/BF00198021. [DOI] [PubMed] [Google Scholar]
- Brock TG, Kaufman PB (1990) Movements in grass shoots. In RL Satter, HL Gorton, TC Vogelmann, eds, The Pulvinus: Motor Organ for Leaf Movement. The American Society of Plant Physiologists, Rockville, MD, pp 59–71
- Brock TG, Lu CR, Ghosheh NS, Kaufman PB. Localization and pattern of graviresponse across the pulvinus of barley Hordeum vulgare. Plant Physiol. 1989;91:744–748. doi: 10.1104/pp.91.2.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush SD. Calcium regulation in plant cells and its role in signaling. Annu Rev Plant Physiol Plant Mol Biol. 1995;46:95–122. [Google Scholar]
- Clifford PE, Reid DM, Pharis RP. Endogenous ethylene does not initiate but may modify geobending—a role for ethylene in autotropism. Plant Cell Environ. 1983;6:433–436. [Google Scholar]
- Fukaki H, Fujisawa H, Tasaka M. Gravitropic response of inflorescence stems in Arabidopsis thaliana. Plant Physiol. 1996;110:933–943. doi: 10.1104/pp.110.3.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring CA, Williams DA, Coby SH, Parish RW. Phototropism and geotropism in maize coleoptiles are spatially correlated with increases in cytosolic free calcium. Nature. 1990;345:528–530. doi: 10.1038/345528a0. [DOI] [PubMed] [Google Scholar]
- Gross J, Sauter M. Effects of 1-N-naphthylphthalamic acid and lanthanum on the polar transport of auxin and calcium in corn coleoptiles. In: Kutacek M, Bandurski RS, Krekule J, editors. Proceedings of Symposium, Czechoslovakia, Physiology and Biochemistry of Auxin in Plants. Dordrecht, The Netherlands: SPB Academic Publishing; 1988. pp. 233–239. [Google Scholar]
- Halevy AH, Mayak S. Senescence and postharvest physiology of cut flowers, part 2. Hortic Rev. 1981;3:59–143. [Google Scholar]
- Jackson C, Hall JL. A fine structural analysis of auxin-induced elongation of cucumber hypocotyls, and the effects of calcium antagonists and ionophores. Ann Bot. 1993;72:193–204. [Google Scholar]
- Kaufman PB, Pharis RP, Reid MD, Beall FD. Investigations into the possible regulation of negative gravitropic curvature in intact Avena sativa plants and in isolated stem segments by ethylene and gibberellin. Physiol Plant. 1985;65:237–244. doi: 10.1111/j.1399-3054.1985.tb02389.x. [DOI] [PubMed] [Google Scholar]
- Kim D, Kaufman PB. Basis for changes in auxin-sensitivity of Avena sativa (oat) leaf-sheath pulvini during the gravitropic response. J Plant Physiol. 1995;145:113–120. doi: 10.1016/s0176-1617(11)81856-0. [DOI] [PubMed] [Google Scholar]
- Kiss JZ, Guisinger MM, Miller AJ, Atackhouse KS. Reduced gravitropism in hypocotyls of starch-deficient mutants of Arabidopsis. Plant Cell Physiol. 1997;38:518–525. doi: 10.1093/oxfordjournals.pcp.a029199. [DOI] [PubMed] [Google Scholar]
- Knight MR, Smith SM, Trewavas AJ. Wind-induced plant motion immediately increases cytosolic calcium. Proc Natl Aca Sci USA. 1992;89:4967–4971. doi: 10.1073/pnas.89.11.4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohji J, Hagimoto H, Masuda Y. Georeaction of the flower stalk in a poppy, Papaver rhoeas L. Plant Cell Physiol. 1979;20:375–386. [Google Scholar]
- Legué V, Blancaflor E, Wymer C, Perbal G, Fantin D, Gilroy S. Cytoplasmic free Ca2+ in Arabidopsis roots changes in response to touch but not gravity. Plant Physiol. 1997;114:789–800. doi: 10.1104/pp.114.3.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Hagen G, Guilfoyle TJ. Gene expression from an auxin-inducible promoter supports the Cholodny-Went theory on tropisms. Plant Cell. 1991;3:1167–1175. doi: 10.1105/tpc.3.11.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meicenheimer RD, Nackid TA. Gravitropic response of kalanchoe stems. Int J Plant Sci. 1994;155:395–404. [Google Scholar]
- Migliaccio F, Galston AW. On the nature and origin of the calcium asymmetry arising during gravitropic response in etiolated pea epicotyls. Plant Physiol. 1987;85:542–547. doi: 10.1104/pp.85.2.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philosoph-Hadas S, Meir S, Rosenberger I, Halevy AH. Control and regulation of the gravitropic response of cut flowering stems during storage and horizontal transport. Acta Hortic. 1995;405:343–350. [Google Scholar]
- Philosoph-Hadas S, Meir S, Rosenberger I, Halevy AH. Regulation of the gravitropic response and ethylene biosynthesis in gravistimulated snapdragon spikes by calcium chelators and ethylene inhibitors. Plant Physiol. 1996;110:301–310. doi: 10.1104/pp.110.1.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickard BG. Roles of hormones, protons and calcium in geotropism. In: Pharis RP, Reid DM, editors. Encyclopedia of Plant Physiology, Vol II. Berlin: Springer-Verlag; 1985. pp. 193–281. [Google Scholar]
- Polisensky DH, Braam J. Cold-shock regulation of the Arabidopsis TCH genes and the effects of modulating intracellular calcium levels. Plant Physiol. 1996;111:1271–1279. doi: 10.1104/pp.111.4.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poovaiah BW, McFadden JJ, Reddy ASN. The role of calcium ions in gravity signal perception and transduction. Physiol Plant. 1987;71:401–407. doi: 10.1111/j.1399-3054.1987.tb04363.x. [DOI] [PubMed] [Google Scholar]
- Rock CD, Quatrano RS. Lanthanide ions are agonists of transient gene expression in rice protoplasts and act in synergy with ABA to increase Em gene expression. Plant Cell Rep. 1996;15:371–376. doi: 10.1007/BF00232374. [DOI] [PubMed] [Google Scholar]
- Rorabaugh PA, Salisbury FB. Gravitropism in higher plant shoots. VI. Changing sensitivity to auxin in gravistimulated soybean hypocotyls. Plant Physiol. 1989;91:1329–1338. doi: 10.1104/pp.91.4.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux SJ, Serlin BS. Cellular mechanisms controlling light-stimulated gravitropism: role of calcium. CRC Crit Rev Plant Sci. 1987;5:205–236. doi: 10.1080/07352688709382240. [DOI] [PubMed] [Google Scholar]
- Sack FD. Plastids and gravitropic sensing. Planta. 1997;203:S63–S68. doi: 10.1007/pl00008116. [DOI] [PubMed] [Google Scholar]
- Salisbury FB. Gravitropism: changing ideas. Hortic Rev. 1993;15:233–278. [Google Scholar]
- Saunders MJ (1990) Calcium and plant hormone action. In J Roberts, C Kirk, M Venis, eds, Hormone Perception and Signal Transduction in Animals and Plants. Society for Experimental Biology, Cambridge, UK, pp 271–283 [PubMed]
- Sinclair W, Trewavas AJ. Calcium in gravitropism. A re-examination. Planta. 1997;203:S85–S90. doi: 10.1007/pl00008120. [DOI] [PubMed] [Google Scholar]
- Smalle J, Haegman M, Kurepa J, van Montagu M, van der Straeten D. Ethylene can stimulate hypocotyl elongation in the light. Proc Natl Acad Sci USA. 1997;94:2756–2781. doi: 10.1073/pnas.94.6.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song I, Lu CR, Brock TG, Kaufman PB. Do starch statoliths act as the gravisensors in cereal grass pulvini? Plant Physiol. 1988;86:1155–1162. doi: 10.1104/pp.86.4.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staves MP. Cytoplasmic streaming and gravity sensing in Chara internodal cells. Planta. 1997;203:S79–S84. doi: 10.1007/pl00008119. [DOI] [PubMed] [Google Scholar]
- Strudwick NJ, Phillips TJ, Scott IM. Expression of the abnormal gravitropism phenotypes Creep and Ageotropum during development in pea. J Plant Physiol. 1997;150:588–591. doi: 10.1016/s0176-1617(97)80323-9. [DOI] [PubMed] [Google Scholar]
- Tester M. Plant ion channels: whole-cell and single-channel studies. New Phytol. 1990;114:305–340. doi: 10.1111/j.1469-8137.1990.tb00403.x. [DOI] [PubMed] [Google Scholar]
- Trewavas AJ (ed) (1992) Tropism forum: what remains of the Cholodny-Went theory? (A multi-author discussion). Plant Cell Environ 15: 757–794
- Vitha S, Yang M, Kiss JZ, Sack FD. Light promotion of hypocotyl gravitropism of a starch-deficient tobacco mutant correlates with plastid enlargement and sedimentation. Plant Physiol. 1998;116:495–502. doi: 10.1104/pp.116.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler RM, Salisbury FB. Gravitropism in higher plant shoots. I. A role for ethylene. Plant Physiol. 1981;67:686–690. doi: 10.1104/pp.67.4.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler RM, White RG, Salisbury FB. Gravitropism in higher plant shoots. IV. Further studies on participation of ethylene. Plant Physiol. 1986;82:534–542. doi: 10.1104/pp.82.2.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woltering EJ. Regulation of ethylene biosynthesis in gravistimulated Kniphofia (hybrid) flower stalks. J Plant Physiol. 1991;138:443–449. [Google Scholar]