Abstract
Introduction
Agonists at the mu opioid receptor (MOR) are widely recognized for their effects on reward and pain. Although prior studies have attributed some of these effects to MORs on GABA neurons in the ventral tegmental area (VTA), recent studies have identified a region of particularly strong MOR immunostaining residing caudal to the VTA, in a region denoted the rostromedial tegmental nucleus (RMTg).
Methods
Hence, we examined whether rats would self-administer small doses (50–250 pmol) of the selective MOR agonist endomorphin-1 (EM1) into the RMTg and adjacent sites. EM1 was chosen due to its short half-life, thus limiting drug spread, and due to its presence endogenously in brain neurons, including some afferents to the RMTg.
Results
The highest rates of EM1 self-administration occurred within 0.5 mm of the RMTg center, in a region roughly 0.8–1.6 mm caudal to the majority of VTA DA neurons. In contrast, self-administration rates were much lower in the adjacent VTA, interpeduncular nucleus, central linear nucleus, or median raphe nucleus. Furthermore, EM1 infusions into the RMTg, but not surrounding regions, produced conditioned place preference, while EM1 infusions into the RMTg but not anterior VTA markedly reduced formalin-induced pain behaviors. EM1 effects were mimicked by infusions of the GABA agonist muscimol into the same region, consistent with EM1 having inhibitory actions on its target neurons.
Conclusion
These results implicate a novel brain region in modulating MOR influences on both appetitive and aversive behavior.
Keywords: Opioids, Reward, Pain, Endomorphin, RMTg, tVTA, Habenula
Introduction
Agonists at the mu opioid receptor (MOR) strongly influence both pain and reward (Basbaum and Fields 1984; Devine and Wise 1994; Le Merrer et al. 2009; Negus et al. 1993) via receptors that are distributed widely throughout the brain and spinal cord (Ding et al. 1996; Mansour et al. 1994). MORs in or near the ventral tegmental area (VTA) have been a topic of particular interest, as this region is enriched in dopamine (DA) neurons projecting to forebrain regions implicated in reward and motivation (Ikemoto 2007; Wise and Bozarth 1987), and numerous studies have implicated MORs in the VTA in reward and pain. For example, MOR agonist injections into the VTA region support self-administration (Devine and Wise 1994; Welzl et al. 1989; Zangen et al. 2002), produce conditioned place preference (CPP) (Bals-Kubik et al. 1993; Nader and van der Kooy 1997; Shippenberg et al. 1993), and markedly reduce pain behaviors induced by formalin injections into the hindpaw (Altier and Stewart 1998, 1999; Manning et al. 1994; Morgan and Franklin 1991).
While numerous studies have hypothesized that MOR agonists influence GABAergic interneurons in the VTA (Johnson and North 1992), few studies have distinguished actions in the VTA from other nearby small midbrain structures. One exception is a notable study that found that the endogenous MOR agonist endomorphin-1 (EM1) was more rewarding in the posterior than anterior VTA (Zangen et al. 2002). Although that study did not examine sites yet further caudal, we recently found high levels of MOR immunoreactivity extending up to 1–2 mm posterior to the VTA (Jhou et al. 2009b), in a region denoted the rostromedial tegmental nucleus (RMTg), and also called the tail of the VTA (Kaufling et al. 2009). The RMTg is a recently identified brain region exhibiting distinct neuroanatomical, physiological, and behavioral properties, and that consists of GABAergic neurons that project intensely to midbrain DA neurons (Balcita-Pedicino et al. 2011; Jhou et al. 2009a) while also receiving a strong input from the lateral habenula and playing central roles in motivated behavior (Hong et al. 2011; Jhou 2005; Jhou et al. 2009a, b; Kaufling et al. 2009; Metzger et al. 2010). A rapidly growing number of recent electrophysiological studies implicate the RMTg in mediating MOR agonist effects on DA neurons (Jalabert et al. 2011; Lecca et al. 2011a, b; Matsui and Williams 2011) (Kaufling and Aston-Jones, unpublished observations), but little is known about the behavioral implications of these findings. Hence, we tested whether small doses of EM1 injected in or near the RMTg are reinforcing, as measured by intracranial self-administration (ICSA) and CPP. We used EM1 due to its short half-life in brain tissue, thus limiting effects of diffusion (Fichna et al. 2007; Perlikowska et al. 2009), and because it is endogenously produced in the brain, making it potentially relevant to mechanisms of endogenous opioid function. We also examined EM1 effects on formalin-induced pain, in order to test the hypothesis that appetitive and aversive behaviors might be modulated by similar substrates. Finally, because of evidence that MOR agonists inhibit RMTg neurons (Lecca et al. 2011a; Matsui and Williams 2011), and that GABAa agonists in this region may be rewarding (Ikemoto et al. 1998), we also examined whether effects of EM1 would be mimicked by the GABAa agonist muscimol.
Methods
Animals
Male albino Wistar rats (275–350 g; Harlan Laboratories) were used for all experiments. All protocols were conducted under National Institutes Health (NIH) Guidelines using the NIH handbook Animals in Research and were approved by the Animal Care and Use Committee (National Institute on Drug Abuse, Intramural Research Program, Baltimore, MD). We used 38 rats for ICSA experiments, 26 rats for CPP experiments, and 12 rats for formalin pain tests.
Surgeries
Guide cannulae (24 gauge, Plastics One) were implanted at least 5 days prior to behavioral sessions. RMTg coordinates were: AP −6.2 to −6.8, DV −7.5, and RL −1.8, while VTA coordinates were: AP −5.2 to −5.8, DV −8.1, and RL −1.8, and control sites lateral to the RMTg used RL=3.0. Cannulae for the formalin pain experiment were bilateral, while all other experiments used unilateral cannulae.
Intracranial self-administration
After implantation of guide cannulae, rats received daily 90-min self-administration sessions in which 75-nl doses of drug were delivered into the brain via an injector cannula as previously described (Ikemoto and Sharpe 2001). After an initial acclimation session, injection reservoirs were filled with 0.4 mg/ml EM1 (0.65 mM), 2 mg/ml EM1 (3.3 mM), or 19.5 mg/ml muscimol (100 µM), yielding 50, 250, or 7.5 pmol/infusion, respectively. The order of drugs tested followed a fixed and repeating daily cycle, in which one session of ACSF infusions was followed on the next daily session by a session of EM1 self-administration (250 nmol/ 75 nl/infusion) at the same site. In 16 rats, muscimol was self-administered in the next daily session after EM1, while in seven rats, self-administration of a lower EM1 dose (50 pmol/infusion) was examined prior to the higher dose of EM1. After one cycle of drugs was completed, the injector cannula lengths were increased by 0.5 mm, and the cycle repeated, hence, testing multiple sites in each rat along a vertical track.
Immunohistochemistry for MOR and TH
Immunostaining procedures were as previously described (Chou et al. 2002). Primary antibodies were rabbit polyclonal against MOR (1:10,000 dilution; Gramsch Laboratories, Schwabhausen, Germany) or mouse monoclonal antibodies against tyrosine hydroxylase (TH) (1:10,000 dilution; MAB5280, Millipore Inc.).
In situ hybridization for prepronociceptin
In situ hybridization was performed as previously described (Chou et al. 2001; Simmons et al. 1989) using digoxigeninlabeled riboprobes transcribed from plasmid containing the prepronociceptin (Pnoc) gene (generous gift of Dr. Stanley Watson). Digoxigenin-labeling was then visualized using an alkaline-phosphatase (AP) reaction with nitro-blue tetrazolium and 5-bromo-4-chloro-3′-indoylphosphate.
Conditioned place preference
Animals were conditioned to the place preference apparatus (Med Associates Inc., St. Albans, VT) as previously described (Ikemoto and Donahue 2005). In brief, the drug-paired chamber was counterbalanced in all groups, and on the first day, animals explored all chambers freely for 15 min, constituting a baseline preference score (seconds spent in the drug-paired chamber, minus seconds spent in the opposite chamber). Over the next 4 days, animals received intracranial ACSF injections (1 nmol in 300 nl, delivered over 20 s) each morning (between 10:00 and 11:00 a.m.) using a syringe pump (Harvard Apparatus, Holliston, MA) and an acutely inserted 29-gauge injector cannula (Plastics One, Roanoke, VA). After a 2-min wait, injectors were removed and rats placed into one chamber (designated the “unpaired” chamber) for 15 min. Later that day (between 3:00 and 4:00 p.m.), animals received intracranial EM1 infusions (1 nmol in 300 nl) and were placed into the opposite, “paired” chamber for the same duration. On the sixth day, animals explored all chambers for 15 min without drug. The post-conditioning preference score was calculated in the same manner as the pre-conditioning score, and the “preference shift” was calculated as the post-conditioning preference score minus the pre-conditioning score.
Anatomical assessment of cannula locations and TH, Pnoc expression
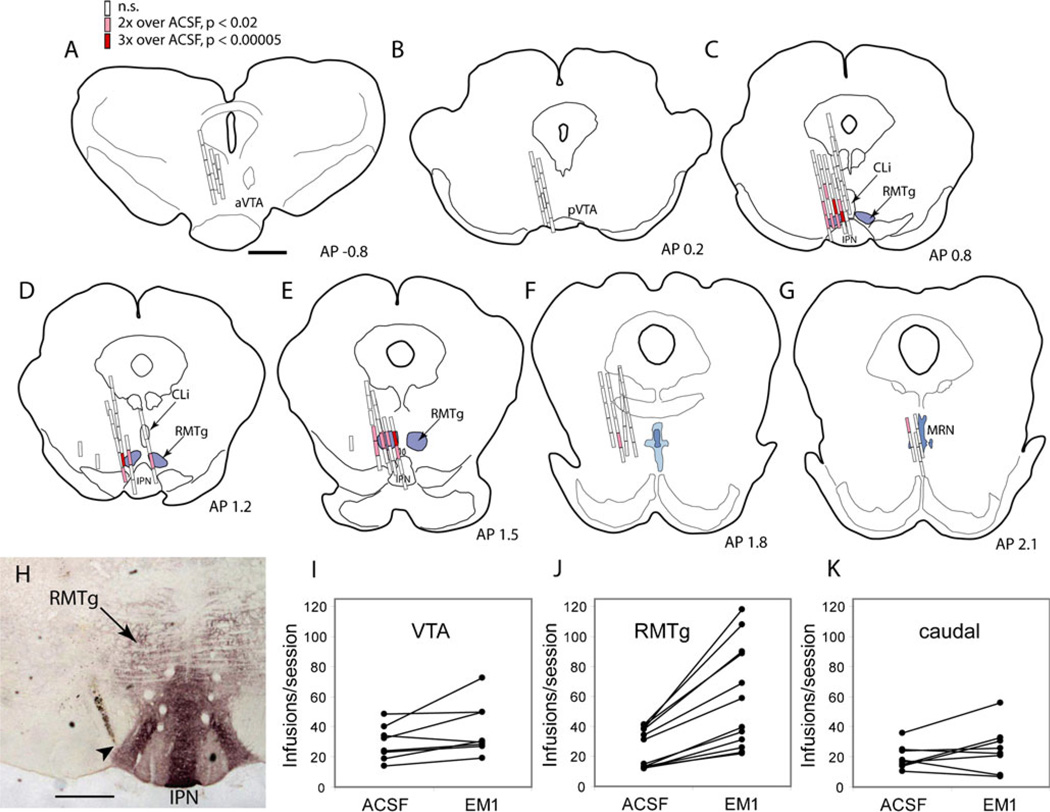
Rats were perfused transcardially with 10 % formalin. Brains were extracted and equilibrated overnight in 20 % sucrose, and then sliced into 40-µm coronal sections. For cannula localization, every fourth section was immunostained for MOR, and AP coordinates determined relative to a coronal reference plane passing through the rostral tip of the interpeduncular nucleus (IPN). To assist in determining AP coordinates, we also sliced two normal adult rat brains into 40-µm coronal sections, and mounted every fourth section stained for MOR, producing a set of “reference” brain sections 160 µm apart, which were photographed and used as templates to which individual cannula sites were compared, guided also by anatomical landmarks such as the IPN, decussation of the superior cerebellar peduncle, and anterior tegmental nucleus. These coordinates were used to group cannula sites into one of several 400-µm bins for subsequent statistical analysis. Drawings of brain sections (Fig. 1a–g; Figs. S1 and S2) were derived from the same reference photographs used to estimate AP coordinates.
Fig. 1.
EM1 self-administration data for individual animals and sessions. a–g Individual ICSA infusion sites are shown in coronal sections arranged from rostral to caudal. Open rectangles represent sites where infusion rates for endomorphin-1 (EM1) were not higher than ACSF (p>0.02). Lighter filled rectangles indicate sites where EM1 infusion rates were two to three times ACSF rates (p<0.02), while darker filled rectangles indicate infusion rates greater than three times ACSF rates (p<0.00005). Concentric dashed rings outline areas within 0.5, 1.0, and 1.5 mm radius of RMTg center. h Coronal brain section shows MOR immunoreactivity (dark reaction product), which demarcates the RMTg; immunoreactivity is also elevated in the adjacent interpeduncular nucleus (IPN). Cannula tip just lateral to RMTg is indicated by arrowhead. Leverpressing rates for ACSF or EM1 in individual rats with cannulae in the VTA (i), within 0.5 mm of the RMTg center (j), or at sites caudal to the RMTg (k). Scale bars=1 mm (a–g) or 0.5 mm (h). aVTA anterior ventral tegmental area, pVTA posterior ventral tegmental area
For quantification of TH and Pnoc, four brains were sliced into six or 12 series of 40-µm sections, i.e., yielding series containing sections 240 or 480 µm apart. One series from each brain was immunostained for TH, while an adjacent series was processed for in situ hybridization to reveal Pnoc. We then estimated each section’s AP coordinate using the same “reference” template photographs used for cannula localization, and then categorize each section into one of the 400-µm anatomic bins used to group cannula sites. TH and Pnoc cells were counted in a square region 0.75 µm high, and extending from the midline to 0.75 mm lateral, and were summed over left and right hemispheres.
Formalin-induced pain
One week prior to testing, animals were implanted with bilateral guide cannulae as in ICSA experiments. Immediately before sessions, we gave infusions of ACSF, EM1 (2 mg/ml) or muscimol (100 µM) (counterbalanced order) into the RMTg or VTA. Infusions consisted of an initial bolus (1 nmol EM1 in 300 µl, or 500 nl muscimol) delivered over 60 s, followed by an additional 100 pmol/min (for EM1) or 50 nl/min (for muscimol) administered over the next 25 min to maintain a relatively constant drug concentration. The formalin pain test was then administered as previously described (Dubuisson and Dennis 1977). Briefly, we injected 50 µl of 2.5 % formalin (Sigma-Aldrich) into the plantar surface of the hindpaw (counterbalanced left/right), and scored behavior for 24 min using a numerical rating scale as follows: Rating 0, injected paw firmly on floor and bearing weight, or used in locomotion without favoring. Rating 1, paw lightly contacts floor, or used in locomotion with limp. Rating 2, paw elevated off floor. Rating 3, animal licks, bites, or shakes affected paw. Seven and 14 days later, rats received additional formalin pain tests into alternating paws with counterbalanced drug order.
Statistical analysis
Because multiple cannula sites were tested in each animal, ICSA data variability derives from differences in site as well as between animals. To reduce between-animal variability, all calculations involving multiple animals were done using ICSA rates normalized to ACSF infusion rates for that particular animal. Unless otherwise indicated, comparisons of groups were made using a one-way analysis of variance (ANOVA) using Statistica (StatSoft Inc., Tulsa, OK), and post-hoc tests performed using a Student’s t-test with Bonferroni correction.
Because each animal was tested for EM1 ICSA at multiple sites, the possibility existed that drug reward in particular sessions might elevate responding in subsequent sessions due to conditioning. However, prior results suggest that any conditioning effects extinguish quickly (Zangen et al. 2002), and in the present study, infusion rates during ACSF sessions were not significantly higher after EM1 sessions that produced high responding rates than after EM1 sessions producing low responding rates (p=0.56, paired two-tailed t-test), i.e., conditioning effects between consecutive sessions were not detectable, and unlikely to affect our results.
Results
Demarcation of intracranial infusion sites
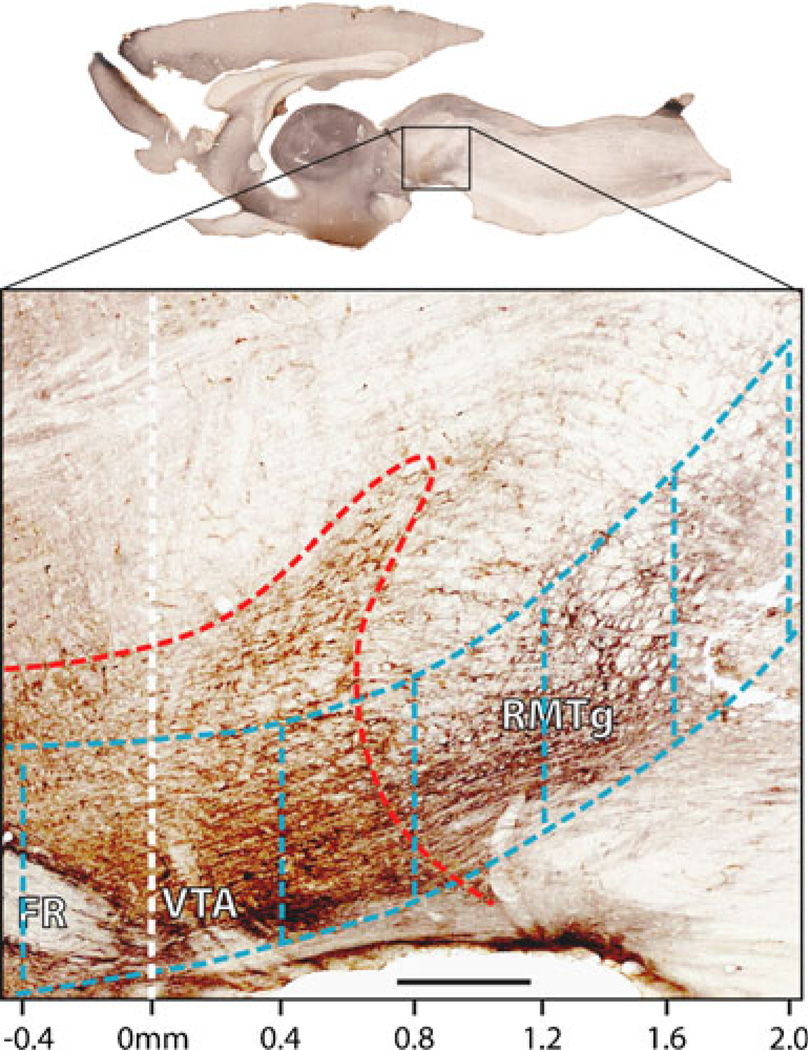
Of 38 rats tested for EM1 ICSA, seven were excluded from analysis due to very low rates of leverpressing (below 10 per session during ACSF sessions). In the remaining rats, 135 distinct midbrain sites were tested (Fig. 1). Cannula placements were compared to MOR immunostaining (Fig. 1h), and the anterior–posterior (AP) coordinate of each cannula site was determined relative to the rostral tip of the IPN (Fig. 2, white vertical line).
Fig. 2.
Parasagittal map of regions used in anterior–posterior analysis. Section roughly 0.5 mm lateral to midline plane shows brown immunostaining for tyrosine hydroxylase (TH; red dashed outline), and bluish black immunostaining for MOR, indicating RMTg region. Scale along bottom indicates anterior–posterior (AP) distance relative to rostral IPN tip (not visible in this section). Blue dashed outlines delineate a series of 0.4-mm-thick rostrocaudal slabs that form a column of tissue passing through the VTA, RMTg, and sites further caudal. Six slabs are visible from eight used for analysis. Scale bar: 400 µm
Rats self-administered an average of 27±3 (mean± standard error) infusions of ACSF vehicle per session, a value which did not differ between the RMTg, VTA, and the adjacent CLi (p=0.72, ANOVA). At sites within 0.5 mm of the RMTg center, EM1 self-administration rates were invariably higher for EM1 than ACSF vehicle (Fig. 1j), an effect not seen in the VTA or sites just caudal to the RMTg (Fig. 1i,k). Because individuals with higher baseline ACSF infusion rates also showed proportionally greater EM1-induced increases (Fig. 1j), for statistical analyses all infusion rates were normalized to the ACSF infusion rate for that particular animal.
Comparisons between infusion sites
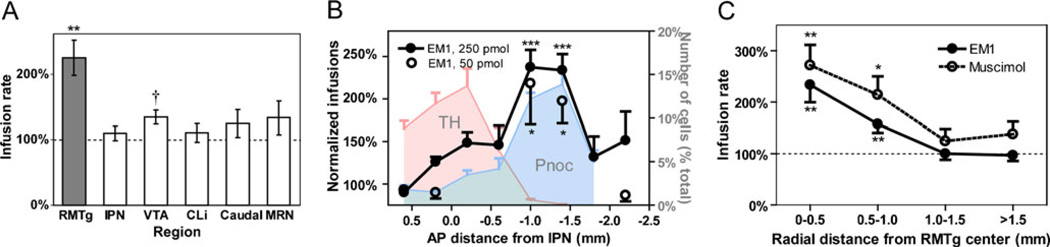
EM1 self-administration rates were elevated to 225±27 % of vehicle rates for sites in the RMTg, a highly significant increase (p=0.0004; Fig. 3a). This elevation was not present for adjacent sites in the IPN (n=6, p=0.43), median raphe nucleus (MRN, n=6, p=0.24), and CLi (n=6, p=0.5; Fig. 3a). EM1 self-administration rates into the VTA were mildly elevated, to 135±11 % of vehicle rates (n=8, p=0.012), but after adjusting for multiple comparisons, this change did not meet criteria for statistical significance.
Fig. 3.
EM1 is self-administered into the RMTg more avidly than adjacent regions. a EM1 self-administration rates are elevated in the RMTg (filled bar) but not VTA, IPN, CLi, or MRN (open bars). b EM1 self-administration rates vary with anterior–posterior coordinate, and are shown for the 50 pmol/infusion dose (open symbols) and 250 pmol/infusion dose (filled symbols and lines). On the same graph are plotted the distribution of tyrosine hydroxylase (TH)-immunoreactive neurons (leftmost shaded region, right-hand y-axis), a marker of DA neruons, and the distribution of prepronociceptin (Pnoc) (rightmost shaded region), a marker of RMTg neurons. c EM1 and muscimol are self-administered at similar rates into the RMTg, and both rates decline with increasing distance from the RMTg center. **p<0.002, *p<0.01, †p<0.02
We next compared EM1 self-administration rates across rostral–caudal sites residing within a 1-mm diameter column of tissue passing through the VTA, RMTg, and sites immediately caudal (Fig. 2, blue dashed lines). We segmented this column of tissue into five coronal slabs corresponding to the “anterior VTA” (AP distances −0.8 to 0 mm relative to reference plane), “posterior VTA” (AP 0 to 0.8 mm), “anterior RMTg” (AP 0.8 to 1.2 mm), “posterior RMTg” (AP 1.2 to 1.6 mm) and “caudal sites” (AP >1.6 mm). EM1 infusion rates varied significantly between groups (ANOVA, F4,22 = 7.9, p=0.00042), with the highest rates seen in the anterior and posterior RMTg (Fig. 3b), which reached 243±29 % and 213±15 % over vehicle infusion rates; both values were significantly higher than ACSF infusion rates (p=0.0001 and p=7×10−5, t-test, respectively), and also significantly higher than EM1 infusion rates into the VTA or regions entirely caudal to the RMTg (p<0.05 each comparison, Tukey HSD post-hoc test).
We also examined the anterior–posterior distribution of markers for DA and RMTg neurons. Using tissue from four rats, we counted the number of neurons expressing TH at the same coronal levels used to categorize infusion sites. Most TH neurons resided anteriorly to the sites where EM1’s behavioral effects were highest (Fig. 3b, light grey-shaded region; Table 1). In adjacent sections, we counted neurons expressing in situ hybridization for prepronociceptin (Pnoc), a gene that encodes the opioid-like peptide nociceptin (also called orphanin FQ), and that colocalizes heavily with existing RMTg markers (Morales et al. 2011). Pnoc neurons were most numerous at AP coordinates 0.8–1.6 mm caudal to the reference plane, matching the regions of highest MOR immunoreactivity where EM1 behavioral effects were highest (Fig. 3b, dark grey-shaded region; Table 1).
Table 1.
Cell counts of markers of DA and RMTg neurons
| AP (mm) | TH cells/section | Pnoc cells/section |
|---|---|---|
| 1.0 | 70±28 | 10±10 |
| .6 | 186±18 | 16±4 |
| .2 | 246±24 | 14±2 |
| −0.2 | 288±44 | 30±4 |
| −0.6 | 134±56 | 36±10 |
| −1.0 | 12±4 | 104±6 |
| −1.4 | 2±0 | 120±16 |
| −1.8 | 0±0 | 42±12 |
Counts of neurons expressing tyrosine hydroxylase (TH) immunohistochemistry or prepronociceptin (Pnoc) mRNA are given per 40-µm section, as a function of anterior–posterior (AP) distance from the rostral edge of the interpeduncular nucleus
Comparison of different doses
Most rats were tested using a 250 pmol/infusion dose of EM1, but in six rats we also tested self-administration of a lower dose (50 pmol/infusion). In nine sessions with these animals, infusion sites were within 0.5 mm of the RMTg center (Fig. S1). Prior work showed that this lower dose produced smaller increases in self-administration rates than the larger dose when infused into the posterior VTA (Zangen et al. 2002). However, in the present study, self-administration rates into the RMTg were 229±13 % of ACSF rates, not significantly different from infusion rates for the higher dose (p=0.52, paired t-test), again consistent with the hypothesis that EM1 is more effective in the RMTg than VTA.
Similar effects of MOR and GABA agonists in the RMTg
We had hypothesized that MOR agonists’ rewarding effects are mediated by the inhibition of RMTg neurons; hence, we examined whether rats would self-administer the GABA agonist muscimol into the RMTg. Indeed, self-administration of muscimol within 0.5 mm of the RMTg center (Fig. S2) occurred at 272±40 % of ACSF rates, a significant increase over vehicle (p=0.001, n=11; Fig. 3c), and similar to the rates seen for EM1. At progressively farther distances from the RMTg center, ICSA rates for both muscimol and EM1 declined steadily (Fig. 3c).
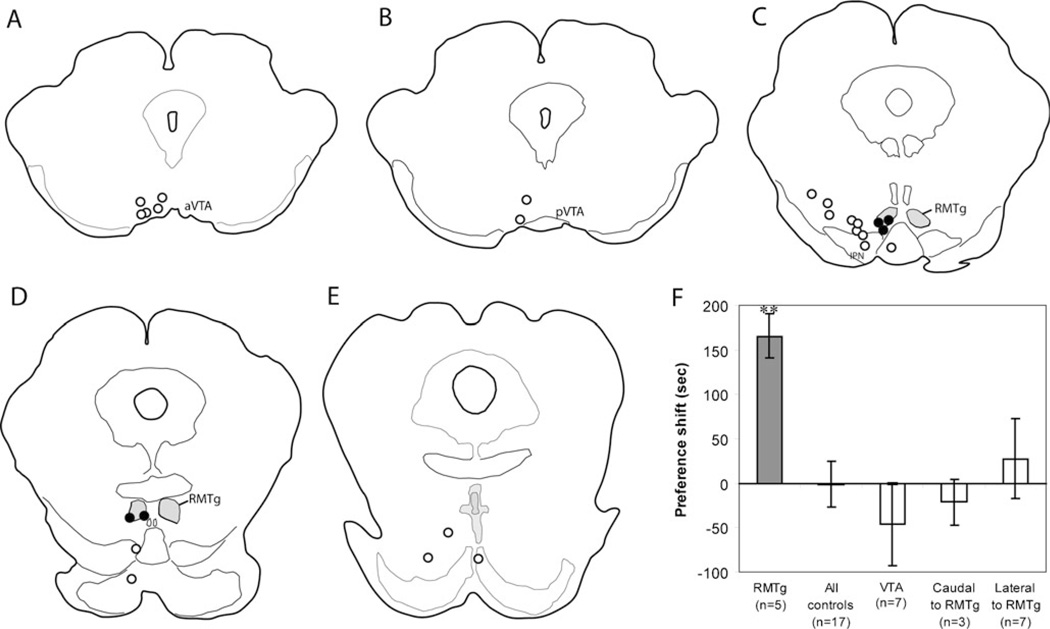
Conditioned place preference after EM1 infusions into the RMTg
Although we had hypothesized a rewarding effect of EM1, elevated ICSA rates can be due to either rewarding effects of drug, or acute motor activating effects (or both). Hence, we examined whether EM1 infusions into the RMTg produce CPP, a paradigm in which animals are tested free of drug, largely avoiding acute drug effects. In a new group of 24 rats, five rats had cannula sites residing within 0.5 mm of the RMTg center (Fig. 4), while the remaining 19 rats had cannula placements caudal, lateral, or rostral to the RMTg. After conditioning, RMTg-injected rats spent 166±25 s longer in the EM1-paired chamber, relative to their preconditioning baseline preference, a significant increase (p= 0.003; Fig. 4). Preference scores for rats with cannulae in the VTA (n=7), or sites lateral to the RMTg (n=7) were not different from baselines (p=0.29, and 0.78, respectively). Three rats with cannula sites caudal to the RMTg, and two sites ventral to the RMTg, did not constitute sufficiently large groups for statistical analysis, but when all control sites were pooled into a single group, no preference (or aversion) for the EM1-paired chamber was seen (p=0.9, n=19).
Fig. 4.
a–e Map of cannula locations for place preference experiment, in order from rostral to caudal. Sites within 0.5 mm of the RMTg center are indicated by filled circles; other sites are indicated by open circles. f Place preference is seen for EM1 injection sites within 0.5 mm of the RMTg center (filled bar), but not sites rostral, caudal, or lateral (open bars). Notably, seven sites in the VTA did not elicit significant conditioned place preference. **p=0.003
EM1 infusions into the RMTg markedly attenuate formalin-induced pain
Numerous studies suggest that rewarding and aversive stimuli are processed by overlapping brain substrates. Hence, we tested whether EM1 infusions into the RMTg would modulate formalin-induced pain behaviors. In six rats with cannulae targeted at the RMTg, five had cannulae tips residing within a 0.5-mm radius of the RMTg center (delineated by MOR staining). The sixth site was rostral and dorsal to this region, and was discarded from further analysis. As the 24-min test sessions exceeded the expected halflife of EM1 in brain tissue, initial bolus infusions (1 nmol) were followed by 100 pmol/min of drug delivered throughout the session, a rate similar to that seen in most EM1 ICSA sessions, and intended to produce relatively constant drug concentrations during the session.
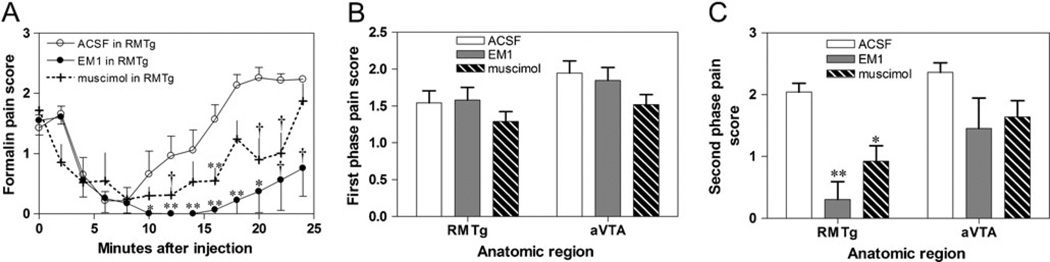
When hindpaw formalin injections were accompanied by vehicle in the RMTg, pain behaviors were elevated in two distinct phases, consistent with prior reports (Dubuisson and Dennis 1977) (Fig. 5a). Hence, we calculated average pain scores during two time windows, the first spanning 0–4 min post-injection, and the second spanning 16–24 min post-injection. Prior studies noted that opioids do not attenuate the first phase of pain (Dubuisson and Dennis 1977), and we saw similar effects after EM1 infusions into the RMTg, which produced a marked 85 % reduction in formalin pain scores during the second phase (from 2.0±0.17 to 0.31± 0.29, p=0.0016, t-test; Fig. 5a,c), but no significant difference in the first phase (p=0.78; Fig. 5a,b). Muscimol infusions into the RMTg produced a more modest, but still significant 55 % decrease in second phase pain scores relative to ACSF infusions (p=0.008; Fig. 5a,c).
Fig. 5.
a Formalin injections into the plantar surface of the hindpaw elicit pain behaviors in two temporal phases (open circles). The first is not influenced by continuous EM1 infusion into the RMTg (b, grey filled bar), while the second phase is strongly attenuated (c, grey filled bar). Infusions of muscimol produced similar results but with a somewhat lesser attenuation of pain behaviors (a plus symbols and dashed traces, b–c hashed bars). Infusions into the anterior VTA (aVTA) did not produce significant reductions in pain scores. **p<0.002, p<0.01, †p<0.05
Effects of EM1 and muscimol in rostral VTA are weaker than in RMTg
We next placed bilateral cannulae into the rostral VTA in a separate group of six rats. Histological reconstruction showed that five pairs of cannulae resided in the rostral VTA, while the sixth resided caudally, and was excluded from further analysis. Neither EM1 nor muscimol infusions into the rostral VTA altered pain scores in the first phase (p=0.40 and 0.46, respectively; Fig. 5b). In the second phase, EM1 and muscimol produced effects that did not reach significance, but were likely trending toward reductions (42 % reduction in formalin pain after EM1, p=0.06 and 24 % reduction after muscimol, p=0.09) (Fig. 5c). Hence, a role for the VTA appears possible, but appears to be weaker than the RMTg role.
Discussion
We found that rats self-administered EM1 into the RMTg much more avidly than into the VTA, IPN, CLi, MRN, and other nearby sites. EM1 infusions into the RMTg region, but not adjacent sites, also elicited CPP, while EM1 infusions into the RMTg produced larger reductions in formalin pain than injections into the rostral VTA. These results are consistent suggest that the most effective sites for these actions are not in the VTA, but rather at sites more caudal. Furthermore, we found that muscimol and EM1 had similar effects on both ICSA and pain behaviors in the RMTg, consistent with prior electrophysiological findings that MOR agonists uniformly inhibit neurons in the RMTg region (Lecca et al. 2011a; Matsui and Williams 2011) and that the RMTg mediates opioid induced disinhibitory effects on DA neurons (Jalabert et al. 2011; Lecca et al. 2011a, b; Matsui and Williams 2011). Prior studies had also found that morphine-inhibited neurons in the RMTg are activated by habenular stimulation and aversive sensory stimuli (Lecca et al. 2011a), again consistent with previously identified properties of RMTg neurons (Hong et al. 2011), and consistent with a link between opioid effects, RMTg functions, and motivated behavior.
Methodological concerns
Because of the proximity of the RMTg to other regions having distinct anatomical, and functional properties, we tried to minimize drug spread using small injections of a short-acting agonist. However, some drug necessarily spread to distal sites, with initial diffusion likely occurring preferentially in a dorsal direction along the cannula track. This could be a concern as the periaqueductal gray (PAG), a known site of opioid actions, resides 2–3 mm dorsal to the RMTg. However, we found that self-administration rates for EM1, although elevated within the RMTg, were never elevated at sites just 0.5 mm ventral, suggesting that EM1 dorsal spread is less than 0.5 mm. ICSA rates within the PAG also were not elevated above vehicle, further suggesting that diffusion into the PAG is not a likely explanation of elevated ICSA rates. Although muscimol’s longer duration of effect could have led to greater diffusion than with EM1, our previous study that investigated self-administration effects of muscimol in the dorsal and median raphe nuclei (Liu and Ikemoto 2007) suggests that muscimol’s effects are confined with 0.5 mm of these regions.
Although EM1 is less extensively studied than synthetic opioids such as morphine, it is notably produced endogenously, e.g., by tuberomammillary neurons in the posterior hypothalamus (Greco et al. 2008; Martin-Schild et al. 1999), which project to the RMTg (Jhou et al. 2009a; Fig. 5O in cited work). Hence, the current results may suggest a possible novel mechanism of action of endogenous opioids.
Relationship to studies of the VTA
Numerous prior studies had shown behavioral effects of MOR agonists in the VTA, albeit often without use of anatomic controls, or other measures to control for drug spread. For example, early ICSA studies often used morphine (Bozarth and Wise 1981b), which remains active for hours when injected into the brain (Houdi et al. 1996), while in contrast EM1’s half-life in brain homogenates is estimated at 6 min (Perlikowska et al. 2009), and its effects on motivated behaviors dissipate within 15 min after intracranial injections (Fichna et al. 2007). Although few prior studies had examined multiple locations around the VTA, one notable study (Zangen et al. 2002) found that EM1 was self-administered more avidly into the posterior than anterior VTA, and produced CPP in the posterior but not anterior VTA, strikingly consistent with the current findings.
Our finding that EM1 into the VTA did not produce CPP differs from prior studies, again possibly due to our use of smaller doses of shorter-acting drugs, shorter conditioning sessions, and more detailed anatomic analyses. Again, some prior studies used morphine with 30- to 45-min-long CPP conditioning sessions (Nader and van der Kooy 1997; Olmstead and Franklin 1997), again increasing the likelihood of diffusion, whereas we used 15-min conditioning sessions and a relatively low 1.0 nmol dose of EM1. Although we saw somewhat lower levels of place preference than previous reports (Olmstead and Franklin 1997), effects were still consistent and highly significant. One other study did report CPP after injections of small doses (1.6 nmol) of EM1 into the VTA (Terashvili et al. 2004), but their injection sites resided at posterior levels likely to overlap the rostral portion of the RMTg. Hence, the current results may be more consistent with existing literature than initially apparent.
Interaction of RMTg with other sites regulating motivated behavior
Prior studies had shown that opioid effects on both reward and analgesia can be blocked by DA receptor antagonists (Altier and Stewart 1998; Bozarth and Wise 1981a; David et al. 2002; Morgan and Franklin 1991; Shippenberg et al. 1993). However, non-DA mechanisms may also be important, as studies by van der Kooy and colleagues note that morphine reward in drug-naïve animals is dependent on the pedunculopontine nucleus (PPTg) (Nader and van der Kooy 1997; Vargas-Perez et al. 2009). Notably, the RMTg projects strongly to both DA neurons and to the PPTg (Jhou et al. 2009b; Lavezzi et al. 2011; Lavezzi and Zahm 2011), and further studies are needed to determine whether these targets mediate the observed effects. The RMTg also receives direct afferents from at least two regions, the ventral PAG and lateral habenula (Jhou et al. 2009b; Kaufling et al. 2009), where opioid injections produce analgesia and/or reward (Cohen and Melzack 1985; Manning et al. 1994; Olmstead and Franklin 1997; Yaksh et al. 1976). Yet another site residing in the posterior hypothalamus 1–2 mm rostral to the VTA has been implicated in opioid analgesia (Manning and Franklin 1998; Olmstead and Franklin 1997), but the current study did not examine sites this far rostral. Hence, further studies are needed to examine relationships of the RMTg to a larger network of MOR-sensitive brain regions regulating both appetitive and aversive behavior.
Supplementary Material
Acknowledgments
We acknowledge Toni Shippenberg and Vicky Minney for helpful technical advice. This work was supported by the Intramural Research Program of the National Institute on Drug Abuse, National Institutes of Health.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00213-012-2753-6) contains supplementary material, which is available to authorized users.
Conflict of interest The authors declare no competing financial interests with the work described in this paper.
Contributor Information
Thomas C. Jhou, Email: tomjhou@gmail.com, Behavioral Neuroscience Branch, National Institute on Drug Abuse, National Institutes of Health, Department of Health and Human Services, Baltimore, MD, USA; Department of Neurosciences, Medical University of South Carolina, 173 Ashley Avenue, Charleston, SC 29425, USA.
Sheng-Ping Xu, Behavioral Neuroscience Branch, National Institute on Drug Abuse, National Institutes of Health, Department of Health and Human Services, Baltimore, MD, USA.
Mary R. Lee, Neuroimaging Research Branch, National Institute on Drug Abuse, National Institutes of Health, Department of Health and Human Services, Baltimore, MD, USA
Courtney L. Gallen, Neuroimaging Research Branch, National Institute on Drug Abuse, National Institutes of Health, Department of Health and Human Services, Baltimore, MD, USA
Satoshi Ikemoto, Behavioral Neuroscience Branch, National Institute on Drug Abuse, National Institutes of Health, Department of Health and Human Services, Baltimore, MD, USA.
References
- Altier N, Stewart J. Dopamine receptor antagonists in the nucleus accumbens attenuate analgesia induced by ventral tegmental area substance P or morphine and by nucleus accumbens amphetamine. J Pharmacol Exp Ther. 1998;285:208–215. [PubMed] [Google Scholar]
- Altier N, Stewart J. The role of dopamine in the nucleus accumbens in analgesia. Life Sci. 1999;65:2269–2287. doi: 10.1016/s0024-3205(99)00298-2. [DOI] [PubMed] [Google Scholar]
- Balcita-Pedicino JJ, Omelchenko N, Bell R, Sesack SR. The inhibitory influence of the lateral habenula on midbrain dopamine cells: ultrastructural evidence for indirect mediation via the rostromedial mesopontine tegmental nucleus. J Comp Neurol. 2011;519:1143–1164. doi: 10.1002/cne.22561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bals-Kubik R, Ableitner A, Herz A, Shippenberg TS. Neuroan-atomical sites mediating the motivational effects of opioids as mapped by the conditioned place preference paradigm in rats. J Pharmacol Exp Ther. 1993;264:489–495. [PubMed] [Google Scholar]
- Basbaum AI, Fields HL. Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Annu Rev Neurosci. 1984;7:309–338. doi: 10.1146/annurev.ne.07.030184.001521. [DOI] [PubMed] [Google Scholar]
- Bozarth MA, Wise RA. Heroin reward is dependent on a dopaminergic substrate. Life Sci. 1981a;29:1881–1886. doi: 10.1016/0024-3205(81)90519-1. [DOI] [PubMed] [Google Scholar]
- Bozarth MA, Wise RA. Intracranial self-administration of morphine into the ventral tegmental area in rats. Life Sci. 1981b;28:551–555. doi: 10.1016/0024-3205(81)90148-x. [DOI] [PubMed] [Google Scholar]
- Chou TC, Lee CE, Lu J, Elmquist JK, Hara J, Willie JT, Beuckmann CT, Chemelli RM, Sakurai T, Yanagisawa M, Saper CB, Scammell TE. Orexin (hypocretin) neurons contain dynorphin. J Neurosci. 2001;21:RC168. doi: 10.1523/JNEUROSCI.21-19-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou TC, Bjorkum AA, Gaus SE, Lu J, Scammell TE, Saper CB. Afferents to the ventrolateral preoptic nucleus. J Neurosci. 2002;22:977–990. doi: 10.1523/JNEUROSCI.22-03-00977.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SR, Melzack R. Morphine injected into the habenula and dorsal posteromedial thalamus produces analgesia in the formalin test. Brain Res. 1985;359:131–139. doi: 10.1016/0006-8993(85)91420-9. [DOI] [PubMed] [Google Scholar]
- David V, Durkin TP, Cazala P. Differential effects of the dopamine D2/D3 receptor antagonist sulpiride on self-administration of morphine into the ventral tegmental area or the nucleus accumbens. Psychopharmacology (Berl) 2002;160:307–317. doi: 10.1007/s00213-001-0981-2. [DOI] [PubMed] [Google Scholar]
- Devine DP, Wise RA. Self-administration of morphine, DAMGO, and DPDPE into the ventral tegmental area of rats. J Neurosci. 1994;14:1978–1984. doi: 10.1523/JNEUROSCI.14-04-01978.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding YQ, Kaneko T, Nomura S, Mizuno N. Immunohistochemical localization of mu-opioid receptors in the central nervous system of the rat. J Comp Neurol. 1996;367:375–402. doi: 10.1002/(SICI)1096-9861(19960408)367:3<375::AID-CNE5>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Dubuisson D, Dennis SG. The formalin test: a quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats. Pain. 1977;4:161–174. doi: 10.1016/0304-3959(77)90130-0. [DOI] [PubMed] [Google Scholar]
- Fichna J, Janecka A, Piestrzeniewicz M, Costentin J, do Rego JC. Antidepressant-like effect of endomorphin-1 and endomorphin-2 in mice. Neuropsychopharmacol. 2007;32:813–821. doi: 10.1038/sj.npp.1301149. [DOI] [PubMed] [Google Scholar]
- Greco MA, Fuller PM, Jhou TC, Martin-Schild S, Zadina JE, Hu Z, Shiromani P, Lu J. Opioidergic projections to sleep-active neurons in the ventrolateral preoptic nucleus. Brain Res. 2008;1245:96–107. doi: 10.1016/j.brainres.2008.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Jhou TC, Smith M, Saleem KS, Hikosaka O. Negative reward signals from the lateral habenula to dopamine neurons are mediated by rostromedial tegmental nucleus in primates. J. Neurosci. 2011 doi: 10.1523/JNEUROSCI.1384-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houdi AA, Kottayil S, Crooks PA, Butterfield DA. 3-O-acetylmorphine-6-O-sulfate: a potent, centrally acting morphine derivative. Pharmacol Biochem Behav. 1996;53:665–671. doi: 10.1016/0091-3057(95)02067-5. [DOI] [PubMed] [Google Scholar]
- Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev. 2007;56:27–78. doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S, Donahue KM. A five-minute, but not a fifteen-minute, conditioning trial duration induces conditioned place preference for cocaine administration into the olfactory tubercle. Synapse. 2005;56:57–59. doi: 10.1002/syn.20124. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Sharpe LG. A head-attachable device for injecting nanoliter volumes of drug solutions into brain sites of freely moving rats. J Neurosci Methods. 2001;110:135–140. doi: 10.1016/s0165-0270(01)00428-9. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Murphy JM, McBride WJ. Regional differences within the rat ventral tegmental area for muscimol self-infusions. Pharmacol Biochem Behav. 1998;61:87–92. doi: 10.1016/s0091-3057(98)00086-0. [DOI] [PubMed] [Google Scholar]
- Jalabert M, Bourdy R, Courtin J, Veinante P, Manzoni OJ, Barrot M, Georges F. Neuronal circuits underlying acute morphine action on dopamine neurons. Proc Natl Acad Sci U S A. 2011;108:16446–16450. doi: 10.1073/pnas.1105418108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhou T. Neural mechanisms of freezing and passive aversive behaviors. J Comp Neurol. 2005;493:111–114. doi: 10.1002/cne.20734. [DOI] [PubMed] [Google Scholar]
- Jhou TC, Fields HL, Baxter MG, Saper CB, Holland PC. The rostromedial tegmental nucleus (RMTg), a GABAergic afferent to midbrain dopamine neurons, encodes aversive stimuli and inhibits motor responses. Neuron. 2009a;61:786–800. doi: 10.1016/j.neuron.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhou TC, Geisler S, Marinelli M, Degarmo BA, Zahm DS. The mesopontine rostromedial tegmental nucleus: a structure targeted by the lateral habenula that projects to the ventral tegmental area of Tsai and substantia nigra compacta. J Comp Neurol. 2009b;513:566–596. doi: 10.1002/cne.21891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SW, North RA. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci. 1992;12:483–488. doi: 10.1523/JNEUROSCI.12-02-00483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufling J, Veinante P, Pawlowski SA, Freund-Mercier MJ, Barrot M. Afferents to the GABAergic tail of the ventral tegmental area in the rat. J Comp Neurol. 2009;513:597–621. doi: 10.1002/cne.21983. [DOI] [PubMed] [Google Scholar]
- Lavezzi HN, Zahm DS. The mesopontine rostromedial tegmental nucleus: an integrative modulator of the reward system. Basal Ganglia. 2011;1:191–200. doi: 10.1016/j.baga.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavezzi HN, Parsley KP, Zahm DS. Mesopontine rostromedial tegmental nucleus neurons projecting to the dorsal raphe and pedunculopontine tegmental nucleus: psychostimulant-elicited Fos expression and collateralization. Brain Struct Funct. 2011 doi: 10.1007/s00429-011-0368-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Merrer J, Becker JA, Befort K, Kieffer BL. Reward processing by the opioid system in the brain. Physiol Rev. 2009;89:1379–1412. doi: 10.1152/physrev.00005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecca S, Melis M, Luchicchi A, Ennas MG, Castelli MP, Muntoni AL, Pistis M. Effects of drugs of abuse on putative rostromedial tegmental neurons, inhibitory afferents to midbrain dopamine cells. Neuropsychopharmacology. 2011a;36:589–602. doi: 10.1038/npp.2010.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecca S, Melis M, Luchicchi A, Muntoni AL, Pistis M. Inhibitory inputs from rostromedial tegmental neurons regulate spontaneous activity of midbrain dopamine cells and their responses to drugs of abuse. Neuropsychopharmacology. 2011b doi: 10.1038/npp.2011.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning BH, Franklin KB. Morphine analgesia in the formalin test: reversal by microinjection of quaternary naloxone into the posterior hypothalamic area or periaqueductal gray. Behav Brain Res. 1998;92:97–102. doi: 10.1016/s0166-4328(97)00130-7. [DOI] [PubMed] [Google Scholar]
- Manning BH, Morgan MJ, Franklin KB. Morphine analgesia in the formalin test: evidence for forebrain and midbrain sites of action. Neurosci. 1994;63:289–294. doi: 10.1016/0306-4522(94)90023-x. [DOI] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Burke S, Meng F, Thompson RC, Akil H, Watson SJ. Mu, delta, and kappa opioid receptor mRNA expression in the rat CNS: an in situ hybridization study. J Comp Neurol. 1994;350:412–438. doi: 10.1002/cne.903500307. [DOI] [PubMed] [Google Scholar]
- Martin-Schild S, Gerall AA, Kastin AJ, Zadina JE. Differential distribution of endomorphin 1- and endomorphin 2-like immunoreactivities in the CNS of the rodent. J Comp Neurol. 1999;405:450–471. [PubMed] [Google Scholar]
- Matsui A, Williams JT. Opioid-sensitive GABA inputs from rostromedial tegmental nucleus synapse onto midbrain dopamine neurons. J Neurosci. 2011;31:17729–17735. doi: 10.1523/JNEUROSCI.4570-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger M, Goncalves L, Sego C. Immunohistochemical and topographical characterization of the rostromedial tegmental nucleus. Soc Neurosci Abstr 916.25. 2010 [Google Scholar]
- Morales M, Wang H, Zhang P, Ikemoto S, Jhou TC. Expression of prepronociceptin in the rostromedial tegmentum (RMTg) Soc Neurosci Abstr. 2011 [Google Scholar]
- Morgan MJ, Franklin KB. Dopamine receptor subtypes and formalin test analgesia. Pharmacol Biochem Behav. 1991;40:317–322. doi: 10.1016/0091-3057(91)90560-o. [DOI] [PubMed] [Google Scholar]
- Nader K, van der Kooy D. Deprivation state switches the neurobiological substrates mediating opiate reward in the ventral tegmental area. J Neurosci. 1997;17:383–390. doi: 10.1523/JNEUROSCI.17-01-00383.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Henriksen SJ, Mattox A, Pasternak GW, Portoghese PS, Takemori AE, Weinger MB, Koob GF. Effect of antagonists selective for mu, delta and kappa opioid receptors on the reinforcing effects of heroin in rats. J Pharmacol Exp Ther. 1993;265:1245–1252. [PubMed] [Google Scholar]
- Olmstead MC, Franklin KB. The development of a conditioned place preference to morphine: effects of microinjections into various CNS sites. Behav Neurosci. 1997;111:1324–1434. doi: 10.1037//0735-7044.111.6.1324. [DOI] [PubMed] [Google Scholar]
- Perlikowska R, Gach K, Fichna J, Toth G, Walkowiak B, do-Rego JC, Janecka A. Biological activity of endomorphin and [Dmt1] endomorphin analogs with six-membered proline surrogates in position 2. Bioorg Med Chem. 2009;17:3789–3794. doi: 10.1016/j.bmc.2009.04.046. [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, Bals-Kubik R, Herz A. Examination of the neurochemical substrates mediating the motivational effects of opioids: role of the mesolimbic dopamine system and D-1 vs. D-2 dopamine receptors. J Pharmacol Exp Ther. 1993;265:53–59. [PubMed] [Google Scholar]
- Simmons DM, Arriza JL, Swanson LW. A complete protocol for in situ hybridization of messenger RNAs in brain and other tissues with radio-labeled single-stranded RNA probes. J Histotechnol. 1989;3:169–181. [Google Scholar]
- Terashvili M, Wu HE, Leitermann RJ, Hung KC, Clithero AD, Schwasinger ET, Tseng LF. Differential conditioned place preference responses to endomorphin-1 and endomorphin-2 microinjected into the posterior nucleus accumbens shell and ventral tegmental area in the rat. J Pharmacol Exp Ther. 2004;309:816–824. doi: 10.1124/jpet.103.059287. [DOI] [PubMed] [Google Scholar]
- Vargas-Perez H, Ting AKR, van der Kooy D. Different neural systems mediate morphine reward and its spontaneous withdrawal aversion. Eur J Neurosci. 2009;29:2029–2034. doi: 10.1111/j.1460-9568.2009.06749.x. [DOI] [PubMed] [Google Scholar]
- Welzl H, Kuhn G, Huston JP. Self-administration of small amounts of morphine through glass micropipettes into the ventral tegmental area of the rat. Neuropharmacology. 1989;28:1017–1023. doi: 10.1016/0028-3908(89)90112-3. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev. 1987;94:469–492. [PubMed] [Google Scholar]
- Yaksh TL, Yeung JC, Rudy TA. Systematic examination in the rat of brain sites sensitive to the direct application of morphine: observation of differential effects within the periaqueductal gray. Brain Res. 1976;114:83–103. doi: 10.1016/0006-8993(76)91009-x. [DOI] [PubMed] [Google Scholar]
- Zangen A, Ikemoto S, Zadina JE, Wise RA. Rewarding and psychomotor stimulant effects of endomorphin-1: anteroposterior differences within the ventral tegmental area and lack of effect in nucleus accumbens. J Neurosci. 2002;22:7225–7233. doi: 10.1523/JNEUROSCI.22-16-07225.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.