Abstract
Rats emit ultrasonic vocalizations (USVs) in a variety of contexts, and it is increasingly clear that USVs reflect more complex information than mere positive and negative affect states. We sought to examine USVs in a common model of addiction and relapse, the self-administration/reinstatement paradigm, in order to gain insight into subjective states experienced by rats during various types of methamphetamine seeking. We measured three subtypes of “50kHz” USVs [flats, trills, and non-trill frequency modulated USVs (FMs)], as well as long and short duration “22kHz” USVs, during self-administration and extinction training, and during reinstatement elicited by cues, a methamphetamine prime, cues + prime, or the pharmacological stressor yohimbine. During self-administration and extinction, rats emitted many flats and FMs, (and short duration “22kHz” USVs on day 1 of self-administration), but few trills. In contrast, methamphetamine priming injections potently enhanced FMs and trills, and trill production was correlated with the degree of methamphetamine + cue-elicited reinstatement. Cues alone yielded increases only in flat USVs during reinstatement, though a subset of rats displaying strong cue-induced reinstatement also emitted long duration, aversion-related “22kHz” USVs. Although yohimbine administration caused reinstatement, it did not induce “22kHz” USVs in methamphetamine-experienced or methamphetamine-naïve rats (unlike footshock stress, which did induce long duration “22kHz” USVs). These findings demonstrate heterogeneity of rat USVs emitted during different types of methamphetamine seeking, and highlight their potential usefulness for gaining insight into the subjective states of rats in rodent models of drug addiction and relapse.
Keywords: addiction, ultrasonic vocalizations, reinstatement, methamphetamine, cues, stress
1. Introduction
In clinical studies, subjects can be queried about the subjective effects of drugs, as well as the affective states and craving they experience during situations that cause relapse to drug use, such as exposure to drug-associated cues, stressors, or “priming” doses of the drug itself [1–3]. In contrast, an inherent limitation of animal models of addiction has been the lack of similar indices of subjective drug effects and drug seeking states.
Behavioral neuroscientists using animal models have access to a wide range of tasks modeling addictive behavior and relapse. One prominent model is the self-administration/reinstatement paradigm. As in humans, drug cues, stressors, and drug primes cause reinstatement of drug seeking in rats [4–6], and therefore can be used to examine a key aspect of addiction—its chronic relapsing nature. However, considerable ambiguity exists regarding the subjective states experienced during drug seeking and relapse, especially in animals. In humans, exposure to drug cues in the absence of drug availability causes both pleasurable, drug-like effects [7–10], as well as subjective distress and negative affect [11–17]. In contrast, the affective states experienced by rodent subjects remain unclear. This issue is of considerable theoretical importance for understanding the psychological substrates of relapse, given that some have emphasized the role of negative affect as a relapse risk [18, 19], while others have instead emphasized the incentive motivational properties of drug cues, which need not entail stress-like states [20–24].
Measurement of spontaneously emitted ultrasonic vocalizations (USVs) could offer an opportunity to examine the subjective states of rats in addiction paradigms. Adult rats emit USVs in many situations, which have traditionally been grouped into two main categories—“50kHz” (usually associated with positive affective or motivational states), and “22kHz” (usually associated with negative affective states)[25–30].
However, mounting evidence suggests that USVs are much more than simple appetitive and aversive signals, but are in fact complex affective and communicative signals that can reflect motivation, anticipation, aggression, social communication, aspects of sexual behavior, aversion, pain, drug withdrawal, and many other states [27, 30–35]. “50kHz” vocalizations occur in at least 14 different subtypes [33], though differences between the functions of most of these are unknown. However, there is evidence that “50kHz” USVs varying little in frequency over time (flats), and those characterized by frequency modulation over time (either with or without a rapidly oscillating “trill” pattern) are differentially produced based upon behavioral context and due to experimental manipulations. For example, administration of psychostimulant drugs preferentially elicits trills and other non-trill frequency-modulated vocalizations (FMs), production of which sensitize with repeated drug administration [33, 36–39], and they have been proposed to reflect positive affective states [27, 28]. FMs and trills are also emitted preferentially in “sign tracking” animals during a cocaine conditioned place preference task, and therefore may reflect incentive salience of rewards and their cues [40]. In addition, “22kHz” USVs are emitted in both long and short durations, which may reflect stronger vs. weaker aversion, respectively [32, 41, 42].
While there has been some limited exploration of rodent USVs emitted during cocaine self-administration [41, 43–45] and reinstatement/relapse [43], no studies to date have examined USVs during methamphetamine taking or seeking, or differences between production of USV subtypes in these tasks. As such, we sought to examine the affective states of rats as measured with spontaneously emitted USVs during methamphetamine self-administration, extinction, and different types of reinstatement.
2. Material and Methods
2.1 Subjects
Male Long-Evans rats (n=15, initial weight 250–300g, Charles River Laboratories, Raleigh, NC, USA) were individually housed upon arrival from the vendor in a temperature- and humidity-controlled vivarium on a reverse 12hr light-dark cycle (lights off at 06:00). All experimental procedures occurred between 07:00 and 16:00. In the home cage, rats had access to water ad libitum and were maintained on a controlled diet (20–25g/day) of standard rat chow (Harlan, Indianapolis, IN, USA) for the duration of each experiment. All experimental procedures were approved by the Institutional Animal Care and Use Committee of the Medical University of South Carolina and conformed to Federal guidelines as described in the “Guide for the Care and Use of Laboratory Rats” of the Institute of Laboratory Animal Resources on Life Sciences, National Research Council.
2.2 Drugs
Methamphetamine hydrochloride (Sigma Chemical, St. Louis, MO, USA) was used for self-administration and primed reinstatement procedures. Drugs used for anesthesia in rats that underwent self-administration and reinstatement procedures were ketamine (Vedco Inc, St. Joseph, MO, USA), xylazine (Lloyd Laboratories, Shenandoah, IA, USA), Equithesin (sodium pentobarbital 4mg/kg, chloral hydrate 17mg/kg, 21.3mg/kg magnesium sulfate heptahydrate dissolved in 44% propylene glycol, 10% ethanol solution), and ketorolac (Sigma Chemical, St. Louis, MO, USA). After each self-administration session, catheters were flushed with cefazolin (Schein Pharmaceuticals, Florham Park, NJ, USA) and heparin (Elkins-Sinn, Cherry Hill, NJ, USA). Catheter patency was verified with methohexital sodium as needed (Eli Lilly, Indianapolis, IN, USA). Yohimbine hydrochloride (Sigma Chemical, St. Louis, MO, USA) was used for stress-induced reinstatement.
2.3 Surgery
Animals were anesthetized with IP injections of ketamine (66mg/kg), xylazine (1.3mg/kg), and equithesin (0.5ml/kg). Ketorolac (2.0mg/kg, IP) was given immediately prior to surgery as an analgesic. Chronic indwelling catheters were constructed as described previously [46]. The end of the catheter was inserted into the right jugular vein and was secured to surrounding tissue with sutures. The catheter ran subcutaneously and exited on the rat’s back, posterior to the shoulder blades. An antibiotic solution of cefazolin (10mg/0.1ml) was given post-surgery and during recovery along with 0.1ml of 70U/ml heparinized saline. During self-administration, rats received an IV infusion (0.1ml) of 10U/ml heparinized saline before each session. After each session, catheters were flushed with cefazolin and heparinized saline. As necessary, catheter patency was verified with methohexital sodium (10mg/ml dissolved in 0.9% physiological saline), a short-acting barbiturate that produces a rapid loss of muscle tone when administered intravenously. No rats were excluded for loss of catheter patency. Methohexital sodium was administered 0–1 times/rat following self-administration sessions (i.e. 22hrs prior to the next session) on rare occasions if methamphetamine intake appeared low.
2.4 Behavioral Training and Testing Procedures
Rats lever pressed for methamphetamine in standard Plexiglas self-administration chambers that were individually enclosed in a melamine sound-attenuating chamber with a ventilation fan (Med-Associates Inc., St. Albans, VT, USA), linked to a computerized data collection program (MED-PC, Med Associates). The chambers were equipped with two levers, a white stimulus light above each lever, a tone generator, and a white house light.
Following three 2hr acclimation sessions in which animals were exposed to the operant conditioning chamber (only the house light was illuminated), rats self-administered methamphetamine (20μg/infusion, dissolved in sterile 0.9% physiological saline) during daily 2hr sessions on a FR1 schedule of reinforcement. At the start of each session, the catheter was connected to a liquid swivel (Instech, Plymouth Meeting, PA, USA) via polyethylene 50 tubing (0.023“ ID × 0.05” OD) encased in steel spring leashes (Plastics One Inc., Roanoke, VA, USA). A house light signaled the initiation of the session and remained illuminated throughout it. Presses on the active lever resulted in a 2sec activation of the infusion pump (50μl bolus) and a 5sec presentation of a stimulus complex (white stimulus light above the active lever and activation of the tone generator; 4.5kHz, 78dB). After each infusion, responses on the active lever were recorded and included in analyses, but had no consequences during a 20sec timeout period. Inactive lever responses were also recorded, but had no consequences. All self-administration sessions were conducted 6 days/week to criterion (15 sessions ≥10 infusions per session) for a total period of 15–21 days. Following chronic self-administration, and before the first reinstatement test, animals underwent seven or more daily 2hr extinction sessions [mean ± SEM = 7.71±0.21; range= 7–9 days]. During extinction sessions, responses on both levers were recorded, but had no consequences (no cues or methamphetamine were delivered).
Once active lever responding extinguished to criteria (minimum of seven extinction sessions with ≤25 active lever responses for two consecutive days), all animals underwent four 2hr reinstatement tests. Prior to methamphetamine and methamphetamine + cue reinstatement tests, animals received an injection of methamphetamine hydrochloride (1mg/kg dissolved in 0.9% physiological saline, IP, immediately prior to testing). Prior to the yohimbine reinstatement test, animals received an injection of yohimbine hydrochloride (2.5mg/kg dissolved in water, IP, 30min prior to testing), a dose that has been shown to be sufficient to reinstate drug-seeking for various drugs including methamphetamine [47–53], and to produce physiological indicators of stress in rats (e.g., increases in plasma corticosterone, arterial blood pressure, and heart rate)[53, 54]. During reinstatement testing, responses on the active lever either resulted in a 5sec presentation of the previously methamphetamine-paired light + tone cues in the absence of response-contingent methamphetamine infusions (“cue” and “methamphetamine + cue” reinstatement tests) or no programmed consequences (“yohimbine” and “methamphetamine” reinstatement tests). Reinstatement tests were conducted in randomized order with no animals receiving all four tests in the same order, and extinction sessions were given between each reinstatement test until animals returned to criterion (≤25 active lever responses per session for two consecutive days; m ± SEM extinction days after each reinstatement test=2.87±0.34).
2.5 Yohimbine in Methamphetamine Naïve Rats
To examine whether the pharmacological stressor yohimbine would induce “22kHz” USVs outside the context of reinstatement, a group of methamphetamine naïve, male Long-Evans (n=4) rats were acclimated to operant chambers for two, 1hr sessions. On a subsequent day, rats were injected with yohimbine (2.5mg/kg, IP), 30min prior to a 30min test period, during which USVs were recorded. During acclimation and test sessions, only the house light was illuminated in the operant chamber (i.e., no levers were extended).
2.6 Footshock Stress in Methamphetamine Naïve Rats
To examine whether exposure to footshock would induce “22kHz” USVs outside a reinstatement context, a drug-naïve group of male Long-Evans rats (n=3) were acclimated to operant chambers during daily 2hr sessions. During acclimation and test sessions, only the house light was illuminated in the operant chamber (i.e., no levers were extended). After five acclimation sessions, the animals underwent a single footshock exposure test. The first 15min of this test was the same as acclimation sessions, followed by 15min of intermittent footshock exposure (VI-40 sec schedule of delivery, 0.5mA intensity, 0.5sec duration). USVs were recorded and analyzed for the 15min of intermittent shock administration.
2.7 USV Recording
All USV recordings occurred in the Med-Associates chambers described above. Condenser ultrasound microphones (frequency range: 10–200kHz; CM16/CMPA, Avisoft Bioacoustics, Berlin, Germany) were positioned outside chambers behind a mesh screen, between the response levers at approximately head-level of the rat (~7cm above the chamber floor). Recordings were made on an UltraSoundGate 416H data acquisition device (Avisoft Bioacoustics; sampling rate 250kHz; 16-bit resolution).
Analysis of USVs was performed using Avisoft SASLab Pro (version 4.2, Avisoft Bioacoustics). Spectrograms were generated with a fast Fourier transform length of 512 points and an overlap of 75% (FlatTop window, 100% frame size). Spectrograms had a frequency resolution of 490Hz and a time resolution of 0.5ms [33, 37]. USVs recorded during the first 30min of each self-administration, extinction, and reinstatement session were quantified and analyzed. Since the vast majority of USVs are produced in the early part of each 2hr session [observed in a previous report measuring USVs during reinstatement [43], in other paradigms [55, 56], and here], we only analyzed USVs in the first 30min of each test period.
2.8 USV Classification
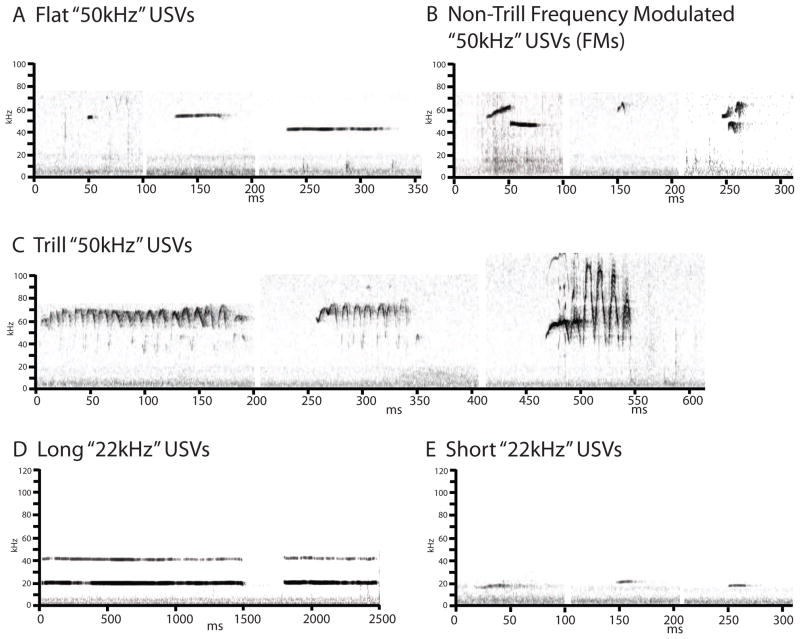
Observers blind to experimental conditions were trained to classify USVs into 5 categories, modified from Wright et al [33] and Barker et al [41] (Figure 1). “Flat” USVs were defined as those with mean frequencies between 30–90kHz, which lasted at least 10ms, and which had a mean slope between −0.2 and 0.2kHz/ms *“flat” and “short” from [33]]. Trill and non-trill frequency modulated USVs were at least 10ms, and had ≥10ms components with mean slopes of >0.2kHz/ms, or <−0.2kHz/ms. “Trills” were defined as frequency modulated USVs between 30–90kHz which contained rapid frequency modulation in a ~15ms “inverted U” shaped pattern, emitted in an oscillatory pattern [at least 2 cycles; “trill,” flat-trill combination,” “trill with jumps,” and “composite” categories from [33]]. Non-trill frequency modulated USVs (FMs) were defined as those frequency modulated USVs which did not contain characteristics of trills (“complex,” “upward/downward ramp,” “split,” “step up/down,” “multi-step,” and “inverted-U” from [33]). “22kHz” USVs were characterized as being either long “22kHz” or short “22kHz” based upon their duration and mean frequency. All “22kHz” USVs had mean frequencies between 18–30kHz. Short “22kHz” USVs were defined as those within this range with durations between 10–300ms. Long “22kHz” USVs were those with durations more than 300ms. Figure 1 shows examples of each of these USV subtypes.
Figure 1.
Examples of “50kHz” (flat, FM, and trills) and “22kHz” (long and short) USVs, captured from screen shots of spectrographs displayed at 490Hz with a time resolution of 0.5ms.
2.9 Analysis and Statistics
Repeated measures ANOVAs and paired sample t-tests were used to examine methamphetamine seeking (active lever pressing) during self-administration, extinction, and reinstatement sessions. To examine differences in the production of subtypes of high frequency USVs within a behavioral test (e.g., cue-induced reinstatement), repeated measures ANOVAs with Bonferroni corrected posthoc tests were used on the total numbers of each USV subtype emitted. The same analyses were conducted to compare emission of long vs. short “22kHz” USVs during each session. To compare differences in the emission of each USV subtype (flat, FM, trill, long “22kHz,” short “22kHz”) during different behavioral tests (e.g. extinction vs. cue-induced reinstatement), one-way ANOVAs with Fisher’s least significant difference posthoc tests were employed, and Greenhouse-Geisser corrections were employed to account for violations in normality assumptions of ANOVAs (Levine’s test for variance homogeneity) if necessary. A between subjects analysis was used here to accommodate some USV data lost due to hardware malfunction, as missing data points precludes use of within-subjects analyses. One animal’s data was lost on days 1 & 15 of self-administration, 4 were lost on day 1 of extinction, 2 on day 7 of extinction, 3 on cue reinstatement, 3 on methamphetamine reinstatement, 2 on yohimbine reinstatement, and 4 on cue + methamphetamine reinstatement. One-way ANOVAs were also used to examine the proportion of all USVs emitted by each rat on a given session that belonged to each subtype (Figure 3D). To quantify this measure, the total number of USVs emitted by each rat on each test was calculated, and the percentage of this total consisting of flats, FMS, etc. was computed for each rat. To examine correlations between lever pressing and USV production, Pearson r correlations were employed.
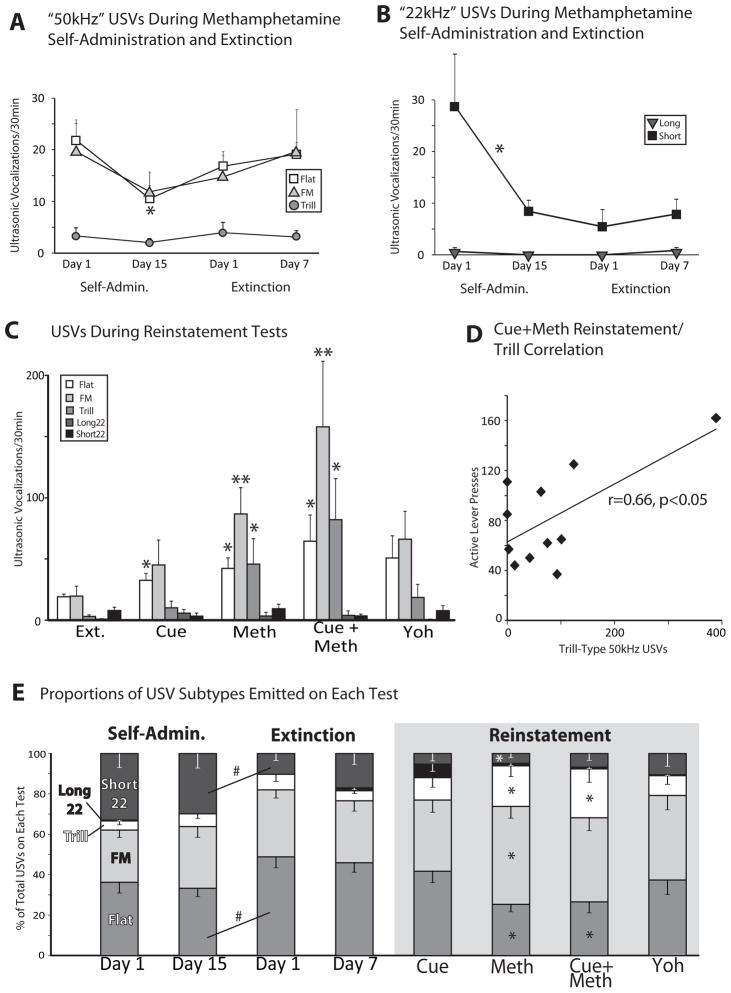
Figure 3.
(A) Mean (± SEM) “50kHz” USVs during the first and last days of methamphetamine self-administration and extinction days 1 and 7. *Decrease from self-admin day 1 to 15 in flats, n=14, one-way ANOVA, p<0.05. (B) Mean (± SEM) “22kHz” USVs during the first and last days of methamphetamine self-administration and extinction days 1 and 7. *Decrease from self-administration day 1 to 15 in short “22kHz” USVs, n=14, one-way ANOVA, p<0.05. (C) Mean (± SEM) “50kHz” and “22kHz” USVs during extinction day 7 (Ext.), cue reinstatement (Cue), methamphetamine prime (Meth), cues + methamphetamine (Cue+Meth), or yohimbine (Yoh). *Increase from extinction levels, ns=11–13, one-way ANOVA, p<0.05; **p<0.01. (D) Pearson correlation between trills (X-axis) and methamphetamine seeking (active lever presses in first 30min of reinstatement session; Y-axis) on cue+methamphetamine test day. (E) Proportion of all USVs emitted on each test day that were of each subtype. Mean (± SEM) proportion of USVs emitted by each rat that were of each USV subtype [short “22kHz,” long “22kHz,” trill, non-trill frequency modulated (FM), and flat]. #Difference between self-administration day 15 and extinction day 1, ns=11–14, one-way ANOVA, p<0.05. *Difference between reinstatement day and extinction day 7, ns=11–13, one-way ANOVA, p<0.05
3. Results
3.1 Self-Administration, Extinction, and Reinstatement
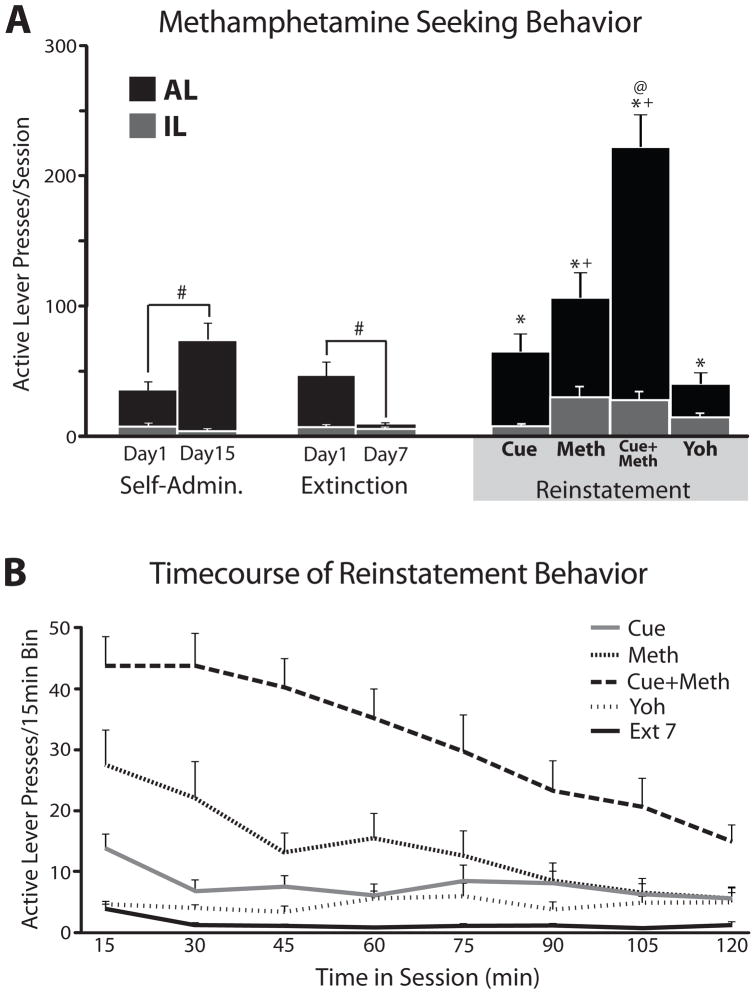
Animals readily acquired methamphetamine self-administration, with increases in active lever pressing from day 1 [35.0±6.4] to day 15 [73.9±12.4; t14=3.42, p<0.01; Figure 2]. Methamphetamine intake also increased from day 1 [1.31±0.21mg/kg; ] to day 15 of self-administration [2.46±0.13; t14=7.24, p<0.001]. Animals took 1–18 methamphetamine infusions in the first 30 min of self-administration day 1 [8.33±1.35], and a total of 10–60 infusions over the whole 2hr session (m=27.40±3/29). On day 15 of self-administration, animals took 32–167 infusions over the 2hr session (m=51.60±8.83). Over the course of subsequent extinction trials, methamphetamine seeking dramatically declined, from 46.2±10.6 lever presses/day on day 1 of extinction, to 8.8±1.5 by the last day of extinction [t14=3.74, p<0.01; days to criterion 7.71±0.21; Figure 2]. Compared to prior extinction responding, cues, methamphetamine prime, methamphetamine + cue, and yohimbine all significantly reinstated methamphetamine seeking (repeated measures ANOVA: F4,56=61.9, p<0.001; pressing on last day of extinction vs. cue reinstatement: t14=4.49, p=0.001; vs. methamphetamine prime: t14=5.03, p<0.001; vs. methamphetamine + cues: t14=8.83, p<0.001; vs. yohimbine: t14=4.45, p=0.001; Figure 2). Methamphetamine + cues elicited the most methamphetamine seeking, with greater reinstatement than methamphetamine alone (t14=5.41, p<0.001), cues alone (t14=6.74, p<0.001), or yohimbine (t14=7.08, p<0.001). Methamphetamine alone also elicited more drug seeking than yohimbine (t14=3.17, p<0.01), while cue-induced and yohimbine-induced reinstatement caused statistically equivalent levels of reinstatement.
Figure 2.
(A) Mean (+SEM) lever responding during the first and last days of methamphetamine self-administration (LEFT), extinction (MIDDLE) and reinstatement by methamphetamine-paired cues (Cue), methamphetamine-prime (Meth), cue+methamphetamine (Cue+Meth), or yohimbine (Yoh; RIGHT). Active lever presses are shown in black bars, while inactive levers are shown in grey bars. Significant differences in responding between self-administration and extinction days (#), significant reinstatement relative to the last day of extinction (*), difference in reinstatement behavior compared to Yoh day (+), and differences between Cue+Meth and other reinstatement groups (@) are indicated (p<0.05; n=15; repeated measures ANOVA; ps < 0.05). (B) Time course of active lever pressing within each reinstatement session (cue, methamphetamine, cue+methamphetamine, yohimbine), and the last day of extinction training (Ext 7) are displayed in 15min bins for these 2hr sessions.
3.2 “50kHz” USVs During Self-Administration and Extinction
On day 1 of self-administration, animals emitted comparable numbers of flat and FM vocalizations, with few trills (repeated measures ANOVA: F2,26=7.40, p<0.01; FMs vs trills: t13=3.37, p=0.01; flats vs. trills: t13=4.06, p=0.001; Figure 3A). By the 15th day of self-administration, a similar pattern of USV production was still observed, with more flats and FMs than trills (repeated measures ANOVA: F2,26=5.96, p<0.01). However, over the course of self-administration, the number of flats emitted decreased (one-way ANOVA on flats, day 1 vs day 15: F1,26=5.84, p<0.05), while FM and trill production was statistically comparable (Fs<1.4; Figure 3A).
Surprisingly, removing response contingent methamphetamine infusions and cues during extinction had few effects on emission of USVs when compared to prior self-administration tests (Figure 3A). Relative to the last day of self-administration, the number of flats, FMs, or trills emitted was unchanged on the first day of extinction (Fs<2.70, n.s.; Figure 3A), though the proportion of all USVs which were flat did increase across these days (F1,23=5.32, p<0.05; Figure 3E). Further extinction training also failed to change USV production, with no differences in the total numbers, or proportion of any subtype of “50kHz” USV produced between days 1 and 7 of extinction (Fs<0.50, n.s.).
3.3 “50kHz” USVs During Reinstatement
USVs observed during reinstatement tests varied based both upon the type of reinstatement, and the subtype of USV (Figure 3C). During cue-induced reinstatement, only flats were significantly increased compared to the last day of extinction (Flats: F1,23=5.13, p<0.05; FM & trills: Fs<1.8, n.s.). For methamphetamine primed reinstatement, flats, FMs, and trills were all increased from extinction levels (flats: F1,23=7.29, p<0.05; FMs: F1,23=9.08, p<0.01; trills: F1,23=4.59, p<0.05), with greater proportions of FMs and trills, and a lower proportion of flats than on extinction day 7 (FMs: F1,23=5.44, p<0.05; trills: F1,23=8.12, p<0.01; flats: F1,23=11.54, p<0.01). For methamphetamine + cue reinstatement, all three types of high frequency USVs were also increased from extinction levels (flats: F1,22=5.29, p<0.05; FMs: F1,22=7.67, p=0.01; trills: F1,22=6.67, p<0.05), and the proportion of trills increased (F1,22=9.36, p<0.01), while the proportion of flats decreased (F1,22=7.39, p=0.01) from late extinction. In addition, the number of trills emitted on this session was correlated with the number of active lever presses, indicating that the animals with the most methamphetamine + cue-induced drug seeking emitted the most trill USVs (Figure 3D). For yohimbine-induced reinstatement, no type of high frequency USV was significantly increased from extinction levels, although the number of flats and FMs emitted trended toward increasing (flats: F1,12=3.00, p=0.10; FMs: F1,15=3.7, p=0.073; Trills: F1,12=2.06, n.s.). No other significant correlations between USV subtype production and methamphetamine seeking behavior was observed on other reinstatement sessions.
The number of trills emitted significantly varied between reinstatement types (one-way ANOVA on trills during each type of reinstatement: F3,44=2.78, p=0.05), with more trills seen during the methamphetamine + cue session than after either cues alone (p=0.01) or yohimbine (p<0.05), but not more than methamphetamine alone (p=0.19), suggesting a specific potentiation of trills after methamphetamine priming injections. No significant differences between the number of flats or FMs emitted in different reinstatement tests were observed, though a trend to this effect was seen for FMs during methamphetamine prime, and methamphetamine + cue reinstatement tests (flats: F3,44=0.80, n.s.; FMs: F3,44=2.35, p=0.086).
3.4 “22kHz” USVs During Self-Administration and Extinction
We observed a number of “22kHz” USVs during methamphetamine self-administration and extinction (Figure 4C). Far more “22kHz” USVs were emitted on the first day of self-administration than on the last day (main effect of day: F1,13=4.82, p<0.05), and the vast majority of these were short “22kHz” USVs (first day: t13=2.91, p=0.01; last day: t13=4.10, p=0.001; Figure 3C). In fact, rats emitted more short “22kHz” USVs than trills on self-administration day 1 (t13=2.49, p<0.05). On day 1 of self-administration, all rats emitted at least one short “22kHz,” but only 1 rat emitted any long “22kHz” USVs, and by the last day of self-administration, 71% of rats emitted at least one short “22kHz,” while none emitted long “22kHz” USVs.
Figure 4.
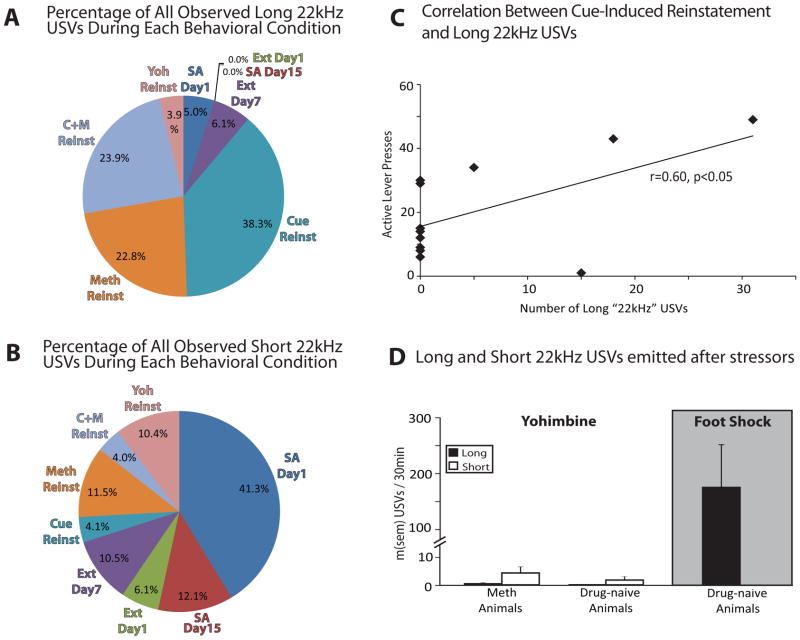
Percentage of all observed long (A) and short “22kHz” USVs (B) emitted during each of the behavioral contexts tested here: Methamphetamine self-administration days 1&15 (SA Day 1 &15), extinction days 1&7 (Ext Day 1&7), and cue, methamphetamine (Meth), cue+methamphetamine (C+M), and yohimbine (Yoh), reinstatement (Reinst). (C) Pearson correlation between active lever (AL) pressing (1st 30min of cued reinstatement session) and the number of long “22kHz” USVs emitted. (D) Mean (± SEM) long and short “22kHz” USVs/30min emitted after yohimbine during reinstatement testing in methamphetamine-experienced animals (Meth Animals), after yohimbine in drug-naïve animals, or after 15min of foot shock in drug naïve animals (right).
Like higher frequency USVs, the number of short “22kHz” USVs emitted did not change from late self-administration to subsequent extinction training (no difference between last day of self-administration vs. day 1 of extinction; F1,22=0.60, n.s.), though the proportion of short “22kHz” USVs as a percentage of all USVs did decrease significantly between these days (Figure 3E). “22kHz” USV production was not altered over the course of extinction (no change from day 1 to day 7 of extinction; F1,22=0.20, n.s.). Long “22kHz” USVs were totally absent on late self-administration and initial extinction sessions, but some animals (23%) did begin emitting low numbers by the last day of extinction (trend toward increase from extinction day 1 to day 7: F1,22=2.96, p=0.099).
3.5 “22kHz” USVs During Reinstatement Testing
During most reinstatement tests, both long and short “22kHz” USVs were emitted at similar levels to late extinction. The overall levels of long and short 22kHz USVs during reinstatement were generally far lower than for “50kHz” USVs (Figure 3E). However, the proportion of short “22kHz” USVs during methamphetamine primed reinstatement decreased from extinction levels (F1,23=4.11, p=0.05; Figure 3E). In contrast, during cue-induced reinstatement a trend toward increased numbers of long “22kHz” USVs was observed (F1,23=2.95, p=0.099; Figure 3C), and ~40% of all long “22kHz” USVs observed on any session were emitted during cue-induced reinstatement tests, with an additional ~25% on cue + methamphetamine tests (a total of ~65% of long “22kHz” USVs were emitted on sessions in which cues were presented in the absence of methamphetamine; Figure 4A). Interestingly, only 33% of animals emitted any long “22kHz” USVs during cue-induced reinstatement, but these were the animals showing the greatest cue-induced reinstatement (correlation between active lever presses and long “22kHz” USVs: r=0.60, p<0.05; Figure 4C).
Though the pharmacological stressor yohimbine caused significant reinstatement of methamphetamine seeking, it failed to increase production of either long or short “22kHz” USVs (Fs<1.00, n.s.). Emission of long and short “22kHz” USVs after yohimbine was statistically comparable to other reinstatement tests (Figure 3C), and consisted of less than 4% of all observed long, and 11% of all short “22kHz” USVs (Figures 4A&B). We also verified that the lack of yohimbine-induced “22kHz” USVs was not due to prior methamphetamine experience, because IP injections of yohimbine in drug-naïve animals failed to elicit significant numbers of either long or short “22kHz” USVs (t3=2.10, n.s. Figure 4D). In contrast, intermittent footshock robustly elicited the production of long (not short) “22kHz” USVs (Figure 4D), showing a clear contrast between USV production after yohimbine vs. footshock stressors.
3.6 Characteristics of USVs During Reinstatement
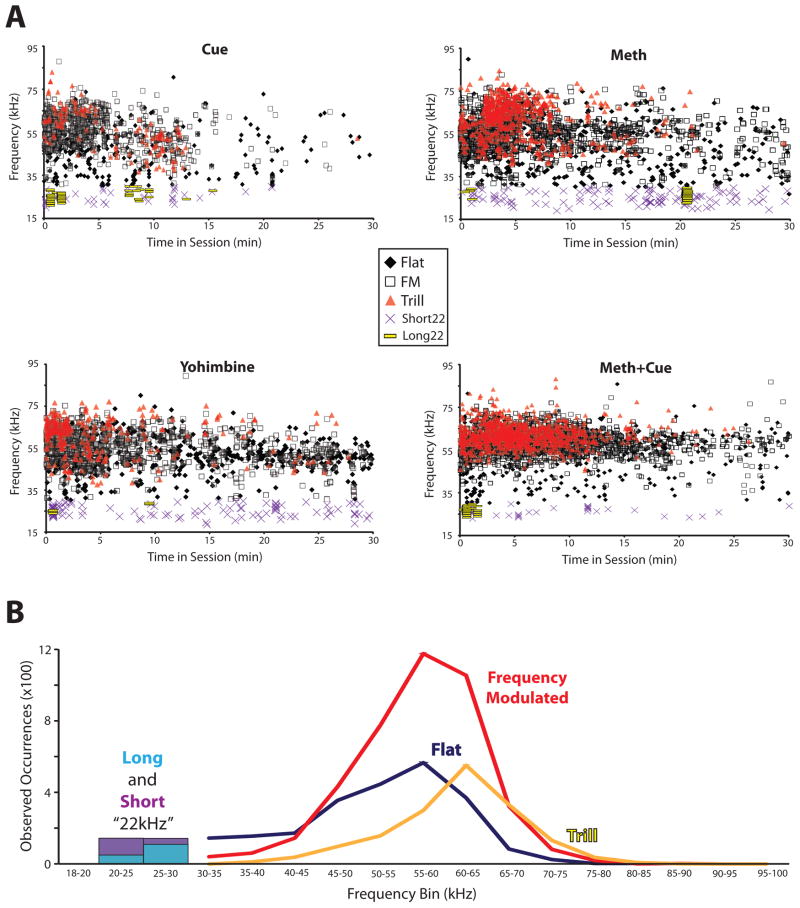
We further sought to characterize subtypes of USVs based on their mean frequencies, as well as their patterns of emission during the first 30 min of reinstatement tests. First, we plotted each USV emitted by rats based on its mean frequency (Y axis) and time it was emitted within each of the four reinstatement sessions (X axis; Figure 5A). We observed that most USVs were emitted in the early part of each session (first 10–15 min), and that all “50kHz” USV subtypes contained considerable variability in their mean frequencies. To further quantify the range of frequencies at which USVs were emitted, we recorded the mean frequency of each observed USV, and plotted a histogram reflecting the occurrences of USVs emitted at each frequency (within 5kHz bins ranging from 18 to 100kHz; Figure 5B). No differences were observed in the frequencies at which USV subtypes were emitted between different types of reinstatement, so sessions were combined for this analysis. We observed that the modal frequency for flat and FM USVs was between 55 and 60kHz, but that both of these USV types had considerable range outside these frequencies, between 30 and 85kHz. Trills had a somewhat higher modal frequency, between 60–65kHz, and ranging between 35 and 85kHz. This finding replicates previous reports that “50kHz” USVs are not always emitted at 50kHz, but instead have a substantial range, centered at between 55–65kHz [26, 32, 55].
Figure 5.
(A) Frequency (kHz) of “50kHz” and “22kHz” USVs during each reinstatement test as a function of time (min) in cue, methamphetamine (Meth), yohimbine, or cue+methamphetamine (Meth+Cue) tests. (B) Histogram showing total occurrences of USVs emitted at various frequency ranges during reinstatement tests. The total number of long “22kHz,” short “22kHz,” flat, trill, and non-trill frequency modulated USVs emitted by rats during reinstatement tests are plotted based upon their mean frequencies falling into 5kHz bins ranging from 18kHz to 100kHz (X-Axis).
4. Discussion
The present data support the argument that ultrasonic vocalizations (USVs) may be useful for exploring the subjective state of rats in models of addiction. We compared patterns of methamphetamine seeking behavior to the production of several subcategories of “50kHz” USVs [flat, trill, and non-trill frequency modulated (FM)], as well as long or short duration “22kHz” USVs. These USVs were expressed differentially in the behavioral situations examined here: methamphetamine self-administration, extinction, and several types of reinstatement of methamphetamine seeking. Reinstatement was primarily associated with elevated numbers of high frequency USVs, and the different stimuli used to elicit reinstatement behavior (methamphetamine prime, cues, methamphetamine + cues, or the pharmacological stressor yohimbine) yielded distinct patterns of USV production. We also found that aversion-related “22kHz” USVs were most commonly emitted during the first experience of self-administering methamphetamine, and during cue-induced reinstatement in a subset of rats particularly susceptible to reinstatement by cues. These data show that rodent USVs can be a useful dependent variable in experiments measuring drug seeking behaviors, potentially providing information about the subjective states of rats during drug seeking.
4.1 Patterns of High Frequency “50kHz” USV Production
Recent studies have shown that rat USVs are much more complex than simple reward and aversion signals [26, 27, 31, 33, 55, 57, 58]. First, “50kHz” USVs are emitted in an apparently stress-related manner during aggressive encounters, suggesting they may not always convey purely positive affect [59–67]. Second, “50kHz” USVs are heterogeneous—for example experimenter-administered psychostimulant drugs (including amphetamines, cocaine, and caffeine) elicit not all types of USVs, but specifically those in frequency modulated and/or trill patterns [33, 38]. In addition, repeated IV amphetamine results in sensitization of trill production as well as locomotion, again suggesting that relatively high-dose stimulants increase frequency modulated USVs in particular [36, 39], mediated at least in part by norepinephrine and dopamine signaling [37, 68]. These findings suggest that careful examination of USV production during a variety of behaviors can lend insight into both the meanings of USVs, and the subjective experiences of rats.
Three previous reports have examined production of USVs during self-administration of cocaine, with mixed results. Browning et al [43] reported an increase in “50kHz” USVs over the course of acquisition of cocaine self-administration. In apparent contrast, Maier et al [45] reported that “50kHz” USVs are elicited during initial cocaine self-administration, but that these vocalizations decreased over the course of subsequent self-administration days. Both of these studies also reported a decrease in high frequency USV production in subsequent extinction sessions. A third study reported that high-dose self-administered cocaine elicited primarily “50kHz” flat and frequency modulated USVs [41]. In contrast, when animals were instead allowed to self-administer a lower cocaine dose (precluding attainment of preferred blood cocaine levels), they instead primarily emitted USVs in the 18–33kHz range.
In the present experiment, we found that flat “50kHz” USVs declined from early to late methamphetamine self-administration, but did not decrease further upon subsequent extinction training. Trill and non-trill FMs were produced at consistent rates during self-administration and extinction. Unlike previous studies measuring “50kHz” USVs during cocaine self-administration and extinction, we did not observe significant changes in “50kHz” USV production between methamphetamine self-administration and subsequent extinction [43, 45]. These mixed results could reflect differences between cocaine and methamphetamine, or differences between studies in self-administered drug dosages, training schedules, rat strains, USV classification strategies, or other procedural factors.
Only one previous report examined USV production during reinstatement of drug seeking [43]. There, cue-induced and cocaine-primed reinstatement of cocaine seeking yielded increases in “50kHz” USVs, especially in the first 5 min of reinstatement sessions. However, subtypes of “50kHz” USVs were not separately analyzed in this study, nor were “22kHz” USVs. Here, we found increased production of flat “50kHz” USVs during cue, methamphetamine primed, and cue + methamphetamine prime reinstatement, as well as a trend toward increased flats during yohimbine reinstatement (compared to late extinction levels; Figure 3C). Non-trill FM USVs were increased by methamphetamine primed and cue + methamphetamine reinstatement, and a trend toward increased FMs was also seen during yohimbine-induced reinstatement.
In contrast, trills were only increased from extinction levels during methamphetamine primed and cue + methamphetamine primed reinstatement sessions. In addition, when animals received a methamphetamine prime and also response-contingent cue presentations (which elicited very high levels of reinstatement), trill production was also correlated with methamphetamine seeking. However, methamphetamine did not always induce trill production. Few trills were emitted when animals were allowed to self-administer multiple, smaller dose infusions of methamphetamine. Instead, they primarily emitted flat and non-trill FM USVs, as well as short-duration “22kHz” USVs during their initial self-administration session. These results strongly support the notion that flat and frequency modulated (especially trill) USVs are emitted in different behavioral contexts.
In particular, trills seem to be produced when animals are in very strong reward seeking states [26, 40, 55], especially when stimuli such as levers and cues, or even play or sexual partners, are imbued with high levels of incentive salience [27, 40, 62, 68, 69]. Alternatively, trills could reflect a similar subjective state to the “rush” experienced by humans injecting high doses of stimulant drugs, which is likely to be related to the positive affective states previously proposed to underlie frequency modulated USVs [27, 70].
In contrast, increased production of flat and non-trill FM “50kHz” USVs were produced in similar patterns to one another during self-administration, extinction, and reinstatement. Both were emitted at higher rates than trills during self-administration and extinction, their emission rates did not change between self-administration and extinction, and emission of both was increased in multiple reinstatement tests. Flat “50kHz” USVs have been hypothesized to be less related to positive affect or strong motivated states than other USVs [26, 27, 32, 55], and our data seem to support this idea. However, we also found that non-trill FMs were emitted in broadly similar patterns to flat USVs during self-administration and extinction, and to some extent also in reinstatement (see Figure 3). Since we combined many different patterns of non-trill frequency modulation observed in a prior report [33] into our “FM” category, it is very possible that further parsing of different types of non-trill frequency modulation will reveal further meaningful variation within these USVs in future studies. In sum, the present findings strongly support the importance of examining subtypes of “50kHz” USVs when examining the relationship of these vocalizations to appetitive behaviors.
4.2 Low Frequency “22kHz” USVs
It has long been known that rats emit USVs in the ~18–30kHz range when exposed to stressful or aversive situations [25, 26, 28–30, 63], and these low frequency USVs come in at least two varieties—based not upon their fundamental frequency, but on their duration. Canonical “alarm call” type USVs are typically quite long (at least 300ms), and are emitted in situations including intermittent footshock (Figure 4D), drug withdrawal, and predatory threat (among others) [30, 34, 66, 71]. Brudzynski and colleagues initially reported that low frequency USVs are also emitted at much shorter durations (sometimes as short as 10ms) during mild stressors such as experimenter touch, or after intra-hypothalamic injections of carbachol [32, 72, 73]. Other than substantial differences in the duration of these “22kHz” USVs, they otherwise appear to be similar in terms of spectral characteristics and frequency range (between ~18–30kHz), though they may also be of a lower amplitude than longer “22kHz” USVs [Figure 1 and [55]]. Barker et al [41] recently reported that short, low frequency USVs are also frequently emitted during established self-administration of a low dose of cocaine, when animals are unable to attain their preferred blood levels of cocaine.
In the present experiment, we found that large numbers of short duration USVs in the “22kHz” range were emitted when animals first began to self-administer methamphetamine. These short “22kHz” USVs were emitted in the same time period as higher frequency USVs, potentially reflecting the mixed rewarding/aversive properties of initial experiences with stimulant drugs including amphetamines [32, 74–77].
However, it is notable that rats rarely emitted longer, “alarm call” type “22kHz” USVs in the self-administration or extinction context, as they frequently did when intermittently shocked (e.g., Figure 4D). Long “22kHz” USVs were also sometimes seen during a cue-induced reinstatement test, especially in animals showing the strong cue-induced reinstatement (Figure 4C). Indeed, over 60% of all long “22kHz” USVs observed at any time were produced during reinstatement sessions in which cues were present (Figure 4A). This could reflect the presence of a negative affective state experienced by a subset of rats when exposed to previously drug-associated cues in the absence of additional drugs. Similarly, humans exposed to drug cues in the absence of drugs themselves also often report negative affective states [2, 11, 12, 14–17]. This supports the notion that USVs can be a useful, translationally-relevant window into the affective state of rodents performing addiction-related behaviors.
The pharmacological stressor yohimbine failed to increase long or short “22kHz” USVs, showing that these vocalizations are not unconditionally induced by all stressful stimuli. In fact, yohimbine has previously been reported to reduce footshock-elicited “22kHz” USVs in rats at similar doses [78, 79], which was interpreted as evidence that yohimbine can be anxiolytic via its actions at serotonin or other receptors [78]. However, yohimbine induces panic-like states in humans and animals [80, 81], and activates physiological stress-like responses in rats at comparable or lower doses to those used here [53, 54, 82]. In addition, yohimbine (like other stressors such as footshock) induces reinstatement of drug seeking [[47–53]; Figure 2], while anxiolytic drugs conversely reduce reinstatement [83]. Rather than emitting stress-associated “22kHz” USVs, yohimbine-induced reinstatement tended to increase emission of flat and FM “50kHz” USVs over extinction levels (Figure 3C). This reinforces the previously reported finding that yohimbine [like other noxious drugs [84]] is markedly different from other stressors, including shock, predators or their odors, social defeat, drug withdrawal, and playback of “22kHz” USVs emitted by other animals—all of which elicit long “22kHz” USVs [29, 30, 34, 60, 84].
4.3 “50kHz” Vocalizations are Heterogeneous
Careful examinations of rodent USVs using computerized analysis software have conclusively shown these vocalizations to be very complex, and are emitted in over a dozen different patterns (not to mention many more potentially meaningful combinations) and at a variety of frequencies [27, 32, 33, 85–87]. Therefore, we sought to further quantify the mean frequencies of the USV subtypes examined here (Figure 5B). While both flat and FM USVs were most commonly emitted at mean frequencies between 50–60kHz, trills tended to be emitted at higher frequencies than other categories of USVs (60–65kHz), as previously reported [26, 33]. This finding should reinforce the fact that “50kHz” USVs are complex and heterogeneous in numerous important ways, including in their mean frequencies [26, 56, 62, 69, 88]. In addition, it should discourage researchers planning similar experiments from relying upon USV detection systems which filter out USVs emitted outside a restricted range (e.g. 50–55kHz), such as commercially available “bat detectors.”
4.4 Limitations of the Present Study
While the present report adds several intriguing findings to the literature, it also has several limitations. First, we divided USVs into categories based on evidence for meaningful differences between flat, trill and other frequency modulated “50kHz” USVs, as well as long and short “22kHz” USVs. As mentioned above, Wright et al [33] reported that at least 14 categories of “50kHz” USVs exist, which we did not separately analyze. Unfortunately, manual USV categorization based upon such subtle differences in spectral patterns is extremely time consuming, and the time required to perform these analyses is considerable. Therefore, we reiterate the call made by Wright et al [33] for the development of an automated system to accurately categorize USVs based upon their patterns of frequency and intensity over time, which might well lend additional insight into the full range of information contained within this rodent self-report measure. Unfortunately, such USV pattern analysis software is presently unavailable/unpublished.
Though we categorized USVs only during early and late self-administration and extinction, it is possible that examining USVs emitted over the entire course of acquisition of self-administration and extinction would have yielded additional insights into the meaning of these vocalizations. For example, changes in USV production during the transition of self-administration behavior from goal-oriented to habitual (which has been related to the onset of addiction) would be of particular interest, as would examination of USVs emitted during self-administration of different doses of methamphetamine. In addition, we measured USVs only in the first 30 min of each session, and although the majority of USVs are emitted during the early part of similar tests [43], additional analyses of USVs over longer periods would be of interest.
The present study did not include a control group in which animals were allowed to self-administer saline instead of methamphetamine. Such a control group would have allowed additional examination of USVs elicited by non-specific factors such as mere exposure to operant boxes, sensory stimuli, primes, stressors, or experimenter handling. This is important, as many factors including housing conditions, presence of bedding in test chambers, presence of a cage mate, IP injections, and food restriction can all influence USV production [31, 55, 56, 58, 89–92]. However, the fact that we observed markedly different patterns of USV production during different behavioral conditions (including reinstatement tests conducted in randomized order) strongly suggests that USVs measured here primarily reflected different subjective states experienced by animals in the various behavioral conditions.
Finally, the present study relied upon a design necessitated by the self-administration/reinstatement paradigm, in which self-administration is followed by extinction trials, followed by reinstatement tests. While this design allowed us to examine USVs in a well-established and frequently used behavioral paradigm, it could potentially have yielded order effects in our measurement of USV production. Future experiments might examine the effects of order in this paradigm, or compare USV production during each session to an immediately preceding baseline period.
4.5 Conclusions
We found that different types of methamphetamine seeking resulted in distinctive patterns of USV production. During self-administration and extinction, mostly flat and non-trill FM “50kHz” USVs were observed, and we found no changes in “50kHz” USV production between self-administration and extinction conditions. Initial methamphetamine self-administration also resulted in many short duration “22kHz” USVs, potentially reflecting mildly aversive properties of initial methamphetamine exposure. A relatively high-dose methamphetamine priming injection yielded trills and other frequency modulated “50kHz” USVs (particularly when cues were also present), concurrently with strong reinstatement of methamphetamine seeking. In addition, trill production was correlated with the degree of reinstatement elicited by cues + methamphetamine prime. In contrast, flat “50kHz” USVs were commonly produced in all reinstatement conditions, regardless of the stimulus which triggered methamphetamine seeking, suggesting a less specific function of these USVs. Interestingly, the degree of cue-induced reinstatement (in the absence of methamphetamine) was also correlated with production of longer, aversion-related “22kHz” USVs, potentially reflecting a frustration-like affective state similar to that reported in humans exposed to drug cues in the absence of drugs. The pharmacological stressor yohimbine failed to induce long or short aversion-related “22kHz” USVs during reinstatement, or in naïve animals, conclusively showing that not all reinstatement-eliciting stressors cause “22kHz” USVs. In sum, we conclude that examination of USVs can yield important insights into the psychological states of rats which are not accessible by traditional measures of operant drug seeking or other trained behaviors. In this way, rat USVs may therefore may serve as a useful bridge between clinical and preclinical addiction studies.
Research Highlights.
Rat ultrasonic vocalizations (USVs) were measured during methamphetamine seeking
Reinstatement elicited more high frequency USVs than other behaviors
Different types of reinstatement elicited different types of USVs
“22 kHz” USVs were not elicited by a pharmacological stressor
USVs contain complex information, and are a useful measure for addiction studies
Acknowledgments
We thank Clifford Chan for assistance with testing. These studies were funded by F32 DA026692, P50 DA016511, P20 DA022658, R21 DA032005, R01 DA021690, and C06 RR015455.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Childress AR, Hole AV, Ehrman RN, Robbins SJ, McLellan AT, O’Brien CP. Cue reactivity and cue reactivity interventions in drug dependence. NIDA research monograph. 1993;137:73–95. [PubMed] [Google Scholar]
- 2.Sinha R, Catapano D, O’Malley S. Stress-induced craving and stress response in cocaine dependent individuals. Psychopharmacology. 1999;142:343–51. doi: 10.1007/s002130050898. [DOI] [PubMed] [Google Scholar]
- 3.Jaffe JH, Cascella NG, Kumor KM, Sherer MA. Cocaine-induced cocaine craving. Psychopharmacology. 1989;97:59–64. doi: 10.1007/BF00443414. [DOI] [PubMed] [Google Scholar]
- 4.de Wit H, Stewart J. Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology. 1981;75:134–43. doi: 10.1007/BF00432175. [DOI] [PubMed] [Google Scholar]
- 5.Kalivas PW, McFarland K. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology. 2003;168:44–56. doi: 10.1007/s00213-003-1393-2. [DOI] [PubMed] [Google Scholar]
- 6.Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology. 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- 7.Robbins SJ, Ehrman RN, Childress AR, O’Brien CP. Relationships among physiological and self-report responses produced by cocaine-related cues. Addictive behaviors. 1997;22:157–67. doi: 10.1016/s0306-4603(96)00007-x. [DOI] [PubMed] [Google Scholar]
- 8.Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O’Brien CP. Limbic activation during cue-induced cocaine craving. The American journal of psychiatry. 1999;156:11–8. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Westman EC, Behm FM, Rose JE. Dissociating the nicotine and airway sensory effects of smoking. Pharmacology, biochemistry, and behavior. 1996;53:309–15. doi: 10.1016/0091-3057(95)02027-6. [DOI] [PubMed] [Google Scholar]
- 10.Brauer LH, Behm FM, Lane JD, Westman EC, Perkins C, Rose JE. Individual differences in smoking reward from de-nicotinized cigarettes. Nicotine & tobacco research: official journal of the Society for Research on Nicotine and Tobacco. 2001;3:101–9. doi: 10.1080/14622200123249. [DOI] [PubMed] [Google Scholar]
- 11.Tiffany ST, Cox LS, Elash CA. Effects of transdermal nicotine patches on abstinence-induced and cue-elicited craving in cigarette smokers. Journal of consulting and clinical psychology. 2000;68:233–40. doi: 10.1037//0022-006x.68.2.233. [DOI] [PubMed] [Google Scholar]
- 12.McClernon FJ, Kozink RV, Rose JE. Individual differences in nicotine dependence, withdrawal symptoms, and sex predict transient fMRI-BOLD responses to smoking cues. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2008;33:2148–57. doi: 10.1038/sj.npp.1301618. [DOI] [PubMed] [Google Scholar]
- 13.Fox HC, Hong KI, Siedlarz K, Sinha R. Enhanced sensitivity to stress and drug/alcohol craving in abstinent cocaine-dependent individuals compared to social drinkers. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2008;33:796–805. doi: 10.1038/sj.npp.1301470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee DC, Myers CS, Taylor RC, Moolchan ET, Berlin I, Heishman SJ. Consistency of subjective responses to imagery-induced tobacco craving over multiple sessions. Addictive behaviors. 2007;32:2130–9. doi: 10.1016/j.addbeh.2007.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davidson D, Tiffany ST, Johnston W, Flury L, Li TK. Using the cue-availability paradigm to assess cue reactivity. Alcoholism, clinical and experimental research. 2003;27:1251–6. doi: 10.1097/01.ALC.0000080666.89573.73. [DOI] [PubMed] [Google Scholar]
- 16.Preston KL, Epstein DH. Stress in the daily lives of cocaine and heroin users: relationship to mood, craving, relapse triggers, and cocaine use. Psychopharmacology. 2011;218:29–37. doi: 10.1007/s00213-011-2183-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sinha R, Fuse T, Aubin LR, O’Malley SS. Psychological stress, drug-related cues and cocaine craving. Psychopharmacology. 2000;152:140–8. doi: 10.1007/s002130000499. [DOI] [PubMed] [Google Scholar]
- 18.Koob GF. Neurobiology of addiction. Toward the development of new therapies. Annals of the New York Academy of Sciences. 2000;909:170–85. doi: 10.1111/j.1749-6632.2000.tb06682.x. [DOI] [PubMed] [Google Scholar]
- 19.Sinha R. Modeling Relapse Situations in the Human Laboratory. Current topics in behavioral neurosciences. 2011 doi: 10.1007/7854_2011_150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stewart J, de Wit H, Eikelboom R. Role of unconditioned and conditioned drug effects in the self-administration of opiates and stimulants. Psychol Rev. 1984;91:251–68. [PubMed] [Google Scholar]
- 21.Bindra D. How adaptive behavior is produced: a perceptual-motivation alternative to response reinforcement. Behavioral and Brain Sciences. 1978;1:41–91. [Google Scholar]
- 22.Bindra D. A motivational view of learning, performance, and behavior modification. Psychol Rev. 1974;81:199–213. doi: 10.1037/h0036330. [DOI] [PubMed] [Google Scholar]
- 23.Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–91. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 24.Di Chiara G. Nucleus accumbens shell and core dopamine: differential role in behavior and addiction. Behavioural brain research. 2002;137:75–114. doi: 10.1016/s0166-4328(02)00286-3. [DOI] [PubMed] [Google Scholar]
- 25.Brudzynski SM. Communication of adult rats by ultrasonic vocalization: biological, sociobiological, and neuroscience approaches. ILAR journal/National Research Council, Institute of Laboratory Animal Resources. 2009;50:43–50. doi: 10.1093/ilar.50.1.43. [DOI] [PubMed] [Google Scholar]
- 26.Burgdorf J, Kroes RA, Moskal JR, Pfaus JG, Brudzynski SM, Panksepp J. Ultrasonic vocalizations of rats (Rattus norvegicus) during mating, play, and aggression: Behavioral concomitants, relationship to reward, and self-administration of playback. J Comp Psychol. 2008;122:357–67. doi: 10.1037/a0012889. [DOI] [PubMed] [Google Scholar]
- 27.Burgdorf J, Panksepp J, Moskal JR. Frequency-modulated 50 kHz ultrasonic vocalizations: a tool for uncovering the molecular substrates of positive affect. Neuroscience and biobehavioral reviews. 2011;35:1831–6. doi: 10.1016/j.neubiorev.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 28.Knutson B, Burgdorf J, Panksepp J. Ultrasonic vocalizations as indices of affective states in rats. Psychological bulletin. 2002;128:961–77. doi: 10.1037/0033-2909.128.6.961. [DOI] [PubMed] [Google Scholar]
- 29.Litvin Y, Blanchard DC, Blanchard RJ. Rat 22kHz ultrasonic vocalizations as alarm cries. Behavioural brain research. 2007;182:166–72. doi: 10.1016/j.bbr.2006.11.038. [DOI] [PubMed] [Google Scholar]
- 30.Covington HE, 3rd, Miczek KA. Vocalizations during withdrawal from opiates and cocaine: possible expressions of affective distress. European journal of pharmacology. 2003;467:1–13. doi: 10.1016/s0014-2999(03)01558-9. [DOI] [PubMed] [Google Scholar]
- 31.Wohr M, Houx B, Schwarting RK, Spruijt B. Effects of experience and context on 50-kHz vocalizations in rats. Physiology & behavior. 2008;93:766–76. doi: 10.1016/j.physbeh.2007.11.031. [DOI] [PubMed] [Google Scholar]
- 32.Brudzynski SM. Principles of rat communication: quantitative parameters of ultrasonic calls in rats. Behavior genetics. 2005;35:85–92. doi: 10.1007/s10519-004-0858-3. [DOI] [PubMed] [Google Scholar]
- 33.Wright JM, Gourdon JC, Clarke PB. Identification of multiple call categories within the rich repertoire of adult rat 50-kHz ultrasonic vocalizations: effects of amphetamine and social context. Psychopharmacology. 2010;211:1–13. doi: 10.1007/s00213-010-1859-y. [DOI] [PubMed] [Google Scholar]
- 34.Blanchard RJ, Blanchard DC, Agullana R, Weiss SM. Twenty-two kHz alarm cries to presentation of a predator, by laboratory rats living in visible burrow systems. Physiology & behavior. 1991;50:967–72. doi: 10.1016/0031-9384(91)90423-l. [DOI] [PubMed] [Google Scholar]
- 35.Panksepp J, Knutson B, Burgdorf J. The role of brain emotional systems in addictions: a neuro-evolutionary perspective and new ‘self-report’ animal model. Addiction. 2002;97:459–69. doi: 10.1046/j.1360-0443.2002.00025.x. [DOI] [PubMed] [Google Scholar]
- 36.Ahrens AM, Ma ST, Maier EY, Duvauchelle CL, Schallert T. Repeated intravenous amphetamine exposure: rapid and persistent sensitization of 50-kHz ultrasonic trill calls in rats. Behavioural brain research. 2009;197:205–9. doi: 10.1016/j.bbr.2008.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wright JM, Dobosiewicz MR, Clarke PB. alpha- and beta-Adrenergic receptors differentially modulate the emission of spontaneous and amphetamine-induced 50-kHz ultrasonic vocalizations in adult rats. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2012;37:808–21. doi: 10.1038/npp.2011.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simola N, Ma ST, Schallert T. Influence of acute caffeine on 50-kHz ultrasonic vocalizations in male adult rats and relevance to caffeine-mediated psychopharmacological effects. Int J Neuropsychopharmacol. 2010;13:123–32. doi: 10.1017/S1461145709990113. [DOI] [PubMed] [Google Scholar]
- 39.Taracha E, Hamed A, Krzascik P, Lehner M, Skorzewska A, Plaznik A, et al. Inter-individual diversity and intra-individual stability of amphetamine-induced sensitization of frequency-modulated 50-kHz vocalization in Sprague-Dawley rats. Psychopharmacology. 2012 doi: 10.1007/s00213-012-2658-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meyer PJ, Ma ST, Robinson TE. A cocaine cue is more preferred and evokes more frequency-modulated 50-kHz ultrasonic vocalizations in rats prone to attribute incentive salience to a food cue. Psychopharmacology. 2012;219:999–1009. doi: 10.1007/s00213-011-2429-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barker DJ, Root DH, Ma S, Jha S, Megehee L, Pawlak AP, et al. Dose-dependent differences in short ultrasonic vocalizations emitted by rats during cocaine self-administration. Psychopharmacology. 2010;211:435–42. doi: 10.1007/s00213-010-1913-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brudzynski SM. Ultrasonic vocalization induced by intracerebral carbachol in rats: localization and a dose-response study. Behavioural brain research. 1994;63:133–43. doi: 10.1016/0166-4328(94)90084-1. [DOI] [PubMed] [Google Scholar]
- 43.Browning JR, Browning DA, Maxwell AO, Dong Y, Jansen HT, Panksepp J, et al. Positive affective vocalizations during cocaine and sucrose self-administration: a model for spontaneous drug desire in rats. Neuropharmacology. 2011;61:268–75. doi: 10.1016/j.neuropharm.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maier EY, Ma ST, Ahrens A, Schallert TJ, Duvauchelle CL. Assessment of ultrasonic vocalizations during drug self-administration in rats. Journal of visualized experiments: JoVE. 2010 doi: 10.3791/2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maier EY, Abdalla M, Ahrens AM, Schallert T, Duvauchelle CL. The missing variable: ultrasonic vocalizations reveal hidden sensitization and tolerance-like effects during long-term cocaine administration. Psychopharmacology. 2012;219:1141–52. doi: 10.1007/s00213-011-2445-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith RJ, See RE, Aston-Jones G. Orexin/hypocretin signaling at the orexin 1 receptor regulates cue-elicited cocaine-seeking. The European journal of neuroscience. 2009;30:493–503. doi: 10.1111/j.1460-9568.2009.06844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Banna KM, Back SE, Do P, See RE. Yohimbine stress potentiates conditioned cue-induced reinstatement of heroin-seeking in rats. Behavioural brain research. 2010;208:144–8. doi: 10.1016/j.bbr.2009.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gass JT, Olive MF. Reinstatement of ethanol-seeking behavior following intravenous self-administration in Wistar rats. Alcoholism, clinical and experimental research. 2007;31:1441–5. doi: 10.1111/j.1530-0277.2007.00480.x. [DOI] [PubMed] [Google Scholar]
- 49.Le AD, Harding S, Juzytsch W, Funk D, Shaham Y. Role of alpha-2 adrenoceptors in stress-induced reinstatement of alcohol seeking and alcohol self-administration in rats. Psychopharmacology. 2005;179:366–73. doi: 10.1007/s00213-004-2036-y. [DOI] [PubMed] [Google Scholar]
- 50.Shepard JD, Bossert JM, Liu SY, Shaham Y. The anxiogenic drug yohimbine reinstates methamphetamine seeking in a rat model of drug relapse. Biological psychiatry. 2004;55:1082–9. doi: 10.1016/j.biopsych.2004.02.032. [DOI] [PubMed] [Google Scholar]
- 51.Anker JJ, Carroll ME. Reinstatement of cocaine seeking induced by drugs, cues, and stress in adolescent and adult rats. Psychopharmacology. 2010;208:211–22. doi: 10.1007/s00213-009-1721-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bongiovanni M, See RE. A comparison of the effects of different operant training experiences and dietary restriction on the reinstatement of cocaine-seeking in rats. Pharmacology, biochemistry, and behavior. 2008;89:227–33. doi: 10.1016/j.pbb.2007.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Feltenstein MW, See RE. Potentiation of cue-induced reinstatement of cocaine-seeking in rats by the anxiogenic drug yohimbine. Behavioural brain research. 2006;174:1–8. doi: 10.1016/j.bbr.2006.06.039. [DOI] [PubMed] [Google Scholar]
- 54.Suemaru S, Dallman MF, Darlington DN, Cascio CS, Shinsako J. Role of alpha-adrenergic mechanism in effects of morphine on the hypothalamo-pituitary-adrenocortical and cardiovascular systems in the rat. Neuroendocrinology. 1989;49:181–90. doi: 10.1159/000125112. [DOI] [PubMed] [Google Scholar]
- 55.Schwarting RK, Jegan N, Wohr M. Situational factors, conditions and individual variables which can determine ultrasonic vocalizations in male adult Wistar rats. Behavioural brain research. 2007;182:208–22. doi: 10.1016/j.bbr.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 56.Brudzynski SM, Pniak A. Social contacts and production of 50-kHz short ultrasonic calls in adult rats. J Comp Psychol. 2002;116:73–82. doi: 10.1037/0735-7036.116.1.73. [DOI] [PubMed] [Google Scholar]
- 57.Burgdorf J, Panksepp J. The neurobiology of positive emotions. Neuroscience and biobehavioral reviews. 2006;30:173–87. doi: 10.1016/j.neubiorev.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 58.Natusch C, Schwarting RK. Using bedding in a test environment critically affects 50-kHz ultrasonic vocalizations in laboratory rats. Pharmacology, biochemistry, and behavior. 2010;96:251–9. doi: 10.1016/j.pbb.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 59.Tornatzky W, Miczek KA. Alcohol, anxiolytics and social stress in rats. Psychopharmacology. 1995;121:135–44. doi: 10.1007/BF02245600. [DOI] [PubMed] [Google Scholar]
- 60.Thomas DA, Takahashi LK, Barfield RJ. Analysis of ultrasonic vocalizations emitted by intruders during aggressive encounters among rats (Rattus norvegicus) J Comp Psychol. 1983;97:201–6. [PubMed] [Google Scholar]
- 61.Haney M, Miczek KA. Ultrasounds emitted by female rats during agonistic interactions: effects of morphine and naltrexone. Psychopharmacology. 1994;114:441–8. doi: 10.1007/BF02249334. [DOI] [PubMed] [Google Scholar]
- 62.Kaltwasser MT. Startle-inducing acoustic stimuli evoke ultrasonic vocalization in the rat. Physiology & behavior. 1990;48:13–7. doi: 10.1016/0031-9384(90)90253-z. [DOI] [PubMed] [Google Scholar]
- 63.Miczek KA, Weerts EM, Vivian JA, Barros HM. Aggression, anxiety and vocalizations in animals: GABAA and 5-HT anxiolytics. Psychopharmacology. 1995;121:38–56. doi: 10.1007/BF02245590. [DOI] [PubMed] [Google Scholar]
- 64.Sales GD. Ultrasound and aggressive behaviour in rats and other small mammals. Animal behaviour. 1972;20:88–100. doi: 10.1016/s0003-3472(72)80177-5. [DOI] [PubMed] [Google Scholar]
- 65.Tornatzky W, Miczek KA. Behavioral and autonomic responses to intermittent social stress: differential protection by clonidine and metoprolol. Psychopharmacology. 1994;116:346–56. doi: 10.1007/BF02245339. [DOI] [PubMed] [Google Scholar]
- 66.Vivian JA, Miczek KA. Diazepam and gepirone selectively attenuate either 20–32 or 32–64 kHz ultrasonic vocalizations during aggressive encounters. Psychopharmacology. 1993;112:66–73. doi: 10.1007/BF02247364. [DOI] [PubMed] [Google Scholar]
- 67.Takahashi LK, Thomas DA, Barfield RJ. Analysis of ultrasonic vocalizations emitted by residents during aggressive encounters among rats (Rattus norvegicus) J Comp Psychol. 1983;97:207–12. [PubMed] [Google Scholar]
- 68.Ciucci MR, Ma ST, Fox C, Kane JR, Ramig LO, Schallert T. Qualitative changes in ultrasonic vocalization in rats after unilateral dopamine depletion or haloperidol: a preliminary study. Behavioural brain research. 2007;182:284–9. doi: 10.1016/j.bbr.2007.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ciucci MR, Ahrens AM, Ma ST, Kane JR, Windham EB, Woodlee MT, et al. Reduction of dopamine synaptic activity: degradation of 50-kHz ultrasonic vocalization in rats. Behavioral neuroscience. 2009;123:328–36. doi: 10.1037/a0014593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Panksepp J, Burgdorf J. “Laughing” rats and the evolutionary antecedents of human joy? Physiology & behavior. 2003;79:533–47. doi: 10.1016/s0031-9384(03)00159-8. [DOI] [PubMed] [Google Scholar]
- 71.Wohr M, Borta A, Schwarting RK. Overt behavior and ultrasonic vocalization in a fear conditioning paradigm: a dose-response study in the rat. Neurobiology of learning and memory. 2005;84:228–40. doi: 10.1016/j.nlm.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 72.Brudzynski SM, Ociepa D, Bihari F. Comparison between cholinergically and naturally induced ultrasonic vocalization in the rat. Journal of psychiatry & neuroscience: JPN. 1991;16:221–6. [PMC free article] [PubMed] [Google Scholar]
- 73.Brudzynski SM, Holland G. Acoustic characteristics of air puff-induced 22-kHz alarm calls in direct recordings. Neuroscience and biobehavioral reviews. 2005;29:1169–80. doi: 10.1016/j.neubiorev.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 74.Ettenberg A, Geist TD. Animal model for investigating the anxiogenic effects of self-administered cocaine. Psychopharmacology. 1991;103:455–61. doi: 10.1007/BF02244244. [DOI] [PubMed] [Google Scholar]
- 75.Wise RA, Yokel RA, DeWit H. Both positive reinforcement and conditioned aversion from amphetamine and from apomorphine in rats. Science. 1976;191:1273–5. doi: 10.1126/science.1257748. [DOI] [PubMed] [Google Scholar]
- 76.Koob GF. Neural mechanisms of drug reinforcement. Annals of the New York Academy of Sciences. 1992;654:171–91. doi: 10.1111/j.1749-6632.1992.tb25966.x. [DOI] [PubMed] [Google Scholar]
- 77.Ettenberg A. Opponent process properties of self-administered cocaine. Neuroscience and biobehavioral reviews. 2004;27:721–8. doi: 10.1016/j.neubiorev.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 78.Molewijk HE, van der Poel AM, Mos J, van der Heyden JA, Olivier B. Conditioned ultrasonic distress vocalizations in adult male rats as a behavioural paradigm for screening anti-panic drugs. Psychopharmacology. 1995;117:32–40. doi: 10.1007/BF02245095. [DOI] [PubMed] [Google Scholar]
- 79.De Vry J, Benz U, Schreiber R, Traber J. Shock-induced ultrasonic vocalization in young adult rats: a model for testing putative anti-anxiety drugs. European journal of pharmacology. 1993;249:331–9. doi: 10.1016/0014-2999(93)90530-u. [DOI] [PubMed] [Google Scholar]
- 80.Bremner JD, Krystal JH, Southwick SM, Charney DS. Noradrenergic mechanisms in stress and anxiety: II. Clinical studies. Synapse. 1996;23:39–51. doi: 10.1002/(SICI)1098-2396(199605)23:1<39::AID-SYN5>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 81.Southwick SM, Morgan CA, 3rd, Charney DS, High JR. Yohimbine use in a natural setting: effects on posttraumatic stress disorder. Biological psychiatry. 1999;46:442–4. doi: 10.1016/s0006-3223(99)00107-9. [DOI] [PubMed] [Google Scholar]
- 82.See RE, Waters RP. Pharmacologically-induced stress: a cross-species probe for translational research in drug addiction and relapse. Am J Transl Res. 2010;3:81–9. [PMC free article] [PubMed] [Google Scholar]
- 83.Goeders NE, Clampitt DM, Keller C, Sharma M, Guerin GF. Alprazolam and oxazepam block the cue-induced reinstatement of extinguished cocaine seeking in rats. Psychopharmacology. 2009;201:581–8. doi: 10.1007/s00213-008-1326-1. [DOI] [PubMed] [Google Scholar]
- 84.Portavella M, Depaulis A, Vergnes M. 22–28 kHz ultrasonic vocalizations associated with defensive reactions in male rats do not result from fear or aversion. Psychopharmacology. 1993;111:190–4. doi: 10.1007/BF02245522. [DOI] [PubMed] [Google Scholar]
- 85.Holy TE, Guo Z. Ultrasonic songs of male mice. PLoS biology. 2005;3:e386. doi: 10.1371/journal.pbio.0030386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lahvis GP, Alleva E, Scattoni ML. Translating mouse vocalizations: prosody and frequency modulation. Genes, brain, and behavior. 2011;10:4–16. doi: 10.1111/j.1601-183X.2010.00603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.van der Poel AM, Noach EJ, Miczek KA. Temporal patterning of ultrasonic distress calls in the adult rat: effects of morphine and benzodiazepines. Psychopharmacology. 1989;97:147–8. doi: 10.1007/BF00442236. [DOI] [PubMed] [Google Scholar]
- 88.Blanchard RJ, Yudko EB, Blanchard DC, Taukulis HK. High-frequency (35–70 kHz) ultrasonic vocalizations in rats confronted with anesthetized conspecifics: effects of gepirone, ethanol, and diazepam. Pharmacology, biochemistry, and behavior. 1993;44:313–9. doi: 10.1016/0091-3057(93)90467-8. [DOI] [PubMed] [Google Scholar]
- 89.Knutson B, Burgdorf J, Panksepp J. High-frequency ultrasonic vocalizations index conditioned pharmacological reward in rats. Physiology & behavior. 1999;66:639–43. doi: 10.1016/s0031-9384(98)00337-0. [DOI] [PubMed] [Google Scholar]
- 90.Thompson B, Leonard KC, Brudzynski SM. Amphetamine-induced 50 kHz calls from rat nucleus accumbens: a quantitative mapping study and acoustic analysis. Behavioural brain research. 2006;168:64–73. doi: 10.1016/j.bbr.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 91.Wintink AJ, Brudzynski SM. The related roles of dopamine and glutamate in the initiation of 50-kHz ultrasonic calls in adult rats. Pharmacology, biochemistry, and behavior. 2001;70:317–23. doi: 10.1016/s0091-3057(01)00615-3. [DOI] [PubMed] [Google Scholar]
- 92.McGinnis MY, Vakulenko M. Characterization of 50-kHz ultrasonic vocalizations in male and female rats. Physiology & behavior. 2003;80:81–8. doi: 10.1016/s0031-9384(03)00227-0. [DOI] [PubMed] [Google Scholar]