Abstract
Background
Binge drinking is common in young people. Alcoholic beverages vary significantly in their ethanol (EtOH) concentration [alcohol by volume (ABV)]. We previously showed EtOH concentration-dependent activation of the hypothalamic supraoptic nucleus. In the HIV-infected population, incidence of alcohol abuse is close to 50%. We found age-dependent expression of HIV-1 viral proteins in the HIV-1 transgenic (HIV-1Tg) rat. Thus, we hypothesized that there are age- and EtOH concentration-dependent effects of binge drinking in HIV-1-positive individuals.
Methods
Blood EtOH concentration (BEC) was measured in adult F344 rats after gavage (i.g.) administration of water, 20% EtOH, or 52% EtOH. We also compared expression of the HIV-1 viral protein, Tat, in the brain, spleen, and liver of adult and adolescent HIV-1Tg rats following binge i.g. administration of water, 20% EtOH, or 52% EtOH for 3 d (4.8 g/kg per d) using absolute quantitative real-time RT-PCR. In a parallel study, we assessed age-dependent motor function in the HIV-1Tg rats one day after exposure to 20% EtOH using the Open Field test.
Results
BEC was significantly higher in the 52% EtOH-treated F344 rats compared to the 20% EtOH animals at 90 min post treatment. In the adult HIV-1Tg rats, HIV-1 Tat expression (copies per μg of total RNA) was significantly increased in the brain, liver, and spleen of the 52% EtOH group, but not in the 20% EtOH group. However, in the adolescent animals, HIV-1 Tat expression in the 52% EtOH group was increased in the brain and liver, but not in the spleen. A significant reduction in locomotor activity occurred in 20% EtOH-treated adult HIV-1Tg rats compared to the water control, although no difference was observed in the adolescent HIV-1Tg animals.
Conclusion
Our data indicate that binge alcohol drinking can have age- and EtOH concentration-dependent effects in the presence of HIV-1 infection.
Keywords: HIV-1 Tat, blood ethanol concentration, locomotor activity
Introduction
Alcohol abuse is a serious problem in most populations, and has been implicated in the development and progression of many health issues, such as liver and heart disease, depression and sleep disorders, stroke, cancer, and HIV/AIDS (Naimi et al., 2003). Low self-esteem, stress, and personal problems are common factors that can lead to increased alcohol use, and, in the United States (U.S.), one in six individuals will develop a drinking problem (CDC, 2010a). Binge drinking, a common form of acute alcohol abuse, is defined as “a pattern of heavy drinking over a very short time that results in a blood alcohol concentration (BAC) of 0.08 g% or above” (CDC, 2010a; NIAAA, 2011). This is considered to result from consumption of four or more alcoholic drinks in a two hour span, where “14 grams or 0.6 fluid ounces of pure alcohol” is considered to be one drink (CDC, 2010a; NIAAA, 2011). Binge drinking is a high risk factor that can lead to “accidents, behavioral problems, violence, suicide, and exposure to sexually transmitted diseases” among other things (CDC, 2010b; Naimi et al., 2003). Binge drinking in young adults has also been correlated with neurocognitive deficits involving working memory (Courtney and Polich, 2009). In 2009, 15.2% of the U.S. population indulged in binge drinking (CDC, 2010a).
In adolescents and young adults, peer pressure and easy access to alcohol can lead to alcohol abuse, and “binge drinking” is common in the young population (CDC, 2010a; NIAAA, 2011). Young people generally tend to binge drink at parties, partly because of the drinking age limit, but also due to the high cost of alcohol in bars and restaurants (Thomas, 2007).
Alcoholic beverages can contain different amounts of alcohol by volume (ABV), such as 5% ABV in beer, 12% ABV in wine, and 40% ABV in hard liquor, including vodka, rum, and whiskey. In the U.S., the general population prefers beer as their alcoholic beverage of choice; however, a significant number of adolescents and young adults (43.8%) prefer hard liquor (Siegel et al., 2011). Using FOS immunocytochemical staining to detect the sites of neuronal activation in response to treatment with alcohol, our laboratory has shown that, in the rat brain, the hypothalamic supraoptic nucleus (SON) is activated only by ethanol at 32% or higher concentrations (Chang et al., 1995). Activation of the SON, particularly vasopressin neurons involved in osmoregulation, occurs only when the alcohol consumed is at a high concentration, equivalent to that of hard liquor. These findings suggest that different ABV beverages can activate several neuronal pathways leading to a variety of physiological outcomes, including dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis. In the current study, we examined the effects of different concentrations of ethanol (EtOH), at the same dosage, on the time-course of blood ethanol concentration (BEC) levels and also confirm the differential effects on neuronal activation as reported previously (Chang et al., 1995).
Individuals infected with HIV-1, a viral disease that results in immunodeficiency, have a higher probability to abuse addictive substances, including alcohol (Chang et al., 2007a; Petry, 1999), which can further complicate the progression of their disease (Acheampong et al., 2002; Baum et al., 2010; Flora et al., 2005; Naimi et al., 2003). Alcohol’s immunosuppressive effects can increase the acquisition of opportunistic infections in the HIV-1-infected population by suppressing T cell responses and NK cell and macrophage activity, and enhancing T cell apoptosis (Baum et al., 2010). Alcohol has also been shown to alter cytokine responses, reduce macrophage reactive oxygen species production, and decrease CD4+ and CD8+ counts in HIV-1-infected individuals, leading to accelerated AIDS symptoms (Baum et al., 2010; Flora et al., 2005). Conversely, both in vivo and in vitro studies have shown that alcohol, in the presence of the HIV-1 viral protein, Tat, increases production of neuronal cytotoxic factors, such as IL-1β, TNF-α, IL-6, and MCP-1, in brain cells, which can lead to HIV-1 associated neurodegeneration and dementia (Acheampong et al., 2002; Flora et al., 2005; Lawson et al., 2011; Mayne et al., 2000). HIV-1 Tat is a potent trans-activator of HIV-1, and affects the host’s immune functions by modulating production of various cytokines, including IL-10, IL-1, TNF- α, and IL-6 (Buonaguro et al., 1992).
The HIV-1 transgenic rat (HIV-1Tg) used in this study is a rodent model with characteristics and abnormalities similar to HIV-1 patients on highly active anti-retroviral therapy (HAART). These animals carry the entire HIV-1 genome, with the exception of the gag and pol viral replication genes (Chang et al., 2007a; Chang et al., 2007b; Peng et al., 2010; Reid et al., 2001). We have also found that the HIV-1Tg rats have learning and cognitive deficits similar to that in HIV-1 infected patients, which can progress to HIV-1 associated dementia (HAD) and HIV-1 associated neurodegeneration (HAND) (Lashomb et al., 2009; Vigorito et al., 2007). There also appears to be an age-dependent differential expression of HIV-1 viral proteins in the HIV-1Tg rat. In older HIV-1Tg rats, there is increased HIV-1 Tat expression in the cerebellum, striatum, and spinal cord; whereas, in younger HIV-1Tg rats, Tat expression is increased in the prefrontal cortex (Peng et al., 2010).
In this study, we examined the EtOH concentration-dependent effect on the BEC profile in general, and investigated age and EtOH concentration-dependent effects on (a) HIV-1 Tat gene expression using absolute quantitative real-time PCR (aqRT-PCR), and (b) motor deficits using the Open Field test in HIV-1Tg rats.
Materials and Methods
Animals
Adolescent and adult HIV-1Tg and F344 male rats were purchased from Harlan Co. (Indianapolis, IN). The rats were allowed food and water ad libitum except during habituation and experimental testing. At the start of the experiment, the adolescent animals were 30–37 PD old and the adult animals were 75–84 PD old. The experimental protocol was approved by the Institutional Animal Care and Use Committee (IACUC) at Seton Hall University, South Orange, NJ.
Ethanol treatment
(a.) Blood EtOH concentration (BEC) study
Adult F344 rats were assigned into one of three EtOH treatment groups: 0% EtOH (water-control); 20% EtOH; or 52% EtOH (n = 16 for each group). The groups were administered 0%, 20%, or 52% EtOH by gavage (i.g.), for a total of 2.4 g/kg, at 8:00 AM. Animals from each group were sacrificed at 0, 15, 45, and 90 min after treatment (n = 4 for each time point per group), and trunk blood was collected.
(b.) AqRT-PCR and PCR array study
Both adult and adolescent rats were assigned into one of the following groups: HIV-1Tg 0% EtOH (water-control, n = 4); HIV-1Tg 20% EtOH (n = 4); HIV-1Tg 52% EtOH (n = 4); F344 0% EtOH (n = 4); F344 20% EtOH (n = 4); or F344 52% EtOH (n = 4). The groups were administered 0%, 20%, or 52% EtOH i.g. twice a day (8:00 AM and 10:00 AM) for 3 d, for a total of 4.8 g/kg/d. Two hours after the final treatment, the brain, liver, and spleen were harvested for total RNA extraction (n = 4 for each group).
(c.) Locomotor activity study
Rats were assigned into one of the following groups: adolescent HIV-1Tg EtOH (n = 9); adolescent HIV-1Tg water-control (n = 10); adolescent F344 EtOH (n = 9); adolescent F344 water-control (n = 10); adult HIV-1Tg EtOH (n = 6); adult HIV-1Tg water-control (n = 6); adult F344 EtOH (n = 6); adult F344 water-control (n = 7). The EtOH groups were administered 20% EtOH i.g. twice a day (8:00 AM and 10:00 AM) for 3 d, for a total of 4.8 g/kg/d. One day after the last treatment, locomotor activity of each rat was measured.
Blood EtOH concentration (BEC)
Blood was collected after ethanol treatment as described above. BEC was measured on serum samples using an alcohol oxidase-based fluorometric assay kit from BioVision (Mountain View, CA).
Total RNA isolation and reverse transcription
Total RNA was isolated from brain, spleen, and liver homogenates with TRIZOL® (Invitrogen, Carlsbad, CA). RNA was purified using an RNeasy mini-kit (Qiagen, Valencia, CA). Five micrograms of total RNA from each sample were converted into cDNA using Moloney murine leukemia virus (M-MLV) reverse transcriptase (Invitrogen, Carlsbad, CA). Reverse transcription (RT) reactions were incubated in GeneAmp 2400 Thermocycler (Eppendorf, Westbury, NY) for 1 h at 37° C, followed by 10 min at 67° C. Negative controls for the RT step consisted of six randomly chosen samples that were not treated with M-MLV reverse transcriptase.
Absolute quantitative real-time PCR (aqRT-PCR)
(a.) Preparation of HIV-1 Tat standards
Genomic DNA from HIV-1Tg rat liver was isolated using a PureLink Genomic Mini-kit (Invitrogen, Carlsbad, CA). Amplitaq DNA Polymerase [5 U/μl] (Applied Biosystems, Foster, CA) and primers (Table 1) were used to amplify HIV-1 Tat sequences in a thermal cycler for 45 cycles (at 95° C for 30 min, 60° C for 30 min, 72° C for 30 min, and 72° C for 10 min). Presence of HIV-1 Tat-specific sequences was confirmed by gel electrophoresis. Purified sequences were ligated into a pCR II TOPO vector using a TOPO TA Cloning Kit (Invitrogen, Carlsbad, CA), transformed into One Shot chemically competent E. coli cells (Invitrogen, Carlsbad, CA), and incubated overnight on LB-agar/pen-strep/X-gal plates at 37° C. To confirm the presence of HIV-1 Tat inserts, colony PCR was performed and sent for DNA sequence analysis to GeneWiz, Inc. (South Plainfield, NJ). Recombinant plasmids were isolated using the PureLink Quick-Plasmid Miniprep (Invitrogen, Carlsbad, CA). Plasmid DNA was linearized and amplified, using pUCM13 primers (Promega, Madison, WI) [Table 1]. The plasmid DNA was transcribed into RNA with a MEGAscript T7 and SP6 transcription kit from Ambion (Austin, TX). Copy numbers for HIV-1 Tat were calculated and adjusted to produce dilutions from 1011 to 104 copies per μl. One microliter of each standard was reverse transcribed into cDNA. GAPDH standards were prepared by reverse transcribing 1000, 200, 40, and 8 ng of total RNA.
Table 1.
Sequence of primers and probes used for semi-quantitative and real-time PCR
| Primers | Sequence |
|---|---|
| HIV-1 Tat | Forward: 5′-ATGGAGCCAGTATATCCTA-3′ Reverse: 5′-TGCTTTGATATAGAAACTTGATG-3′ Probe: 5′-AAGCGGAGACAGCGACGAAGACCTC-3′ |
| GAPDH | Forward: 5′-AGAGAGAGGCCCTCAGTTGCT-3′ Reverse: 5′-TTGTGAGGGAGATGCTCAGTGT-3′ Probe: 5′-AGTCCCCATCCCAACTCAGCCCC-3′ |
| pUCM13 | Forward: 5′-CGCCAGGGTTTTCCCAGTCACGAC-3′ Reverse: 5′-TCACACAGGAAACAGCTATGAC-3′ |
(b.) Real-time PCR
Real-time PCR was performed with HIV-1 Tat- and GAPDH-specific primers, probes (Table 1), and standards for each experimental sample. The ABI Prism 7900HT Fast Detection System (Applied Biosystems, Foster, CA) was used with the following parameters: 2 min at 50° C, 10 min at 95° C, 15 sec at 95° C, and 1 min at 60° C (40 cycles).
(c.) Analysis
Copy numbers of HIV-1 Tat for each sample were determined using the HIV-1 Tat standard curve, and normalized to GAPDH. The negative controls showed no amplification, indicating no DNA contamination in the RNA preparation.
PCR array
A custom made PCR array kit was used according to the manufacturer’s instructions (SABiosciences, Frederick, MD). Total RNA from the samples was subjected to real-time PCR. Thermocycler parameters were 95° C for 10 min, 95° C for 15 sec, and 60° C for 1 min (40 cycles). Threshold cycle value (Ct) data were analyzed on the manufacturer’s website, (http://www.sabiosciences.com/pcr/arrayanalysis.php). Expression of each gene was normalized to two housekeeping genes. Relative expression, compared to the control group, was calculated using the ΔΔCT method, and >2-fold difference compared to control was considered as differential regulation.
Assessment of locomotor activity
One day after the last ethanol treatment, locomotor activity of each rat was measured in an Open-field chamber, 40 cm w × 40 cm d × 35 cm h (Stoelting Co., Wood Dale, IL). The video image was tracked using ANY-maze software (Stoelting Co., Wood Dale, IL). The distance (cm) the rat traveled was recorded for 25 min.
Statistical analysis
All data were expressed as the mean ± S.E.M. Statistical analysis was performed using GraphPad Prism software. Significance (p value < 0.05) was determined using the student’s t-test or one-way ANOVA with a Dunn’s post hoc test.
Results
Time course of blood EtOH concentration (BEC) following binge treatment with 20% EtOH versus 52% EtOH
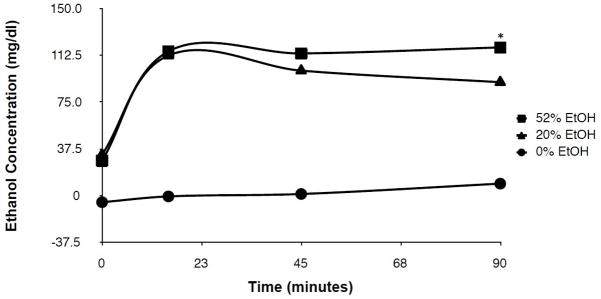
Figure 1 shows the time course of BEC in response to 0%, 20%, and 52% EtOH solutions in adult F344 rats. Peak BEC was reached at 15 min post-treatment for both the 20% (112.7 ± 9.85 mg/dl) and 52% (115.7 ± 6.73 mg/dl) EtOH groups. At 90 min post-treatment, the BEC was significantly lower in the 20% group (91.01 ± 4.07 mg/dl) compared to the 52% group (118.9 ± 9.55 mg/dl).
Figure 1. Time course of blood EtOH concentration following binge treatment with 20% EtOH versus 52% EtOH.
F344 rats were given i.g. treatment of a 0% (water control), 20% EtOH, or 52% EtOH solution (total dose = 2.4 g/kg), and the blood EtOH concentration in serum was determined at 0, 15, 45, and 90 min post treatment. Values represent the mean ± SEM (n = 4 rats for each group per time point). *p< 0.05.
Tissue- and age-dependent expression of HIV-1 Tat in the brain, liver, and spleen of the HIV-1Tg rat
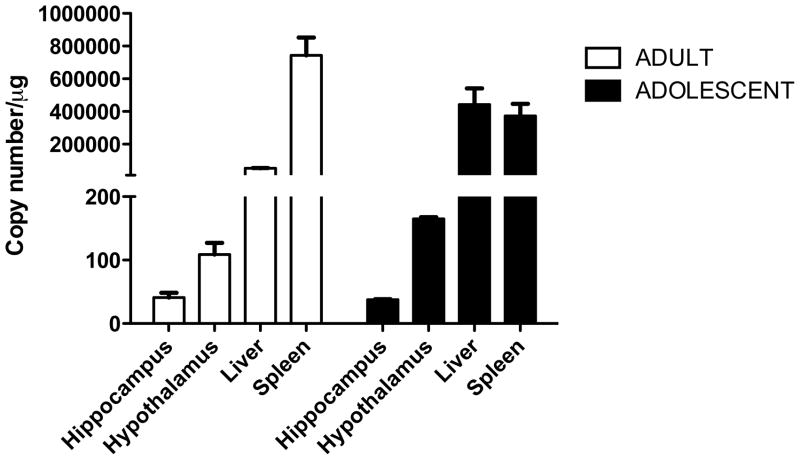
We used a novel aqRT-PCR method to determine HIV-1 Tat expression in adult and adolescent HIV-1Tg rats. In both adult and adolescent rats, HIV-1 Tat expression was lowest in the brain compared to the liver and spleen (Fig. 2).
Figure 2. Tissue- and age-dependent expression of HIV-1 Tat in the brain, liver, and spleen of the HIV-1Tg rat.
The hypothalamus, hippocampus, liver, and spleen of adult (PD 84) and adolescent (PD 30–37) HIV-1Tg rats were harvested and analyzed for HIV-1 Tat expression using a qRT-PCR. Values represent the mean ± SEM (n = 4 rats per tissue type for each age group).
In the adult HIV-1Tg rat brain, HIV-1 Tat expression was lower in the hippocampus (41.10 ± 7.3 copies/μg) than the hypothalamus [90.30 ± 3.0 copies/μg] (Fig. 2A). The liver had moderate HIV-1 Tat expression (5.24 × 104 ± 1.47 × 104 copies/μg). Highest HIV-1 Tat expression among the three tissues was observed in the spleen (6.48 × 105 ± 9.2 × 104 copies/μg).
In the adolescent HIV-1Tg rat brain, HIV-1 Tat expression was lower in the hippocampus (37.69 ± 0.75 copies/μg) than in the hypothalamus [164.9 ± 2.77 copies/μg] (Fig. 2B). In contrast to the adult HIV-1Tg rats, HIV-1 Tat expression in the adolescent liver (4.42 × 105 ± 9.87 × 104 copies/μg) was the highest compared to the spleen (3.73 × 105 ± 7.42 × 104 copies/μg) or brain.
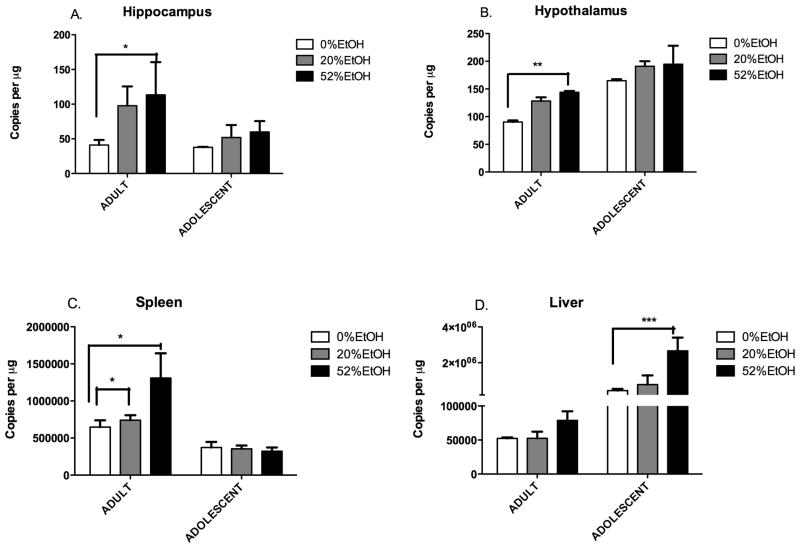
Age- and EtOH concentration-dependent HIV-1 Tat expression in adult and adolescent HIV-1Tg rats
HIV-1 Tat expression was measured in adult and adolescent HIV-1Tg rats binge treated with 20% and 52% EtOH. In adult HIV-1Tg animals, there was a corresponding increase in HIV-1 Tat expression as EtOH concentration increased in all three tissues – brain, liver, and spleen. HIV-1 Tat expression was significantly higher in the brain of the 52% EtOH group (113.4 ± 47.16 copies/μg in the hippocampus; 144 ± 2.374 copies/μg in the hypothalamus) compared to the 20% EtOH (97.71 ± 27.82 copies/μg in the hippocampus; 128.2 ± 6.683 copies/μg in the hypothalamus) or water-control (41.10 ± 7.3 copies/μg in hippocampus; 90.3 ± 2.9 copies/μg in hypothalamus) groups. Likewise, in the spleen (Fig. 3C), the 52% EtOH group showed significantly higher HIV-1 Tat expression (1.31 ×106 ± 3.33 × 105 copies/μg) than the 20% EtOH (7.42 × 105 ± 6.57 × 104 copies/μg) and water-control (6.48 ×105 ± 9.2 × 104 copies/μg) groups. Similarly, in the liver (Fig. 3D), the 52% EtOH group had the highest HIV-1 Tat expression (7.88 × 104 ± 2.3× 104 copies/μg). No difference was observed between the 20% EtOH (5.26 × 104 ± 9.73 × 104 copies/μg) and water-control (5.24 × 104 ± 1.47 × 104 copies/μg) groups.
Figure 3. Age- and EtOH concentration-dependent HIV-1 Tat expression in adult and adolescent HIV-1Tg rats.
HIV-1 Tat expression (copies/μg of total RNA) was measured in the brain, liver, and spleen of adult (PD 84) and adolescent (PD 30–37) HIV-1Tg rats administered (i.g.) 0% (water control), 20% EtOH, or 52% EtOH solution in a 3-d binge regimen (total dose of 4.8 g/kg/day). Values represent the mean ± SEM (n = 4 for each age group per tissue type for each EtOH concentration). *p< 0.05; **p < 0.01; ***p<0.001.
In the adolescent HIV-1Tg animals, elevated HIV-1 Tat expression was seen in the 52% EtOH concentration groups. However, differences between the groups were not statistically significant except in the liver. The 52% EtOH group showed the highest HIV-1 Tat expression in the brain (60 ± 15.56 copies/μg in the hippocampus; 194.7 ± 33.27 copies/μg in the hypothalamus), followed by the 20% EtOH group (51.91 ± 17.93 copies/μg in the hippocampus; 191 ± 9.23 copies/μg in the hypothalamus) and the water-control (37.69 ± 0.75 copies/μg in the hippocampus; 164.9 ±2.77 copies/μg in the hypothalamus) [Fig. 3A, 3B]. The most significant increase in HIV-1 Tat expression was observed in the liver (Fig. 3D), with the 52% EtOH group showing a very high level of expression (2.66 × 106 ± 7.4 × 105 copies/μg), followed by the 20% EtOH (7.81 × 105 ± 5.11 × 104 copies/μg), and water-control (4.42 × 105 ± 9.87 × 104 copies/μg) groups. However, no such trend was observed in the spleen (Fig. 3C). HIV-1 Tat expression in the spleen was similar in the 52% EtOH (3.73 × 105 ± 7.42 × 104 copies/μg), 20% EtOH (3.53 × 105 ± 4.61 × 104 copies/μg) and water-control (3.23 ×105 ± 4.92 × 104 copies/μg) groups.
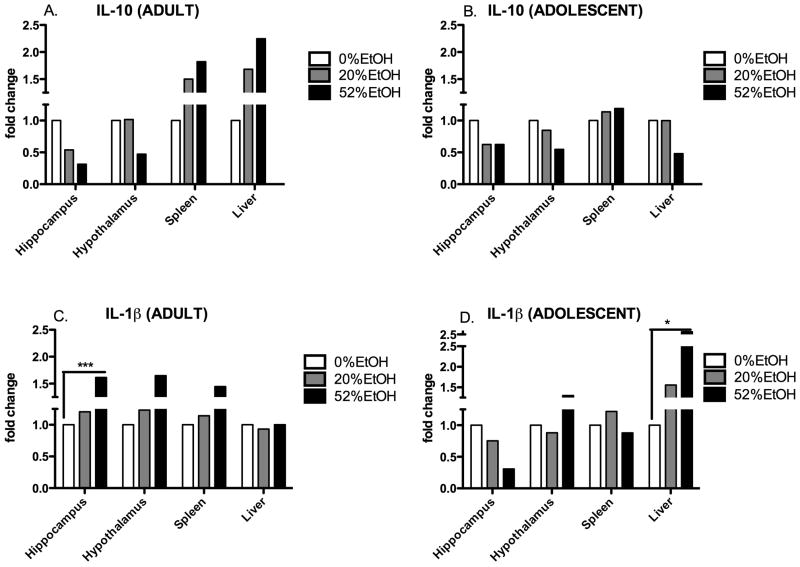
Age- and EtOH concentration-dependent expression of IL-1β and IL-10 in adult and adolescent HIV-1Tg rats
PCR array analysis was used to determine expression of the anti-inflammatory cytokine, IL-10, and the pro-inflammatory cytokine, IL-1β. Expression of IL-10 decreased in the brain in the adult HIV-1Tg rats (0.31-fold in the hippocampus; 0.47-fold in the hypothalamus) in response to the 52% EtOH treatment (Fig. 4A). Conversely, in both the liver and spleen, the increase in IL-10 expression correlated with the increase in EtOH concentration. The 52% EtOH group showed the greatest increase (1.82-fold in the spleen; 2.25-fold in the liver), followed by the 20% EtOH group (1.5-fold in the spleen; 1.68-fold in the liver). In the brain and liver of the adolescent HIV-1Tg rats, there was a strong decrease in the 52% EtOH group in both the brain (0.62-fold in the hippocampus; 0.54-fold in the hypothalamus) and liver (0.47-fold); although the spleen showed a slight increase [<1.2-fold] (Fig. 4B).
Figure 4. Age- and EtOH concentration-dependent expression of IL-1β and IL-10 in adult and adolescent HIV-1Tg rats.
IL-1β and IL-10 expression was measured (fold change) in the brain, liver, and spleen of adult (PD 84) and adolescent (PD 30–37) HIV-1Tg rats treated with 0% (water control), 20% EtOH, and 52% EtOH in a 3-d binge regimen (total dose of 4.8 g/kg/day). Data were calculated using the ΔΔCT method relative to water control (0% EtOH) and represented as fold change (n = 4 rats for each age group per tissue type for each EtOH concentration). *p< 0.05; **p < 0.01; ***p<0.001.
Expression of IL-1β in the brain and spleen of the adult HIV-1Tg rats increased as the EtOH concentration increased (Fig. 4C). The 52% EtOH group showed the greatest increase in both the brain (1.61-fold in the hippocampus; 1.65- fold in the hypothalamus) and spleen (1.45-fold). However, in the liver, IL-1β expression was not dependent on EtOH concentration. In the adolescent HIV-1Tg rats, IL-1β expression did not correlate with EtOH concentration in either the brain or spleen (Fig. 4D). However, in the liver, there was a significant increase (>3-fold) in the 52% EtOH group, followed by a moderate increase (1.6-fold) in the 20% EtOH group.
Age-dependent effect of alcohol (20% EtOH) on the locomotor activity of adult and adolescent HIV-1Tg rats
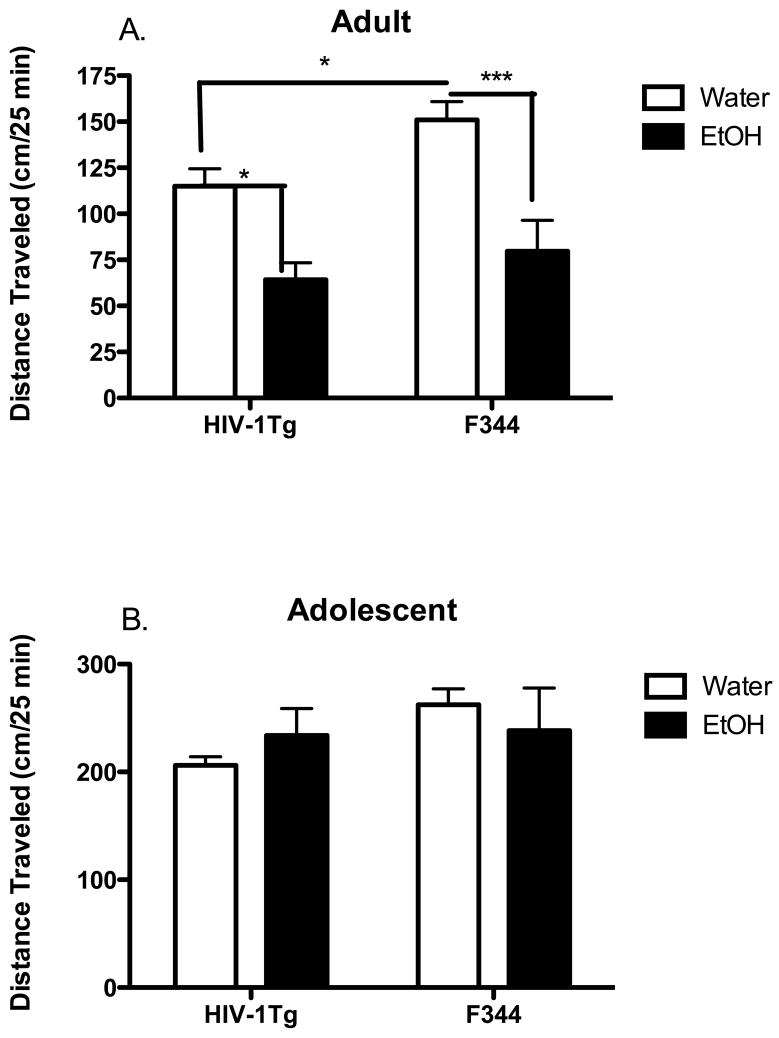
We investigated the age-dependent effect of binge alcohol abuse on locomotor activity in the adult and adolescent HIV-1Tg and F344 (control) rats following treatment with 20% EtOH compared to water (control) using the Open-Field test. In the adult animals (Fig. 5A), locomotor activity was decreased in the HIV-1Tg animals (115 ± 9.45 cm) given water compared to the F344 rats (151 ± 10 cm) given water. In the EtOH treated groups, locomotor activity of both the HIV-1Tg (64.2 ± 9.2 cm) and F344 (79.63 ± 16.8 cm) rats was significantly decreased after 20% EtOH treatment compared to the water-control groups.
Figure 5. Age-dependent effect of alcohol (20% EtOH) on the locomotor activity of adult and adolescent HIV-1Tg rats.
Locomotor activity (distance travelled in cm in 25 min) was assessed in adult (PD 84) and adolescent (PD 30–37) HIV-1Tg and F344 rats treated with 20% EtOH solution using a 3-d binge regimen (total dose of 4.8 g/kg/day). Values represent the mean ± SEM (n = 6–10 rats per treatment for each age group). *p< 0.05; **p < 0.01; ***p<0.001.
In the adolescent animals (Fig. 5B), there was no significant difference in locomotor activity in either the HIV-1Tg or F344 rats following either EtOH (206.2 ± 7.9 cm in HIV-1Tg; 262.4 ± 14.7 cm in F344) or water (233.9 ± 25.1 cm in HIV-1Tg; 238.4 ± 39.5 cm in F344) treatment.
Majority of the 52% EtOH treated rats (both HIV-1Tg and F344) were observed to be unresponsive in terms of physical activity on the day following the final treatment. Thus, no valid data for the locomotor activity of the animals in the 52% EtOH group is available, and we have chosen to present the data for the 20% EtOH group to address the age-dependent difference in locomotor activity.
Discussion
Choice of alcoholic beverage and pattern of drinking not only depend on personal preference, but also on age. Beer is the most popular alcoholic beverage in the U.S., although young people tend to binge drink with hard liquor (Siegel et al., 2011). Therefore, it is important to take into consideration the effect of the relative EtOH concentration of the beverage being consumed. We have previously reported that only 32% and higher EtOH solutions are able to activate the SON in the hypothalamus of the rat brain (Chang et al., 1995). One explanation for this finding is that higher EtOH concentrations induce dehydration, which increases production of vasopressin in the hypothalamus, which is a signal for fluid retention.
To validate our earlier data, we examined the possibility that the BEC profile could have been affected by the EtOH concentration. We observed that the BEC was dependent on the relative EtOH concentration of the alcoholic beverage. BEC peaked in both groups at 15 min post-treatment, indicating that BEC is not dependent on EtOH concentration at an early time point. However, binge treatment with 52% EtOH significantly increased the BEC 90 min after treatment compared to 20% EtOH, even though the total amount of EtOH administered was the same (2.4 g/kg) for both concentrations. These data indicate that the body does experience dehydration following the intake of a high concentration of EtOH, and confirms our previous findings (Chang et al., 1995).
Highly active anti-retroviral therapy (HAART) has been widely successful in limiting the HIV-1 viral load and maintaining a certain CD4+ T cell level, thereby increasing life expectancy (Volberding and Deeks, 2010). However, it cannot inhibit HIV-1 viral protein expression once the virus has entered the cell (Peng et al., 2010; Reid et al., 2001; Teixeira et al., 2011). Among the HIV-1 viral proteins, HIV-1 Tat is the most important regulatory protein since its expression is necessary for transcription of other viral protein genes (Teixeira et al., 2011).
Modulation of host immune responses is an important function of HIV-1 Tat. We found that the expression of HIV-1 Tat was lower in the brain compared to the liver and spleen in both the adult and adolescent HIV-1Tg rats. This could be due to the fact that the heightened immune response in the brain suppresses HIV-1 viral functioning (Persidsky and Poluektova, 2006). Comparing HIV-1 Tat expression in adult and adolescent HIV-1Tg rats, we found that HIV-1 Tat expression in the brain of both age groups was quite similar, with the hypothalamus showing a slightly higher expression than the hippocampus. In the spleen, HIV-1 Tat expression in the adult HIV-1Tg rats was two-fold higher than in the adolescent HIV-1Tg rats. However, in the liver, HIV-1 Tat expression was nine times higher in the adolescent HIV-1Tg rats than in the adult HIV-1Tg rats. Age- and tissue-dependent expression of HIV-1 Tat was also observed in our previous study, where we found that there is an age-dependent expression of viral protein expression, particularly HIV-1 Tat, in the central nervous system (CNS) and peripheral tissues of younger (2–3 mo) and older (10–11 mo) adult HIV–1Tg rats (Peng et al., 2010).
The high correlation between HIV-1 infection and alcohol abuse is a significant clinical problem. Alcohol not only interferes with the treatment of HIV-infected individuals, it can also aggravate the disease condition and lead to a faster development of AIDS (Baum et al., 2010). In addition, alcohol abuse can cause poorer adherence to anti-retroviral medication by further depreciating CD4+ T cell counts and compromising viral load reduction (Baum et al., 2010).
Many studies have suggested that there may be a synergistic effect between HIV-1 viral proteins, particularly HIV-1 Tat, and EtOH on the host’s immune responses and disease pathology (Acheampong et al., 2002; Flora et al., 2005). In this study, we found a correlation between EtOH concentration and HIV-1 Tat expression in the HIV-1Tg rat. HIV-1 Tat expression was greater in the group of HIV-1Tg rats given the higher concentration of EtOH (52%) than in the group given the lower concentration of EtOH (20%).
This HIV-1 Tat expression was further influenced by the age of the rats. In the adult HIV-1Tg rats, the increase in HIV-1 Tat expression was significantly greater in the 52% EtOH group, particularly in the brain and spleen. However, in the adolescent HIV-1Tg rats, elevated HIV-1 Tat expression was noted in the brain and liver, but not in the spleen in the 52% EtOH group.
HIV-1 Tat, in conjunction with EtOH, has been implicated in inducing brain microvascular endothelial cell apoptosis by modulating cytokine secretion (Acheampong et al., 2002). Therefore, an EtOH concentration-dependent increase in HIV-1 Tat expression and a consequent elevation in pro-inflammatory IL-1β expression in the brain could reflect damage to brain cells. In the hippocampus, this damage could lead to the development of HAND (Acheampong et al., 2002). Similarly, hypothalamic neuronal damage can cause dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis. Hormone secretion, such as vasopressin and corticotropin releasing factor (CRF), from the hypothalamus induces the pituitary to secrete adrenocorticotropic hormone (ATCH) that, in turn, stimulates the adrenal gland to release stress hormones, such as cortisol, which, in a negative feedback loop, influences the hypothalamus. Therefore, in the event of HPA axis dysregulation, homeostasis is lost, causing an accumulation of stress hormones that can induce further immunosuppression. EtOH is known to affect the function of the HPA axis, which could be aggravated in the presence of HIV-1 Tat.
Age also influenced HIV-1 Tat expression. HIV-1 Tat and IL-1β expression in the adult HIV-1Tg rats showed a greater dependence on EtOH concentration compared to the adolescent HIV-1Tg rats, suggesting increased vulnerability to HIV-1 Tat and EtOH at a younger age.
In the spleen, the adult HIV-1Tg rats had increased HIV-1 Tat expression, in addition to increased expression of IL-1β and IL-10, when treated with the higher EtOH concentration (52%). However, in the adolescent HIV-1Tg rats, neither HIV-1 Tat nor cytokine expression showed EtOH concentration-dependence, indicating that the spleen of the adolescent HIV-1Tg rat is less susceptible to the damaging effects of alcohol. However, in the liver of the adolescent HIV-1Tg rats, expression of HIV-1 Tat and IL-1β increased significantly with 52% EtOH compared to 20% EtOH. Conversely, in the adult HIV-1Tg rats, there was no significant difference in HIV-1 Tat or IL-1β expression between groups treated with a high (52%) or low (20%) EtOH concentration. This is the reverse of our observations in the brain and spleen, and suggests that the adolescent HIV-1Tg liver is more susceptible to EtOH than the adult HIV-1Tg rat liver. It has been reported that, in adolescents with alcohol use disorders (AUD), the liver secretes elevated levels of liver enzymes, particularly alanine aminotransferase (ALT). Over-secretion of ALT has also been reported in adolescents with hepatitis C virus (HCV) infection (Van der Poorten et al., 2007). Elevated ALT is widely used as a clinical marker for liver injury (Clark, 2001). These reports support our observation that the adolescent liver is more prone to damage, particularly in the presence of EtOH and viral infection.
The data from our studies strongly suggest that HIV-1 Tat expression depends not only on the concentration of EtOH, but also on age. In addition to gene expression, both binge EtOH consumption and age can influence physical performance of motor functions. We investigated age-dependent differences in locomotor activity in HIV-1Tg and F344 (control) rats given a binge treatment with either EtOH or water. In the EtOH-treated groups of both the HIV-1Tg and F344 adult rats, locomotor activity was significantly decreased compared to the respective water-controls. In addition, locomotor activity was decreased significantly in the adult HIV-1Tg rats compared to the F344 rats, suggesting that the presence of HIV-1 Tat and other viral proteins, alone and in conjunction with EtOH, can affect motor function. It is possible that the persistent presence of the virus may alter EtOH metabolism leading to different physiological outcomes in the HIV-1Tg rats. Haorah et al. (2004) reported that activity of the enzyme, CYP2E1, which is required for EtOH metabolism, is significantly low in HIV-1 infected human monocyte-derived macrophages (Haorah et al., 2004). In the adolescent HIV-1Tg and F344 rats, there was no statistical difference between the HIV-1Tg and F344 rats, or between the EtOH treated and water-control groups, indicating that adolescent rats are not as sensitive to the adverse effects of EtOH and are, therefore, prone to consume greater amounts of alcohol, such as is seen in binge drinking of hard liquor. In addition, younger individuals appear to be able to metabolize EtOH more efficiently in comparison to their older counterparts, partly due to the larger size of their liver and the corresponding higher levels of liver enzymes, including those involved in EtOH metabolism (Kim et al., 2003). However, early in our study, we found that binge intake of EtOH, particularly high concentration EtOH, increased HIV-1 Tat expression and modulated cytokine expression in the adolescent HIV-1Tg rats. This suggests that, although, physically, the adolescent HIV-1Tg rats are not affected by binge EtOH exposure, physiologically the adverse effects of binge EtOH drinking may still be experienced. In addition, age-related differences in EtOH metabolism can influence subsequent effects of EtOH.
Our data showed that HIV-1 Tat expression increases with binge exposure to a high EtOH concentration (52%) compared to a low EtOH concentration (20%). These results support our hypothesis that there are EtOH concentration-dependent effects on the expression of HIV-1 viral proteins, such as Tat, which can potentially aggravate HIV-1 disease. Our findings also indicate that there is a correlation between age, EtOH concentration, and HIV-1 Tat expression. Taken together, our findings suggest that the choice of alcoholic beverage can significantly affect the physiological condition of younger people, especially HIV-1 infected individuals.
Acknowledgments
This study was supported, in part, by the National Institutes of Health/National Institute on Alcohol Abuse and Alcoholism (RC2 AA019415) and National Institute on Drug Abuse (K02 DA016149) to Sulie L. Chang. The authors thank Dr. Louaine Spriggs for her helpful critique of the manuscript during its preparation, and Eric J. LeTellier for his assistance with copy editing of the manuscript.
References
- Acheampong E, Mukhtar M, Parveen Z, Ngoubilly N, Ahmad N, Patel C, Pomerantz RJ. Ethanol strongly potentiates apoptosis induced by HIV-1 proteins in primary human brain microvascular endothelial cells. Virology. 2002;304:222–234. doi: 10.1006/viro.2002.1666. [DOI] [PubMed] [Google Scholar]
- Baum MK, Rafie C, Lai S, Sales S, Page JB, Campa A. Alcohol use accelerates HIV disease progression. AIDS Res Hum Retroviruses. 2010;26:511–518. doi: 10.1089/aid.2009.0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonaguro L, Barillari G, Chang HK, Bohan CA, Kao V, Morgan R, Gallo RC, Ensoli B. Effects of the human immunodeficiency virus type 1 Tat protein on the expression of inflammatory cytokines. J Virol. 1992;66:7159–7167. doi: 10.1128/jvi.66.12.7159-7167.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. Alcohol and public health. Vol. 2011. Center for Disease Control and prevention; 2010a. [Google Scholar]
- CDC. Fact sheets: Binge drinking. Vol. 2011. Center for Disease Control and prevention; 2010b. [Google Scholar]
- Chang SL, Beltran JA, Swarup S. Expression of the mu opioid receptor in the human immunodeficiency virus type 1 transgenic rat model. J Virol. 2007a;81:8406–8411. doi: 10.1128/JVI.00155-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SL, Ocasio F, Beltran JA. Immunodeficient Parameters in the HIV-1 Transgenic Rat Model. American Journal of Infectious Diseases. 2007b;3:202–207. [Google Scholar]
- Chang SL, Patel NA, Romero AA. Activation and desensitization of Fos immunoreactivity in the rat brain following ethanol administration. Brain Res. 1995;679:89–98. doi: 10.1016/0006-8993(95)00210-h. [DOI] [PubMed] [Google Scholar]
- Courtney KE, Polich J. Binge drinking in young adults: Data, definitions, and determinants. Psychol Bull. 2009;135:142–156. doi: 10.1037/a0014414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flora G, Pu H, Lee YW, Ravikumar R, Nath A, Hennig B, Toborek M. Proinflammatory synergism of ethanol and HIV-1 Tat protein in brain tissue. Exp Neurol. 2005;191:2–12. doi: 10.1016/j.expneurol.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Haorah J, Heilman D, Diekmann C, Osna N, Donohue TM, Jr, Ghorpade A, Persidsky Y. Alcohol and HIV decrease proteasome and immunoproteasome function in macrophages: implications for impaired immune function during disease. Cellular immunology. 2004;229:139–148. doi: 10.1016/j.cellimm.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Kim YC, Kim SY, Sohn YR. Effect of age increase on metabolism and toxicity of ethanol in female rats. Life sciences. 2003;74:509–519. doi: 10.1016/j.lfs.2003.07.009. [DOI] [PubMed] [Google Scholar]
- Lashomb AL, Vigorito M, Chang SL. Further characterization of the spatial learning deficit in the human immunodeficiency virus-1 transgenic rat. J Neurovirol. 2009;15:14–24. doi: 10.1080/13550280802232996. [DOI] [PubMed] [Google Scholar]
- Lawson MA, Kelley KW, Dantzer R. Intracerebroventricular administration of HIV-1 Tat induces brain cytokine and indoleamine 2,3-dioxygenase expression: A possible mechanism for AIDS comorbid depression. Brain Behav Immun. 2011;25:1569–1575. doi: 10.1016/j.bbi.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayne M, Holden CP, Nath A, Geiger JD. Release of calcium from inositol 1,4,5-trisphosphate receptor-regulated stores by HIV-1 Tat regulates TNF-alpha production in human macrophages. J Immunol. 2000;164:6538–6542. doi: 10.4049/jimmunol.164.12.6538. [DOI] [PubMed] [Google Scholar]
- Naimi TS, Brewer RD, Mokdad A, Denny C, Serdula MK, Marks JS. Binge drinking among US adults. JAMA. 2003;289:70–75. doi: 10.1001/jama.289.1.70. [DOI] [PubMed] [Google Scholar]
- NIAAA. Rethinking Drinking: Alcohol and your health. Vol. 2011. National Institute on Alcohol Abuse and Alcoholism; 2011. [Google Scholar]
- Peng J, Vigorito M, Liu X, Zhou D, Wu X, Chang SL. The HIV-1 transgenic rat as a model for HIV-1 infected individuals on HAART. J Neuroimmunol. 2010;218:94–101. doi: 10.1016/j.jneuroim.2009.09.014. [DOI] [PubMed] [Google Scholar]
- Persidsky Y, Poluektova L. Immune privilege and HIV-1 persistence in the CNS. Immunol Rev. 2006;213:180–194. doi: 10.1111/j.1600-065X.2006.00440.x. [DOI] [PubMed] [Google Scholar]
- Petry NM. Alcohol use in HIV patients: what we don’t know may hurt us. Int J STD AIDS. 1999;10:561–570. doi: 10.1258/0956462991914654. [DOI] [PubMed] [Google Scholar]
- Reid W, Sadowska M, Denaro F, Rao S, Foulke J, Jr, Hayes N, Jones O, Doodnauth D, Davis H, Sill A, O’Driscoll P, Huso D, Fouts T, Lewis G, Hill M, Kamin-Lewis R, Wei C, Ray P, Gallo RC, Reitz M, Bryant J. An HIV-1 transgenic rat that develops HIV-related pathology and immunologic dysfunction. Proc Natl Acad Sci U S A. 2001;98:9271–9276. doi: 10.1073/pnas.161290298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel M, Dejong W, Naimi TS, Heeren T, Rosenbloom DL, Ross C, Ostroff J, Jernigan DH. Alcohol brand preferences of underage youth: results from a pilot survey among a national sample. Subst Abus. 2011;32:191–201. doi: 10.1080/08897077.2011.601250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira C, Gomes JR, Gomes P, Maurel F, Barbault F. Viral surface glycoproteins, gp120 and gp41, as potential drug targets against HIV-1: brief overview one quarter of a century past the approval of zidovudine, the first anti-retroviral drug. Eur J Med Chem. 2011;46:979–992. doi: 10.1016/j.ejmech.2011.01.046. [DOI] [PubMed] [Google Scholar]
- Thomas SP. A dangerous new type of teenage drinking. Issues Ment Health Nurs. 2007;28:119971200. doi: 10.1080/01612840701651447. [DOI] [PubMed] [Google Scholar]
- Van der Poorten D, Kenny DT, Butler T, George J. Liver disease in adolescents: A cohort study of high-risk individuals. Hepatology. 2007;46:1750–1758. doi: 10.1002/hep.21918. [DOI] [PubMed] [Google Scholar]
- Vigorito M, LaShomb AL, Chang SL. Spatial learning and memory in HIV-1 transgenic rats. J Neuroimmune Pharmacol. 2007;2:319–328. doi: 10.1007/s11481-007-9078-y. [DOI] [PubMed] [Google Scholar]
- Volberding PA, Deeks SG. Antiretroviral therapy and management of HIV infection. Lancet. 2010;376:49–62. doi: 10.1016/S0140-6736(10)60676-9. [DOI] [PubMed] [Google Scholar]