Abstract
Rationale
The mechanisms that mediate age differences during nicotine withdrawal are unclear.
Objective
This study compared kappa opioid receptor (KOR) activation in naïve and nicotine-treated adolescent and adult rats using behavioral and neurochemical approaches to study withdrawal.
Methods
The behavioral models used to assess withdrawal included conditioned place and elevated plus maze procedures. Deficits in dopamine transmission in the nucleus accumbens (NAcc) were examined using microdialysis procedures. Lastly, the effects of KOR stimulation and blockade on physical signs produced upon removal of nicotine were examined in adults.
Results
Nicotine-treated adults displayed a robust aversion to an environment paired with a KOR agonist versus naïve adults. Neither of the adolescent groups displayed a place aversion. KOR activation produced an increase in anxiety-like behavior that was highest in nicotine-treated adults versus all other groups. KOR activation produced a decrease in NAcc dopamine that was largest in nicotine-treated adults versus all other groups. Lastly, KOR activation facilitated physical signs of upon removal of nicotine and KOR blockade reduced this effect.
Conclusion
Chronic nicotine enhanced the affective, anxiogenic, and neurochemical effects produced by KOR activation in adult rats. Our data suggest that chronic nicotine elicits an increase in KOR function, and this may contribute to nicotine withdrawal since KOR activation facilitated and KOR blockade prevented withdrawal signs upon removal of nicotine. Given that chronic nicotine facilitated the neurochemical effects of KOR agonists in adults but not adolescents, it is suggested that KOR regulation of mesolimbic dopamine may contribute to age differences in nicotine withdrawal.
Keywords: adolescent, anxiety, conditioned place aversion, development, dopamine, elevated plus maze, nicotinic, mesolimbic, microdialysis, nucleus accumbens
Introduction
Pre-clinical studies have shown that abstinence from chronic nicotine produces a withdrawal syndrome characterized by dysphoria, anxiety, and physical signs of withdrawal (Malin et al. 1993; Markou 2008; O’Dell 2009). Previous work has established that there are robust developmental differences in the behavioral effects of nicotine withdrawal. For example, nicotine-treated adult rats display physical signs of withdrawal following administration of the nicotinic receptor antagonist mecamylamine, and this effect is lower in adolescents (O’Dell et al. 2004 and 2006; Shram et al. 2008). Nicotine-treated adult rats (O’Dell et al. 2007) and mice (Kota et al. 2007) avoid an environment previously paired with nicotine withdrawal as compared to adolescents that display reduced aversive effects of withdrawal. Moreover, nicotine withdrawal produces an increase in anxiety-like behavior that is more robust in adult versus adolescent rats (Wilmouth and Spear 2006).
To date, the mechanisms that mediate developmental sensitivity to the behavioral and neurochemical effects of nicotine withdrawal are not clear. Negative states associated with nicotine withdrawal have been shown to depend, in large part, on dopamine neurotransmission (Grieder et al. 2010). Nicotine withdrawal produces a decrease in extracellular levels of dopamine in the nucleus accumbens (NAcc), a terminal region of the mesolimbic brain reward pathway (Carboni et al 2000; Hildebrand et al. 1998; Rada et al. 2001). Dopamine decreases in the NAcc during withdrawal have been closely linked to the emergence of physical signs produced by withdrawal from chronic nicotine (Hildebrand et al. 1998). With regard to developmental differences, we have shown that nicotine withdrawal decreases dopamine levels in the NAcc, and this effect is larger in adult versus adolescent rats (Natividad et al. 2010). Collectively, these reports demonstrate that the behavioral and neurochemical effects of nicotine withdrawal are reduced during the adolescent period of development.
Previous work has suggested that endogenous opioid systems modulate nicotine withdrawal. For example, naloxone, an opioid receptor antagonist, precipitates somatic signs of nicotine withdrawal at doses selective for mu-opioid receptors (MORs; Malin et al. 1993; Watkins et al. 2000). Interestingly, kappa-opioid receptor (KOR) and MOR agonists produce opposite effects in tests of affective behavior (Bals-Kubik et al. 1993; Mucha and Herz, 1985; Shippenberg et al. 1993) and exert opposing control over mesolimbic dopamine levels (Di Chiara and Imperato 1988; Spanagel et al. 1992). Given the opposite role of these systems, the possibility exists that blockade of MORs elicits nicotine withdrawal, whereas stimulation of KORs may precipitate withdrawal. The present series of studies were conducted as a first step towards examining the role of KOR systems in mediating developmental differences in nicotine withdrawal.
KORs and their endogenous ligands, dynorphin, are highly expressed in neural circuits that control affect, motivation, and stress (Carlezon and Thomas 2009; Shippenberg et al. 2007; Wee and Koob 2010). It has been suggested that KOR activation elicits negative affect via modulation of mesolimbic dopamine transmission, particularly in the NAcc. For example, mesolimbic, but not nigrostriatal or mesocortical, dopaminergic lesions or NAcc D1 dopamine receptor antagonism attenuates aversive effects produced by KOR agonist administration (Shippenberg et al. 1993). Also, NAcc dopamine levels are decreased following systemic (Di Chiara and Imperato 1988; Devine et al. 1993) and intra-NAcc (Chefer and Shippenberg 2006; Spanagel et al. 1992) administration of KOR agonists. KORs are anatomically positioned to alter dopamine transmission via their location on pre-synaptic dopamine terminals in the NAcc where they decrease extracellular dopamine levels by inhibiting dopamine release and increasing dopamine reuptake (Chefer et al. 2005; Spanagel et al. 1992; Thompson et al. 2000).
During abstinence from tobacco, the emergence of negative affective states is believed to contribute to relapse behavior. It has been suggested that chronic drug use dysregulates the KOR system in a manner that modulates negative reinforcement processes that contribute to drug addiction (Berrendero et al. 2010; Bruchas et al. 2010; Brujinzeel 2009; Shippenberg et al. 2007; Wee and Koob 2010). A better understanding of the role of KOR systems in mediating withdrawal may lead to more effective treatments that may guide specialized treatments for tobacco abusers of different ages. However, the manner in which KOR systems are dysregulated following chronic nicotine has not been studied and it is unclear whether these changes mediate nicotine withdrawal. To examine these issues, this study first compared the behavioral and neurochemical effects produced by KOR agonist administration in nicotine-naïve and nicotine-treated adolescent and adult rats using CPA, elevated plus maze (EPM), and microdialysis procedures. In order to further examine the role of KORs in withdrawal, subsequent studies examined whether KOR systems alter physical signs produced by spontaneous nicotine withdrawal in adult rats. Given our previous work, it was hypothesized that chronic nicotine will result in changes in the KOR system that will be manifested in an age-dependent manner.
Materials and Methods
Subjects
Male Wistar rats were fully outbred such that each experimental group consisted of animals from separate litters. Rats were weaned on postnatal day (PND 21) and then housed with 2–3 rats per cage. All rats were housed in a humidity- and temperature-controlled (22°C) vivarium on a 12-hour light/dark cycle (lights on at 8 am) with food and water available ad libitum. Testing was conducted during the light phase of the light/dark cycle. Animals were handled for 4–5 days prior to experimentation. Adolescent and adult rats started experimentation on PND 28 and 60, respectively. All procedures were conducted in adherence to the NIH Guideline for the Care and Use of Laboratory Animals and were approved by our Institutional Animal Care and Use Committee.
CPA
An important factor to consider when assessing nicotine withdrawal with CPA procedures is whether a biased or unbiased design is used. In a biased design, the animal receives repeated mecamylamine administration to precipitate withdrawal in their initially preferred environment. In an unbiased design, the animals are randomly assigned without regard to initial bias for either side of the conditioning apparatus. The majority of studies comparing the aversive effects of nicotine withdrawal in rodents have utilized a biased design because these procedures are sensitive for detecting small shifts in initial preference behavior that can be detected across different experimental conditions (Jackson et al. 2010 and 2009; Malin et al. 2006; Miyata et al., 2011; O’Dell et al. 2007; Suzuki et al. 1999). Biased procedures are commonly used with nicotine because this drug produces mild subjective effects, and it is easier to detects shifts in preferences in a biased chamber versus an unbiased one where the animal does not have an initial preference for one side of the conditioning apparatus (O’Dell and Khroyan, 2009). This is in agreement with another exhaustive review by Le Foll and Goldberg (2005) showing that biased procedures are more suitable for evaluating conditioning effects produced by nicotine. Using biased procedures is important when studying changes in negative affect that are not easy to detect as compared to other drug manipulations that produce robust changes in affective states. Importantly, our laboratory has shown that nicotine-treated adult rats display a significant CPA produced by nicotine withdrawal that is absent in adolescent rats (O’Dell et al. 2007). Given that the focus of the present study is on age differences, we utilized a similar biased CPA design with the same conditioning parameters as our previous work. Lastly, biased conditioning procedures have also been applied to study the aversive effects of KOR agonist administration (Michaels and Holtzman 2008; McLaughlin et al. 2006).
The conditioning apparatus consisted of 2 distinct and adjacent chambers elevated over different types of bedding (21.6 cm wide × 30.5 cm long × 20.3 high). The chambers were constructed from Plexiglas.® One compartment had black and white striped walls and a smooth, perforated floor with chlorophyll bedding beneath it. The other compartment had solid black walls and a rough, perforated floor with pine bedding beneath it. Both compartments were equally illuminated with 1-way mirrors on the front walls.
Adolescent and adult rats (n=5–15 per group) were tested for their initial preference for either of the 2 compartments. On the pre-test day, rats were allowed to shuttle between the 2 compartments for 15 min and time spent in each side was recorded. The initially preferred compartment was defined as the compartment where the animal spent greater than 50% of their time during the pre-test. Animals that spent more than 65% time in the initially preferred side were removed from the study (n=4). The day after the pre-test, the rats were anaesthetized (1–3% isofluorane) and received a sham surgery (control rats) or were prepared with subcutaneous osmotic pumps (nicotine-treated rats). The nicotine pump (Alzet, Inc., model 2mL2) delivered a nicotine dose that has been shown to produce equivalent plasma nicotine levels across these age groups (4.7 mg/kg/day in adolescents and 3.2 mg/kg/day in adults) for 14 days (O’Dell et al. 2006). The doses are expressed as base. Subsequent conditioning procedures with U50,488 were conducted in the presence of nicotine that was continuously administered through the pumps.
Conditioning began after 7 days of nicotine exposure. Rats were injected with (±)U50,488 methanesulfonate (U50,488; 0, 1.5, 2.5, 5, or 7.5 mg/kg; sc; expressed as salt) and were immediately confined to their initially preferred compartment for 30 min. On alternate days, rats were injected with saline (sc) and were placed into their initially non-preferred compartment for 30 min. This 2-day procedure was repeated 4 times over 8 consecutive days. The order of drug treatment was counterbalanced such that half the rats in each treatment group received saline on the first day of conditioning and the other half received U50,488. The day after the last conditioning session (adolescents PND 43; adults PND 75), rats were re-tested for their preference for either compartment for 15 min. A separate group of nicotine-treated adults was pretreated with the KOR antagonist nor-BNI (5 mg/kg; sc; expressed as salt) 2 hrs prior to receiving U50,488 (5 mg/kg, sc) during conditioning. This was done to assess whether the effects produced by U50,488 were KOR-mediated.
EPM
The apparatus consisted of a cross-shaped Plexiglas® platform (elevated 61 cm) with a center platform (15.2 cm × 15.2 cm.), 2 open (22.9 cm long × 15.2 cm wide), and 2 closed (22.9 cm long × 15.2 cm wide × 19.8 cm high) arms located across from each other. The animals were first prepared with pumps containing the same doses of nicotine as the CPA experiment (n=9–16 per group). Following 7 days of nicotine exposure, rats were injected with U50,488 (5 mg/kg; sc). This dose was chosen based on results from the CPA experiments, which revealed that this dose produced maximal developmental differences. This procedure was repeated every other day for 8 days to mimic the injection regimen used in the CPA experiment. Immediately after the 4th and final injection of U50,488, rats were isolated in a rectangular cage for 20 min and then we recorded time spent in the open and closed arms of the EPM for 5 min.
Microdialysis
The goal of the microdialysis experiments was to examine the effects of KOR stimulation on NAcc dopamine across nicotine-treated adolescent and adult rats. These studies were done in separate animals from the CPA experiments because animals in the CPA studies received repeated U50,488 administration during conditioning. Thus, conducting dialysis in the CPA animals would have confounded the results due to repeated administration of U50,488.
Adolescent and adult rats (n=6–9 per group) were anaesthetized with an isofluorane/oxygen mixture (1–3% isofluorane) and prepared with pumps containing the same nicotine doses as the behavioral studies. On the 13th day of nicotine exposure, a unilateral concentric microdialysis probe (CMA, Chelmsford, MA; 2 mm active membrane length) was implanted into the NAcc (from bregma; adolescents: AP +2.2, ML ±0.8, DV −7.1; adults: AP +1.7, ML ±1.4, DV −8.1). The probe was cemented in place with 3 stainless steel screws and dental acrylic cement. The probe was perfused overnight (0.2 μL/min) with aCSF containing (in mM): 145 NaCl, 2.8 KCl, 1.2 CaCl2, 1.2 MgCl2, 5.4 D-glucose, and 0.25 ascorbic acid, pH 7.2–7.4. The following day, the flow rate was changed to 1.0 μL/min and probes were allowed to equilibrate for 1 hr. Samples were collected in 10-min intervals during a 1-hr baseline period, 1-hr following saline (sc), and then for 4 hrs following U50,488 administration (5 mg/kg; sc).
A separate group of adult rats were included that received the same U50,488 treatment as in the behavioral studies. This was done in order to compare the effects of U50,488 following an acute injection of this drug and following pre-treatment with a regimen that is similar to what was used in the behavioral studies. Naïve and nicotine-treated adult rats (n=7 per group) received saline or U50,488 administration every other day, such that the rats received 3 injections of U50,488 prior to dialysis testing. The fourth injection of U50,488 was given on the day of dialysis testing. The rats were implanted with dialysis probes into the NAcc and dopamine levels were monitored following administration of U50,488.
Dialysate samples were diluted with an equal volume of 0.05N perchloric acid to prevent degradation and immediately frozen on dry ice until storage at −70°C. The samples were analyzed using an isocratic HPLC system with electrochemical detection using a mobile phase consisting of (in mM) 60 NaH2PO4, 30 citric acid, 0.1 EDTA, 17% methanol, and 0.035 sodium dodecyl sulfate at pH 2.75. Concentrations of dopamine were estimated using external calibration curves derived from known standards that were prepared on the day of testing.
Physical Signs of Spontaneous Withdrawal
A separate group of adults (n=6–17) was included to examine the effects of a KOR agonist or antagonist on somatic signs produced by spontaneous nicotine withdrawal. Rats received sham surgery or nicotine pumps containing the same nicotine dose as the previous studies. Fourteen days later, the pumps were removed or the rats received a sham surgery. Rats received pretreatment with either vehicle or nor-BNI (5 or 15 mg/kg; sc) prior to surgery. Twenty-four hrs after surgery, the rats received saline or U50,488 (5 mg/kg; sc). A separate group of rats received the nonspecific nicotinic receptor antagonist, mecamylamine (3.0 mg/kg; sc) to include a group that would be expected to show enhanced somatic signs produced by spontaneous nicotine withdrawal. Adolescents were not included in these studies because they do not show spontaneous signs of nicotine withdrawal and we did not expect that U50,488 would enhance withdrawal in adolescents. Twenty-five min later, the animals were placed in a clear Plexiglas® cage to acclimate for 10 min. Somatic signs were then recorded by an observer that was blind to the animals’ prior treatment. The frequency of occurrence of the following signs was recorded for 10 min: eye blinks, body shakes, gasps, writhes, headshakes, ptosis, and teeth chattering. Multiple successive counts of any sign required a distinct pause between episodes. If present continuously, ptosis was counted only once.
Statistics
CPA data were analyzed using difference scores, which reflect the amount of time spent in the initially preferred compartment after conditioning minus before conditioning. Negative values reflect an aversion to the initially preferred chamber. The CPA data were analyzed using separate 2-way ANOVAs for adolescents and adults with pump treatment and dose as between-subject factors. EPM data were analyzed using percent open and closed arm time, which was calculated as the amount of time spent on the open or closed arms in seconds divided by the total time of testing multiplied by 100%. The EPM data were analyzed using separate one-way ANOVAs for adolescents and adults with treatment group as the between-subject factor. The somatic signs data were analyzed using a two-way ANOVA with nicotine treatment and drug condition as between-subject factors. Where appropriate, statistical comparisons were employed using Fisher’s LSD tests (p ≤ 0.05).
For the neurochemical experiments, basal concentrations of dopamine were first analyzed using separate repeated measures ANOVAs for adolescents and adults with pump treatment as a between-subject factor and time as the repeated measure. Subsequent analyses were performed on percent change from baseline values, which was calculated by dividing the raw nM concentration of dopamine in each dialysate by the average baseline value and then multiplying by 100%. Percent changes from baseline values were then analyzed using separate repeated measures ANOVAs for adolescents and adults with pump treatment as a between-subject factor and time as the repeated measure. The area under the curve (AUC) was calculated by subtracting the baseline value for each data point from 100 and summing up all the data points after U50,488 treatment. Significant effects were further analyzed using Fisher’s LSD tests (p ≤ 0.05). A similar analyses was used for adults that received repeated U50,488 pre-treatment.
Results
CPA
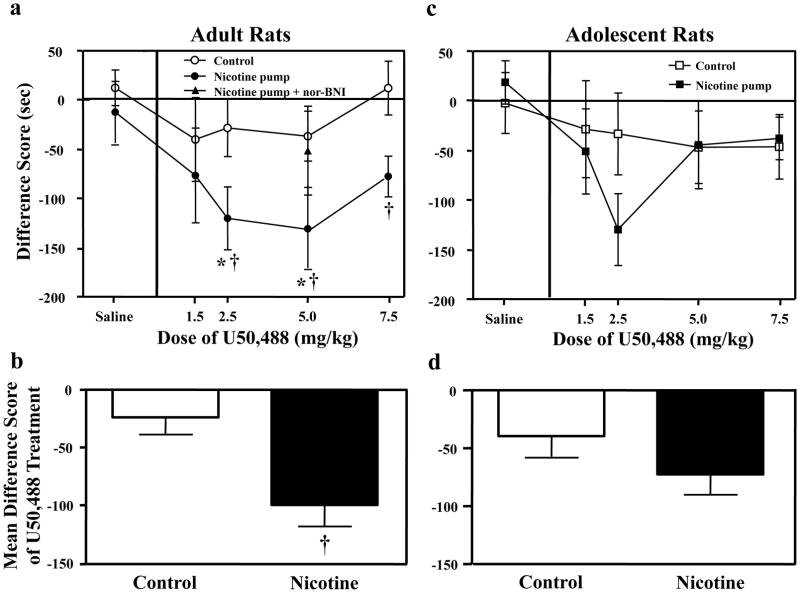
Overall, the results revealed that stimulation of KORs produced CPA in nicotine-treated adults, and this effect was absent in all other groups. Table 1 includes all of the pre- and post-test values across treatment groups. The adult data (Fig. 1a) revealed a main effect of pump treatment [F(1, 69)= 9.5, p ≤ 0.01] showing that the effects of U50,488 are greater in nicotine-treated adult rats. Planned comparisons revealed that nicotine-treated adults that received the 2.5 and 5.0 mg/kg doses of U50,488 displayed a significant CPA relative to nicotine-treated adults that received saline during conditioning (p ≤ 0.05). Similarly, nicotine-treated adults that received 2.5, 5.0 and 7.5 mg/kg displayed a significant CPA relative to U50,488-treated control rats that did not receive nicotine (p ≤ 0.05). Pre-treatment with the KOR antagonist nor-BNI (5 mg/kg) blocked the ability of U50,488 to produce CPA at a dose that produced maximal aversion in nicotine-treated animals. This suggests that the effects of U50,488 are selectively mediated by KORs. In contrast to the adult data, the adolescent data (Fig. 1c) did not reveal any significant effects of pump treatment [F(1, 80)= 0.6, p = 0.5] or dose [F(4, 80)= 1.6, p = 0.2] or interaction of dose and pump treatment [F(4, 80)= 0.9, p = 0.5], suggesting that the adolescent groups did not differ from one another. Given the trend for aversive effects observed in nicotine-treated adolescent rats that received the 2.5 mg/kg dose (p = 0.09), another cohort of animals was added to this group (n=15 in total). Despite this, significant pump treatment effects still did not emerge.
Table 1.
Time spent in the preferred chamber before and after conditioning
| Adult Rats | |||||
|---|---|---|---|---|---|
| Control | Saline | 1.5 | 2.5 | 5.11 | 7.5 |
| Preconditioning time | 520.2 12 | 509.3 20 | 482.7 6 | 493.7 13 | 519.7 15 |
| Post-conditioning time | 532.7 14 | 469.2 43 | 454.9 28 | 456.9 31 | 531.9 18 |
| Nicotine-treated
| |||||
| Preconditioning time | 498.7 14 | 496.1 14 | 515.1 12 | 505.8 12 | 512.6 14 |
| Post-conditioning time | 486.4 26 | 419.5 41 | 394.8 28 | 379.3 35 | 435.7 31 |
| Adolescent Rats | |||||
|---|---|---|---|---|---|
| Control | Saline | 1.5 | 2.5 | 5.0 | 7.5 |
| Pre-conditioning time | 512.2 17 | 489.3 11 | 498.0 14 | 506.8 18 | 512.1 18 |
| Post-conditioning time | 510.0 22 | 460.8 48 | 464.6 47 | 460.0 31 | 465.7 23 |
| Nicotine-treated
| |||||
| Pre-conditioning time | 514.5 15 | 504.9 13 | 519.8 11 | 504.9 12 | 530.1 13 |
| Postcondition ing time | 533.1 32 | 453.9 35 | 389.4 26 | 460.4 34 | 473.6 29 |
Fig. 1.
Data reflect difference scores (± SEM), which represent time spent in the initially non-preferred side after conditioning minus before conditioning. Separate groups of adults received various doses of U50,488 in control [0 (n=9), 1.5 (n=6), 2.5 (n=7), 5 (n=10), or 7.5 n=6) and nicotine-treated groups [0 (n=5), 1.5 (n=7), 2.5 (n=8), 5 (n=10), or 7.5 n=11). Adolescents also received various doses of U50,488 in control [0 (n=7), 1.5 (n=6), 2.5 (n=6), 5 (n=8), or 7.5 (n=7)] and nicotine-treated groups [0 (n=11), 1.5 (n=8), 2.5 (n=15), 5 (n=13), or 7.5 (n=9)]. In adults (Fig. 1a and b), U50,488 produced CPA in a dose-dependent manner in nicotine-treated adult rats relative to their respective saline-treated controls (*p ≤ 0.05) and relative to control rats (†p ≤ 0.05). This effect was blocked in nicotine-treated adults that were pre-treated with the KOR antagonist nor-BNI (5 mg/kg). An analysis of the mean difference score across all doses (Fig. 1b) revealed that nicotine-treated adults displayed higher overall CPA following different doses of U50,488 relative to control adults (†p ≤ 0.05). In adolescents (Fig. 1c and d); however, there were no significant differences across all groups of rats.
Figure 1b and d reflect an average of the difference scores produced by all U50,488 treatment doses. The analysis of these data revealed that nicotine-treated adults showed higher CPA values relative to control adults that did not receive nicotine (p ≤ 0.05). However, this effect was not significant in adolescent rats.
The conditioning parameters that were used for this study were chosen because U50,488 produces marginal CPA in naïve adult rats in this regimen. Thus, these parameters allowed us to examine whether group differences in the aversive effects of U50,488 could be altered by chronic nicotine. Preliminary studies were conducted to ensure that robust CPA could be observed in our laboratory using parameters (U50,488 dose range of 1.25–5.0 mg/kg and 8 morning/evening conditioning sessions) that produce aversive effects in other laboratories (Goktalay et al. 2006; McLaughlin et al. 2006; Morales et al. 2007; Skoubis et al. 2005). The results revealed that U50,488 alone (1.25 and 5.0 mg/kg, sc) produces significant place aversion in naïve adult rats (−113±34) as compared to saline controls (21.6±28) conditioned with 8 morning/evening sessions (n=13; F(1,12)= 5.9, p ≤ 0.05).
EPM
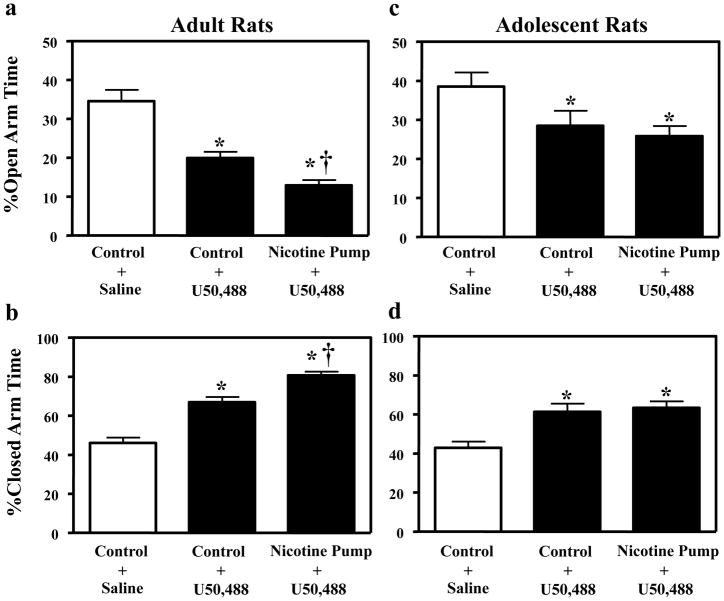
The results revealed that stimulation of KORs elicited anxiety-like behavior that was exacerbated in nicotine-treated adult, but not adolescent rats. The adult data (Fig. 2a and b) revealed a main effect of treatment in both the open [F(2, 43)= 28.0, p ≤ 0.001] and closed [F(2, 43)= 54.6, p ≤ 0.001] arms of the EPM. U50,488 alone produced a decrease in open arm time and an increase in closed arm time in both nicotine-naïve and nicotine-treated adults relative to saline-treated controls (p ≤ 0.05). Nicotine treatment exacerbated the anxiogenic effects of U50,488 relative to nicotine-naïve adults that were treated with U50,488 (p ≤ 0.05). The adolescent data (Fig. 2c and d) revealed a main effect of treatment in the open [F(2, 25)= 4.0, p ≤ 0.05] and closed [F(2, 25)= 9.7, p ≤ 0.001] arms of the EPM. Similar to the adults, U50,488 alone produced a decrease in open arm time and an increase in closed arm time in both nicotine-naïve and nicotine-treated adolescents relative to saline-treated controls (p ≤ 0.05). In contrast to adults; however, nicotine did not exacerbate the U50,488 effect in control versus nicotine-treated adolescents.
Fig. 2.
Data reflect % open and closed arm time (± SEM). Separate groups of adults received saline (n=16) or U50,488 in control (n=14) or nicotine-treated groups (n=16). Adolescents also received saline (n=9) or U50,488 in control (n=9) or nicotine-treated groups (n=10). In adults (Fig. 2a and b), U50,488 (5 mg/kg) decreased time spent on the open arms and increased time spent in the closed arms in both control and nicotine-treated adults relative to rats that received saline (*p ≤ 0.05). This effect was exacerbated in nicotine-treated adults relative to control adults that received U50,488 alone (†p ≤ 0.05). In adolescents (Fig. 2c and d), U50,488 (5 mg/kg) also decreased time spent on the open arms and increased time spent on the closed arms in control and nicotine-treated adolescents relative to saline controls (*p ≤ 0.05). However, this effect was not exacerbated in nicotine-treated adolescents.
In a separate group of animals, an additional control study was conducted to examine whether nicotine exposure induced anxiogenic effects in adolescent (n=13) and adult (n=21) rats relative to their respective saline control groups. In adults, the results revealed that nicotine exposure did not alter percent open [F(1, 19)= 0.1, p =0.75] or closed [F(1, 19)=0.68, p =.42] arm time versus control animals. Similarly, in adolescents, nicotine exposure did not alter percent open [F(1, 11)= .05, p =0.81] or closed [F(1, 11)= .22, p =0.64] arm time versus controls. These data suggest that our nicotine dose parameters did not produce anxiogenic effects that were different across age groups.
Microdialysis
The baseline nM values of NAcc dopamine were as follows: control adolescents (1.6 ± 0.2), nicotine-treated adolescents (2.1 ± 0.3), control adults (2.3 ± 0.4) and nicotine-treated adults (2.4 ± 0.5). Our analysis of baseline NAcc dopamine levels revealed that there were no significant differences in pump treatment for either adolescents [F(1, 11)= 2.4, p = 0.2] or adults [F(1, 15)= 0.02, p = 0.9]. The finding that there were no age differences in basal dopamine values is consistent with a previous report using no-net flux procedures (Frantz et al. 2007). The data were transformed to percent change from baseline levels for further comparisons across treatment conditions.
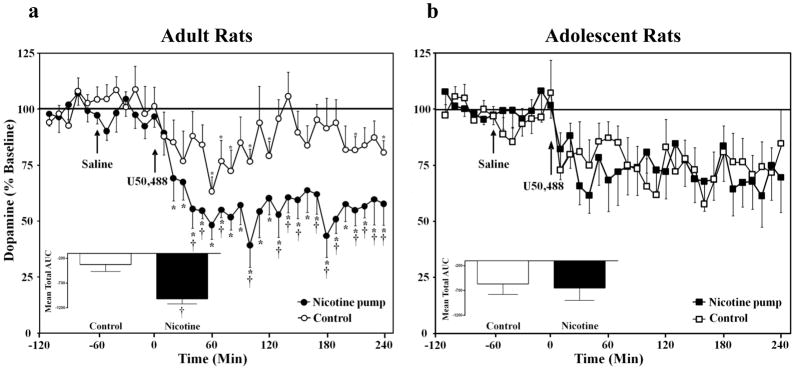
Overall, the results revealed that U50,488 produced a decrease in NAcc dopamine overflow in both control and nicotine-treated rats from both age groups. However, the ability of this drug to decrease dopamine transmission in nicotine-treated adults was larger as compared to their nicotine-naïve counterparts. In contrast, the adolescents did not show these differences in nicotine treatment conditions. Analysis of adult dopamine levels (Fig. 3a) revealed a significant time x pump treatment interaction [F(35, 525)= 2.1, p ≤ 0.001], a main effect of time [F(35, 525)= 7.0, p ≤ 0.001] and a main effect of treatment [F(1, 15)= 14.4, p ≤ 0.01]. Subsequent analyses revealed that nicotine-treated adults displayed decreases in dopamine levels relative to baseline 20–240 min after U50,488 administration (p ≤ 0.05). Similarly, the control adults displayed decreases in dopamine levels relative to baseline 60–80, 100, 120, 210, 240 min after U50,488 administration (p ≤ 0.05). Nicotine-treated adults displayed decreases that were larger relative to adult controls 40–50, 70, 100, 130–150, 170–190, 210–240 min after U50,488 administration (p ≤ 0.05). The AUC analysis (see Fig. 3a inset) revealed that nicotine-treated adults displayed overall lower dopamine levels following U50,488 as compared to adult controls (p ≤ 0.05).
Fig. 3.
Data reflect extracellular dopamine levels in the NAcc expressed as a percentage of basal values (± SEM) in control and nicotine-treated rats. Separate groups of adult control (n=9), adult nicotine-treated (n=8), adolescent control (n=7), or nicotine-treated adolescents (n=6) were included. In adults (Fig. 3a), U50,488 (5 mg/kg) significantly decreased dopamine overflow in control and nicotine-treated rats relative to baseline (*p ≤ 0.05). The decreases in dopamine were larger in nicotine-treated adults as compared to controls (†p ≤ 0.05). The figure insets reflect the mean total AUC (± SEM) following U50,488. These data show that nicotine-treated adults displayed decreases in NAcc dopamine following U50,488 that were lower relative to adult controls (†p ≤ 0.05). However, these effects were not observed in adolescent rats (Fig. 3b).
Analysis of the adolescent dopamine levels (Fig. 3b) also revealed a significant main effect of time [F(35, 385)= 4.5, p ≤ 0.01], with both groups displaying a decrease in NAcc dopamine overflow after U50,488 administration. The decreases in dopamine were similar between the adolescent treatment groups in all of the sampling periods. In contrast to the adult data, the adolescent data did not reveal any significant effects of pump treatment [F(1, 11)= 0.02, p =0.9] or interaction effects [F(35, 385)= 0.8, p = 0.8], suggesting that the adolescent groups did not differ from one another. The AUC analysis (see Fig. 3b inset) revealed that nicotine-treated adolescents displayed decreases in NAcc dopamine levels following U50,488 that were similar as compared to control adolescents (p = 0.9).
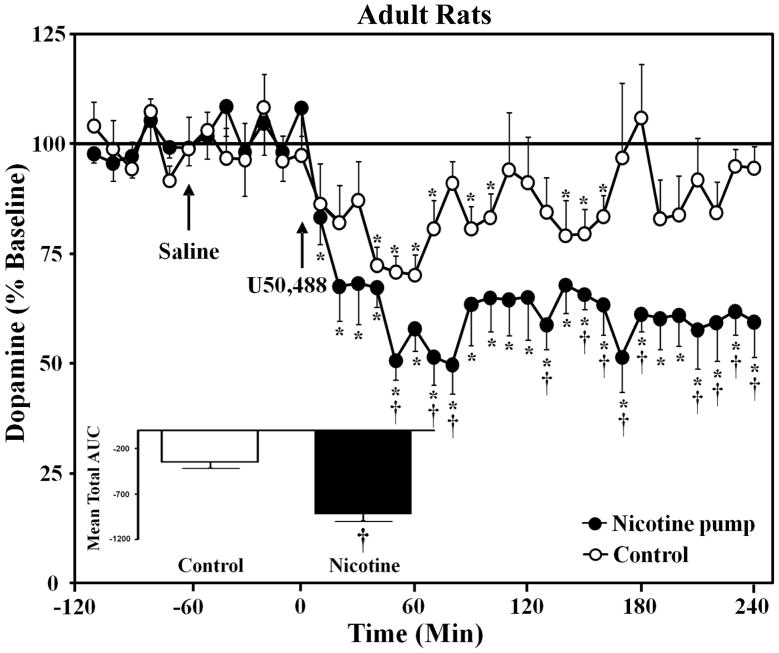
The data in Figure 4 reflect adult rats that received the same U50,488 treatment as in the behavioral studies. This was done in order to compare the effects of U50,488 following an acute injection of this drug and following pre-treatment with 3 injections of U50,488 prior to dialysis testing. The baseline nM values of NAcc dopamine were as follows: control adults (1.0 ± 0.02) and nicotine-treated adults (1.2 ± 0.01). There were no significant differences in pump treatment [F(1, 12)= 1.2, p = 0.3]. During dialysis testing, the 4th injection of U50,488 produced a decrease in NAcc dopamine overflow in both control and nicotine-treated rats from both age groups. The ability of this drug to decrease dopamine transmission in nicotine-treated adults was larger as compared to their nicotine-naïve counterparts. Analysis of the dopamine levels (Fig. 4) revealed a significant time x pump treatment interaction [F(35, 420)= 2.5, p ≤ 0.001]. Subsequent analyses revealed that nicotine-treated adults displayed decreases in dopamine levels relative to baseline 10–240 min after U50,488 administration (p ≤ 0.05). Similarly, the control adults displayed decreases in dopamine levels relative to baseline 40–70, 90–100, 140–160 min after U50,488 administration (p ≤ 0.05). Nicotine-treated adults displayed decreases that were larger relative to adult controls 50, 70–80, 130, 150–180, and 210–240 min after U50,488 administration (p ≤ 0.05). The AUC analysis (see Fig. 4 inset) revealed that nicotine-treated adults displayed overall lower dopamine levels following U50,488 as compared to adult controls (p ≤ 0.05).
Fig. 4.
Data reflect extracellular dopamine levels in the NAcc expressed as a percentage of basal values (± SEM) in control and nicotine-treated rats that were pre-treated with U50,488 as in the behavioral studies. Separate groups of adult control (n=7) and nicotine-treated (n=7) rats were included. U50,488 (5 mg/kg) significantly decreased dopamine overflow in control and nicotine-treated rats relative to baseline (*p ≤ 0.05). The decreases in dopamine were larger in nicotine-treated adults as compared to controls (†p ≤ 0.05). The figure insets reflect the mean total AUC (± SEM) following U50,488. These data show that nicotine-treated adults displayed decreases in NAcc dopamine following U50,488 that were lower relative to adult controls (†p ≤ 0.05).
A comparison of adult rats in Figure 3a and Figure 4 shows that nicotine-treated adults displayed a similar magnitude of decrease in NAcc dopamine following an acute injection of U50,488 (average decrease of 42% relative to baseline) versus following repeated pre-treatment of this drug (average decrease of 38% relative to baseline).
Somatic Signs of Withdrawal in Adults
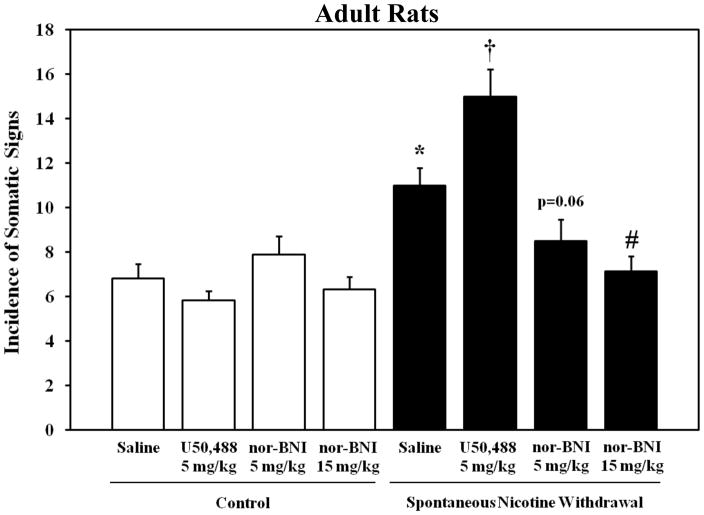
In order to determine whether KOR systems modulate nicotine withdrawal, the effects of KOR stimulation and blockade on the somatic signs of nicotine withdrawal were examined in adults (Fig. 5). Overall, the results revealed that KOR stimulation facilitated somatic signs produced by spontaneous nicotine withdrawal. Also, this effect was attenuated by KOR blockade as compared to nicotine-treated rats receiving saline during withdrawal. The analysis revealed an interaction between nicotine treatment and drug condition [F(2,72)= 13.2, p ≤ 0.001]. Removal of the nicotine pump produced an increase in somatic signs (p ≤ 0.05) that was enhanced by U50,488 (p ≤ 0.05). Importantly, somatic signs produced by nicotine pump removal were dose-dependently blocked in rats that were pre-treated with the high dose of nor-BNI (p ≤ 0.05). There was no significant difference between saline control and nor-BNI pre-treated rats, suggesting that KOR blockade ameliorated somatic signs only in nicotine-treated rats experiencing spontaneous withdrawal. Positive control studies revealed that somatic signs produced by spontaneous nicotine withdrawal were significantly enhanced by administration of the non-selective nicotinic receptor antagonist mecamylamine (data not shown; n=8; mean value=26.6 ± 2.0; p ≤ 0.05).
Fig. 5.
Data reflect the total number of somatic signs exhibited (± SEM) in control and nicotine-treated adults experiencing spontaneous withdrawal. Separate groups of control rats received saline (n=16), U50,488 (n=17) or nor-BNI (5 mg/kg n=8; 15 mg/kg n=6). Another group of nicotine-treated rats received saline (n=10), U50,488 (n=6), or nor-BNI (5 mg/kg n=8; 15 mg/kg n= 8). Spontaneous nicotine withdrawal produced an increase in somatic signs relative to saline controls (*p ≤ 0.05). Administration of U50,488 (5 mg/kg) facilitated spontaneous withdrawal signs (†p ≤ 0.05), whereas pretreatment with nor-BNI (15 mg/kg) blocked spontaneous withdrawal signs (#p ≤ 0.05).
Discussion
To summarize, nicotine-treated adults displayed CPA for an environment paired previously with KOR agonist administration, and this effect was reversed by the selective KOR antagonist nor-BNI. In contrast, the agonist effect was absent in nicotine-naïve adults and both groups of adolescent rats under the present conditions. KOR agonist administration produced anxiety-like behavior in nicotine-naïve animals of both age groups, and this effect was enhanced in nicotine-treated adult, but not adolescent rats. KOR agonist administration also decreased NAcc dopamine overflow in both age groups, and this effect was larger and more prolonged in nicotine-treated adult versus adolescent rats. A similar decrease in dopamine was observed in adult rats that were pre-treated with the same regimen of U50,488 as in the behavioral studies. Subsequent studies further examining the role of KOR systems in withdrawal revealed that KOR stimulation facilitated spontaneous withdrawal signs in nicotine-treated adult rats. Importantly, a KOR antagonist dose-dependently blocked somatic signs produced by spontaneous nicotine withdrawal.
The present study provides a novel contribution to the literature regarding enhanced behavioral and neurochemical effects of KOR stimulation in adult rats that received chronic nicotine versus naïve adult controls. Our finding that chronic nicotine exacerbates the ability of KOR agonists to decrease NAcc dopamine levels suggests that the interaction between nicotine and KOR systems involves dopamine transmission in the mesolimbic dopamine pathway. In support of this hypothesis, previous studies have shown that intact mesolimbic, but not nigrostriatal or mesocortical, dopamine neurotransmission is necessary for KOR agonists to produce aversion (Shippenberg et al. 1993). The precise mechanisms by which chronic nicotine enhances the effects of KOR stimulation on NAcc dopamine levels are presently unclear. However, the following possibilities are offered that can be examined in future studies. First, previous reports show that KOR activation decreases NAcc dialysate dopamine by inhibiting release and increasing dopamine uptake (Chefer et al. 2005; Thompson et al. 2000). Chronic nicotine may, therefore, enhance the ability of KORs to modulate channels involved in neurotransmitter release or cell excitability (i.e. Ca2+ or K+ channels). Second, nicotinic receptor activation has been shown to increase dopamine uptake in terminal regions of the mesocorticolimbic dopamine system (Middleton et al. 2007; Zhu et al. 2009). Chronic nicotine may, therefore, enhance the ability of KORs to increase dopamine uptake. Thus, changes in either of the aforementioned mechanisms may enhance negative affect and anxiety produced by KOR activation via exacerbated decreases in NAcc dopamine.
A major goal of this study was to examine the role of KORs in mediating nicotine withdrawal. To achieve this goal, we implemented behavioral and neurochemical assays typically used to assess withdrawal-like states. These include, negative affect as assessed by CPA procedures, increases in anxiety-like behavior, and decreases in NAcc dopamine transmission, which has been used widely as a neurochemical index of withdrawal from several different drugs of abuse (see Rossetti et al. 1992). Decreases in NAcc dopamine have also been closely linked to the incidence of physical signs of nicotine withdrawal (Hildebrand et al. 1998). However, after close consideration of the data in Figures 1–3, it seemed unclear whether the effects of KOR agonists alone were being amplified by chronic nicotine exposure or were eliciting a withdrawal-like state. Thus, to more conclusively determine the role of KOR systems in withdrawal, we examined the effects of a KOR agonist and antagonist on the emergence of the physical signs of withdrawal following nicotine removal. Our results revealed that KOR stimulation enhanced the somatic signs of withdrawal when nicotine was removed. Importantly, administration of a KOR antagonist blocked the emergence of somatic signs produced by nicotine withdrawal. These data suggest that endogenous KOR systems are necessary for the emergence of the nicotine withdrawal syndrome.
Studies from other laboratories support for the role of KOR systems in mediating nicotine withdrawal. For example, spontaneous withdrawal from nicotine produced anxiety-like behavior and physical signs of withdrawal that are attenuated in adult mice that received a selective KOR antagonist (Jackson et al. 2010). Also, Isola et al. (2008) reported that withdrawal from chronic nicotine injections diminishes striatal dynorphin content and enhances prodynorphin expression relative to saline-treated controls. Additionally, McCarthy et al. (2009) found that striatal KOR coupling decreases over time after nicotine withdrawal, suggesting decreased KOR functionality is associated with the dissipation of nicotine withdrawal. Taken together, these studies support the role of KORs in mediating withdrawal from chronic nicotine exposure.
We propose a theoretical construct whereby chronic nicotine enhances KOR function. Following the removal of nicotine, we suggest that these long-term changes in KOR systems mediate the nicotine withdrawal syndrome. It has been postulated that following chronic exposure to drugs of abuse, there are changes in the reward/aversion neural circuitry that involve a dysregulation of KOR systems in a manner that contributes to the development of drug dependence (Berrendero et al. 2010; Bruchas et al. 2010; Brujinzeel 2009; Walker et al. 2012; Wee and Koob 2010). Dysregulation of the dynorphin/KOR systems following chronic drug exposure is hypothesized to be due to repeated induction of the dynorphin/KOR system that normally counteract the rewarding effects of drugs of abuse (Shippenberg et al. 2007; Tejeda et al. 2012). Our data suggest that this dysregulation involves enhanced KOR modulation of affective/anxiety-like behavior and NAcc dopamine following chronic nicotine exposure. Upon removal of nicotine, enhanced KOR function mediates a nicotine withdrawal syndrome involving the emergence of negative affect, heightened anxiety, and enhanced physical signs. Moreover, our neurochemical data suggest that the mechanism whereby these behavioral manifestations of withdrawal are precipitated involves decreases in NAcc dopamine that are modulated by KOR systems.
Another major contribution of this report is that adolescent rats are less sensitive than adults to KOR activation following nicotine exposure. Our measures of anxiety-like behavior are consistent with of a growing body of literature suggesting that adolescents are less sensitive to the behavioral effects of nicotine withdrawal as compared to adults (Kota et al. 2007; O’Dell 2006; 2007; Shram et al. 2008; Wilmouth and Spear 2006). We recently suggested that these effects are mediated via mesolimbic dopamine systems, since nicotine withdrawal produces a decrease in NAcc dopamine levels that is lower in adolescent versus adult rats (Natividad et al. 2010). The present findings support our hypothesis that dopamine systems play an important role in mediating developmental differences to nicotine withdrawal. It may be suggested that our results are related to underdeveloped KOR systems during adolescence. However, U50,488 produced similar increases in anxiety-like behavior during EPM testing and decreases in NAcc dopamine in nicotine-naïve rats of both age groups. These data suggest that an underdeveloped KOR system may not explain the lack of alterations in KOR function following chronic nicotine in adolescents. Taken together, our results suggest that adolescents do not display enhanced KOR function with chronic nicotine exposure. A lack of changes in KOR systems following chronic nicotine is one potential mechanism by which adolescents may be less sensitive to the effects of withdrawal from this drug.
An important consideration is whether our observed developmental differences are related to differences in drug metabolism. To address this issue, we administered a dose of nicotine in the pumps that was 1.5 times higher in the adolescents than in the adults. The rationale for using a higher dose of nicotine in adolescents is based on work from our laboratory (O’Dell et al. 2006) and others (Trauth et al. 2000; Wilmouth and Spear 2006) demonstrating that a 1.5 fold higher concentration of nicotine is needed to produce equivalent blood plasma levels of nicotine in adolescent and adult rats with nicotine pumps. Our previous work demonstrated that a nicotine dose of 4.7 mg/kg/day in adolescents results in 76.2±7 ng/ml of plasma nicotine and a nicotine dose of 3.2 mg/kg/day in adults results in 65.4±9 ng/ml of plasma nicotine 7 days after the rats were prepared with pumps (O’Dell et al. 2006). The U50,488 treatment began for the behavioral experiments on day 7, such that these age groups had equivalent blood levels of nicotine when the behavioral studies began.
A related issue is whether there are developmental differences in U50,488 metabolism. However, our results revealed that the degree to which this drug produced CPA was similar across a range of doses in naïve rats of both ages. We also observed similar effects of U50,488 in naïve rats of both ages in anxiety-like behavior and decreases in NAcc dopamine during the entire 4-hour sampling period. We acknowledge that chronic nicotine may impact U50,488 metabolism in an age-dependent manner. However, low doses of U50,488 produced the same magnitude of CPA in nicotine-treated adolescents and adults. Taken together, our findings suggest that our observed developmental differences are not likely related to metabolic differences.
The goal of the present study was to determine whether KOR systems are involved in mediating the nicotine withdrawal syndrome in an age-dependent manner. In order to study this question, the effects of kappa agonists were compared in adolescent and adult rats that had received chronic nicotine using procedures that reliably produce nicotine dependence and withdrawal. Our results indicate that the age differences in KOR stimulation are only evident in following chronic nicotine, since drug naïve adolescent and adult rats displayed similar behavioral and neurochemical responses to KOR stimulation. Based on these findings, we suggest that chronic nicotine upregulates KOR systems in adult animals and the changes is KOR systems contribute to the emergence of withdrawal states. Given that our focus is on withdrawal, the present study did not include animals that received acute nicotine because these procedures do not produce dependence and withdrawal. Future studies might examine the degree to which kappa agonists alter the acute behavioral effects of nicotine, and developmental differences might be expected with regard to pain based on the finding that adolescent rats are less sensitive to the analgesic effects of acute nicotine as compared to adults (Kota et al., 2007). The extent to which these developmental differences in pain are mediated via kappa/dynorphin systems remains to be elucidated.
There are several clinical implications of the present studies, as the KOR system has been postulated to serve as a potential therapeutic target for nicotine addiction. Our finding that the anxiogenic effects of KOR agonists are facilitated in nicotine-treated rats has clinical implications for treating anxiety disorders in populations where nicotine abuse is highly co-morbid (Picciotto et al. 2002) and typically precedes and/or triggers the onset of anxiety disorders (Roy-Byrne and Uhde 1988; Cosci et al. 2009). Furthermore, our finding that stimulation of KORs enhances aversive effects in nicotine-treated adults suggests that treatments that activate KORs may induce withdrawal-like states in nicotine-dependent populations. Thus, KOR antagonists may be useful pharmacological tools for ameliorating the negative affective properties of nicotine withdrawal and treat anxiety/panic disorders associated with nicotine abuse. Lastly, our finding that adolescents are less sensitive to the effects produced by KOR activation suggests that treatments that target KOR may be less effective in young tobacco abusers, a hypothesis that warrants future investigation.
Acknowledgments
The authors thank Ivan Torres, Evelyn Escalante, and Arturo Orona for technical assistance. This research was supported by NIDA (R01-DA021274) and the UTEP BBRC-RCMI (5G12RR008124). Funds for student training were also provided by the NIMH Career Opportunities in Research Program (MH 019978-08; HAT), the Ford Foundation (HAT), the National Science Foundation (HAT), the NIH Ruth L. Kirschstein pre-doctoral fellowship program (F31-DA021133; LAN), and the APA Diversity Program in Neuroscience (T32-MH018882; LAN).
References
- Bals-Kubik R, Ableitner A, Herz A, Shippenberg TS. Neuroanatomical sites mediating the motivational effects of opioids as mapped by the conditioned place preference paradigm in rats. J Pharmacol Exp Ther. 1993;264:489–495. [PubMed] [Google Scholar]
- Berrendero F, Robledo P, Trigo JM, Martín-García E, Maldonado R. Neurobiological mechanisms involved in nicotine dependence and reward: Participation of the endogenous opioid system. Neurosci Biobehav Rev. 2010;35:220–231. doi: 10.1016/j.neubiorev.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Land BB, Chavkin C. The dynorphin/kappa opioid system as a modulator of stress-induced and pro-addictive behaviors. Brain Res. 2010;1314:44–55. doi: 10.1016/j.brainres.2009.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijnzeel AW. Kappa-opioid receptor signaling and brain reward function. Brain Res Rev. 2009;62:127–146. doi: 10.1016/j.brainresrev.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carboni E, Bortone L, Giua C, Di Chiara G. Dissociation of physical abstinence signs from changes in extracellular dopamine in the nucleus accumbens and in the prefrontal cortex of nicotine dependent rats. Drug Alcohol Depend. 2000;58:93–102. doi: 10.1016/s0376-8716(99)00064-2. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Thomas MJ. Biological substrates of reward and aversion: a nucleus accumbens activity hypothesis. Neuropharmacology. 2009;56:122–132. doi: 10.1016/j.neuropharm.2008.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chefer VI, Czyzyk T, Bolan EA, Moron J, Pintar JE, Shippenberg TS. Endogenous kappa-opioid receptor systems regulate mesoaccumbal dopamine dynamics and vulnerability to cocaine. J Neurosci. 2005;25:5029–5037. doi: 10.1523/JNEUROSCI.0854-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chefer VI, Shippenberg TS. Paradoxical effects of prodynorphin gene deletion on basal and cocaine-evoked dopaminergic neurotransmission in the nucleus accumbens. Eur J Neurosci. 2006;23:229–238. doi: 10.1111/j.1460-9568.2005.04525.x. [DOI] [PubMed] [Google Scholar]
- Cosci F, Knuts IJ, Abrams K, Griez EJ, Schruers KR. Cigarette smoking and panic: a critical review of the literature. J Clin Psychiatry. 2009;71:606–615. doi: 10.4088/JCP.08r04523blu. [DOI] [PubMed] [Google Scholar]
- Devine DP, Leone P, Pocock D, Wise RA. Differential involvement of ventral tegmental mu, delta and kappa opioid receptors in modulation of basal mesolimbic dopamine release: in vivo microdialysis studies. J Pharmacol Exp Ther. 1993;266:1236–1246. [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frantz KJ, O’Dell LE, Parsons LH. Behavioral and neurochemical responses to cocaine in periadolescent and adult rats. Neuropsychopharmacology. 2007;32:625–637. doi: 10.1038/sj.npp.1301130. [DOI] [PubMed] [Google Scholar]
- Göktalay G, Cavun S, Levendusky MC, Hamilton JR, Millington WR. Glycyl-glutamine inhibits nicotine conditioned place preference and withdrawal. Eur J Pharmacol. 2006;530:95–102. doi: 10.1016/j.ejphar.2005.11.034. [DOI] [PubMed] [Google Scholar]
- Grieder TE, Sellings LH, Vargas-Perez H, Ting-A-Kee R, Siu EC, Tyndale RF, van der Kooy D. Dopaminergic signaling mediates the motivational response underlying the opponent process to chronic but not acute nicotine. Neuropsychopharmacology. 2010;35:943–954. doi: 10.1038/npp.2009.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand BE, Nomikos GG, Hertel P, Schilström B, Svensson TH. Reduced dopamine output in the nucleus accumbens but not in the medial prefrontal cortex in rats displaying a mecamylamine-precipitated nicotine withdrawal syndrome. Brain Res. 1998;779:214–225. doi: 10.1016/s0006-8993(97)01135-9. [DOI] [PubMed] [Google Scholar]
- Isola R, Zhang H, Tejwani GA, Neff NH, Hadjiconstantinou M. Dynorphin and prodynorphin mRNA changes in the striatum during nicotine withdrawal. Psychopharmacology (Berl) 2008;201:507–516. doi: 10.1007/s00213-008-1315-4. [DOI] [PubMed] [Google Scholar]
- Jackson KJ, Carroll FI, Negus SS, Damaj MI. Effect of the selective kappa-opioid receptor antagonist JDTic on nicotine antinociception, reward, and withdrawal in the mouse. Psychopharmacology (Berl) 2010;210:285–294. doi: 10.1007/s00213-010-1803-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kota D, Martin BR, Robinson SE, Damaj MI. Nicotine dependence and reward differ between adolescent and adult male mice. J Pharmacol Exp Ther. 2007;322:399–407. doi: 10.1124/jpet.107.121616. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Goldberg SR. Nicotine induces conditioned place preferences over a large range of doses in rats. Psychopharmacol. 2005;178:481–492. doi: 10.1007/s00213-004-2021-5. [DOI] [PubMed] [Google Scholar]
- Malin DH, Lake JR, Carter VA, Cunningham JS, Wilson OB. Naloxone precipitates nicotine abstinence syndrome in the rat. Psychopharmacology (Berl) 1993;112:339–342. doi: 10.1007/BF02244930. [DOI] [PubMed] [Google Scholar]
- Markou A. Neurobiology of nicotine dependence. Philos Trans R Soc Lond B Biol Sci. 2008;363:3159–3168. doi: 10.1098/rstb.2008.0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaels CC, Holtzman SG. Early postnatal stress alters place conditioning to both mu-and kappa-opioid agonists. J Pharmacol Exp Ther. 2008;325(1):313–8. doi: 10.1124/jpet.107.129908. [DOI] [PubMed] [Google Scholar]
- McCarthy MJ, Zhang H, Neff NH, Hadjiconstantinou M. Nicotine withdrawal and kappa-opioid receptors. Psychopharmacology (Berl) 2009;210:221–229. doi: 10.1007/s00213-009-1674-5. [DOI] [PubMed] [Google Scholar]
- McLaughlin JP, Land BB, Li S, Pintar JE, Chavkin C. Prior activation of kappa opioid receptors by U50,488 mimics repeated forced swim stress to potentiate cocaine place preference conditioning. Neuropsychopharmacol. 2006;31:787–794. doi: 10.1038/sj.npp.1300860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata H, Itasaka M, Kimura N, Nakayama K. Decreases in brain reward function reflect nicotine- and methamphetamine-withdrawal aversion in rats. Curr Neuropharmacol. 2011;9(1):63–7. doi: 10.2174/157015911795017218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton LS, Apparsundaram S, King-Pospisil KA, Dwoskin LP. Nicotine increases dopamine transporter function in rat striatum through a trafficking-independent mechanism. Eur J Pharmacol. 2007;554:128–136. doi: 10.1016/j.ejphar.2006.09.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales L, Perez-Garcia C, Herradon G, Alguacil LF. Place conditioning in a two- or three-conditioning compartment apparatus: a comparative study with morphine and U-50,488. Addict Biol. 2007;12:482–484. doi: 10.1111/j.1369-1600.2007.00071.x. [DOI] [PubMed] [Google Scholar]
- Mucha RF, Herz A. Motivational properties of kappa and mu opioid receptor agonists studied with place and taste preference conditioning. Psychopharmacology (Berl) 1985;86:274–280. doi: 10.1007/BF00432213. [DOI] [PubMed] [Google Scholar]
- Natividad LA, Tejeda HA, Torres OV, O’Dell LE. Nicotine withdrawal produces a decrease in extracellular levels of dopamine in the nucleus accumbens that is lower in adolescent versus adult male rats. Synapse. 2010;64:136–145. doi: 10.1002/syn.20713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dell LE. A psychobiological framework of the substrates that mediate nicotine use during adolescence. Neurpharmacology. 2009;56:263–278. doi: 10.1016/j.neuropharm.2008.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dell LE, Bruijnzeel AW, Ghozland S, Markou A, Koob GF. Nicotine withdrawal in adolescent and adult rats. Ann N Y Acad Sci. 2004;1021:167–174. doi: 10.1196/annals.1308.022. [DOI] [PubMed] [Google Scholar]
- O’Dell LE, Bruijnzeel AW, Smith RT, Parsons LH, Merves ML, Goldberger BA, Richardson HN, Koob GF, Markou A. Diminished nicotine withdrawal in adolescent rats: implications for vulnerability to addiction. Psychopharmacology (Berl) 2006;186:612–619. doi: 10.1007/s00213-006-0383-6. [DOI] [PubMed] [Google Scholar]
- O’Dell LE, Khroyan TV. Rodent models of nicotine reward: what do they tell us about tobacco abuse in humans? Pharmacology Biochemistry and Behavior. 2009;91:481–488. doi: 10.1016/j.pbb.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dell LE, Torres OV, Natividad LA, Tejeda HA. Adolescent nicotine exposure produces less affective measures of withdrawal relative to adult nicotine exposure in male rats. Neurotoxicol Teratol. 2007;29:17–22. doi: 10.1016/j.ntt.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto MR, Brunzell DH, Caldarone BJ. Effect of nicotine and nicotinic receptors on anxiety and depression. Neuroreport. 2002;13:1097–1106. doi: 10.1097/00001756-200207020-00006. [DOI] [PubMed] [Google Scholar]
- Rada P, Jensen K, Hoebel BG. Effects of nicotine and mecamylamine-induced withdrawal on extracellular dopamine and acetylcholine in the rat nucleus accumbens. Psychopharmacology (Berl) 2001;157:105–110. doi: 10.1007/s002130100781. [DOI] [PubMed] [Google Scholar]
- Rossetti ZL, Hmaidan Y, Gessa GL. Marked inhibition of mesolimbic dopamine release: a common feature of ethanol, morphine, cocaine and amphetamine abstinence in rats. Eur J Pharmacol. 1992;221:227–234. doi: 10.1016/0014-2999(92)90706-a. [DOI] [PubMed] [Google Scholar]
- Roy-Byrne PP, Uhde TW. Exogenous factors in panic disorder: clinical and research implications. J Clin Psychiatry. 1988;49:56–61. [PubMed] [Google Scholar]
- Shippenberg TS, Bals-Kubik R, Herz A. Examination of the neurochemical substrates mediating the motivational effects of opioids: role of the mesolimbic dopamine system and D-1 vs. D-2 dopamine receptors. J Pharmacol Exp Ther. 1993;265:53–59. [PubMed] [Google Scholar]
- Shippenberg TS, Zapata A, Chefer VI. Dynorphin and the pathophysiology of drug addiction. Pharmacol Ther. 2007;116:306–321. doi: 10.1016/j.pharmthera.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shram MJ, Siu EC, Li Z, Tyndale RF, Lê AD. Interactions between age and the aversive effects of nicotine withdrawal under mecamylamine-precipitated and spontaneous conditions in male Wistar rats. Psychopharmacology (Berl) 2008;198:181–190. doi: 10.1007/s00213-008-1115-x. [DOI] [PubMed] [Google Scholar]
- Skoubis PD, Lam HA, Shoblock J, Narayanan S, Maidment NT. Endogenous enkephalins, not endorphins, modulate basal hedonic state in mice. Eur J Neurosci. 2005;21:1379–1384. doi: 10.1111/j.1460-9568.2005.03956.x. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Herz A, Shippenberg TS. Opposing tonically active endogenous opioid systems modulate the mesolimbic dopaminergic pathway. Proc Natl Acad Sci U S A. 1992;89:2046–2050. doi: 10.1073/pnas.89.6.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejeda HA, Shippenberg TS, Henriksson R. The dynorphin/κ-opioid receptor system and its role in psychiatric disorders. Cell Mol Life Sci. 2012;69(6):857–96. doi: 10.1007/s00018-011-0844-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AC, Zapata A, Justice JB, Jr, Vaughan RA, Sharpe LG, Shippenberg TS. Kappa-opioid receptor activation modifies dopamine uptake in the nucleus accumbens and opposes the effects of cocaine. J Neurosci. 2000;15(20):9333–9340. doi: 10.1523/JNEUROSCI.20-24-09333.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trauth JA, Seidler FJ, Slotkin TA. An animal model of adolescent nicotine exposure: effects on gene expression and macromolecular constituents in rat brain regions. Brain Res. 2000;867:29–39. doi: 10.1016/s0006-8993(00)02208-3. [DOI] [PubMed] [Google Scholar]
- Walker BM, Valdez GR, McLaughlin JP, Bakalkin G. Targeting dynorphin/kappa opioid receptor systems to treat alcohol abuse and dependence. Alcohol. 2012 doi: 10.1016/j.alcohol.2011.10.006. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins SS, Stinus L, Koob GF, Markou A. Reward and somatic changes during precipitated nicotine withdrawal in rats: centrally and peripherally mediated effects. J Pharmacol Exp Ther. 2000;292:1053–1064. [PubMed] [Google Scholar]
- Wee S, Koob GF. The role of the dynorphin-kappa opioid system in the reinforcing effects of drugs of abuse. Psychopharmacology (Berl) 2010;210:121–35. doi: 10.1007/s00213-010-1825-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmouth CE, Spear LP. Withdrawal from chronic nicotine in adolescent and adult rats. Pharmacol Biochem Behav. 2006;85:648–657. doi: 10.1016/j.pbb.2006.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Apparsundaram S, Dwoskin LP. Nicotinic receptor activation increases [3H]dopamine uptake and cell surface expression of dopamine transporters in rat prefrontal cortex. J Pharmacol Exp Ther. 2009;328:931–939. doi: 10.1124/jpet.108.147025. [DOI] [PMC free article] [PubMed] [Google Scholar]