Abstract
Eukaryotes are constantly fine-tuning their gene expression programs in response to the demands of the environment and the availability of nutrients. Such dynamic regulation of the genome necessitates versatile chromatin architecture. Rapid changes in transcript levels are brought about via a wide range of posttranslational modifications of the histone proteins that control chromatin structure. Many enzymes responsible for these modifications have been identified and they require various metabolic cofactors or substrates for their activity. Herein, we highlight recent developments that have begun to reveal particular cellular metabolites that might in fact be underappreciated regulators of gene expression through their ability to modulate particular histone modifications.
Keywords: epigenetics, histones, acetylation, methylation, acetyl-CoA, SAM
Chromatin architecture and histone modifications
Eukaryotic genomes condense two meters of DNA by over 100,000-fold in order to fit into the limited space of the nucleus of a cell. Such compaction must in turn allow access to the genetic material by various factors that regulate DNA replication, repair, and transcription among numerous other DNA-dependent processes. The hierarchical packaging of DNA into such a dynamic chromatin structure is accomplished by wrapping approximately 146 base pairs of DNA around an octamer containing two copies of each of the histone proteins H2A, H2B, H3 and H4 [1-3]. The amino terminus of each of the histones is rich in lysine residues and sticks out like a tail (Figure 1). These tails as well as interior residues are subject to reversible covalent modifications such as acetylation, methylation, phosphorylation, ubiquitylation, SUMOylation and poly-ADP-ribosylation [4-6]. This assortment of modifications is important for regulating chromatin architecture and accessibility of the underlying DNA to the transcription machinery. In recent years, the race to decipher the logic behind these various histone modifications has led researchers in a surprising new direction. Intriguingly, many of the histone-modifying enzymes employ essential metabolites such as ATP, nicotinamide adenine dinucleotide (NAD+), acetyl-Coenzyme A (acetyl-CoA), and S-adenosylmethionine (SAM or AdoMet) for their function. These substrates are key intermediates of important metabolic pathways and thus provide a means to link gene regulation with cellular metabolism. In this short review, we discuss the emerging body of literature that marries chromatin modifications and gene expression with cellular metabolism. We focus on histone acetylation and methylation, for which there is currently particularly compelling evidence that cellular metabolites influence these modifications of chromatin (Table 1).
Figure 1. Metabolite-driven, rapid changes in gene expression through chromatin plasticity.
Small-molecule metabolites in the cell are involved in the regulation of gene expression. For example, histone acetyltransferases (HATs) use acetyl groups from acetyl-CoA to acetylate lysines (small blue circles) at multiple sites along the histone tails that protrude out of the octamer. This modification facilitates ‘opening’ of the chromatin and allows various transcription factors to access the DNA to turn on gene transcription. By contrast, histone deacetylases (HDACs) remove acetylation marks from histones and return the chromatin to its ‘closed’ confirmation. A subset of HDACs, the sirtuins, could be regulated by NAD+ levels in the cell. Acetyl-CoA is generated by acetyl-CoA synthetases (Acs), and ATP citrate lyase (ACL) by using acetate and citrate as the precursor, respectively. ACL is not present in budding yeast. Production of NAD+ is more complex but its abundance is linked to energy flux through glycolysis and the TCA cycle.
Table 1. Metabolite substrates and cofactors utilized in histone and DNA modifications.
| Metabolite | Enzyme | Effect on transcription | Reaction products |
|---|---|---|---|
| Acetyl-Coenzyme A (Acetyl-CoA) |
Histone acetyltransferases (HATs) | Activation | Coenzyme A |
| Nicotinamide adenine dinucleotide (NAD+) |
Histone deacetylases (Sirtuins) Poly-(ADP-ribose) polymerase (PARP) |
Repression Activation or repression |
O-acetyl-ADP-ribose and nicotinamide |
| S-adenosylmethionine (SAM) |
Histone methyltransferases DNA methyltransferases |
Activation or repression Repression |
S-adenosylhomocysteine |
| Flavin adenine dinucleotide (FAD) |
Histone demethylases (LSD1) | Activation or repression | H2O2 and formaldehyde |
| α-ketoglutarate (α-KG) |
Histone demethylases (JmjC) TET (Ten-Eleven Translocase) proteins |
Activation or repression | Succinate, CO2, and formaldehyde |
A link between chromatin and metabolism first revealed by sirtuins
Gene regulation in eukaryotes is elaborative, and involves highly coordinated molecular events that are frequently rooted at the level of chromatin. Multiple mechanisms contribute to whether a gene is turned on or off. These include regulation of the core transcription machinery, recruitment of transcriptional activators or repressors, and altering chromatin structure via post-translational covalent modification of histone proteins. Of these mechanisms, gene regulation resulting from a change in chromatin architecture has become a widely observed and investigated phenomenon. Studies of chromatin-level gene regulation began with the pioneering work of Allfrey in 1964, when he proposed that active RNA synthesis (transcription) in eukaryotes is closely associated with histone acetylation [4]. The acetylation of histone tails is thought to neutralize the positive charge of lysine side chains, thereby facilitating access to the DNA, and its negatively charged backbone, for transcription factors. Since then, an extensive body of work from various model organisms has provided much of the details on how histone acetylation could result in increased transcriptional activity in vivo [7, 8]. In addition to neutralizing the charges on lysine side chains of histones, acetylation is also an important signal for binding of trans-acting factors and chromatin remodelers. Numerous transcription factors contain a bromodomain, which specifically interacts with acetylated lysine [9-11].
Interestingly, much of the support for the hypothesis that histone acetylation is tightly connected to transcriptional activation came from the opposite side of the histone acetylation coin, histone deacetylation. Studies of transcriptionally ‘silent’ chromatin in Saccharomyces cerevisiae, which includes telomeric regions and the silent mating-type loci, have revealed that the amino-terminal tails of histone H3 and H4 in these chromosomal regions tend to be hypoacetylated relative to the rest of the genome [12-16]. A family of proteins termed ‘silent information regulator’ (SIR/sirtuin) was discovered to be crucial for keeping the desired genomic region in this hypoacetylated form [14, 17-20]. It was subsequently discovered that yeast Sir2, as well as other sirtuins, harbors an enzymatic activity and utilizes NAD+ as a substrate to deacetylate lysine residues on histones [21], converting them back to their unmodified form (Figure 1). In this process, one molecule of NAD+ is converted to nicotinamide and O-acetyl-ADP-ribose, an acceptor of the liberated acetate [22-24].
The discovery that sirtuins are NAD+-dependent deacetylase enzymes created much excitement in the field by suggesting a tantalizing link between cellular NAD+ levels and the regulation of chromatin accessibility and gene expression. Numerous metabolic enzymes, including several in glycolysis and the TCA cycle, utilize NAD+ as an electron acceptor and reduce it to NADH. Thus, the activity of sirtuins could be influenced by fluctuations in free NAD+ levels stemming from changes in cellular metabolism, for example in response to feeding or fasting [25, 26]. Such regulation of Sir2 by NAD+ levels was one of the earliest hints that small molecule metabolites might be important for regulating gene expression through histone modifications. More evidence mounted as the field turned its attention to understanding the biochemical reactions that led to generation of acetylated histones that the histone deacetylases acted upon.
Acetyl-Coenzyme A and histone acetylation
The interest in sirtuins and their role as histone deacetylases raises the question of how these histones become acetylated in the first place. In the mid-1990s, Brownell and Allis identified a 55kDa polypeptide in Tetrahymena themophila that showed histone acetyltransferase (HAT) activity; later, it turned out to be a homolog of the previously identified yeast Gcn5 (general control of amino-acid synthesis 5) transcriptional coactivator [27-29]. Functional characterization of yeast Gcn5 mutants revealed a direct correlation between the ability of the protein to acetylate histones and activate transcription. Subsequently, a flurry of studies led to the discovery of a large number of HAT enzymes, some of which were previously identified as transcriptional coactivators [8, 30-35]. To date, more than 20 distinct proteins have been shown to have intrinsic HAT or lysine acetyltransferase (KAT) activity, dubbing acetylation to be one of the major modifications on histones that affects gene transcription (Figure 1, [36]).
All HATs and KATs identified to date use acetyl-CoA as the acetyl donor for acetylation. Acetyl-CoA is a central metabolite that is involved in many metabolic transformations within the cell. The ‘activated acetate’ moiety is more than just an acetyl group donor for protein acetylation modifications; it also has a well-known, essential role in stitching together components of cellular membranes such as fatty acids and sterols. The acetyl group of acetyl-CoA can also be oxidized via the TCA cycle to reduce NAD+ and FAD to NADH and FADH2, respectively, which subsequently fuel ATP production through the electron transport chain.
Acetyl-CoA can be generated from pyruvate via the pyruvate dehydrogenase (PDH) enzyme complex present in the mitochondria. Acetyl-CoA can also be synthesized by acetyl-CoA synthetase enzymes, which join a molecule of acetate to Coenzyme A in an ATP-dependent reaction [37, 38]. Mitochondrial acetyl-CoA can be exported to the cytosol in the form of citrate, which is converted back to acetyl-CoA (and oxaloacetate) by ATP citrate lyase (ACL) [39-41]. The utilization of acetyl-CoA by acetyltransferases suggests that the production of acetyl-CoA could be important for regulation of acetyltransferases. Only more recently have researchers begun to appreciate the possibility that levels of acetyl-CoA itself could be rate-limiting for specific protein acetylation modifications (also discussed in depth in the following reviews [36, 42, 43]).
A major indication that the enzymes that synthesize acetyl-CoA could be important regulators of chromatin state and gene expression came initially from yeast. Budding yeast synthesize nucleocytosolic pools of acetyl-CoA from acetate using the acetyl-CoA synthetases, Acs1p and Acs2p. ACS1 is expressed under poor carbon sources, whereas ACS2 is essential for rapid growth on glucose [38, 44, 45]. Temperature sensitive acs2 mutants exhibit a near complete loss of H3/H4 acetylation and downregulation of more than 70% of the genome, linking intracellular energy status to gene activity [46]. Moreover, an acs2 mutant exhibited synthetic growth defects when combined with mutations in acetyltransferase enzymes (including Gcn5) [46]. Thus, it became apparent that the metabolic enzymes that control biosynthesis of acetyl-CoA, also supply the acetyl-CoA pool required by histone acetyltransferases. Interestingly, both yeast and human acetyl-CoA synthetases are deacetylated by sirtuins in a nutrient-responsive manner, which allows the enzyme to assume its full activity [47, 48].
Similar to budding yeast, mammalian acetyl-CoA-producing enzymes such as ATP citrate lyase (ACL) can also alter gene transcription by regulating histone acetylation. ACL is a major source of the acetyl-CoA used in histone acetylation under normal growth conditions [41]. RNAi knockdown of ACL severely impaired histone acetylation, whereas non-histone acetylated proteins (such as p53) were not affected by silencing of ACL, suggesting that ACL specifically promotes histone acetylation [41]. siRNA knockdown of ACL also resulted in a significant decrease in the expression of particular genes involved in metabolism, such as glucose transporter (Glut4), hexokinase 2 (HK2), phosphofructokinase (PFK-1), and lactate dehydrogenase-A (LDH-A) [41]. Thus, collectively these findings argue that nutrient uptake and the metabolic state of the cell in terms of energy flux are intertwined with histone acetylation and consequently gene expression.
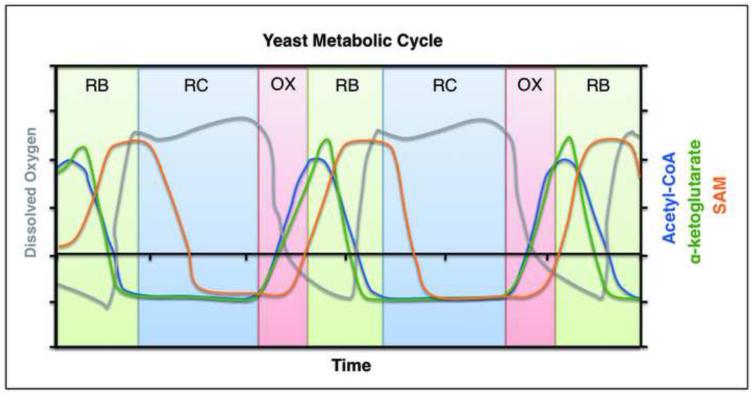
The extent to which glucose availability and acetyl-CoA levels can influence chromatin architecture and cellular growth or proliferation is illustrated by a recent study in yeast, which proposes an attractive logic behind histone acetylation [49]. Using a system wherein a highly synchronized yeast cell population continuously transitions between growth and quiescent-like phases, a direct role for acetyl-CoA in the regulation of specific protein acetylation modifications and transcriptional activation of cellular growth genes was uncovered [45, 49]. The level of intracellular acetyl-CoA and the concomitant acetylation of multiple lysines on histone H3 and H4 was observed to peak during the growth phase (Figure 2) [49, 50]. Addition of ethanol or acetate, which results in a sharp increase in acetyl-CoA levels, was also shown to trigger cells to exit the quiescent-like phase and to enter growth [49]. A proteomic screen using 13C-labelled acetate and subsequent experimentation discovered that several members of the SAGA transcriptional coactivator complex and histones themselves are dynamically acetylated substrates whose acetylation coincides with the increase in acetyl-CoA. Interestingly, chromatin immunoprecipitation (ChIP) experiments revealed that these acetylated histones were present almost exclusively at growth genes. This study outlines a major role for acetyl-CoA in inducing the expression of a particular set of genes (mainly the growth genes) via a specific acetyltransferase complex, SAGA, in response to availability of nutrients in the form of carbon sources [49]. Taken together, these findings make the explicit prediction that intracellular acetyl-CoA levels represent a key barometer of the metabolic state of a cell, which gates access to chromatin as a means to regulate the expression of genes needed for cell growth [42].
Figure 2. Metabolite substrate modifiers of chromatin are dynamic.
Yeast cells undergo robust oscillations in oxygen consumption during continuous growth. They transition between OX (oxidative), RB (reductive, building), and RC (reductive, charging) phases during each metabolic cycle and each phase is characterized by distinct gene expression profiles. The OX phase transcripts predominantly comprise genes involved in growth. During this time, cells increase oxygen consumption and experience a surge in acetyl-CoA and α-ketoglutarate levels, followed by a rapid rise in SAM [50]. The abundance of these metabolites can be very dynamic as a function of the growth and metabolic state of a cell.
It must be reiterated that acetyl-CoA is important for non-histone protein acetylation modifications as well [51]. In fact, since the discovery of p53 as a non-histone target of the p300/CBP acetyltransferase [52], numerous other targets have been identified in yeast and mammals [53-55]. Reversible acetylation of lysines on many non-histone proteins plays a critical role in the regulation of many cellular processes including DNA repair, cell cycle progression, differentiation, replication, and apoptosis [53, 56-59]. As in the case of histones, acetylation of these other targets by KATs could also be a means to metabolically regulate their structure and function [56]. Recent proteomic studies suggest many metabolic enzymes involved in glycolysis, gluconeogenesis, fatty acid biosynthesis, the urea cycle, and glycogen metabolism can also be acetylated, which might influence their activity and/or stability [53, 55, 60]. Hence, the number of proteins that can be acetylated could number in the thousands [53]. While some of these acetylation modifications could be regulated by fluctuations in acetyl-CoA levels, others could be independent of metabolic state and instead regulated by localization or recruitment of the appropriate KAT [42]. The underlying logic and extent by which these numerous reported acetylation modifications are linked to cellular metabolism remains an active area of investigation.
S-adenosylmethionine and histone methylation
Another major post-translational modification of significant biological consequence that might be regulated by the metabolic state of the cell is methylation. DNA, RNA, and proteins can all be subjected to methylation modifications by various methyltransferases, which transfer the methyl group from SAM to an acceptor substrate (Figure 3). In particular, lysine residues on proteins can be mono-, di-, or tri-methylated, while arginine residues can be either mono- or di-methylated [61]. Due to the nature of the arginine side chain, di-methylated arginine can exist in symmetric or asymmetric forms. SAM is generated by SAM synthetases (methionine adenosyl transferase, MAT) by covalently linking an adenosine moiety from ATP to methionine [62]. Upon donating its methyl group during subsequent methylation reactions, SAM is converted to S-adenosylhomocysteine (SAH). If accumulated at a high level, SAH can act as an inhibitor of methyltransferases in the cell. Consequently, SAH is rapidly hydrolyzed to adenosine and homocysteine which can be further converted to cysteine or again back to methionine (Figure 3). The synthesis of methionine from homocysteine is dependent on the folate pathway as a one-carbon donor.
Figure 3. Roles of metabolites in histone methylation and demethylation.
Methylation of histones and DNA play key roles in keeping the genome in a transcriptionally silent state both locally and globally. Some histone methylation modifications can be activating. Such events are potentially linked to cellular metabolism via S-adenosylmethionine (SAM), which provides the necessary methyl groups for methyltransferases to catalyze histone/DNA methylation as well as non-histone protein methylation (small orange circles indicate methylation marks). Histones are methylated on lysine and arginine residues by histone methyltransferases, HMTs. SAM is produced by SAM synthetases (methionine adenosyl transferase, MAT) via the addition of an adenosine moiety from ATP to methionine. In mammals, dietary folate can be enzymatically converted to 5-methyltetrahydrofolate (5-MTHF). The transfer of a methyl group from 5-MTHF to homocysteine requires Vitamin B12 and results in the synthesis of methionine. Upon donating its high-energy methyl group, SAM becomes S-adenosylhomocysteine (SAH) that is a potent inhibitor of methyltransferases unless broken down by SAH hydrolase (SAHH) into adenosine and homocysteine. Homocysteine can act as a precursor for methionine or cysteine synthesis. Histone demethylases (HDMs) remove methyl groups from histones. Lysine-specific histone demethylase (LSD1) uses FAD as a cofactor whereas JmjC-domain-containing HDMs use α-ketoglutarate (α-KG) as a substrate.
Recently, SAM synthetase (MAT) in mammals has been linked to gene repression specifically through regulation of histone methylation. The production of SAM by the promoter-bound MAT isozyme, MatIIα, was reported to modulate the activity of methyltransferases involved in transcriptional control [63]. Through a proteomic approach, more than one hundred MatIIα-interacting proteins were uncovered, including transcription factor MafK (musculoaponeurotic fibrosarcoma oncogene homolog K), components of Swi/Snf (switch/sucrose non-fermentable), NuRD (nucleosome remodeling and histone deacetylase complex) nucleosome remodelers, PARP-1 (poly (ADP-ribose) polymerase), and PcG (polycomb group) proteins [63]. A single point mutation abolishing the SAM synthetase activity of MatIIα reversed the transcriptional repression that is normally in place for the MatIIα target gene heme oxygenase-1 (HO-1). A sharp decrease in dimethylation of H3K4 and H3K9 was observed following MatIIα knockdown [63]. ChIP experiments revealed that histone methyltransferases function as mediators of SAM-dependent transcriptional repression. The methyltransferases that are specifically under regulation by SAM have not been identified, but several (i.e., G9a, Ehmt, and MLL1/KMT2A) have been implicated [63]. Nonetheless, this study provides an example of how metabolic enzymes can localize to chromatin and synthesize metabolites that might locally function as substrates or cofactors for histone modifiers, resulting in changes in gene expression (Figure 3).
α-ketoglutarate and histone demethylation
Methylated histones are demethylated by histone demethylases (HDMs), which utilize α-ketoglutarate (α-KG, also known as 2-oxoglutarate) as a substrate to demethylate mono-, di-, or tri-methylated histones. α-KG is a TCA cycle intermediate that is generated from isocitrate by isocitrate dehydrogenase (IDH) (Figure 3). Interestingly, it was recently observed that greater than 75% of gliomas harbor a mutation in one of the two isoforms of IDH genes, IDH1 and IDH2 [64]. These mutations occur in the substrate binding pocket: Arg132 in IDH1 and Arg140 and Arg172 in IDH2. As a result, these enzymes are unable to convert isocitrate to α-KG. Instead, they aberrantly produce 2-hydroxyglutarate (2-HG), which can function as a competitive inhibitor of histone demethylases by occupying the same space in the binding pocket as α-KG [65, 66]. It was recently reported that accumulation of 2-HG resulted in increased levels of methylated H3K9 and H3K27, which are associated with transcriptional repression [67]. These changes could be counteracted by supplementing cells with cell-permeable octyl-α-KG [65].
Knockdown of IDH1 is also accompanied by a reduction in the activity of another α-KG-dependent enzyme, TET2 (Ten-Eleven Translocation 2), which is a 5-methylcytosine (5-mC) hydroxylase that utilizes molecular oxygen to transfer a hydroxyl group to 5-methylcytosine, generating 5-hydroxymethylcytosine (5-hmC) [68]. In IDH mutants, there was a global decrease in 5-hydroxymethylcytosine and an increase in 5-methylcytosine, the substrate of TET2 hydroxylation [68]. These effects could impact DNA demethylation and contribute to alterations in gene expression and epigenetic states.
Other histone modifications that could be influenced by metabolites
In addition to the metabolites discussed above, numerous other less appreciated metabolic intermediates might also play roles in regulating histone modifications and gene expression. Flavin adenine dinucleotide (FAD), which serves as a cofactor in many oxidative reactions including mitochondrial fatty acid β-oxidation and in the respiratory chain, is also an important cofactor for demethylation of histones [69]. FAD has been shown to alter the activity of lysine specific histone demethylase LSD1, which specifically removes methyl groups from mono- and dimethylated H3K4 or H3K9 through an FAD-dependent oxidation reaction (Figure 3, [69, 70]). This results in context-dependent activation or repression. Inhibition of LSD1 by either siRNA-mediated knockdown or selective inhibitors results in activation of metabolic genes, mitochondrial respiration, and lipolysis in adipocytes, suggesting a crucial role for LSD1 in repression of these metabolic genes [71]. Importantly, this de-repression pattern in the knockdown was dependent on FAD, as disruption of cellular FAD synthesis exerted similar effects.
In addition to sirtuins, NAD+ is also utilized by poly (ADP-ribose) polymerases (PARP), a family of enzymes that have a crucial role during DNA repair and cell death. PARPs utilize NAD+ to transfer ADP-ribose to histones, non-histone proteins, and PARP itself. Poly-ADP-ribosylation of histones and PARP results in decondensation and opening of chromatin structure, thus promoting gene transcription [72-74]. Sirtuins have also been found to catalyze ADP-ribosylation of proteins [73, 75]. The dependence of both sirtuins and PARPs on NAD+ suggests possible competition between the two enzymes and between open and closed states of chromatin.
O-GlcNAcylation of histones has also received much attention recently (reviewed in [76]). UDP-GlcNAc, a metabolic intermediate from the hexosamine biosynthetic pathway, serves as the sugar donor for O-GlcNAcylation. Mass spectrometry methods have been used to map O-GlcNAcylation to the core sequence of H2A, H2B, H3 and H4 of both transcriptionally active and repressed genes [76]; interestingly, many of these sites are known phosphorylation sites. Thus, O-GlcNAcylation can influence the substrate preference of histone-modifying enzymes and could indirectly alter gene expression patterns.
Lastly, because these substrates and cofactors used in histone modifications are derived from various nutrients, it follows that gene regulatory mechanisms and chromatin state (e.g., methylation patterns) can be significantly influenced by environmental factors and diet [77]. As one example, the conversion of homocysteine to methionine (which feeds into SAM production) is catalyzed by methionine synthase, which is dependent on 5-methyltetrahydrofolate, a bioactive folate metabolite, and Vitamin B12 (Figure 3) [62]. Therefore, SAM levels might also be influenced by diet and consequently lead to epigenetic changes by influencing methylation states [78, 79].
Concluding remarks
The intimate, reciprocal regulation between gene expression outputs and cellular metabolic state is undoubtedly highly beneficial for the cell. There is now evidence to suggest that chromatin has been selected to function as a ‘metabolic’ gatekeeper of the genome. The wrapping of DNA around histones in a default repressive state might enable a cell to closely couple nutrient availability (by controlling access to the DNA via the requirement of metabolites as histone modifiers) to the regulation of a variety of cellular outputs (by controlling the expression of particular modules of genes). Research in the past few years has placed this complex network of metabolism, chromatin architecture, and gene expression at center stage. Studies discussed in this review highlight the possibility that histone acetylation, deacetylation, methylation, and demethylation are under the control of several key metabolic regulators: Acetyl-CoA, NAD+, SAM, and α-KG. Indeed, it is likely more than coincidence that cells have chosen to regulate access to their DNA via histone modifications that are dependent on key intermediates of metabolism. The list of known histone modifications includes numerous other types of modifications and is continually expanding, but it remains to be seen which of these additional modifications might also be coordinated with cellular metabolism via a specified logic.
The subcellular localization and compartmentalization of metabolite pools might represent an important mechanism that contributes to regulation of chromatin architecture and gene expression. For example, there are at least two potential pools of acetyl-CoA in the cell: mitochondrial and nucleocytoplasmic. The nucleocytoplasmic pool of acetyl-CoA is clearly important for histone acetylation, and the mitochondrial pool of acetyl-CoA purportedly cannot compensate for the lack of a nucleocytoplasmic pool of acetyl-CoA in budding yeast [46]. To date, only one biosynthetic enzyme of the key metabolic regulators mentioned in this review, MatII-α, has been found in a chromatin-bound state and shown to interact with relevant histone remodelers. The benefit of generating such metabolites locally via a chromatin-bound enzyme could be to enable a particular modification or regulatory event to be independent or insulated from the bulk metabolic activities of the cell. Developing reliable cellular metabolite ‘sensors’ might help reveal the subcellular organization of metabolism and metabolite pools.
Finally, as the field proceeds to uncover additional metabolic enzymes that modify chromatin structure, it also remains to be seen which of these key metabolites will steal the spotlight and emerge as master regulators of gene expression. Perhaps certain transcription factors and transcriptional coactivators will ultimately be slaves to these metabolites themselves. The logic behind the numerous modifications of histones must now account for what appears to be intentional and intimate connections to cellular metabolism.
Acknowledgments
Due to space constraints, we apologize to those whose important work in this broad field could not be discussed or cited. We also thank the anonymous reviewers for numerous helpful suggestions in improving this article. B.P.T. wishes to acknowledge funding support from award R01GM094314 from the National Institute of General Medical Sciences, the Burroughs Wellcome Fund, the David and Lucile Packard Foundation, the Damon Runyon Cancer Research Foundation, and CPRIT.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Salma Kaochar, Department of Biochemistry, University of Texas Southwestern Medical Center, 5323 Harry Hines Blvd. Dallas, TX 75390-9038.
Benjamin P. Tu, Department of Biochemistry, University of Texas Southwestern Medical Center, 5323 Harry Hines Blvd. Dallas, TX 75390-9038
References
- 1.Kornberg RD. Chromatin structure: a repeating unit of histones and DNA. Science. 1974;184:868–871. doi: 10.1126/science.184.4139.868. [DOI] [PubMed] [Google Scholar]
- 2.Luger K, et al. Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature. 1997;389:251–260. doi: 10.1038/38444. [DOI] [PubMed] [Google Scholar]
- 3.Kornberg RD, Lorch Y. Twenty-five years of the nucleosome, fundamental particle of the eukaryote chromosome. Cell. 1999;98:285–294. doi: 10.1016/s0092-8674(00)81958-3. [DOI] [PubMed] [Google Scholar]
- 4.Allfrey VG, et al. Acetylation and Methylation of Histones and Their Possible Role in the Regulation of Rna Synthesis. Proc Natl Acad Sci U S A. 1964;51:786–794. doi: 10.1073/pnas.51.5.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shilatifard A. Chromatin modifications by methylation and ubiquitination: implications in the regulation of gene expression. Annu Rev Biochem. 2006;75:243–269. doi: 10.1146/annurev.biochem.75.103004.142422. [DOI] [PubMed] [Google Scholar]
- 6.Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403:41–45. doi: 10.1038/47412. [DOI] [PubMed] [Google Scholar]
- 7.Shahbazian MD, Grunstein M. Functions of site-specific histone acetylation and deacetylation. Annu Rev Biochem. 2007;76:75–100. doi: 10.1146/annurev.biochem.76.052705.162114. [DOI] [PubMed] [Google Scholar]
- 8.Lee KK, Workman JL. Histone acetyltransferase complexes: one size doesn’t fit all. Nat Rev Mol Cell Biol. 2007;8:284–295. doi: 10.1038/nrm2145. [DOI] [PubMed] [Google Scholar]
- 9.Filippakopoulos P, et al. Histone recognition and large-scale structural analysis of the human bromodomain family. Cell. 2012;149:214–231. doi: 10.1016/j.cell.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dhalluin C, et al. Structure and ligand of a histone acetyltransferase bromodomain. Nature. 1999;399:491–496. doi: 10.1038/20974. [DOI] [PubMed] [Google Scholar]
- 11.Zeng L, Zhou MM. Bromodomain: an acetyl-lysine binding domain. FEBS Lett. 2002;513:124–128. doi: 10.1016/s0014-5793(01)03309-9. [DOI] [PubMed] [Google Scholar]
- 12.Braunstein M, et al. Transcriptional silencing in yeast is associated with reduced nucleosome acetylation. Genes Dev. 1993;7:592–604. doi: 10.1101/gad.7.4.592. [DOI] [PubMed] [Google Scholar]
- 13.Braunstein M, et al. Efficient transcriptional silencing in Saccharomyces cerevisiae requires a heterochromatin histone acetylation pattern. Mol Cell Biol. 1996;16:4349–4356. doi: 10.1128/mcb.16.8.4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gottschling DE. Gene silencing: two faces of SIR2. Curr Biol. 2000;10:R708–711. doi: 10.1016/s0960-9822(00)00714-4. [DOI] [PubMed] [Google Scholar]
- 15.Kimura A, et al. Chromosomal gradient of histone acetylation established by Sas2p and Sir2p functions as a shield against gene silencing. Nature genetics. 2002;32:370–377. doi: 10.1038/ng993. [DOI] [PubMed] [Google Scholar]
- 16.Suka N, et al. Sir2p and Sas2p opposingly regulate acetylation of yeast histone H4 lysine16 and spreading of heterochromatin. Nature genetics. 2002;32:378–383. doi: 10.1038/ng1017. [DOI] [PubMed] [Google Scholar]
- 17.Rine J, Herskowitz I. Four genes responsible for a position effect on expression from HML and HMR in Saccharomyces cerevisiae. Genetics. 1987;116:9–22. doi: 10.1093/genetics/116.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lustig AJ. Mechanisms of silencing in Saccharomyces cerevisiae. Curr Opin Genet Dev. 1998;8:233–239. doi: 10.1016/s0959-437x(98)80146-9. [DOI] [PubMed] [Google Scholar]
- 19.Chien CT, et al. Targeting of SIR1 protein establishes transcriptional silencing at HM loci and telomeres in yeast. Cell. 1993;75:531–541. doi: 10.1016/0092-8674(93)90387-6. [DOI] [PubMed] [Google Scholar]
- 20.Ivy JM, et al. Cloning and characterization of four SIR genes of Saccharomyces cerevisiae. Mol Cell Biol. 1986;6:688–702. doi: 10.1128/mcb.6.2.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Imai S, et al. Denu JM. Linking chromatin function with metabolic networks: Sir2 family of NAD(+)-dependent deacetylases. Trends Biochem Sci. 2003;28:41–48. doi: 10.1016/s0968-0004(02)00005-1. [DOI] [PubMed] [Google Scholar]
- 22.Denu JM. Linking chromatin function with metabolic networks: Sir2 family of NAD(+)-dependent deacetylases. Trends Biochem Sci. 2003;28:41–48. doi: 10.1016/s0968-0004(02)00005-1. [DOI] [PubMed] [Google Scholar]
- 23.Borra MT, et al. Substrate specificity and kinetic mechanism of the Sir2 family of NAD+-dependent histone/protein deacetylases. Biochemistry. 2004;43:9877–9887. doi: 10.1021/bi049592e. [DOI] [PubMed] [Google Scholar]
- 24.Sauve AA. Sirtuin chemical mechanisms. Biochim Biophys Acta. 2010;1804:1591–1603. doi: 10.1016/j.bbapap.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fulco M, et al. Sir2 regulates skeletal muscle differentiation as a potential sensor of the redox state. Mol Cell. 2003;12:51–62. doi: 10.1016/s1097-2765(03)00226-0. [DOI] [PubMed] [Google Scholar]
- 26.Ying W. NAD+/NADH and NADP+/NADPH in cellular functions and cell death: regulation and biological consequences. Antioxid Redox Signal. 2008;10:179–206. doi: 10.1089/ars.2007.1672. [DOI] [PubMed] [Google Scholar]
- 27.Brownell JE, Allis CD. An activity gel assay detects a single, catalytically active histone acetyltransferase subunit in Tetrahymena macronuclei. Proc Natl Acad Sci U S A. 1995;92:6364–6368. doi: 10.1073/pnas.92.14.6364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kleff S, et al. Identification of a gene encoding a yeast histone H4 acetyltransferase. J Biol Chem. 1995;270:24674–24677. doi: 10.1074/jbc.270.42.24674. [DOI] [PubMed] [Google Scholar]
- 29.Brownell JE, et al. Tetrahymena histone acetyltransferase A: a homolog to yeast Gcn5p linking histone acetylation to gene activation. Cell. 1996;84:843–851. doi: 10.1016/s0092-8674(00)81063-6. [DOI] [PubMed] [Google Scholar]
- 30.Bannister AJ, Kouzarides T. The CBP co-activator is a histone acetyltransferase. Nature. 1996;384:641–643. doi: 10.1038/384641a0. [DOI] [PubMed] [Google Scholar]
- 31.Mizzen CA, et al. The TAF(II)250 subunit of TFIID has histone acetyltransferase activity. Cell. 1996;87:1261–1270. doi: 10.1016/s0092-8674(00)81821-8. [DOI] [PubMed] [Google Scholar]
- 32.Parthun MR, et al. The major cytoplasmic histone acetyltransferase in yeast: links to chromatin replication and histone metabolism. Cell. 1996;87:85–94. doi: 10.1016/s0092-8674(00)81325-2. [DOI] [PubMed] [Google Scholar]
- 33.Spencer TE, et al. Steroid receptor coactivator-1 is a histone acetyltransferase. Nature. 1997;389:194–198. doi: 10.1038/38304. [DOI] [PubMed] [Google Scholar]
- 34.Robert F, et al. Global position and recruitment of HATs and HDACs in the yeast genome. Mol Cell. 2004;16:199–209. doi: 10.1016/j.molcel.2004.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamamoto T, Horikoshi M. Novel substrate specificity of the histone acetyltransferase activity of HIV-1-Tat interactive protein Tip60. J Biol Chem. 1997;272:30595–30598. doi: 10.1074/jbc.272.49.30595. [DOI] [PubMed] [Google Scholar]
- 36.Albaugh BN, et al. KAT(ching) metabolism by the tail: insight into the links between lysine acetyltransferases and metabolism. Chembiochem. 2011;12:290–298. doi: 10.1002/cbic.201000438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Virgilio C, et al. Cloning and disruption of a gene required for growth on acetate but not on ethanol: the acetyl-coenzyme A synthetase gene of Saccharomyces cerevisiae. Yeast. 1992;8:1043–1051. doi: 10.1002/yea.320081207. [DOI] [PubMed] [Google Scholar]
- 38.Van den Berg MA, Steensma HY. ACS2, a Saccharomyces cerevisiae gene encoding acetyl-coenzyme A synthetase, essential for growth on glucose. Eur J Biochem. 1995;231:704–713. doi: 10.1111/j.1432-1033.1995.tb20751.x. [DOI] [PubMed] [Google Scholar]
- 39.Srere PA. The citrate cleavage enzyme. I. Distribution and purification. J Biol Chem. 1959;234:2544–2547. [PubMed] [Google Scholar]
- 40.Srere PA, Lipmann F. An enzymatic reaction between citrate, adenosine triphosphate and coenzyme A1. Journal of the American Chemical Society. 1953;75:4874. [Google Scholar]
- 41.Wellen KE, et al. ATP-citrate lyase links cellular metabolism to histone acetylation. Science. 2009;324:1076–1080. doi: 10.1126/science.1164097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cai L, Tu BP. On Acetyl-CoA as a Gauge of Cellular Metabolic State. Cold Spring Harb Symp Quant Biol. 2011;76:195–202. doi: 10.1101/sqb.2011.76.010769. [DOI] [PubMed] [Google Scholar]
- 43.Wellen KE, Thompson CB. A two-way street: reciprocal regulation of metabolism and signalling. Nat Rev Mol Cell Biol. 2012;13:270–276. doi: 10.1038/nrm3305. [DOI] [PubMed] [Google Scholar]
- 44.Kratzer S, Schuller HJ. Carbon source-dependent regulation of the acetyl-coenzyme A synthetase-encoding gene ACS1 from Saccharomyces cerevisiae. Gene. 1995;161:75–79. doi: 10.1016/0378-1119(95)00289-i. [DOI] [PubMed] [Google Scholar]
- 45.Tu BP, et al. Logic of the yeast metabolic cycle: temporal compartmentalization of cellular processes. Science. 2005;310:1152–1158. doi: 10.1126/science.1120499. [DOI] [PubMed] [Google Scholar]
- 46.Takahashi H, et al. Nucleocytosolic acetyl-coenzyme a synthetase is required for histone acetylation and global transcription. Mol Cell. 2006;23:207–217. doi: 10.1016/j.molcel.2006.05.040. [DOI] [PubMed] [Google Scholar]
- 47.Hallows WC, et al. Sirtuins deacetylate and activate mammalian acetyl-CoA synthetases. Proc Natl Acad Sci U S A. 2006;103:10230–10235. doi: 10.1073/pnas.0604392103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Starai VJ, et al. Sir2-dependent activation of acetyl-CoA synthetase by deacetylation of active lysine. Science. 2002;298:2390–2392. doi: 10.1126/science.1077650. [DOI] [PubMed] [Google Scholar]
- 49.Cai L, et al. Acetyl-CoA induces cell growth and proliferation by promoting the acetylation of histones at growth genes. Mol Cell. 2011;42:426–437. doi: 10.1016/j.molcel.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tu BP, et al. Cyclic changes in metabolic state during the life of a yeast cell. Proc Natl Acad Sci U S A. 2007;104:16886–16891. doi: 10.1073/pnas.0708365104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Polevoda B, Sherman F. The diversity of acetylated proteins. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-5-reviews0006. reviews0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gu W, Roeder RG. Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell. 1997;90:595–606. doi: 10.1016/s0092-8674(00)80521-8. [DOI] [PubMed] [Google Scholar]
- 53.Choudhary C, et al. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science. 2009;325:834–840. doi: 10.1126/science.1175371. [DOI] [PubMed] [Google Scholar]
- 54.Lin YY, et al. Protein acetylation microarray reveals that NuA4 controls key metabolic target regulating gluconeogenesis. Cell. 2009;136:1073–1084. doi: 10.1016/j.cell.2009.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim SC, et al. Substrate and functional diversity of lysine acetylation revealed by a proteomics survey. Mol Cell. 2006;23:607–618. doi: 10.1016/j.molcel.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 56.Megee PC, et al. Histone H4 and the maintenance of genome integrity. Genes Dev. 1995;9:1716–1727. doi: 10.1101/gad.9.14.1716. [DOI] [PubMed] [Google Scholar]
- 57.Liu L, et al. p53 sites acetylated in vitro by PCAF and p300 are acetylated in vivo in response to DNA damage. Mol Cell Biol. 1999;19:1202–1209. doi: 10.1128/mcb.19.2.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marzio G, et al. E2F family members are differentially regulated by reversible acetylation. J Biol Chem. 2000;275:10887–10892. doi: 10.1074/jbc.275.15.10887. [DOI] [PubMed] [Google Scholar]
- 59.Morris L, et al. Regulation of E2F transcription by cyclin E-Cdk2 kinase mediated through p300/CBP co-activators. Nat Cell Biol. 2000;2:232–239. doi: 10.1038/35008660. [DOI] [PubMed] [Google Scholar]
- 60.Zhao S, et al. Regulation of cellular metabolism by protein lysine acetylation. Science. 2010;327:1000–1004. doi: 10.1126/science.1179689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bedford MT, Clarke SG. Protein arginine methylation in mammals: who, what, and why. Mol Cell. 2009;33:1–13. doi: 10.1016/j.molcel.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Finkelstein JD. Methionine metabolism in mammals. J Nutr Biochem. 1990;1:228–237. doi: 10.1016/0955-2863(90)90070-2. [DOI] [PubMed] [Google Scholar]
- 63.Katoh Y, et al. Methionine adenosyltransferase II serves as a transcriptional corepressor of Maf oncoprotein. Mol Cell. 2011;41:554–566. doi: 10.1016/j.molcel.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 64.Yan H, et al. IDH1 and IDH2 mutations in gliomas. The New England journal of medicine. 2009;360:765–773. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chowdhury R, et al. The oncometabolite 2-hydroxyglutarate inhibits histone lysine demethylases. EMBO Rep. 2011;12:463–469. doi: 10.1038/embor.2011.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dang L, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2009;462:739–744. doi: 10.1038/nature08617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lu C, et al. IDH mutation impairs histone demethylation and results in a block to cell differentiation. Nature. 2012;483:474–478. doi: 10.1038/nature10860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Figueroa ME, et al. Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation. Cancer Cell. 2010;18:553–567. doi: 10.1016/j.ccr.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shi Y, et al. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell. 2004;119:941–953. doi: 10.1016/j.cell.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 70.Forneris F, et al. Histone demethylation catalysed by LSD1 is a flavin-dependent oxidative process. FEBS Lett. 2005;579:2203–2207. doi: 10.1016/j.febslet.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 71.Hino S, et al. FAD-dependent lysine-specific demethylase-1 regulates cellular energy expenditure. Nat Commun. 2012;3:758. doi: 10.1038/ncomms1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gomez M, et al. PARP1 Is a TRF2-associated poly(ADP-ribose)polymerase and protects eroded telomeres. Mol Biol Cell. 2006;17:1686–1696. doi: 10.1091/mbc.E05-07-0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hawse WF, Wolberger C. Structure-based mechanism of ADP-ribosylation by sirtuins. J Biol Chem. 2009;284:33654–33661. doi: 10.1074/jbc.M109.024521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schreiber V, et al. Poly(ADP-ribose): novel functions for an old molecule. Nat Rev Mol Cell Biol. 2006;7:517–528. doi: 10.1038/nrm1963. [DOI] [PubMed] [Google Scholar]
- 75.Tanny JC, et al. An enzymatic activity in the yeast Sir2 protein that is essential for gene silencing. Cell. 1999;99:735–745. doi: 10.1016/s0092-8674(00)81671-2. [DOI] [PubMed] [Google Scholar]
- 76.Hanover JA, et al. Bittersweet memories: linking metabolism to epigenetics through O-GlcNAcylation. Nat Rev Mol Cell Biol. 2012;13:312–321. doi: 10.1038/nrm3334. [DOI] [PubMed] [Google Scholar]
- 77.Edwards TM, Myers JP. Environmental exposures and gene regulation in disease etiology. Environ Health Perspect. 2007;115:1264–1270. doi: 10.1289/ehp.9951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dolinoy DC, et al. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc Natl Acad Sci U S A. 2007;104:13056–13061. doi: 10.1073/pnas.0703739104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cropley JE, et al. Germ-line epigenetic modification of the murine A vy allele by nutritional supplementation. Proc Natl Acad Sci U S A. 2006;103:17308–17312. doi: 10.1073/pnas.0607090103. [DOI] [PMC free article] [PubMed] [Google Scholar]