Abstract
A novel approach to porous polymer monoliths hypercrosslinked to obtain large surface areas and modified with zwitterionic functionalities through the attachment of gold nanoparticles in a layered architecture has been developed. The capillary columns were used for the separation of small molecules in hydrophilic interaction liquid chromatography mode. First, a monolith with a very large surface area of 430 m2/g was prepared by hypercrosslinking from a generic poly(4-methylstyrene-co-vinylbenzene chloride-co-divinylbenzene) monolith via a Friedel-Crafts reaction catalyzed with iron chloride. Free radical bromination then provided this hypercrosslinked monolith with 5.7 at% Br that further reacted with cystamine under microwave irradiation, resulting in a product containing 3.8 at% sulfur. Clipping the disulfide bonds with tris(2-carboxylethyl) phosphine liberated the desired thiol groups that bind the first layer of gold nanoparticles. These immobilized nanoparticles were an intermediate ligand enabling the attachment of polyethyleneimine as a spacer followed by immobilization of the second layer of gold nanoparticles which were eventually functionalized with zwitterionic cysteine. This layered architecture, prepared using 10 nm nanoparticles, contains 17.2 wt% Au, more than twice than that found in the first layer alone. Chromatographic performance of these hydrophilic monolithic columns was demonstrated with the separation of mixtures of nucleosides and peptides in HILIC mode. A column efficiency of 51,000 plates/m was achieved for retained analyte cytosine.
Porous polymer monoliths containing large-through pores were introduced 20 years ago.1 The first generation of monoliths exhibited small surface areas of only a few tens of m2/g which made them an ideal stationary phase for the fast separations of large molecules. However, the absence of small pores represented a challenge in achieving efficient separations of small molecules. Several studies have been published describing attempts to circumvent this problem, including (i) optimization the polymerization conditions, (ii) polymerizations of a single cross-linker, (iii) use of high reaction temperature, (iv) termination of polymerization before completion, and to prepare in a single step polymer-based monolithic columns possessing both large through pores and a multiplicity of small pores.2
We recently demonstrated second generation monoliths exhibiting surface areas as large as several hundred m2/g that are prepared using a two-step approach that included (i) preparation of a poly(styrene-co-vinylbenzene chloride-co-divinylbenzene) monolith and (ii) its in-situ hypercrosslinking via a Friedel-Crafts alkylation.3 These monoliths enabled excellent separations of small molecules but only in the reversed phase mode as dictated by their hydrophobic chemistry. Use of hypercrosslinked monoliths in other chromatographic modes has yet to be demonstrated.
In an unrelated study,4 we discovered that gold nanoparticles (GNP) attached to the pore surface are excellent intermediate ligands since they can be readily modified using the advantage of the high affinity between gold and thiols.5 This approach led to the application of monoliths in a variety of modes including reversed phase, ion exchange, and affinity chromatography.4,6 All these reports described columns prepared by a simple attachment of a monolayer of GNP to the pore surface of the first generation of monoliths. However, these monoliths are not suitable for the separation of small molecules and the conjugates were mostly used for the separation of biological molecules.
In this communication, for the first time, we describe the combination of hypercrosslinked large surface area polymer-based monoliths with gold nanoparticles attached through layered architecture and functionalized with hydrophilic functionalities. The effectiveness of these novel capillary columns is demonstrated with their application in hydrophilic interaction chromatography (HILIC) of small polar analytes including nucleosides and peptides.
EXPERIMENTAL SECTION
Complete description of chemicals, materials, instrumentation, and preparation of thiol-containing hypercrosslinked monolithic capillary columns is available in the Supporting Information.
RESULTS AND DISCUSSION
Preparation of thiol groups containing monolith
The generic poly(4-methylstyrene-co-vinylbenzene chloride-co-divinylbenzene) monolith was prepared in a capillary and hypercrosslinked via a Friedel-Crafts alkylation (Figure S-1 in the Supporting Information). This reaction formed a plethora of mesopores within the monolith. This was confirmed by a dramatic change in the specific surface area calculated from nitrogen adsorption/desorption in the dry state that increased from only 32 m2/g typical of the generic monolith to 430 m2/g found for its hypercrosslinked counterpart. Figure S-2 illustrates the effect of hypercrosslinking on the reversed phase separation of alkylbenzenes. While the separation using a generic monolith is poor, a significant improvement in both retention and efficiency is observed after the hypercrosslinking. Using the mobile phase comprised of acetonitrile, tetrahydrofuran, and water, increased the column efficiency for retained analyte toluene from 5,000 to 60,000 plates/m.
Although an enhancement in separation ability of the hypercrosslinked column was achieved, it did not go beyond the reversed phase separation mode. Therefore, additional functionalization of the pore surface using free radical bromination was needed to enable their modification, thus manipulating the surface chemistry. While energy dispersive X-ray spectroscopy (EDS) revealed no bromine signal in both generic and hypercrosslinked monoliths, the presence of 5.7 at% bromine in the monolith was detected after the bromination reaction. (Figure S-3).
The next step then included reaction of brominated monolith with cystamine that was carried out at a temperature of 80°C for 16 h. However, only 1.2 at% sulfur was detected in the cystamine modified monolith. An increase to 2.5 at% was observed when the reaction ran at a temperature of 120°C. Repeating this reaction with fresh cystamine solution for several times led to a further increase in the yield. As illustrated in Table S1, 3.9 at% sulfur was detected in monolith MIII after repeating the modification process 4 times. Further cycles did not lead to a further increase in the content of sulfur. Although a monolith with a high content of sulfur can be obtained using this approach, it requires 64 h to reach the maximum conversion using heating to 120°C. Clearly, this process is time-consuming and less convenient. Ju et al. reported that microwaves facilitate the reaction between aromatic bromine derivatives and amines.7 Inspired by this work, we also used the simpler, more efficient, and straightforward approach to functionalize the brominated monolith with cystamine that included reaction in a microwave oven. EDS revealed up to 3.8 at% sulfur in monolith MIV modified with cystamine using irradiation with microwaves for only 30 min (Table S-1), which is comparable with monolith MIII that required reaction for 64 h. Repeating the reaction upon further microwave irradiation did not afford any increase in sulfur content. This confirms that the reaction with cystamine reached its maximum. Finally, snipping the disulfide bond using TCEP liberated the desired thiol groups.
Functionalization with gold nanoparticles
The thiol-containing monolith is an ideal support for immobilization of gold nanoparticles. We pumped GNP though this monolith until the saturation of the pore surface with gold was achieved. The gold content of GNP-functionalized monoliths was determined by EDS. Consistent with our previous study, gold content increased with the increase in GNP size.4c As shown in Table S-2 in the Supporting Information, use of the smallest 5 nm particles resulted in monolith M5 that contained only 1.2 wt% gold while monolith M15 with immobilized 15 nm particles produced 28.1 wt%, the highest quantity of gold obtained. It is likely that due to the large curvature of smaller GNP, a multipoint attachment to a multitude of thiol groups located at the uneven surface is only possible on certain surface locations possessing the desired geometrical shapes. As the size of the gold particles increases, it is easier for them to find larger arrays of thiol functionalities with which they can interact.4c The cross-sectional segments of GNP-modified monoliths were imaged using scanning electron microscopy (SEM). The micrographs shown in Figure 1 confirm the sparser surface coverage with both 5 and 10 nm gold nanoparticle monolayers in the monoliths, which is consistent with the EDS result. In contrast, 15 nm GNP forms a denser, almost continuous layer on the pore surface.
Figure 1.
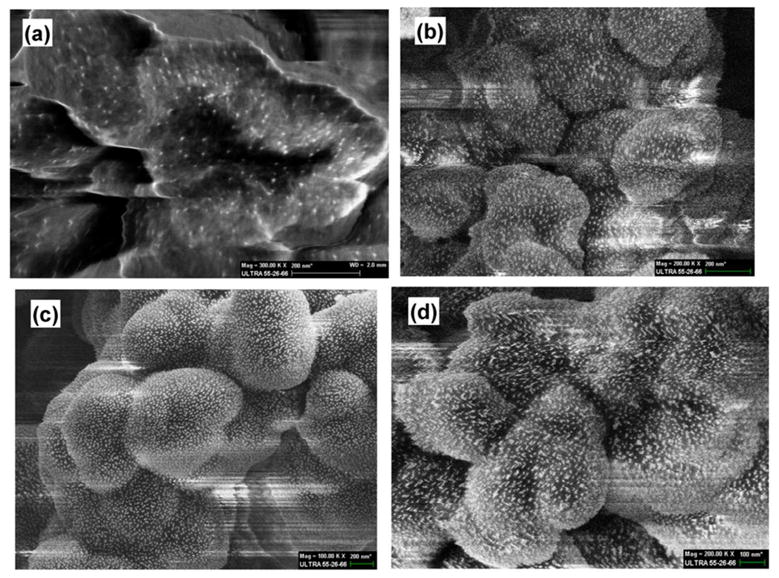
Scanning electron micrographs of the internal structures of the hypercrosslinked poly(4-methylstyrene-co-vinylbenzene chloride-co-divinylbenzene) monoliths attached with 5 (a), 10 (b), 15 (c) nm gold nanoparticles monolayer, and 10 nm gold nanoparticle dual-layer (d).
Preparation of monolith for HILIC
Alpert 8 introduced the term hydrophilic interaction chromatography for a chromatographic method, which involves use of polar, typically highly hydrophilic stationary phases and a mobile phase consisting of high percentage of organic solvent. HILIC, which has experienced an enormous growth during the last decade, has proven to be a powerful separation technique, and a solid alternative for reversed phase chromatography. It enables separation for polar compounds, such as carbohydrates, peptides, proteins, natural products, and polar pharmaceuticals.9
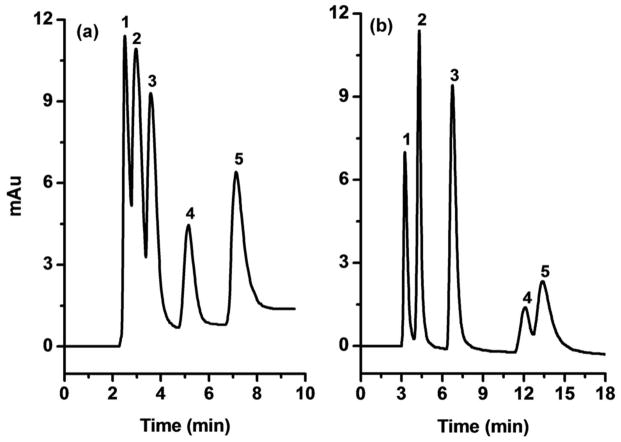
Cysteine is a naturally zwitterionic compound that we utilized for the preparation of monoliths suitable for the HILIC separations. This amino acid was attached through its thiol groups to the gold nanoparticles (Figure S-4). The monolithic column was used for the isocratic separation of nucleosides in HILIC mode. It is worth noting that none of the monoliths that did not contain cysteine enabled good separation for nucleosides, due to the lack of the desired hydrophilicity. Almost all the analytes were eluted in a single peak at the column dead volume. This situation changed dramatically after the functionalization of GNP with cysteine. Figure 2 shows that all five nucleosides were retained and separated in monolith M15 functionalized with cysteine. Figure 2 also shows that due to the sparse surface coverage of monolith M10 with 10 nm GNP, the amount of cysteine functionalities is too low to achieve efficient HILIC separation. The HILIC separation performance was even worse for monolith M5 with 5 nm GNP for the same reason. Although selectivity of cysteine functionalized monolith M15 was good, the column efficiency for nucleosides is only about 30,000 plates/m. This is likely due to the competing hydrophobicity of the uncovered hypercrosslinked polystyrene surface within the monolithic column still present even after functionalization with cysteine.
Figure 2.
HILIC isocratic separation of nucleosides using cysteine functionalized monoliths with a monolayer of 10 (a), and 15 nm gold nanoparticles (b). Conditions: Columns: 159 mm × 100 μm i.d. (a), 168 mm × 100 μm i.d. (b). mobile phase, 25 mmol/L ammonium formate (pH 3.2) in 90:10 vol% acetonitrile-water; flow rate, 0.5 μL/min; UV detection 254 nm; temperature 25°C. Peaks: thymine (1), adenosine (2), cytidine (3), cytosine (4), guanosine (5).
Effect of polyethyleneimine
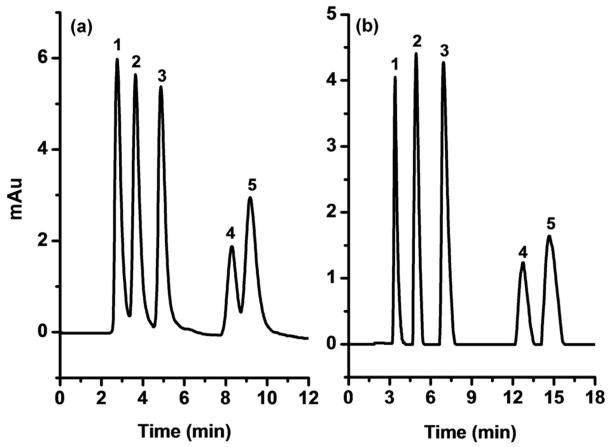
Polyethyleneimine is known to be an excellent anion-exchange ligand, which also exhibits good hydrophilicity.10 To further improve the column efficiency, we attached PEI 70,000 onto the monolith modified with bare 10 nm GNP. A comparison of Figures 2a and 3a demonstrates the favorable effect of PEI on the separation of nucleosides. The attachment of PEI produces columns exhibiting better performance than those modified with cysteine alone mainly because of the formation of a dense hydrophilic polymer layer on the surface of the gold nanoparticles. Positive effect of a solvated, gel-like layer covering the pore surface within a monolith on the chromatographic performance in the reversed phase separations of small molecules was observed previously.11 This explanation is also supported by an experiment in which we used a low molecular weight polyethyleneimine (MW 2,000), which afforded columns with a separation performance inferior to that obtained with its high molecular weight counterpart because it cannot form the desired solvated layer. Another support for this speculation is inferior performance of the column after functionalization of the monolayer GNP monolith with compounds bearing single functionality, cysteamine and 3-mercaptopropionic acid.
Figure 3.
HILIC isocratic separation of nucleosides using PEI functionalized 10 nm GNP monolayer monolith (a), and PEI-cysteine functionalized 10 nm GNP dual-layer monolith (b). Columns: 100 μm i.d. × 171 (a) and 165 mm (b). For other conditions see Fig. 2.
Coverage with gold nanoparticles in layered architecture
Finally, we prepared monolithic columns with a layered architecture using procedure shown in Figure S-4 in the Supporting Information. We embedded the second layer of gold nanoparticles after modification with PEI which served as a spacer enabling construction of a dual-layer of gold nanoparticles that were then functionalized with cysteine.
This approach formed a hydrophilic layer consisting of both PEI and cysteine functionalities held together via interactions with GNP. EDS analysis indicated the presence of 17.2 wt% Au in dual-layer monolith M10 containing 10 nm GNP, which was more than twice of that found for GNP containing the single layer (Table S-2). The SEM image in Figure 2d also shows the much denser coverage on the pore surface with GNP, which is consistent with the EDS result. Interestingly, coverage of the pore surface within monolith M5 with 5 nm GNP remained poor even after construction of the second GNP layer, and it contains only 2.7 wt% Au. This monolithic column was not suitable for the separation of nucleosides (Figure S-5). Unfortunately, the dual layer approach could not be used with 15 nm GNP since the application of the second layer led to a significant increase in the column back pressure that exceeded the maximum pressure limit of the pump.
The monolithic column prepared using the dual-layer approach with 10 nm GNP that included PEI and cysteine functionalities was again tested in isocratic HILIC for separation of nucleosides. The chromatogram in Figure 3b confirms that this modification further increased retention of the analytes manifested by longer retention times. The column efficiency calculated for cytosine was 51,000 plates/m at a flow velocity of 0.3 μL/min corresponding to the minimum at the vanDeemter plot.
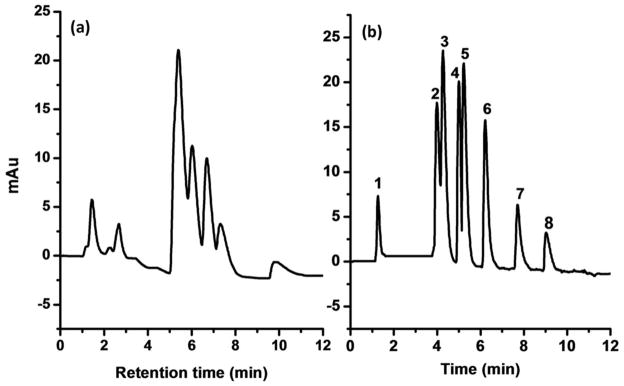
The positive effect of the dual layer architecture was also demonstrated with the HILIC separation of peptides using a gradient elution with a decreasing percentage of acetonitrile. A comparison of chromatograms in Figure 4 confirms a significant increase in retention and better separation of peptides using the dual layer stationary phase compared to the column featuring only a single layer chemistry.
Figure 4.
HILIC separation of peptides using cysteine functionalized 10 nm GNP monolayer monolith (a), and PEI-cysteine functionalized 10 nm GNP dual-layer monolith (b). Conditions: Columns: 159 mm 100 μm i.d. (a), 165 mm × 100 μm i.d. (b). mobile phase: A - 10 mmol/L triethylammonium phosphate buffer (pH 2.8), B - 5 vol.% of 10 mmol/L triethylammonium phosphate buffer (pH 2.8) in acetonitrile, gradient from 100 to 60% B in A in 10 min, flow rate 1.0 μL/min, UV detection 210 nm, temperature 25°C. Peaks: Impurity (1), Phe-Gly-Phe-Gly (2), Val-Try-Val (3), Gly-Phe (4), Gly-Leu (5), Gly-Try (6), Lys-Val (7), Gly-Gly-Gly (8).
In a control experiment, we subjected generic poly(4-methylstyrene-co-vinylbenzene chloride-co-divinylbenzene) monoliths without hypercrosslinking to the same modification steps, which first formed the single layer. This functionalization process afforded a monolith that contained only 4.1 wt% Au, which was much less than 7.3% that was obtained with the monolith that was first hypercrosslinked. The small surface area typical of the generic monolith cannot accommodate any larger quantities of GNP. Formation of the second 10 nm GNP layer on this monolith afforded 9.5 wt% Au, an amount which was again inferior to that found previously. Application of these monolithic columns for isocratic HILIC separation of nucleosides is shown in Figure S-6 in the Supporting Information. Although a certain separation can be achieved that is again better for the column featuring the dual layer architecture, the best efficiency achieved for cytosine at the minimum of the van Deemter curve is only 7,000 plates/m. These control experiments clearly demonstrate the need for hypercrosslinking of the monolith prior to its functionalization.
CONCLUSIONS
Combination of hypercrosslinking of hydrophobic poly(4-methylstyrene-co-vinylbenzene chloride-co-divinylbenzene) monoliths with a formation of hydrophilic layered structure including gold nanoparticles embedded in a polyethyleneimine layer, and functionalized with cysteine, enabled the preparation of very efficient monolithic stationary phase for the separation of small molecules in HILIC mode. Indeed, the technique presented here is more complex than single step reactions. However, our results further demonstrate the versatility of functionalization approaches for monoliths relying on gold nanoparticles, which serve as a “universal” intermediate ligand that several research groups are currently developing.
Supplementary Material
Acknowledgments
All experimental and characterization work was performed at the Molecular Foundry, Lawrence Berkeley National Laboratory. This work as well as Z.L. and F.S. were supported by the Office of Science, Office of Basic Energy Sciences, Scientific User Facilities Division of the U.S. Department of Energy, under Contract No. DE-AC02-05CH11231. The financial support of Y.L. by a grant from NIH (GM48364) is gratefully acknowledged.
Footnotes
The authors declare no competing financial interest.
References
- 1.Hjertén S, Liao JL, Zhang R. J Chromatogr A. 1989;473:273–275. [Google Scholar]; Tennikova TB, Svec F, Belenkii BG. J Liquid Chromatogr. 1990;13:63–70. [Google Scholar]; Svec F, Fréchet JMJ. Anal Chem. 1992;54:820–822. [Google Scholar]
- 2.Svec F. J Chromatogr A. 2012;1228:250–262. doi: 10.1016/j.chroma.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Urban J, Svec F, Fréchet JMJ. J Chromatogr A. 2010;1217:8212–8221. doi: 10.1016/j.chroma.2010.10.100. [DOI] [PMC free article] [PubMed] [Google Scholar]; Urban J, Svec F, Fréchet JMJ. Anal Chem. 2010;82:1621–1623. doi: 10.1021/ac100008n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.(a) Xu Y, Cao Q, Svec F, Fréchet JMJ. Anal Chem. 2010;82:3352–3358. doi: 10.1021/ac1002646. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Cao Q, Xu Y, Liu F, Svec F, Fréchet JMJ. Anal Chem. 2010;82:7416–7421. doi: 10.1021/ac1015613. [DOI] [PubMed] [Google Scholar]; (c) Lv YQ, Alejandro Maya F, Fréchet JMJ, Svec F. J Chromatogr A. 2012 doi: 10.1016/j.chroma.2012.04.007. [DOI] [Google Scholar]
- 5.Guihen E, Glennon JD. Anal Lett. 2003;36:3309–3336. [Google Scholar]; Zhong ZY, Male KB, Luong JHT. Anal Lett. 2003;36:3097–3118. [Google Scholar]
- 6.Connolly D, Twamley B, Paull B. Chem Commun. 2010;46:2109–2111. doi: 10.1039/b924152c. [DOI] [PubMed] [Google Scholar]; Alwael H, Connolly D, Clarke P, Thompson R, Twamley B, O’Connor B, Paull B. Analyst. 2011;136:2619–2628. doi: 10.1039/c1an15137a. [DOI] [PubMed] [Google Scholar]
- 7.Ju YH, Varma RS. J Org Chem. 2006;71:135–141. doi: 10.1021/jo051878h. [DOI] [PubMed] [Google Scholar]
- 8.Alpert AJ. J Chromatogr A. 1990;499:177–196. doi: 10.1016/s0021-9673(00)96972-3. [DOI] [PubMed] [Google Scholar]
- 9.Hemstrom P, Irgum K. J Sep Sci. 2006;29:1784–1821. doi: 10.1002/jssc.200600199. [DOI] [PubMed] [Google Scholar]; Jiang Z, Smith NW, Liu Z. J Chromatogr A. 2011;1218:2350–2361. doi: 10.1016/j.chroma.2011.02.024. [DOI] [PubMed] [Google Scholar]; Ngoc PD, Jonsson T, Irgum K. J Chromatogr A. 2011;1218:5880–5891. doi: 10.1016/j.chroma.2011.06.037. [DOI] [PubMed] [Google Scholar]
- 10.Kitagawa N. J Chromatogr A. 1988;443:133–141. doi: 10.1016/s0021-9673(00)94788-5. [DOI] [PubMed] [Google Scholar]
- 11.Nischang I, Teasdale I, Bruggemann O. J Chromatogr A. 2010;1217:7514–7522. doi: 10.1016/j.chroma.2010.09.077. [DOI] [PubMed] [Google Scholar]; Lv YQ, Lin ZX, Svec F. Analyst. 2012;137:4114–4118. doi: 10.1039/c2an35706b. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.